Abstract
The effect of mutations in the Src homology 2 (SH2) domain of theBCR/ABL oncogene on leukemogenesis was tested in a quantitative murine bone marrow transduction/transplantation assay that accurately models human Philadelphia-positive B-lymphoid leukemia and chronic myeloid leukemia (CML). The SH2 domain was not required for induction of B-lymphoid leukemia in mice byBCR/ABL. Under conditions where the p190 and p210 forms ofBCR/ABL induce fatal CML-like myeloproliferative disease within 4 weeks, p210 SH2 mutants induced CML-like disease in some mice only after a significant delay, with other recipients succumbing to B-lymphoid leukemia instead. In contrast, p190 BCR/ABL SH2 point and deletion mutants rapidly induced CML-like disease. These results provide the first direct evidence of significant differences in cell signaling by the Bcr/Abl tyrosine kinase between these distinct leukemias. Contrary to previous observations, high levels of phosphatidylinositol 3-kinase (PI 3-kinase) activity in primary malignant lymphoblasts and myeloid cells from recipients of marrow transduced with the BCR/ABL SH2 mutants were found. Hence, the decreased induction of CML-like disease by the p210BCR/ABL SH2 mutants is not due to impaired activation of PI 3-kinase.
Introduction
The Bcr/Abl fusion protein is the product of the t(9;22) Philadelphia chromosome translocation found in the human leukemias chronic myeloid leukemia (CML) and acute lymphoblastic leukemia.1 Bcr/Abl exists in 2 major forms, p190 and p210, generated by distinct breakpoints within the BCR gene on chromosome 22. Relative to c-Abl, the Bcr/Abl proteins exhibit elevated tyrosine kinase activity2,3 and have gained the ability to transform fibroblasts,2 factor-dependent hematopoietic cells,3 and primary bone marrow-derived B-lymphoid cells4 in vitro. In a retroviral bone marrow transduction/transplantation model, p190 and p210 induce an identical fatal CML-like myeloproliferative disease in recipients of transduced marrow when donors are pretreated with 5-fluorouracil (5-FU).5 Mice with the CML-like illness succumb within 4 weeks after transplantation and exhibit massive expansion of maturing myeloid cells, principally neutrophils, with involvement of peripheral blood, bone marrow, spleen, liver, and lungs. Analysis of proviral integration shows the CML-like disease to be polyclonal and involve multiple myeloid and B-lymphoid lineages, implicating a target cell that is an early multipotential progenitor or stem cell.5In contrast, when donors are not treated with 5-FU, recipients of p190- and p210-transduced marrow develop a mixture of CML-like disease, B-lymphoid leukemia, and macrophage tumors.5 p190 is more potent than p210 for induction of B-lymphoid leukemia, a result anticipated by in vitro studies.4 Bcr/Abl-induced B-lymphoid leukemia develops between 4 and 12 weeks after transplant, is monoclonal or oligoclonal by proviral integration and involves only the B-lymphoid lineage,5 suggesting a lineage-restricted target cell similar to that characterized for Abelson murine leukemia virus,6 which requires additional events in addition toBCR/ABL transduction for full malignant transformation. Thus, the phenotype of the leukemia induced by BCR/ABL in primary bone marrow depends critically on the type of cell that is transduced, and it is plausible that the leukemogenic process in these distinct cells may be quite different.
Bcr/Abl constitutively activates several cell signaling pathways that contribute to transformation, including activation of Myc,7 Ras,8 c-Raf-1,9MAPK/ERK,10 SAPK/JNK,11STAT,12,13 NF-κB,14 and PI 3-kinase.15,16 In Bcr/Abl-transformed hematopoietic cells, the total PI 3-kinase activity in whole cell extracts is increased about 60% relative to serum- and IL-3–starved parental cells.17 Activated PI 3-kinase is present in antiphosphotyrosine immunoprecipitates in complexes containing tyrosine-phosphorylated Shc,18 c-Cbl,19CrkL,20 and Bcr/Abl along with the adapter protein Grb2.19 In these complexes, engagement of tandem SH2 domains of the p85 regulatory subunit of PI 3-kinase by tyrosine-phosphorylated pYXXM motifs leads to activation of p110 lipid kinase activity.21 Although Bcr/Abl tyrosine kinase activity is required for activation of PI 3-kinase, direct binding of the p85 SH2 domains to Bcr/Abl is not.17 Alternatively, PI 3-kinase may be directly stimulated by binding of activated Ras.22
The role of the Src homology 2 (SH2) domain of Bcr/Abl in transformation has been extensively studied. Mutation of the highly conserved FLVRES motif in the phosphotyrosine binding site blocks binding of the Abl SH2 domain to tyrosine phosphorylated proteins in vitro,23 and significantly impairs transformation of fibroblasts by SH3-deleted c-Abl23 and by p190 Bcr/Abl, which is restored by overexpression of c-Myc.24 SH2 is not required for transformation of cytokine-dependent hematopoietic cell lines3,25,26 or primary bone marrow B-lymphoid cells by Bcr/Abl in vitro,27 but the latter exhibit decreased and delayed tumor formation in SCID mice. A recent study reported that p210 Bcr/Abl SH2 mutants were completely defective in activation of PI 3-kinase and the downstream serine/threonine kinase Akt in 32D cells,28 and induced less hepatic and splenic myeloid infiltration in SCID mouse recipients of transduced bone marrow, relative to p210 wild-type. Cotransduction of marrow with retroviruses expressing a p210 SH2 deletion mutant and a constitutively active mutant of Akt increased spleen size and liver involvement by myeloid cells in recipients. However, it was not clear whether the myeloid infiltration represented acute myeloid leukemia or a CML-like process, whether the disorder was fatal or transplantable, and whether the myeloid cells contained the BCR/ABL gene or were a reactive process. The role of the Bcr/Abl SH2 domain in lymphoid and myeloid leukemogenesis is therefore unclear. In this study, we have tested the leukemogenic activity of Bcr/Abl SH2 mutants in a bone marrow transduction/transplantation system under conditions that accurately model either Philadelphia-positive B-lymphoid leukemia or CML.
