Abstract
A novel intravenous liposomal formulation of all-transretinoic acid (ATRA) was evaluated in 69 patients with acute promyelocytic leukemia (APL): 32 new diagnoses, 35 relapses, and 2 oral ATRA failures. Liposomal ATRA (90 mg/m2) was administered every other day until complete remission (CR) or a maximum of 56 days. Treatment following CR was liposomal ATRA with or without chemotherapy. In an intent-to-treat (ITT) analysis of all patients, CR rates were 62%, 70%, and 20% in newly diagnosed, group 1 first relapses (ATRA naive or off oral ATRA more than or equal to 1 year), or group 2 relapses (second or subsequent relapse or first relapses off oral ATRA less than 1 year), respectively. In 56 evaluable patients (receiving 4 or more doses), CR rates for the same groups were 87% (20 of 23), 78% (14 of 18), and 23% (3 of 13). Remission failure in newly diagnosed patients was not from resistant disease. Several patients in CR became polymerase chain reaction (PCR) negative for promyelocytic leukemia/retinoic acid receptor-alpha (PML/RARα) after liposomal ATRA alone. Toxicity was generally mild, most commonly headaches (67.5%). Eighteen patients (26%) had ATRA syndrome develop during induction. One-year survival of ITT patients was 62%, 56%, and 20% for newly diagnosed, group 1, and group 2, respectively. The medium duration of CR has not yet been reached and was 18 and 5.5 months in the same groups. These results demonstrate that liposomal ATRA is effective in inducing CR in newly diagnosed or group 1 APL patients. It provides a reliable dosage of ATRA for patients with APL unable to swallow or absorb medications and can induce molecular remissions without chemotherapy.
Introduction
The standard induction for patients with newly diagnosed acute promyelocytic leukemia (APL) is currently oral all-trans retinoic acid (ATRA) either alone or in combination with chemotherapy.1-5 ATRA alone can induce a hematologic complete remission (CR) in nearly all patients with APL.1-9 Failures are due primarily to disease-related bleeding rather than primary drug resistance.2,10,11Furthermore, patients with newly diagnosed APL treated with ATRA plus chemotherapy achieve higher disease-free and overall survival rates than patients treated with chemotherapy alone.1-4 In patients with relapsed APL, ATRA can induce a second remission, which is more likely to occur in ATRA-naive patients than in those previously treated with the drug.9,12-14 ATRA induces differentiation of APL cells into mature granulocytes that subsequently undergo apoptosis with induction of CR.15 The effect of ATRA is most likely mediated through the APL-specific promyelocytic leukemia/retinoic acid receptor-alpha (PML/RARα) fusion protein, resulting from the translocation between chromosomes 17 and 15.15 16
ATRA is currently available for clinical use only in an oral form. However, its therapeutic blood concentration drops with continuous administration (usually within 1 to 6 weeks) to levels that are inadequate to support differentiation of APL cells.17,18Recently, a liposomal formulation of ATRA (ATRAGEN, Aronex Pharmaceuticals, Inc, The Woodlands, TX) for intravenous (IV) administration was developed, in part, to overcome the reduction in drug levels that occurs with oral administration. Lipophilic agents such as ATRA are incompatible for IV administration but can be given by this route when encapsulated with liposomes.19 The liposomal delivery system potentially alters the tissue distribution and pharmacologic profile of ATRA.20,21 When given intravenously, it also bypasses the initial hepatic clearance associated with repeated oral ATRA administration.22Compared with standard dosages of oral ATRA, liposomal ATRA yields higher peak blood concentrations that are not reduced after periods of administration lasting 15 days or longer20,23 (oral communication, Wall, Douer, August 1998). In vitro, liposomal ATRA and nonliposomal ATRA were equally effective in inducing differentiation of leukemia cells from patients with APL.19
Oral ATRA treatment may also be problematic in patients with APL who cannot swallow or absorb capsules, patients with a nasogastric tube, or small children. Oral ATRA is a soft-gelatin capsule containing the drug in a mixture of glycerin and soybean oil. Removing the oil-based capsule content and mixing it with a fluid for administration, in these situations, is very difficult and may result in a suboptimal drug dosage. Intravenous ATRA would provide a more reliable and easy-to-administer alternative.
In a phase I dose-escalation study, the maximum-tolerated dose (MTD) of liposomal ATRA was 140 mg/m2.20 At this dosage, the liposomal formulation was well tolerated, with a safety profile similar to that of oral ATRA. The dose of liposomal ATRA selected for phase II APL trials was 90 mg/m2 (the dosage level at which the first APL patients achieved CR), with an option to escalate to 110 mg/m2 in the setting of inadequate clinical response.
In a previous study of newly diagnosed APL, performed in a single institution, liposomal ATRA was shown to have activity in inducing and maintaining CRs without chemotherapy.24 The current report summarizes the results of several prospective clinical trials using IV liposomal ATRA for patients with newly diagnosed/previously untreated, or relapsed APL.
Patients and methods
Patients
Patients with APL aged 2 years or older were eligible. Study entry requirements included bilirubin less than or equal to 2 mg/dL, SGOT/SGPT less than or equal to 3 times the upper normal range, and creatinine less than or equal to 2 mg/dL. Pregnant or lactating women and HIV-positive patients were ineligible. A total of 69 patients with APL were enrolled in 5 multi-institutional, nonrandomized, open-label studies (Table 1). Newly diagnosed, previously untreated patients (except hydroxyurea for leukocytosis) were enrolled on 2 phase II studies (003 and 005). Results of study 005 were recently reported.24 Relapsed patients were enrolled on a phase II study (002). We also included, in the current analysis, relapsed patients from the phase I study (001), who had received an equal dose of liposomal ATRA that was used later in the phase II studies. Another trial, compassionate plea study (004), was opened for newly diagnosed and relapsed patients or patients who had failed oral ATRA and were switched to the liposomal form. Approval was obtained from the local institutional review boards for these studies. Informed consent was provided according to the Declaration of Helsinki.
Clinical features of intent-to-treat patients
| Characteristic . | Study number* . | Total . | ||||
|---|---|---|---|---|---|---|
| (001) . | (002) . | (003) . | (005) . | (004) . | ||
| No. of patients | 3 | 28 | 13 | 15 | 10 | 69 |
| Newly diagnosed | 0 | 0 | 13 | 15 | 4 | 32 |
| Prior treatment | 3 | 28 | 0 | 0 | 6† | 37 |
| Enrollment (mo/y) | 3/93-10/95 | 5/95-5/99 | 8/97-5/99 | 8/97-5/99 | 8/97-5/99 | |
| Age (y) | ||||||
| Median | 24 | 44 | 34 | 54 | 28 | 44 |
| Range | 24-48 | 19-70 | 5-82 | 10-77 | 11-64 | 5-82 |
| Males (no.) | 1 | 18 | 6 | 10 | 5 | 40 (58%) |
| Race (no.) | ||||||
| White | 1 | 13 | 5 | 11 | 3 | 33 (48%) |
| Latino | 2 | 10 | 3 | 4 | 6 | 25 (36%) |
| Black | 0 | 3 | 0 | 0 | 0 | 3 (4%) |
| Asian | 0 | 0 | 0 | 0 | 0 | 0 (0%) |
| Other | 0 | 2 | 5‡ | 0 | 1 | 8 (12%) |
| WBC (cells/μL)1-153 | ||||||
| Median | 1.1 | 2.2 | 2.0 | 5.1 | 15.1 | 2.5 |
| Range | 0.8-1.2 | 0.6-16.4 | 0.5-73.3 | 0.4-42 | 0.9-77 | 0.4-77 |
| > 10 000 (no. pts.) | 0 | 2 | 2 | 7 | 6 | 17 |
| Characteristic . | Study number* . | Total . | ||||
|---|---|---|---|---|---|---|
| (001) . | (002) . | (003) . | (005) . | (004) . | ||
| No. of patients | 3 | 28 | 13 | 15 | 10 | 69 |
| Newly diagnosed | 0 | 0 | 13 | 15 | 4 | 32 |
| Prior treatment | 3 | 28 | 0 | 0 | 6† | 37 |
| Enrollment (mo/y) | 3/93-10/95 | 5/95-5/99 | 8/97-5/99 | 8/97-5/99 | 8/97-5/99 | |
| Age (y) | ||||||
| Median | 24 | 44 | 34 | 54 | 28 | 44 |
| Range | 24-48 | 19-70 | 5-82 | 10-77 | 11-64 | 5-82 |
| Males (no.) | 1 | 18 | 6 | 10 | 5 | 40 (58%) |
| Race (no.) | ||||||
| White | 1 | 13 | 5 | 11 | 3 | 33 (48%) |
| Latino | 2 | 10 | 3 | 4 | 6 | 25 (36%) |
| Black | 0 | 3 | 0 | 0 | 0 | 3 (4%) |
| Asian | 0 | 0 | 0 | 0 | 0 | 0 (0%) |
| Other | 0 | 2 | 5‡ | 0 | 1 | 8 (12%) |
| WBC (cells/μL)1-153 | ||||||
| Median | 1.1 | 2.2 | 2.0 | 5.1 | 15.1 | 2.5 |
| Range | 0.8-1.2 | 0.6-16.4 | 0.5-73.3 | 0.4-42 | 0.9-77 | 0.4-77 |
| > 10 000 (no. pts.) | 0 | 2 | 2 | 7 | 6 | 17 |
WBC = white blood cell count; pts = patients.
Phase I (001): relapse; Phase II: 002 (relapse); 003 and 005 (previously untreated, newly diagnosed); 004 (compassionate plea).
Two of these patients failed oral all-trans retinoic acid (ATRA) and, were switched to the liposomal form.
Includes one patient with ethnicity unknown.
WBC at presentation before any treatment and before hydroxyurea or leukaphersis.
All relapsed patients were divided into 2 groups. Group 1 consisted of patients who might still be sensitive to ATRA, including first relapses who had received oral ATRA 1 year or more previously or who had never received ATRA. Group 2 included patients in second or subsequent relapses or first relapses who had received oral ATRA within 1 year; these patient are usually resistant to oral ATRA.10
Induction treatment
In all 5 studies, liposomal ATRA (90 mg/m2) was administered IV over 30 minutes every other day until a CR was achieved or for a maximum of 56 days.20 The dosage could be increased to 110 mg/m2 if no signs of clinical improvement were seen after 5 doses as evidenced by hemorrhage and/or coagulopathy and without extramedullary toxicities grade greater than 2 or if no clinical response was seen after 14 doses. If any extramedullary toxicity greater than or equal to grade 3 developed (with the exception of toxicity related to infection), liposomal ATRA was withheld until the toxicity(ies) improved to less than or equal to grade 1; then administration of drug was resumed at 75% of the original dosage. No chemotherapy was given during induction therapy.
Remission treatment
Patients who achieved CR received consolidation treatment. Newly diagnosed patients on study 003 and relapses in group 1 were treated over a total period of 6 months with chemotherapy alternating monthly with liposomal ATRA. The chemotherapy regimens differed between institutions and liposomal ATRA was administered 3 times per week at the final induction dose. This was followed by a 12-month course of liposomal ATRA monotherapy maintenance as in the consolidation cycles. Relapsed group 2 patients in CR received only liposomal ATRA 3 times per week for 6 months. In study 005, newly diagnosed patients who remained polymerase chain reaction (PCR) negative for PML/RARα received liposomal ATRA alone without chemotherapy for 9 months. Chemotherapy was added if the PCR assay results converted to positive.24
Liposomal ATRA
The drug is a sterile, lyophilized powder in a 100-cc vial, reconstituted with 0.9% sodium chloride to form a liposomal suspension with a final ATRA concentration of 2 mg/mL.
Pathology
Patients were permitted to enter the trials based on a morphologic definition of APL according to the FAB M3 or M3 variant morphologic criteria.24 A central slide review was conducted by a single hematopathologist (J.M.B.) for most patients, with the exception of those on study 005. The reviewer's determination of the diagnosis, CR, and relapse was in agreement with the institutional conclusion in all 54 patients reviewed. In 45 of the 69 patients, the diagnosis was confirmed by the t(15;17) translocation, in 42 patients by PCR positive for PML/RARα transcripts, and in 61 patients by either t(15;17) or positive PCR. In 7 patients, these tests were not performed, and the diagnosis was established by typical morphology of the leukemic cells. One oral ATRA failure patient had M3-like morphology with normal chromosomes and negative PCR (details described below).