Materials and methods
Cell lysates and PI 3-kinase assays
For analysis of hematopoietic cell lines, early passages (passage 4 or earlier, maintained in culture for less than 2 weeks) of polyclonal populations of cells transformed by p210 Bcr/Abl and the SH2 mutants were used. All cells were exposed to low serum (1% FCS) in the absence of IL-3 for 8 hours before harvest. For analysis of malignant lymphoblasts, pathologically involved lymph nodes and pleural effusion cells were collected from diseased mice at autopsy, and erythrocytes were lysed with NH4Cl solution before lysis of leukocytes. Wright-Giemsa staining of cytospin preparations of the populations indicated that more than 80% of the cells were lymphoblasts. As a control, B220+ cells were isolated from spleens of 2 mice transplanted 8 weeks earlier with uninfected marrow, using anti-B220 monoclonal antibody and the MicroMACS system (Miltenyi Biotec, San Fernando, CA), as described.5 FACS analysis of the purified population revealed more than 98% to be B220+. Whole cell lysates were prepared and PI 3-kinase activity measured in 1000-fold diluted whole cell extracts and antiphosphotyrosine immunoprecipitates as described.29 Briefly, cells were lysed at 2 to 4 × 107 cells/mL of buffer containing 1% Nonidet P-40, 50 mmol/L HEPES pH 7.5, 10% glycerol, 0.5 mmol/L EDTA, 5 mmol/L Na3VO4 and protease inhibitors (10 μg/mL leupeptin and aprotinin, 5 μg/mL pepstatin and PMSF), clarified by centrifugation at 12 000g, and stored at −80°C until use. The reaction mixture contained sonicated standard phospholipid with equal parts PS, PI, and PIP2 at a concentration of 0.2 mg/mL in a 50-μL reaction, incubated at 37°C for 20 minutes after addition of 150 μmol/L and 0.55 MBq (15 μCi) γ-[32P]-ATP. Reactions were terminated by addition of methanol:1 N HCl (1:1), phospholipids extracted twice with chloroform, and fractionated by thin-layer chromatography and autoradiography. PI 3-kinase activity was expressed as picomoles (pmol) of phosphate incorporated into PI 3,4,5-P3, and presented as fold increase relative to activity in control cells.
Western blotting
Extracts from peripheral blood leukocytes or spleen cells (predominantly maturing myeloid cells) were prepared by direct boiling as described,5 fractionated by SDS-PAGE, transferred to nitrocellulose membranes, and blotted with phospho-specific antibodies. Activation-specific antibodies for Akt, ERK, and JNK were obtained from New England BioLabs (Beverly, MA) and used according to the manufacturer's instructions. Anti-c-Myc antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), and anti-Abl monoclonal antibody Ab-3 from Calbiochem (San Diego, CA). Antiphospho-STAT5 antibodies were the kind gift of Dr David Frank, Dana-Farber Cancer Institute, Boston, MA.
Bone marrow transduction/transplantation
Generation and titering of retroviral stocks was as previously described5; all stocks had titers of 3 to 5 × 106 neomycin resistant colony-forming units per milliliters and gave equivalent proviral copy number, assessed by Southern blotting on transduction of NIH 3T3 cells or primary bone marrow. Balb/c mice (Taconic Farms, Germantown, NY) were used for all experiments. For induction of CML-like disease with BCR/ABL, marrow was harvested from male donors 4 days after treatment with 200 mg/kg 5-fluorouracil, prestimulated in vitro in medium containing 5% WEHI-3B cell-conditioned medium, 6 ng/mL IL-3, 10 ng/mL IL-6, and 50 ng/mL SCF (all from Peprotech, Rocky Hill, NJ). Subsequently, marrow was subjected to 2 rounds of cosedimentation with retroviral stocks in the same medium containing 2 μg/mL polybrene. Recipient female mice were prepared by 2 doses of 450 cGy gamma irradiation, and transduced marrow transplanted by intravenous injection of 0.5 × 106 cells per animal.
For induction of B-lymphoid leukemia by BCR/ABL, marrow from donors that had not been 5-FU–treated was used, the prestimulation step was omitted, and cells subjected to a single round of cosedimentation with retroviral stock in medium containing 5% WEHI-3B-conditioned medium and 10 ng/mL IL-7. Immediately after transduction, cells were transplanted into lethally irradiated (2 × 450 cGy) syngeneic female recipient mice (1 × 106 cells each) or plated for Whitlock/Witte culture in RPMI 1640 medium supplemented with 10% fetal calf serum, 200 μmol/L l-glutamine, 50 μmol/L 2-mercaptoethanol, and penicillin/streptomycin.
For negative selection of B220-expressing cells from p210 R1053K-transduced bone marrow, erythrocytes were lysed by addition of NH4Cl solution, leukocytes were washed and incubated with monoclonal antibody 6B2 (unconjugated, from Pharmingen, San Diego, CA) at 1.0 μg/106 cells. Antibody was removed by pelleting cells through a cushion of fetal calf serum, cell pellets resuspended and goat-antirat microbeads (Miltenyi Biotec) added, and incubated at 10°C for 15 minutes. Cells were then pelleted, washed, and subjected to 2 rounds of negative selection on a MicroMACS column with flow resistor, according to the manufacturer's instructions. Approximately 2 × 105 purified B220-negative transduced marrow cells were transplanted per animal into sublethally irradiated (450 cGy) female Balb/c mice.
Results
SH2 mutation decreases the in vitro kinase activity of Bcr/Abl
Previous studies showed that mutation of the Abl SH2 domain reduced the tyrosine kinase activity of p210 Bcr/Abl in vitro,3 and demonstrated decreased tyrosine phosphorylation of cellular proteins in fibroblasts24 and hematopoietic cell lines3,25,26 expressing Bcr/Abl SH2 mutants. To determine the relative effects of mutation of SH2 on the tyrosine kinase activities of p190 and p210 Bcr/Abl, we performed immune complex kinase assays on wild-type and SH2-mutated p190 and p210 (Figure 1). In agreement with previous reports,2,3 5 we found the in vitro kinase activity of wild-type p190 to be higher than wild-type p210, with both Bcr/Abl proteins significantly more active than c-Abl. Point mutation in the FLVRES motif decreased the kinase activity of both p190 and p210 Bcr/Abl by about 50%, whereas complete deletion of SH2 had a more dramatic effect on Bcr/Abl kinase activity, reducing p190 activity to near the level of c-Abl and p210 to significantly below that of c-Abl (Figure 1).
SH2 mutation decreases Bcr/Abl tyrosine kinase activity in vitro.
Bcr/Abl proteins were expressed by transfection of 293T cells and labeled in vivo with 35[S]-l-methionine. Lysates were immunoprecipitated with anti-Abl antisera and immune complexes incubated with γ-32[P]-ATP and GST-Crk substrate, as described.5 (A) 35[S] label, indicating relative levels of expression of the different Abl proteins. (B) 32[P] label. The position of the GST-Crk substrate is indicated by the arrowhead. The kinase activity of the different Bcr/Abl proteins relative to c-Abl after correction for levels of expression is shown at the top. (C) Coomassie blue stain demonstrating equal amounts of the GST-Crk substrate in all reactions.
SH2 mutation decreases Bcr/Abl tyrosine kinase activity in vitro.
Bcr/Abl proteins were expressed by transfection of 293T cells and labeled in vivo with 35[S]-l-methionine. Lysates were immunoprecipitated with anti-Abl antisera and immune complexes incubated with γ-32[P]-ATP and GST-Crk substrate, as described.5 (A) 35[S] label, indicating relative levels of expression of the different Abl proteins. (B) 32[P] label. The position of the GST-Crk substrate is indicated by the arrowhead. The kinase activity of the different Bcr/Abl proteins relative to c-Abl after correction for levels of expression is shown at the top. (C) Coomassie blue stain demonstrating equal amounts of the GST-Crk substrate in all reactions.