PCR method
Response criteria
Complete response required no clinical and laboratory evidence of disseminated intravascular coagulation (DIC), morphologically normal bone marrow (ie, less than 5% blasts and less than 8% promyelocytes, without abnormal promyelocytes), platelet count more than 100 000 cells/μL, and neutrophil count more than 1000 cells/μL. All 69 enrolled patients who received at least 1 dose of liposomal ATRA were included in an ITT analysis. Administration of liposomal ATRA was stopped in 13 patients during the first week of treatment, after 3 doses or less, because of death or irreversible organ damage, usually from brain or lung hemorrhage. A separate analysis for response was performed for the 56 remaining patients who received 4 or more doses of liposomal ATRA (evaluable group).
Toxicity criteria
Adverse effects were graded by the NCI common toxicity criteria. ATRA syndrome was determined by respiratory distress, pleural effusion, fever, and interstitial pulmonary infiltrates unrelated to infection or hemorrhage. Patients could continue liposomal ATRA with dexamethasone 10 mg every 12 hours for 3 or more days and then tapered. Alternatively, ATRA was held, dexamethasone was initiated, and ATRA reinstated at the same dosage or a 25% dosage reduction if ATRA syndrome decreased to grade 2 or less. The drug was discontinued if ATRA syndrome manifestations persisted or worsened after the initiation of steroids.
Statistical methods
Survival was calculated from the first day on study drug to time of death. Duration of remission was calculated from day of CR to relapse. Time to achieve CR was calculated from the first day on study drug to day of CR determination. Survival, duration of remission, and time to achieve CR were estimated by Kaplan-Meier curves.
Prognostic factors for induction of CR were investigated with linear regression analysis. The Cox proportional hazard regression analysis was used to determine prognostic factors for survival and duration of remission.28 Univariate analysis of linear regression (for CR) or Cox proportional hazard regression models (for survival and duration of remission) were used for each individual prognostic variable. All factors found to be marginally significant (P < .25) were included in the multivariate logistic model (for CR) and Cox regression models (for the duration of remission and survival). In the multivariate model, a stepwise approach was used; the factors with the greatest nonsignificant P-value were removed at each step, and the significance level wasP < .05. All statistics were set at 2-tailedP-values.
Results
Patient features
Sixty-nine patients were enrolled in all the studies. The median age was 44 years (range, 5 to 82). The median white blood cell (WBC) count at presentation, before treatment and before hydroxyurea or leukaphersis, was 2500 cells/μL (range, 400 to 77 000); 17 patients presented with a WBC count greater than 10 000 cells/μL. Patient features by individual study are shown in Table 1.
Remission induction in newly diagnosed patients
The overall CR rate for the intent-to-treat group of all 32 newly diagnosed patients was 62% (95% CI: 44%, 79%) (Table2). No patient had resistant disease; all 12 patients who did not achieve a CR failed because of early death. Nine patients died after receiving 3 or less doses from the following causes: brain hemorrhage occurring before or within the first 6 days after starting liposomal ATRA (5 patients), lung hemorrhage on days 2 and 3 of treatment (2 patients), DIC and massive pulmonary embolism that had occurred 6 days earlier on the second day of treatment (one patient), and sepsis on day 3 of treatment (one patient). Three additional patients died after receiving liposomal ATRA for 13 to 16 days: one patient died on day 26 (from a pulmonary hemorrhage that occurred on day 2) after 7 doses of liposomal ATRA; a 78-year-old patient died of DIC and multiple organ failure after 7 doses of liposomal ATRA; and one patient died of ATRA syndrome on day 19. In 6 patients, the dosage of liposomal ATRA was modified during induction; 5 of 6 entered CR. In 2 patients, the dosage was increased to 110 mg/m2 after dose 5, and in 4 patients the dosage was reduced by 25% because of adverse effects. The 2 patients who were not tested for cytogenetics or PCR did not enter CR.
Response to induction therapy
| . | Newly diagnosed . | . | Relapses* . | Oral ATRA Failure† . | Total . | |
|---|---|---|---|---|---|---|
| Group 1 . | Group 2 . | |||||
| Intent-to-treat patients | ||||||
| n = 32 | n = 35 | n = 20 | n = 15 | n = 2 | n = 69 | |
| No. remission (%) | 20 (62%) | 17 (49%) | 14 (70%) | 3 (20%) | 1 (50%) | 38 (57%) |
| Early death | 12 | 8 | 2 | 6 | 0 | 20 |
| Resistant | 0 | 10 | 4 | 6 | 1 | 11 |
| Evaluable patients | ||||||
| n = 23 | n = 31 | n = 18 | n = 13 | n = 2 | n = 56 | |
| No. remission (%) | 20 (87%) | 17 (55%) | 14 (71%) | 3 (23%) | 1 (50%) | 38 (63%) |
| Early death | 3 | 6 | 2 | 4 | 0 | 9 |
| Resistant | 0 | 8 | 2 | 6 | 1 | 9 |
| . | Newly diagnosed . | . | Relapses* . | Oral ATRA Failure† . | Total . | |
|---|---|---|---|---|---|---|
| Group 1 . | Group 2 . | |||||
| Intent-to-treat patients | ||||||
| n = 32 | n = 35 | n = 20 | n = 15 | n = 2 | n = 69 | |
| No. remission (%) | 20 (62%) | 17 (49%) | 14 (70%) | 3 (20%) | 1 (50%) | 38 (57%) |
| Early death | 12 | 8 | 2 | 6 | 0 | 20 |
| Resistant | 0 | 10 | 4 | 6 | 1 | 11 |
| Evaluable patients | ||||||
| n = 23 | n = 31 | n = 18 | n = 13 | n = 2 | n = 56 | |
| No. remission (%) | 20 (87%) | 17 (55%) | 14 (71%) | 3 (23%) | 1 (50%) | 38 (63%) |
| Early death | 3 | 6 | 2 | 4 | 0 | 9 |
| Resistant | 0 | 8 | 2 | 6 | 1 | 9 |
Group 1: includes first-relapse patients who had not received oral all-trans retinoic acid (ATRA) for at least 1 year before relapse and 4 ATRA naive relapses. All ATRA naive patients achieved CR. Group 2: second or subsequent remission or in first relapse but received ATRA within 1 year before enrollment.
These 2 patients were newly diagnosed who had failed oral ATRA and were switched to the liposomal form.
In a separate analysis of the evaluable group of 23 patients (Tables 2and 3) who received 4 or more doses of liposomal ATRA (excluding the 9 patients who died early), the CR rate was 87% (95% CI: 73%, 100%). The CR rate of 22 APL patients whose diagnosis was confirmed either by cytogenetics or PCR was 91%. The median time to CR was 36 days (range, 21 to 65 days).
Patients evaluable for response
| . | Newly diagnosed . | Prior treatment . |
|---|---|---|
| Patients (no.) | 23 | 33 |
| Study no. | ||
| 001 | 0 | 3 |
| 002 | 0 | 25 |
| 003 | 12 | 0 |
| 005 | 10 | 0 |
| 004 | 1 | 53-150 |
| Age (y) | ||
| Median | 44.5 | 38.2 |
| Range | 5.5-82.1 | 11.4-70.2 |
| Male (no.) | 14 | 18 |
| WBC at entry (cells/μL) | ||
| Median | 1.7 | 2.0 |
| Range | 0.4-73.3 | 0.6-15.6 |
| >10 000 (no. pts) | 5 | 3 |
| . | Newly diagnosed . | Prior treatment . |
|---|---|---|
| Patients (no.) | 23 | 33 |
| Study no. | ||
| 001 | 0 | 3 |
| 002 | 0 | 25 |
| 003 | 12 | 0 |
| 005 | 10 | 0 |
| 004 | 1 | 53-150 |
| Age (y) | ||
| Median | 44.5 | 38.2 |
| Range | 5.5-82.1 | 11.4-70.2 |
| Male (no.) | 14 | 18 |
| WBC at entry (cells/μL) | ||
| Median | 1.7 | 2.0 |
| Range | 0.4-73.3 | 0.6-15.6 |
| >10 000 (no. pts) | 5 | 3 |
WBC = white blood cell count; pts = patients.
2 patients failed oral all-trans retinoic acid (ATRA).
Remission induction in previously treated patients
In the ITT analysis, the CR rate in group 1 patients (first relapse and ATRA naı̈ve or those who had not received oral ATRA for at least 1 year before relapse) was 70% (95% CI: 46%, 88%). For group 2 patients (second or subsequent relapse or in first relapse but had received ATRA within 1 year before enrollment), the CR rate was 20% (95% CI: 4%, 48%). None of the 5 group 2 patients in second or subsequent relapse achieved a CR. Among the 18 relapsed patients who did not achieve CR, 10 patients (29%) had resistant disease, and 8 patients (23%) were classified as early deaths. Resistance to liposomal ATRA was less common among relapsed group 1 patients (20%) than group 2 patients (40%). In 8 of the 35 patients, the dosage of liposomal ATRA was modified during the induction. It was increased in 6 patients, and one patient achieved CR; and reduced by 25% in 2 patients, one of whom achieved CR. Of the 5 patients who were not tested for cytogenetics or PCR, 2 patients achieved a CR.
A separate analysis was performed on an evaluable group of 31 patients (Tables 2 and 3), who received 4 or more doses of liposomal ATRA. Excluded from this analysis were 2 patients who died of brain hemorrhages on days 5 and 8 and 2 patients in a third and fourth relapse with severe DIC and multiple organ failure after 3 doses and died on days 14 and 9, respectively. In group 1 patients, the CR rate was 78% (95% CI: 52%, 93%). When the 4 ATRA naive patients were excluded from group 1, the CR rate for patients previously treated with oral ATRA was 71% (95% CI: 42%, 91%). In contrast, the CR rate for group 2 was only 23% (95% CI: 5%, 39%); only 3 of 13 evaluable patients in this group achieved CR. The median time to CR was 42 days (range, 28 to 62 days) and 50 days (range, 49 to 81 days) for groups 1 and 2, respectively.
Two patients with newly diagnosed disease had failed to respond to oral ATRA therapy and were switched to liposomal ATRA. One Jehovah's witness had worsening of DIC after 21 days of oral ATRA, refused blood products, and achieved a CR after switching to liposomal ATRA. It is possible that she did not receive sufficient oral ATRA. A second patient had 90% blasts in the bone marrow after 26 days of oral ATRA, received 8 doses (17 days) of liposomal ATRA without an effect, and was removed from the study. Her blood drug levels remained stable between days 1 and 15 of liposomal ATRA treatment. She showed typical M3 bone marrow morphology, immune phenotype of CD33+, and HLA DR negative but had normal chromosomes and was PCR negative for PML/RARα. This patient may be similar to previously described atypical APL patients with M3-like morphology but without the PML/RARα rearrangement who do not respond to ATRA.1 29
Molecular remission
In 23 patients, PCR for PML/RARα was performed on the first bone marrow sample that confirmed the CR at completion of induction therapy. In 11 patients (48%), the PCR was negative: 64% (on days 27 to 81 from starting treatment) and 22% (on days 28 and 50), by the low- and high-sensitivity assays, respectively (Table4). PCR negativity by the low-sensitivity assay appeared more frequent at hematologic CR in newly diagnosed than relapsing patients. Follow-up high-sensitivity PCR testing performed during CR in 7 patients who did not receive chemotherapy showed that, at 3 to 4 months from staring liposomal ATRA, all were negative; 6 patients were in study 005 continuing liposomal ATRA monotherapy during CR and one patient was in study 003, stopping liposomal ATRA at CR and starting consolidation chemotherapy later. In addition, the PCR results were negative in 65% of all 26 CR patients tested at any time during CR but had not received chemotherapy, by combining the results at CR plus results obtained later in CR (Table 4).