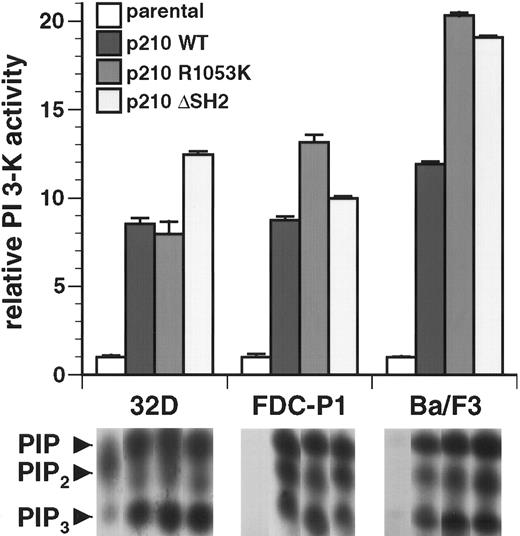
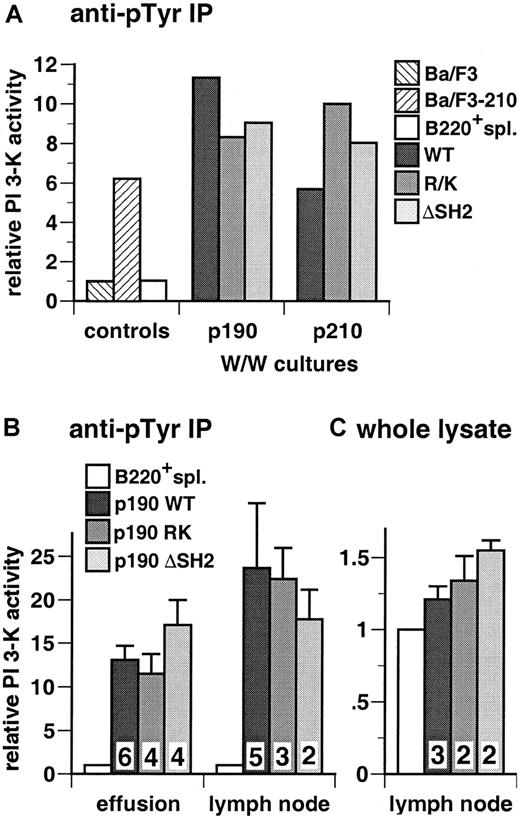
The Bcr/Abl SH2 domain is not required for activation of PI 3-kinase in factor-dependent myeloid and lymphoid cell lines
The SH2 domain of Bcr/Abl is not required for transformation of IL-3–dependent B-lymphoid (Ba/F3) and myeloid (FDCP-1, 32D cl3) cell lines to become independent of IL-3 for survival and growth.3,25 26 In antiphosphotyrosine immunoprecipitates, extracts from each of the 3 cell lines transformed with wild-type p210 exhibited an 8- to 11-fold increase in PI 3-kinase activity relative to parental cells (Figure 2). In all 3 cell types, extracts from cells early after transformation by the p210 R1053K and p210 ΔSH2 mutants showed prominent PI 3-kinase activity, equal to or greater than that detected in wild-type p210-transformed cells (Figure 2). Similar results were obtained when PI 3-kinase activity was measured in whole cell lysates (data not shown). These results demonstrate that the SH2 domain of Bcr/Abl is not required for activation of PI 3-kinase in hematopoietic cell lines.
The SH2 domain is not required for activation of PI 3-kinase in hematopoietic cell lines by p210 Bcr/Abl.
Top: PI 3-kinase activity in antiphosphotyrosine immunoprecipitates from lysates of the 3 indicated parental cell lines and polyclonal populations of each line transformed with p210 wild-type or the indicated SH2 mutants was determined and expressed as fold increased activity relative to parental cells. Data represent mean and standard deviation from 3 independent experiments. Similar results were obtained with assays of anti-p85 immunoprecipitates or whole cell lysates (data not shown). Bottom: Representative autoradiographs of thin-layer chromatograms of lipid kinase assays from these cell lines. Only the region containing phosphorylated PI products is reproduced. The positions of PIP, PIP2, and PIP3 are indicated by arrowheads at left; only the PI 3,4,5-P3(PIP3) species is exclusively generated by PI 3-kinase and was used to calculate activity.
The SH2 domain is not required for activation of PI 3-kinase in hematopoietic cell lines by p210 Bcr/Abl.
Top: PI 3-kinase activity in antiphosphotyrosine immunoprecipitates from lysates of the 3 indicated parental cell lines and polyclonal populations of each line transformed with p210 wild-type or the indicated SH2 mutants was determined and expressed as fold increased activity relative to parental cells. Data represent mean and standard deviation from 3 independent experiments. Similar results were obtained with assays of anti-p85 immunoprecipitates or whole cell lysates (data not shown). Bottom: Representative autoradiographs of thin-layer chromatograms of lipid kinase assays from these cell lines. Only the region containing phosphorylated PI products is reproduced. The positions of PIP, PIP2, and PIP3 are indicated by arrowheads at left; only the PI 3,4,5-P3(PIP3) species is exclusively generated by PI 3-kinase and was used to calculate activity.