PCR results for PML/RARα expression in CR bone marrow samples after treatment with liposomal ATRA
| . | No. of patients PCR negative/no. of patients tested (%) . | |
|---|---|---|
| Low-sensitivity PCR (10−3 level) . | High-sensitivity PCR (10−4level) . | |
| At time of CR confirmation4-150 | 9/14 (64%) | 2/9 (22%) |
| Newly diagnosed | 5/6 (83%) | 1/8 (12%) |
| Relapsed | 4/8 (50%) | 1/1 |
| Any time at CR without chemotherapy4-151 | 9/14 (64%) | 8/12 (67%) |
| Newly diagnosed | 5/6 (83%) | 7/11 (64%) |
| Relapsed | 4/8 (50%) | 1/1 |
| . | No. of patients PCR negative/no. of patients tested (%) . | |
|---|---|---|
| Low-sensitivity PCR (10−3 level) . | High-sensitivity PCR (10−4level) . | |
| At time of CR confirmation4-150 | 9/14 (64%) | 2/9 (22%) |
| Newly diagnosed | 5/6 (83%) | 1/8 (12%) |
| Relapsed | 4/8 (50%) | 1/1 |
| Any time at CR without chemotherapy4-151 | 9/14 (64%) | 8/12 (67%) |
| Newly diagnosed | 5/6 (83%) | 7/11 (64%) |
| Relapsed | 4/8 (50%) | 1/1 |
PCR = polymerase chain reaction; PML/RARα = promyelocytic leukemia/retinoic acid receptor-alpha; CR = complete remission; ATRA = all-trans retinoic acid.
PCR was performed on first bone marrow sample that confirmed the CR, on days 21 to 81 (median, day 43) from start of liposomal ATRA.
PCR was assayed anytime during CR before patients received chemotherapy combining the results at CR plus results obtained later during CR. Tests were performed on days 21 to 132 (median, day 53) from start of liposomal ATRA.
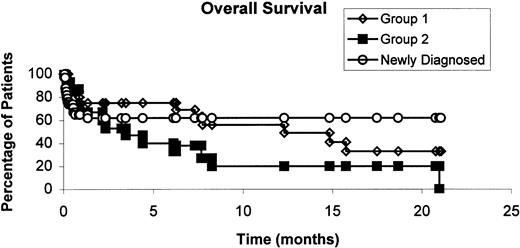
Overall survival
The median survival of all 69 enrolled patients was 12 months. The median survival has not yet been reached in newly diagnosed patients, and was 12 months for group 1 patients, and 3 months for group 2 patients. The probability of survival at 1 year for all enrolled patients is shown in Figure 1. For the 56 evaluable patients, it was 87%, 62.5%, and 23% for newly diagnosed, group 1, and group 2 patients, respectively (P = .0067 by log rank, data not shown).
Overall survival curve of all the enrolled patients according to disease status at enrollment.
The probability of survival at 1 year was 62%, 56%, and 20% for newly diagnosed, relapses group 1 and group 2, respectively.
Overall survival curve of all the enrolled patients according to disease status at enrollment.
The probability of survival at 1 year was 62%, 56%, and 20% for newly diagnosed, relapses group 1 and group 2, respectively.
Remission duration
All 38 patients who achieved CR received postinduction treatment; 12 patients did not complete the scheduled postremission protocol because of: relapse (4 patients), bone marrow transplantation (3 patients), withdrawal (3 patients), removal for adverse effects (one patient), or noncompliance (one patient). The median remission duration was 17.5 months. For newly diagnosed patients, the median has not yet been reached, but was 18 and 5.5 months for groups 1 and 2 relapses, respectively.
Prognostic factors for complete remission
The 10 baseline variables for CR are shown in Table5. In the ITT population of 69 patients, only intubation status was predictive of poor response compared to patients who were not intubated (multivariate analysis, odds ratio 0.017, P = .0005). Because all 13 patients who received 3 or less doses of liposomal ATRA died early and 9 of these 13 patients (69%) were intubated, we looked at prognostic factors of patients who had received the drug for a longer duration (4 or more doses) and a separate analysis was performed with the 56 evaluable patients (Table5). With univariate analysis, relapse status, prior oral ATRA, and intubation status were predictive of a low CR rate. In the stepwise logistic regression multivariate analysis, the relapse status and intubation status had an independent adverse prognostic impact on CR rate.
Univariate and multivariate analysis for complete remission (evaluable patients)
| Prognostic factor . | Univariate . | Multivariate . | ||
|---|---|---|---|---|
| P-value . | N . | Odd ratio . | P-value . | |
| Age (y)5-150 | .4434 | |||
| < 45 | ||||
| > 45 | ||||
| Baseline WBC (cells/μL)5-150 | .6215 | |||
| < 5 000 | ||||
| > 5 000 | ||||
| Sex | .6799 | |||
| Female | ||||
| Male | ||||
| Relapse status group | .00003 | 56 | 0.014 | .0015 |
| Newly diagnosed | ||||
| Relapse (Group 1) | ||||
| Relapse (Group 2) | ||||
| Prior oral ATRA | .0027 | |||
| No | ||||
| Yes | ||||
| Prior ATRA syndrome | .1123 | |||
| No | ||||
| Yes | ||||
| Current ATRA syndrome | .3420 | |||
| No | ||||
| Yes | ||||
| Baseline performance status | .9083 | |||
| Intubation | .019 | 56 | 0.249 | .0002 |
| No | ||||
| Yes | ||||
| DIC | .3243 | |||
| No | ||||
| Yes | ||||
| Prognostic factor . | Univariate . | Multivariate . | ||
|---|---|---|---|---|
| P-value . | N . | Odd ratio . | P-value . | |
| Age (y)5-150 | .4434 | |||
| < 45 | ||||
| > 45 | ||||
| Baseline WBC (cells/μL)5-150 | .6215 | |||
| < 5 000 | ||||
| > 5 000 | ||||
| Sex | .6799 | |||
| Female | ||||
| Male | ||||
| Relapse status group | .00003 | 56 | 0.014 | .0015 |
| Newly diagnosed | ||||
| Relapse (Group 1) | ||||
| Relapse (Group 2) | ||||
| Prior oral ATRA | .0027 | |||
| No | ||||
| Yes | ||||
| Prior ATRA syndrome | .1123 | |||
| No | ||||
| Yes | ||||
| Current ATRA syndrome | .3420 | |||
| No | ||||
| Yes | ||||
| Baseline performance status | .9083 | |||
| Intubation | .019 | 56 | 0.249 | .0002 |
| No | ||||
| Yes | ||||
| DIC | .3243 | |||
| No | ||||
| Yes | ||||
ATRA = all-trans retinoic acid; WBC = white blood cell count; DIC = disseminated intravascular coagulation.