The Bcr/Abl SH2 domain is not required for transformation of primary bone marrow B-lymphoid progenitors in vitro or for induction of B-lymphoid leukemia in mice
To develop a model in which BCR/ABL induces exclusively B-lymphoid leukemia, we transduced bone marrow from non–5-FU–treated donor mice without prestimulation or prolonged incubation in cytokines (described in “Materials and methods”). Transduced cells were then either immediately cultured under conditions (Whitlock-Witte cultures) favoring propagation of B-lymphoid cells or transplanted into lethally irradiated syngeneic recipient mice. The p190-transduced marrow cultures reached maximal density (more than 1 × 106cells/mL) of B220+ lymphoblasts within 7 days, with p210-transduced cultures delayed by 2 to 3 days, in agreement with previous observations4; the decrease in time required to reach confluence in our experiments is likely a consequence of increased transduction efficiency due to higher virus titers. We observed no decrease in the efficiency or kinetics of transformation by the p190 and p210 SH2 point and deletion mutants relative to the respective wild-type oncogenes, and no significant difference in the growth rates of the different populations (data not shown). These data confirm that the SH2 domain is dispensable for in vitro transformation of primary B-lymphoid cells by BCR/ABL.27
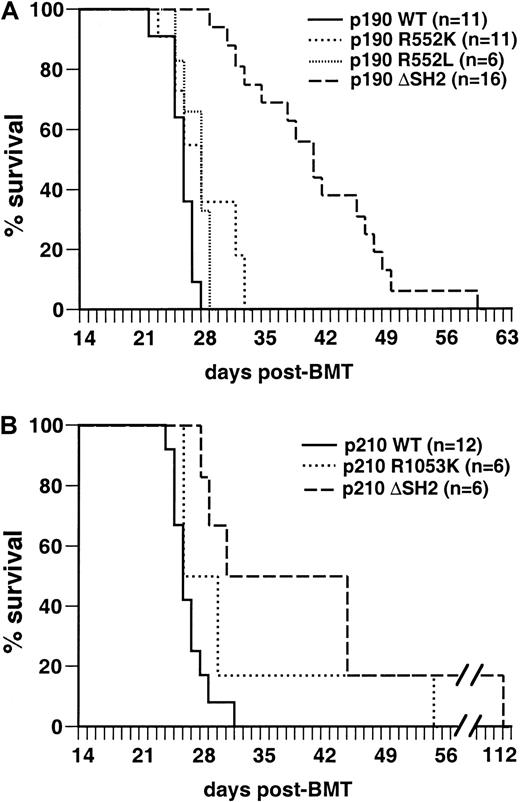
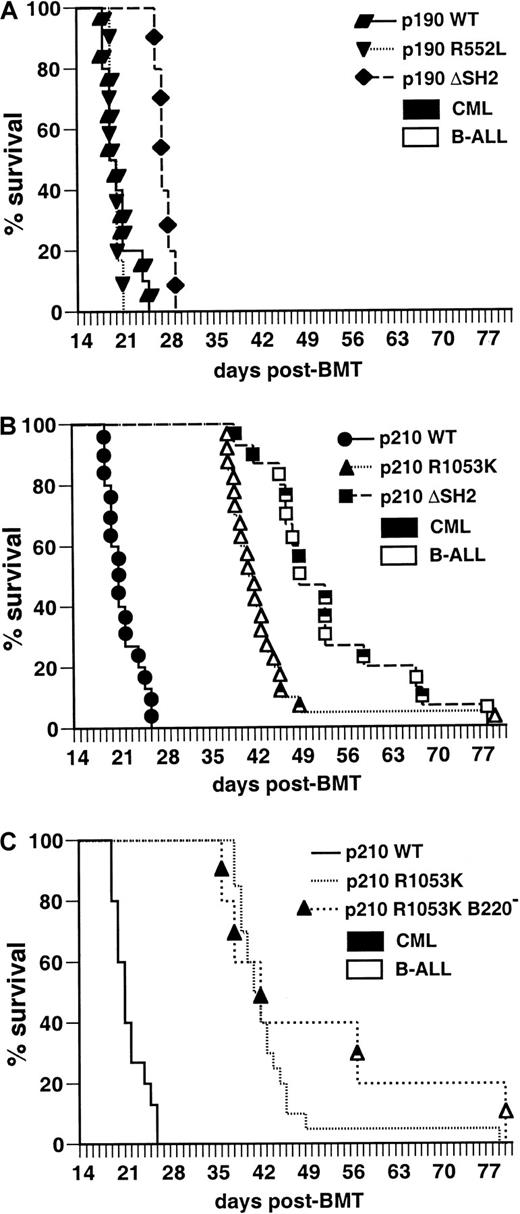
Mice transplanted with marrow transduced by wild-type p190BCR/ABL under these conditions developed B-lymphoid leukemia with 100% incidence within 4 weeks after transplantation (Figure3A), characterized by circulating malignant B220+ lymphoblasts, lymphadenopathy, moderate splenomegaly (average weight 0.25 g), normal thymus, and a hemorrhagic malignant pleural effusion that was the cause of death. The clinicopathologic features of the disease were identical to those previously observed in this model system.5 Recipients of marrow transduced with p190 Bcr/Abl containing an FLVRES point mutation (R552K or R552L) developed identical leukemias with the same latency, whereas p190 with a complete deletion of SH2 (ΔSH2) also developed B-lymphoid leukemia but survived somewhat longer (Figure 3A) and tended to exhibit bulkier lymphadenopathy. Similar results were obtained with the p210 SH2 mutants (Figure 3B). The B-lymphoid leukemias were oligoclonal by proviral integration (Figure4) and were efficiently transplanted, with no significant difference in survival of secondary recipients of p190 wild-type or SH2 mutant-induced leukemias (data not shown). In selected mice, we isolated macrophages and neutrophils and found that DNA from these myeloid cells lacked the proviral integrations found in the lymphoblasts (data not shown). This finding confirms that the target cell for the B-lymphoid leukemias is lineage-restricted,5 and that myeloid cells are not part of the leukemic process in these animals. Because the R552L mutation creates a new XhoI restriction enzyme recognition site in the BCR/ABL provirus, we were able to verify by Southern blot analysis that the FLVRES mutation had not reverted to wild-type in malignant cells transduced with the p190 R552L mutant (data not shown).
The SH2 domain of p190 Bcr/Abl is not required for induction of B-lymphoid leukemia in mice.
Kaplan-Meier survival curve for recipients of marrow transduced with p190 BCR/ABL wild-type and the indicated SH2 mutants (panel A, top) and p210 BCR/ABL wild-type and SH2 mutants (panel B, bottom). Several independent transplantation experiments were carried out for each mutant with similar outcome, and the results are combined here. The number of individual mice in each arm is indicated. All animals developed B-lymphoid leukemia that was identical to that previously described,5 except that the majority of p190 ΔSH2-transduced mice had bulky lymphadenopathy at autopsy, with the size of individual lymph nodes from 5 to 12 mm and the combined weight of inguinal, axillary, and cervical nodes often exceeding 1 g (average, 0.65 ± 0.27 g versus 0.27 ± 0.15 g for p190 wild-type). The survival of mice receiving p190 ΔSH2-transduced marrow was significantly longer (P < .0001, Mantel-Cox test), whereas there was no significant difference in survival between p190 wild-type, p190 R552K, and p190 R552L.
The SH2 domain of p190 Bcr/Abl is not required for induction of B-lymphoid leukemia in mice.
Kaplan-Meier survival curve for recipients of marrow transduced with p190 BCR/ABL wild-type and the indicated SH2 mutants (panel A, top) and p210 BCR/ABL wild-type and SH2 mutants (panel B, bottom). Several independent transplantation experiments were carried out for each mutant with similar outcome, and the results are combined here. The number of individual mice in each arm is indicated. All animals developed B-lymphoid leukemia that was identical to that previously described,5 except that the majority of p190 ΔSH2-transduced mice had bulky lymphadenopathy at autopsy, with the size of individual lymph nodes from 5 to 12 mm and the combined weight of inguinal, axillary, and cervical nodes often exceeding 1 g (average, 0.65 ± 0.27 g versus 0.27 ± 0.15 g for p190 wild-type). The survival of mice receiving p190 ΔSH2-transduced marrow was significantly longer (P < .0001, Mantel-Cox test), whereas there was no significant difference in survival between p190 wild-type, p190 R552K, and p190 R552L.