Age as a continuous parameter P = .4434; WBC as a continuous parameter P = .6215.
Prognostic factors for remission duration
In the univariate analysis of patients in CR (Table6), the relapse status and prior ATRA syndrome were predictive of shorter remission duration; but in the multivariate analysis, only relapse status was an independent prognostic indicator for duration of remission.
Univariate and multivariate analysis for remission duration (evaluable patients)
| Prognostic factor . | Univariate . | Multivariate . | ||
|---|---|---|---|---|
| P-value . | N . | Risk ratio . | P-value . | |
| Age (y)6-150 | .2987 | |||
| < 45 | ||||
| > 45 | ||||
| Baseline WBC (cells/μL)6-150 | .2933 | |||
| < 5000 | ||||
| > 5000 | ||||
| Sex | .6741 | |||
| Female | ||||
| Male | ||||
| Relapse status group | .0153 | 38 | 2.42 | .0153 |
| Newly diagnosed | ||||
| Relapse (group 1) | ||||
| Relapse (group 2) | ||||
| Prior oral ATRA | .1741 | |||
| No | ||||
| Yes | ||||
| Prior ATRA syndrome | .0063 | |||
| No | ||||
| Yes | ||||
| Current ATRA syndrome | .9923 | |||
| No | ||||
| Yes | ||||
| Baseline performance status | .1750 | |||
| Intubation | .9928 | |||
| No | ||||
| Yes | ||||
| DIC | .0465 | |||
| No | ||||
| Yes | ||||
| Prognostic factor . | Univariate . | Multivariate . | ||
|---|---|---|---|---|
| P-value . | N . | Risk ratio . | P-value . | |
| Age (y)6-150 | .2987 | |||
| < 45 | ||||
| > 45 | ||||
| Baseline WBC (cells/μL)6-150 | .2933 | |||
| < 5000 | ||||
| > 5000 | ||||
| Sex | .6741 | |||
| Female | ||||
| Male | ||||
| Relapse status group | .0153 | 38 | 2.42 | .0153 |
| Newly diagnosed | ||||
| Relapse (group 1) | ||||
| Relapse (group 2) | ||||
| Prior oral ATRA | .1741 | |||
| No | ||||
| Yes | ||||
| Prior ATRA syndrome | .0063 | |||
| No | ||||
| Yes | ||||
| Current ATRA syndrome | .9923 | |||
| No | ||||
| Yes | ||||
| Baseline performance status | .1750 | |||
| Intubation | .9928 | |||
| No | ||||
| Yes | ||||
| DIC | .0465 | |||
| No | ||||
| Yes | ||||
See Table 5 for abbreviations.
Age as a continuous parameter P = .2453; WBC as a continuous parameter P = .3126.
Toxicity
ATRA syndrome was diagnosed in 18 patients (26%) during the induction phase, classified as grade 3 or 4 in 10 patients, and was fatal in one patient. The latter patient had dyspnea, cough, pleural effusion, tachycardia, and a worsening of bilateral lung densities that were attributed to ATRA syndrome. Liposomal ATRA was stopped, but dexamethasone administration may have been tapered off too fast.
Thirty-five patients (51%) had peripheral leukocytosis (WBC > 10 000 cells/μL) during treatment. Their median peak leukocyte count was 42 000/μL and the highest peak was 154 500/μL. Ten of the 35 patients (28%) with leukocytosis had ATRA syndrome develop. Eleven of the 17 patients (65%) with ATRA syndrome had leukocytosis during treatment (range 12 800 to 99 700/μL) compared with 24 of the 52 patients (46%) without the syndrome (range 12 400 to 154 500/μL); the difference was not statistically significant (P = .15; Fisher exact test). The median peak WBC count during treatment among patients with ATRA syndrome was 15 500/μL (range 2700 to 99 700/μL) and was higher compared with 9700/μL (range 900 to 154 000/μL) in patients without ATRA syndrome; the difference was of borderline statistical significance (P = .076, 1-tailed Mann-Whitney test).
The most frequent adverse event was headache (67%). Hypertriglyceridemia ranging from 166 to 1209 mg/dL (median, 334 mg/dL) was noted in 23 patients (33%); in 4 patients (5.8%), the concentration was above 800 mg/dL. Four patients (5.8%) had documented pseudotumor cerebri manifesting by severe headache, papilledema, increased cerebrospinal pressure on lumbar puncture, and absence of a structural cranial lesion by computed tomography (CT) or magnetic resonance imaging (MRI). The frequency of other side effects is shown in Table 7. The majority of adverse events were mild to moderate (grades 1 or 2) (Table 7).
Adverse effects of liposomal ATRA in the 69 patients enrolled
| Adverse effect . | Total no. (%) . | Grade 3/4 no. (%) . |
|---|---|---|
| ATRA syndrome | 18 (26%) | 10 (15%) |
| Leukocytosis (WBC > 10 000 cells/μL) | 35 (51%) | |
| Headache | 46 (67%) | 10 (15%) |
| Dry skin | 23 (33%) | |
| Hypertriglyceridemia | 23 (33%) | 4 (5.8%) |
| Fever | 18 (26%) | |
| Nausea | 13 (19%) | |
| Stomatitis | 11 (16%) | |
| Vomiting | 10 (15%) | |
| Chelitis | 9 (13%) | |
| Exfoliative dermatitis | 9 (13%) | |
| Rash | 8 (12%) | |
| Myalgia | 8 (12%) | |
| Liver enzymes abnormalities | 7 (10%) | |
| Bone pain | 6 (9%) | 2 (3%) |
| Arthralgia | 5 (7%) | |
| Elevated LDH | 4 (6%) | |
| Pseudotumor cerebri | 4 (6%) | 3 (4%) |
| Hypercholesteremia | 4 (6%) | |
| Chills | 4 (6%) | |
| Pruritus | 3 (4%) | |
| Diarrhea | 2 (3%) |
| Adverse effect . | Total no. (%) . | Grade 3/4 no. (%) . |
|---|---|---|
| ATRA syndrome | 18 (26%) | 10 (15%) |
| Leukocytosis (WBC > 10 000 cells/μL) | 35 (51%) | |
| Headache | 46 (67%) | 10 (15%) |
| Dry skin | 23 (33%) | |
| Hypertriglyceridemia | 23 (33%) | 4 (5.8%) |
| Fever | 18 (26%) | |
| Nausea | 13 (19%) | |
| Stomatitis | 11 (16%) | |
| Vomiting | 10 (15%) | |
| Chelitis | 9 (13%) | |
| Exfoliative dermatitis | 9 (13%) | |
| Rash | 8 (12%) | |
| Myalgia | 8 (12%) | |
| Liver enzymes abnormalities | 7 (10%) | |
| Bone pain | 6 (9%) | 2 (3%) |
| Arthralgia | 5 (7%) | |
| Elevated LDH | 4 (6%) | |
| Pseudotumor cerebri | 4 (6%) | 3 (4%) |
| Hypercholesteremia | 4 (6%) | |
| Chills | 4 (6%) | |
| Pruritus | 3 (4%) | |
| Diarrhea | 2 (3%) |
See Table 5 for abbreviations.