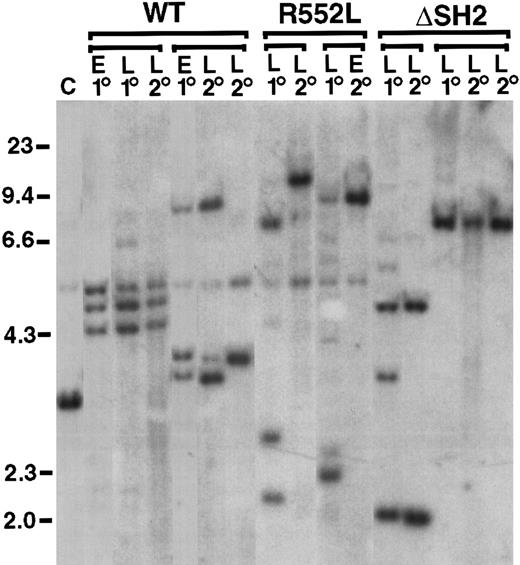
Oligoclonal disease in primary mice with p190-induced B-lymphoid leukemia, with clonal restriction in secondary recipients.
Genomic DNA from lymphoblasts from pleural effusion (E) or lymph node (L) from a primary diseased animal (1°) or secondary transplant recipients of lymph node-derived lymphoblasts (2°) were digested withBglII and hybridized with a radioactive probe from the neomycin resistance gene. Groups of primary and secondary recipients are indicated by brackets; in some cases, 2 different secondary recipients of the same primary leukemia are shown. Tumor cell DNA from secondary transplant recipients often contained only a subset of the proviral clones observed in the primary tumor, suggesting that secondary transplantation selects for growth of particular clones, perhaps reflecting continued evolution of p190-transformed lymphoid cells to a more aggressive state. Control DNA (C) from a cell line containing a single BCR/ABL provirus is on the left, as are molecular size markers in kilobases (kb).
Oligoclonal disease in primary mice with p190-induced B-lymphoid leukemia, with clonal restriction in secondary recipients.
Genomic DNA from lymphoblasts from pleural effusion (E) or lymph node (L) from a primary diseased animal (1°) or secondary transplant recipients of lymph node-derived lymphoblasts (2°) were digested withBglII and hybridized with a radioactive probe from the neomycin resistance gene. Groups of primary and secondary recipients are indicated by brackets; in some cases, 2 different secondary recipients of the same primary leukemia are shown. Tumor cell DNA from secondary transplant recipients often contained only a subset of the proviral clones observed in the primary tumor, suggesting that secondary transplantation selects for growth of particular clones, perhaps reflecting continued evolution of p190-transformed lymphoid cells to a more aggressive state. Control DNA (C) from a cell line containing a single BCR/ABL provirus is on the left, as are molecular size markers in kilobases (kb).
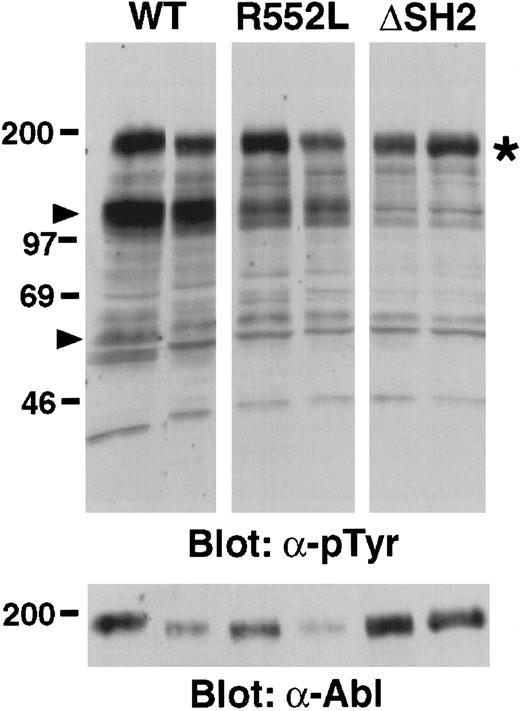
The Bcr/Abl SH2 mutants exhibit decreased kinase activity in vivo but no defect in PI 3-kinase activation in malignant lymphoblasts
In primary malignant lymphoblasts isolated from mice transduced with p190 BCR/ABL and the SH2 mutants, we observed a fairly consistent global decrease in the tyrosine phosphorylation of cellular proteins in cells transformed by the p190 SH2 point mutants, and a further decrease with the SH2 deletion mutant (Figure5). As previously observed,3 24 there was a prominent decrease in the tyrosine phosphorylation of proteins of molecular mass around 110 to 120 kd and 62 kd, but relatively similar levels of tyrosine phosphorylation of Bcr/Abl itself. We did not observe any novel tyrosine phosphorylated species in cells transduced with the p190 SH2 mutants.
Decreased in vivo tyrosine kinase activity of p190
BCR/ABL SH2 mutants. Proteins from malignant pleural effusion-derived lymphoblasts from 2 different primary recipients of marrow transduced with p190 BCR/ABL wild-type (WT), R552K, or ΔSH2 were blotted with monoclonal antibodies against phosphotyrosine (upper panel) or Abl (bottom panel). Equal amounts of protein were loaded in each lane by Ponceau-S staining of the membrane, and equivalent amounts of p46 and p52 Shc proteins were detected in each lane after immunoblotting with anti-Shc antibodies (data not shown). Molecular size markers (kd) are at left. Arrowheads indicate the position of p110-120 and p62 proteins whose tyrosine phosphorylation is greatly decreased in the SH2 mutant-transduced cells. The position of Bcr/Abl is indicated by the asterisk. The decreased size of the p190 ΔSH2 protein (about 1 kd) is not apparent under these conditions.
Decreased in vivo tyrosine kinase activity of p190
BCR/ABL SH2 mutants. Proteins from malignant pleural effusion-derived lymphoblasts from 2 different primary recipients of marrow transduced with p190 BCR/ABL wild-type (WT), R552K, or ΔSH2 were blotted with monoclonal antibodies against phosphotyrosine (upper panel) or Abl (bottom panel). Equal amounts of protein were loaded in each lane by Ponceau-S staining of the membrane, and equivalent amounts of p46 and p52 Shc proteins were detected in each lane after immunoblotting with anti-Shc antibodies (data not shown). Molecular size markers (kd) are at left. Arrowheads indicate the position of p110-120 and p62 proteins whose tyrosine phosphorylation is greatly decreased in the SH2 mutant-transduced cells. The position of Bcr/Abl is indicated by the asterisk. The decreased size of the p190 ΔSH2 protein (about 1 kd) is not apparent under these conditions.
To determine whether the BCR/ABL SH2 mutants were defective in activation of PI 3-kinase activity in lymphoid cells, we prepared extracts from primary bone marrow B-lymphoid cells immediately after in vitro transformation by BCR/ABL wild-type and the SH2 mutants. We observed prominent activation of PI 3-kinase in cells transformed by wild-type p190 and p210 BCR/ABL and no significant or consistent decrease in PI 3-kinase activity with the SH2 point and deletion mutants (Figure 6A). We also examined PI 3-kinase activation in malignant lymphoblasts isolated from lymph nodes or pleural effusion from mice transduced with p190 BCR/ABL wild-type and the SH2 mutants. As a control, we determined PI 3-kinase activity in extracts of B220+splenocytes isolated from mice 8 weeks after transplantation with untransduced bone marrow. In antiphosphotyrosine immunoprecipitates, we found high levels of PI 3-kinase activity in lymphoblasts from wild-type p190-transduced mice relative to control B220+splenocytes, and similar levels of activated PI 3-kinase with the p190 R552K, R552L, and ΔSH2 mutants (Figure 6B). Determination of total cellular PI 3-kinase activity by assay of whole cell lysates demonstrated a typical 20% to 30% increase in the activity of p190 wild-type-transformed cells relative to control B220+splenocytes and similar increases for the SH2 mutants (Figure 6C). These results demonstrate that, despite the lower in vivo kinase activity of the Bcr/Abl SH2 mutants, there is no decrease in activation of PI 3-kinase in malignant lymphoid cells expressing these mutants.
Activation of PI 3-kinase in malignant lymphoblasts from mice with p190-induced B-lymphoid leukemia.
(A) Whitlock-Witte (W/W) cultures. Primary bone marrow cells were transduced with BCR/ABL wild-type and SH2 FLVRES (RK = R552K for p190 and R1053K for p210) and deletion (ΔSH2) mutants and cultured in vitro as described in “Materials and methods.” Stromal-independent populations of lymphoblasts were readily established, cell extracts prepared 12 to 14 days after transduction, and assayed for PI 3-kinase activity in antiphosphotyrosine immunoprecipitates. As controls, Ba/F3 parental cells or a population transformed with p210 BCR/ABL(Ba/F3-210), and B220+ splenocytes isolated from Balb/c mice 8 weeks after transplantation with untransduced donor marrow (B220+ spl.) were used. Results are representative of 3 independent experiments and expressed as fold increase in PI 3-kinase activity over that present in parental Ba/F3 cells. (B) Primary malignant lymphoblasts from diseased mice. Lysates from cells isolated from pleural effusion (left) or malignant lymph nodes (right) were assayed for PI 3-kinase activity in antiphosphotyrosine immunoprecipitates. Wright-Giemsa staining of cytospin preparations indicated that all populations were more than 80% lymphoblasts (data not shown). Results represent the mean and standard error of PI 3-kinase activity in tumor cell populations from several independent animals for each p190 genotype, as indicated by the numbers at the bottom, expressed as fold increase over control B220+splenocytes (B220+ spl.). The difference in mean lymph node PI 3-kinase activity between p190 wild-type and p190 ΔSH2 was not significant (P = .66, Student t test). (C) Whole cell lysate assay. PI 3-kinase activity was measured in diluted whole cell lysates from lymph node-derived lymphoblasts and control B220+ splenocytes, as described in “Materials and methods.” Results represent the mean and standard error for PI 3-kinase activity in lymphoblasts from several independent mice, as indicated, expressed as fold increase over B220+splenocytes.
Activation of PI 3-kinase in malignant lymphoblasts from mice with p190-induced B-lymphoid leukemia.
(A) Whitlock-Witte (W/W) cultures. Primary bone marrow cells were transduced with BCR/ABL wild-type and SH2 FLVRES (RK = R552K for p190 and R1053K for p210) and deletion (ΔSH2) mutants and cultured in vitro as described in “Materials and methods.” Stromal-independent populations of lymphoblasts were readily established, cell extracts prepared 12 to 14 days after transduction, and assayed for PI 3-kinase activity in antiphosphotyrosine immunoprecipitates. As controls, Ba/F3 parental cells or a population transformed with p210 BCR/ABL(Ba/F3-210), and B220+ splenocytes isolated from Balb/c mice 8 weeks after transplantation with untransduced donor marrow (B220+ spl.) were used. Results are representative of 3 independent experiments and expressed as fold increase in PI 3-kinase activity over that present in parental Ba/F3 cells. (B) Primary malignant lymphoblasts from diseased mice. Lysates from cells isolated from pleural effusion (left) or malignant lymph nodes (right) were assayed for PI 3-kinase activity in antiphosphotyrosine immunoprecipitates. Wright-Giemsa staining of cytospin preparations indicated that all populations were more than 80% lymphoblasts (data not shown). Results represent the mean and standard error of PI 3-kinase activity in tumor cell populations from several independent animals for each p190 genotype, as indicated by the numbers at the bottom, expressed as fold increase over control B220+splenocytes (B220+ spl.). The difference in mean lymph node PI 3-kinase activity between p190 wild-type and p190 ΔSH2 was not significant (P = .66, Student t test). (C) Whole cell lysate assay. PI 3-kinase activity was measured in diluted whole cell lysates from lymph node-derived lymphoblasts and control B220+ splenocytes, as described in “Materials and methods.” Results represent the mean and standard error for PI 3-kinase activity in lymphoblasts from several independent mice, as indicated, expressed as fold increase over B220+splenocytes.