All 69 enrolled patients were evaluated for toxicity.
Discussion
This study demonstrates that liposomal ATRA, as single agent, has activity in both newly diagnosed and relapsed patients with APL. When all enrolled newly diagnosed, untreated patients were considered, the CR rate in the ITT analysis was 62%, which is lower than the reported 72% to 95% with oral ATRA alone.1,2,10,11,30 However, our group of newly diagnosed patients included a particularly high proportion of patients with a very poor prognosis. Nine patients (28%) stopped treatment after receiving only 1 to 3 doses of liposomal ATRA, primarily because of severe bleeding in the brain or the lung. All 9 of these patients were intubated and unconscious at time of study entry. Furthermore, 12 of the 32 newly diagnosed patients (37%) presented with a WBC count of more than or equal to 10 000 cells/μL. In comparison, in large oral ATRA studies, elevated WBC count was reported in 21% to 28% of patients.1-3 A high WBC count at diagnosis is considered a poor risk factor, mostly for life-threatening bleeding,30-32 as also observed in our study. A WBC count of more than 10 000 cells/μL was measured at presentation in 11 (73%) of our 15 patients with brain or lung bleeding, but only in 7% (4 of 54) without such bleedings. The high number of patients presenting with very poor prognosis is partially attributable to the availability of an IV formulation that permitted physicians to specifically select to treat with liposomal ATRA patients who could not swallow capsules and would have otherwise been unable to receive oral ATRA.
After excluding the patients who had received 3 or less doses (all were intubated), the CR rate in the 23 evaluable newly diagnosed patients was 87%, and in patients with WBC counts of less than 10 000 cells/μL the CR rate was 89% (16 of 18). These results compare favorably with reported large-scale clinical studies using oral ATRA for induction therapy in newly diagnosed APL patients.1,30Although early deaths were not excluded from the analysis of the oral ATRA trials, the comparison is valid for several reasons. First, it is unclear from the published reports if and how intubated or unconscious patients were started on oral ATRA. Second, it is unknown whether all consecutive patients with APL were included in the trials or whether attending physicians decided not to enroll certain intubated or unconscious patients because oral medications could not be administered. These patients are most likely to die early from brain or lung bleeding, thus potentially reducing the CR rate. Primary resistance to oral ATRA is very unusual in APL with the t(15;17) translocation. In the large European APL93 trial, only one patient failed treatment as a result of resistant disease.2Primary resistance to liposomal ATRA was not seen among newly diagnosed patients, and failure was due to early death, primarily from brain or lung bleeding.
Patients with APL in first CR, treated with a combination of oral ATRA and chemotherapy, relapse at a rate of approximately 30%.1,2,30 Successful reinduction with ATRA, after using the drug in first induction, has been reported only in a limited number of patients. Our studies provide additional information in this setting. For patients in first relapse (1 year after stopping oral ATRA), liposomal ATRA was an effective salvage agent, yielding a CR rate of 62.5% in the ITT analysis. The CR rate was 71% when the 2 patients who died early and received 3 or less doses of liposomal ATRA were excluded. In contrast, only 20% of patients in first relapse who had taken oral ATRA during the preceding year achieved a second CR with liposomal ATRA. Furthermore, no patients in second or subsequent relapse achieved CR. These conclusions are similar to or more favorable than those of smaller studies with oral ATRA retreatment reported by Chen et al14 and Warrell et al.9
Our study included only 4 ATRA-naive patients in first relapse and all achieved a second CR with liposomal ATRA, which compares favorably with oral ATRA in this setting.7,10,14,33 For example, in 30 ATRA-naive patients reported by Warrell et al,9 the rate of second CR with oral ATRA was 83%; this is equivalent to the CR rate with oral ATRA in newly diagnosed patients.33
Comparatively, both oral and liposomal formulations of ATRA appear very effective in first-relapsed ATRA-naive patients, probably at a similar rate to that of newly diagnosed patients. In addition, liposomal ATRA can be used effectively for retreatment in first-relapse patients, provided that oral ATRA has not been given for at least 1 year.
It is not clear if patients resistant to oral ATRA would respond to IV liposomal ATRA. In fact, patients in second relapse or after a short first remission who are usually resistant to oral ATRA were also the group most resistant to liposomal ATRA. The 2 patients who failed oral ATRA and were switched to the liposomal form cannot answer this question; one patient responded but could have received insufficient oral ATRA, whereas the patient who failed both forms of ATRA had an M3-like leukemia without PML-RARα.
Intravenous liposomal ATRA was safe and well tolerated. The most serious side effect was ATRA syndrome, reported in 26% of the patients. ATRA syndrome has been reported in 6% to 27% of patients in recent studies of oral ATRA with or without concomitant chemotherapy.1,11,34-39 Similarly to oral ATRA, the syndrome occurred with the liposomal compound early in the induction and even immediately after the first dose of liposomal ATRA.34 With both ATRA forms, ATRA syndrome was not seen in remission.35 The WBC count during induction tended to be higher among patients with ATRA syndrome but did not reach statistical significance when compared with those without the syndrome. ATRA syndrome was fatal in one patient (5%) compared with a 5% to 9% mortality rate with oral ATRA.1,5,11,34,35 Early intervention with high-dosage corticosteroids is usually effective in this complication.36,37 In addition, several investigators use chemotherapy to reduce the rising WBC count associated with this syndrome.2 5 A valid comparison is difficult to make using existing data on oral ATRA because of different criteria used for classifying respiratory distress as ATRA syndrome, methods of prophylaxis, and effective early intervention. However, it appears that liposomal ATRA is not associated with a higher rate of ATRA syndrome than that reported for oral ATRA.