The phosphotyrosine-binding function of the Abl SH2 domain is required for efficient induction of CML-like myeloproliferative disease in mice by p210 BCR/ABL, but not p190
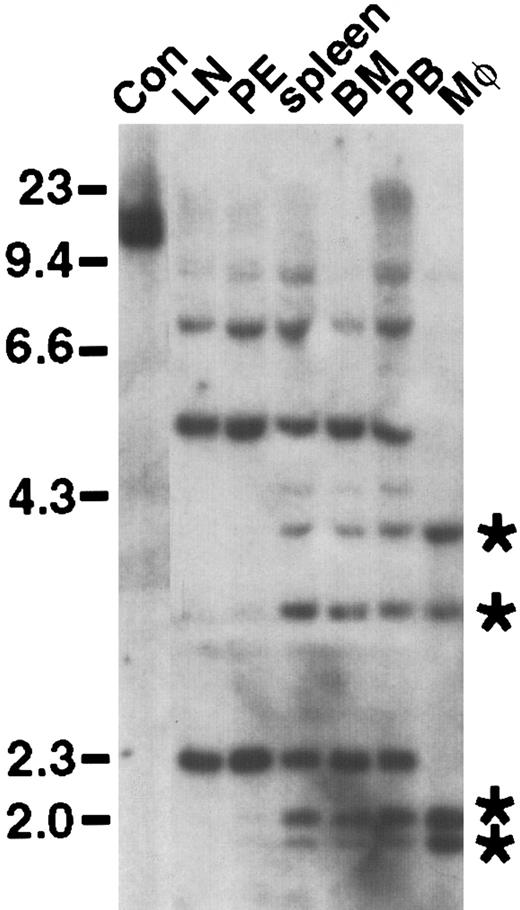
We next tested the leukemogenicity of the Bcr/Abl SH2 mutants under conditions (described in “Materials and methods”) in which wild-type p210 and p190 Bcr/Abl induce CML-like myeloproliferative disease in 100% of transplant recipients within 4 weeks (Figure7A,B).5Recipients of marrow transduced with the p210 ΔSH2 mutant exhibited prolonged survival (median 8.5 weeks; Figure 7B). Some recipients developed typical CML-like illness5 between 6 and 8 weeks after transplant, with greatly increased peripheral blood neutrophils, hepatosplenomegaly with myeloid cell infiltration, and pulmonary parenchymal infiltration and hemorrhage, with the pulmonary disease the immediate cause of death. Other mice developed fatal B-lymphoid leukemia between 7 and 11 weeks after transplant, characterized by peripheral lymphadenopathy, a hemorrhagic malignant pleural effusion and infiltration of spleen and marrow with malignant B220+lymphoblasts. This leukemia was histopathologically identical to that observed with the mice in Figure 3, with the immediate cause of death being the pleural effusion. A third of the recipient mice developed both diseases simultaneously (Figure 7B); because of the distinctive clinicopathologic features of the 2 malignancies, such animals were easily recognized. Analysis of genomic DNA from malignant myeloid cells by Southern blotting demonstrated the presence of 4 to 14 independent proviral clones (average 7) contributing to the CML-like disease (data not shown); in animals with both diseases, different sets of proviral clones were detected in lymphoblasts and myeloid cells (Figure8), in accordance with previous observations that the target cells for these diseases are distinct.5 These results demonstrate that the Abl SH2 domain is not absolutely required for induction of CML-like disease by p210 BCR/ABL, but the efficiency of disease induction is lower in the absence of SH2.
The phosphotyrosine-binding function of the Abl SH2 domain is required for efficient induction of CML-like disease in mice by p210
BCR/ABL but not by p190. Kaplan-Meier survival curves for recipients of marrow transduced withBCR/ABL wild-type and the indicated SH2 mutants; the individual mice in each arm are designated by the symbols. Several independent transplantation experiments were carried out for each mutant with similar outcome, and the results combined here. (A) Survival curve for recipients of p190 BCR/ABL wild-type (WT)- and SH2 mutant-transduced marrow (p190 WT = 10, p190 R552L = 6, and p190 ΔSH2 = 5); all mice developed CML-like disease (closed symbols). There was no significant difference in survival between p190 WT and p190 R552L recipients, whereas the increased survival of p190 ΔSH2 recipients relative to WT was of borderline significance (P = .005, Mantel-Cox test). (B) Survival curve for recipients of p210 BCR/ABL WT and SH2 mutant-transduced marrow (p210 WT = 15, p210 R1053K = 20, p210 ΔSH2 = 15). All recipients of p210 WT-transduced marrow developed CML-like disease (closed symbols), whereas recipients of marrow transduced with the p210 R1053K SH2 mutant developed predominantly B-lymphoid leukemia (open symbols). Recipients of p210 ΔSH2-transduced marrow developed CML-like disease, B-lymphoid leukemia, or both malignancies simultaneously (indicated by the mixed symbols). Mice were diagnosed with simultaneous B-lymphoid leukemia and CML-like disease if they exhibited the cardinal clinicopathologic features of each disease (bloody pleural effusion and lymphadenopathy for B-lymphoid leukemia; peripheral blood neutrophil count more than 50 × 103/μL, splenomegaly more than 0.5 gm, and pulmonary parenchymal hemorrhage for CML-like disease) to an extent that both malignancies contributed to premorbidity or death. The survival of mice receiving marrow transduced with either p210 SH2 mutant was significantly longer than that of recipients of p210 wild-type-transduced marrow (P < .0001, Mantel-Cox test). (C) Survival curve for recipients of p210 R1053K-transduced marrow that was depleted of B220-expressing cells before transplantation. The survival curves for recipients of p210 R1053K-transduced unfractionated marrow and for p210 WT-transduced marrow (identical to those in panel B) are reproduced for comparison.
The phosphotyrosine-binding function of the Abl SH2 domain is required for efficient induction of CML-like disease in mice by p210
BCR/ABL but not by p190. Kaplan-Meier survival curves for recipients of marrow transduced withBCR/ABL wild-type and the indicated SH2 mutants; the individual mice in each arm are designated by the symbols. Several independent transplantation experiments were carried out for each mutant with similar outcome, and the results combined here. (A) Survival curve for recipients of p190 BCR/ABL wild-type (WT)- and SH2 mutant-transduced marrow (p190 WT = 10, p190 R552L = 6, and p190 ΔSH2 = 5); all mice developed CML-like disease (closed symbols). There was no significant difference in survival between p190 WT and p190 R552L recipients, whereas the increased survival of p190 ΔSH2 recipients relative to WT was of borderline significance (P = .005, Mantel-Cox test). (B) Survival curve for recipients of p210 BCR/ABL WT and SH2 mutant-transduced marrow (p210 WT = 15, p210 R1053K = 20, p210 ΔSH2 = 15). All recipients of p210 WT-transduced marrow developed CML-like disease (closed symbols), whereas recipients of marrow transduced with the p210 R1053K SH2 mutant developed predominantly B-lymphoid leukemia (open symbols). Recipients of p210 ΔSH2-transduced marrow developed CML-like disease, B-lymphoid leukemia, or both malignancies simultaneously (indicated by the mixed symbols). Mice were diagnosed with simultaneous B-lymphoid leukemia and CML-like disease if they exhibited the cardinal clinicopathologic features of each disease (bloody pleural effusion and lymphadenopathy for B-lymphoid leukemia; peripheral blood neutrophil count more than 50 × 103/μL, splenomegaly more than 0.5 gm, and pulmonary parenchymal hemorrhage for CML-like disease) to an extent that both malignancies contributed to premorbidity or death. The survival of mice receiving marrow transduced with either p210 SH2 mutant was significantly longer than that of recipients of p210 wild-type-transduced marrow (P < .0001, Mantel-Cox test). (C) Survival curve for recipients of p210 R1053K-transduced marrow that was depleted of B220-expressing cells before transplantation. The survival curves for recipients of p210 R1053K-transduced unfractionated marrow and for p210 WT-transduced marrow (identical to those in panel B) are reproduced for comparison.
Distinct target cells for p210 ΔSH2-induced CML-like disease and B-lymphoid leukemia.
Genomic DNA from the indicated hematopoietic tissues and lineages (LN, lymph node; PE, pleural effusion; BM; bone marrow; PB, peripheral blood; Mφ, macrophages) from an animal with simultaneous CML-like disease and B-lymphoid leukemia was digested withBglII and hybridized with a radioactive probe from the neomycin resistance gene. Control DNA (Con) from a cell line containing a single BCR/ABL provirus is in the first lane on the left. DNA size markers (in kb) are at left, whereas the positions of 4 unique proviral clones in peripheral blood neutrophils and peritoneal macrophages are indicated by asterisks at the right.