Other adverse events were similar to those reported with oral ATRA, and most were mild to moderate. The most common adverse effect was transient headache, occurring in 73% of the patients, more frequently during induction but did not result in treatment discontinuation. Liposomal ATRA is associated with pseudotumor cerebri, which occurred in 5.8% of our patients compared with 9% with oral ATRA.40 41 Thus, despite the more direct IV route of administration and the higher blood levels achieved compared with oral ATRA, the liposomal formulation does not appear to adversely affect the safety profile of the drug.
In our studies, newly diagnosed patients and those in first relapse more than 1 year after discontinuing oral ATRA received consolidation with chemotherapy plus liposomal ATRA, followed by liposomal ATRA maintenance. Although the follow-up period is short, the preliminary results on overall survival and remission duration in these 2 patient groups are encouraging. At 1 year, the rate of continuous CR in newly diagnosed patients compares favorably with oral ATRA plus chemotherapy.1 2 The remission duration of patients in second relapse or relapse and off oral ATRA for short periods is poor. In multivariate analysis, only the relapse status at time of starting liposomal ATRA was predictive for longer remission.
ATRA is a standard and crucial part of the induction treatment in APL. Although the results of induction treatment with oral ATRA are very good, IV liposomal ATRA has potential advantages, particularly in certain situations. First, IV ATRA can be administered more reliably to small children who cannot easily swallow capsules. Second, an IV formulation would be the only reliable way of delivering ATRA to patients with APL who are unconscious or intubated, as it is extremely difficult to administer the contents of ATRA capsules via a nasogastric tube. Among the 69 patients enrolled in our studies, 16 (23%) were intubated at study entry and unable to take oral medications. Although the overall response rate was very poor in this group, one patient did achieve a CR. Third, absorption of oral ATRA could be less consistent in patients who receive oral ATRA in conjunction with induction chemotherapy if it results in vomiting and intestinal mucosal damage. Although the concomitant administration of chemotherapy and oral ATRA during induction results in very good overall long-term outcome, optimal drug blood levels after parenteral treatment might further improve these results. Fourth, a more stable blood concentration produced by liposomal ATRA may permit more efficient maintenance therapy than oral ATRA. Recently, 2 studies reported the value of ATRA maintenance in patients with newly diagnosed APL.1 2
In general, ATRA is easier to administer as capsules than IV and the overall good outcome reported with oral ATRA, suggests that compliance is not an important issue. However, compliance of certain patients with hematologic malignancies can be problematic42; IV administration is less dependent on this factor.
Another potential advantage of liposomal ATRA is its ability to produce molecular remissions without chemotherapy. In patients with newly diagnosed APL, using a PCR assay with a sensitivity level of 10−4, Estey et al24 reported that liposomal ATRA monotherapy induced molecular remissions within 3 months of hematologic remission. In several patients, PCR negativity persisted for months without adding chemotherapy. In contrast, oral ATRA alone appears not to induce PCR negativity.10,26,43 44
Because we did not give chemotherapy during induction, we could evaluate the frequency of molecular remissions after liposomal ATRA alone in both newly diagnosed and relapsing patients. Our trials were initiated before 1998, when we introduced the high-sensitivity PCR assay; therefore, many patients were tested with a low-sensitivity assay (10−3 level). With this low-sensitivity PCR, the rate of molecular remission with ATRA alone appeared to be higher with the liposomal than with the oral form. A molecular remission at the time of CR determination, after induction with one cycle of liposomal ATRA and before chemotherapy, was found in 50% and 85% of our relapsed and newly diagnosed patients, respectively. In comparison, Miller et al,45 using the same low-sensitivity assay,27 reported that none of their 10 relapsed patients induced and maintained solely with oral ATRA achieved PCR negativity, even after several months of therapy. Further, in the same study,45 among 22 newly diagnosed patients induced with oral ATRA alone, a negative PCR was detected at the time of achieving CR in only one patient (5%).
Follow-up PCR testing after liposomal ATRA alone was limited, because eventually most of our CR patients received consolidation chemotherapy. However, in 7 patients follow-up PCR was performed during CR without chemotherapy, and all were negative by the high-sensitivity assay. By 4 months, a total of 8 (67%) of 12 patients tested with the high-sensitivity assay at or during CR were PCR negative without chemotherapy; 6 were in study 005 and continued liposomal ATRA monotherapy in remission, which may explain a higher rate of molecular remission seen later during CR, as previously reported.24However, 2 patients (one newly diagnosed and one relapsed) were PCR negative at the time of CR with the high-sensitivity assay after one cycle of liposomal ATRA. There are no published PCR data on newly diagnosed patients maintained with oral ATRA alone, but they all relapsed early.6,14 Also, no data with a PCR sensitivity level of 10−4 are available at CR after induction with oral ATRA alone, but only after induction with oral ATRA plus chemotherapy.4 Achieving a molecular remission with liposomal ATRA is a potential advantage, because a molecular remission in APL is a likely marker for longer survival.44
Our results indicate that IV liposomal ATRA is as least as effective as oral ATRA in achieving and maintaining CR in newly diagnosed and first-relapse APL patients with a similar safety profile. Because of several potential advantages, liposomal ATRA is a useful alternative for ATRA therapy in patients with APL.
Acknowledgments
We thank Helen Wren and Kristy Watkins, RN, for their assistance with data collection.
Supported by a research grant from Aronex Pharmaceuticals, Inc.
Two of the authors, K.B. and T.W., declare a financial interest in the company whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dan Douer, Division of Hematology, USC/Norris Cancer Center, 1441 Eastlake Ave, Room 3460, Los Angeles, CA 90033; e-mail: douer_d@mikey.hsc.usc.edu