Distinct target cells for p210 ΔSH2-induced CML-like disease and B-lymphoid leukemia.
Genomic DNA from the indicated hematopoietic tissues and lineages (LN, lymph node; PE, pleural effusion; BM; bone marrow; PB, peripheral blood; Mφ, macrophages) from an animal with simultaneous CML-like disease and B-lymphoid leukemia was digested withBglII and hybridized with a radioactive probe from the neomycin resistance gene. Control DNA (Con) from a cell line containing a single BCR/ABL provirus is in the first lane on the left. DNA size markers (in kb) are at left, whereas the positions of 4 unique proviral clones in peripheral blood neutrophils and peritoneal macrophages are indicated by asterisks at the right.
Surprisingly, all but 2 of the mice transplanted with marrow transduced by the p210 SH2 point mutant (R1053K) died from B-lymphoid leukemia around 6 to 7 weeks after transplant (Figure 7B), with little or no gross evidence of CML-like disease at autopsy. About half of these recipients also had small macrophage tumors confined to the liver that did not contribute to cause of death (data not shown). The remaining 2 mice developed mixed CML-like disease and B-lymphoid leukemia. At first glance, these results suggest that the p210 SH2 FLVRES point mutant is more defective for myeloid leukemogenesis than p210 with complete deletion of SH2. However, analysis of recipients of p210 R1053K-transduced marrow between 3 and 6 weeks after transplant (Table1) revealed that most exhibited palpable splenomegaly and peripheral blood leukocytosis (70 to 200 × 103/μL) with excess neutrophils preceding the development of B-lymphoid leukemia. This suggests that the R1053K mutant is also able to inefficiently induce myeloproliferative disease, but the subsequent rapid emergence of B-lymphoid leukemia obscures the CML-like disease by the time of death. To demonstrate this, potential target cells for BCR/ABL-induced B-lymphoid leukemia were removed from p210 R1053K-transduced bone marrow by negative selection of cells expressing B220 antigen. Recipients of B220-negative p210 R1053K-transduced marrow developed predominantly CML-like disease with latency similar to that of the B-lymphoid leukemia (Figure 7C), confirming that the p210 R1053K mutant is capable of inducing delayed CML-like disease if lymphoid leukemogenesis by this mutant is attenuated. Interestingly, although recipients with CML-like disease in Figure 7C lacked the lymphadenopathy and pleural effusion characteristic of B-lymphoid leukemia, all exhibited minor populations of B-lymphoid blasts in peripheral blood and spleen (data not shown), suggesting that not all target cells forBCR/ABL-induced B-lymphoid leukemia express B220.
Clinicopathologic features of recipients of p210 R1053K-transduced marrow before death
| Mouse . | Day after transplantation . | |||
|---|---|---|---|---|
| Day 22 . | Day 28 . | Day 35 . | Death (day) . | |
| p210 R1053K #2 | (day 38) | |||
| Pb WBC* | ND | 99 000 | ND | 4 000 |
| Differential† | 33% myeloid | 90% lymphoblasts | ||
| Spleen size‡ | ++ | 0.34 g | ||
| p210 R1053K #4 | (day 40) | |||
| Pb WBC* | ND | 345 000 | ND | ND |
| Differential† | 14% myeloid | |||
| Spleen size‡ | +++ | 0.41 g | ||
| p210 R1053K #5 | (day 41) | |||
| Pb WBC* | ND | 195 000 | ND | 4 800 |
| Differential† | 31% myeloid | 90% lymphoblasts | ||
| Spleen size‡ | +++ | 0.42 g | ||
| p210 R1053K #6 | (day 42) | |||
| Pb WBC* | 67 000 | ND | 90 000 | ND |
| Differential† | 30% myeloid | 60% myeloid | ||
| Spleen size‡ | ++ | ++ | 0.5 g | |
| Mouse . | Day after transplantation . | |||
|---|---|---|---|---|
| Day 22 . | Day 28 . | Day 35 . | Death (day) . | |
| p210 R1053K #2 | (day 38) | |||
| Pb WBC* | ND | 99 000 | ND | 4 000 |
| Differential† | 33% myeloid | 90% lymphoblasts | ||
| Spleen size‡ | ++ | 0.34 g | ||
| p210 R1053K #4 | (day 40) | |||
| Pb WBC* | ND | 345 000 | ND | ND |
| Differential† | 14% myeloid | |||
| Spleen size‡ | +++ | 0.41 g | ||
| p210 R1053K #5 | (day 41) | |||
| Pb WBC* | ND | 195 000 | ND | 4 800 |
| Differential† | 31% myeloid | 90% lymphoblasts | ||
| Spleen size‡ | +++ | 0.42 g | ||
| p210 R1053K #6 | (day 42) | |||
| Pb WBC* | 67 000 | ND | 90 000 | ND |
| Differential† | 30% myeloid | 60% myeloid | ||
| Spleen size‡ | ++ | ++ | 0.5 g | |
WBC = white blood cell; Pb = peripheral blood; ND = not done.
Peripheral blood leukocyte count (per μL).
Percentage of maturing myeloid cells (CD11b+) or lymphoblasts (B220+) assessed by morphology or FACS.
Assessed by palpation: ++ easily palpable; +++ greatly enlarged.
In contrast to the p210 SH2 mutants, there was no defect in induction of CML-like disease by the corresponding p190 SH2 point mutant R552L, and only a slight delay for induction of CML-like disease by p190 ΔSH2 (Figure 7A). Therefore, although there is no significant difference in induction of CML-like disease between wild-type p210 and p190 BCR/ABL,5 mutation of SH2 reveals a difference in potency between the 2 oncogenes.
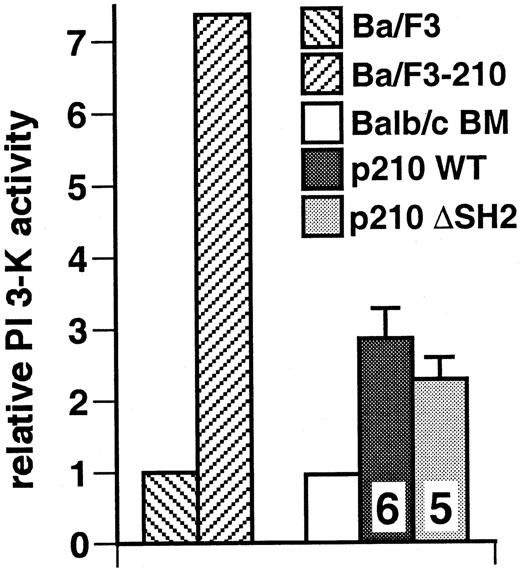
The Bcr/Abl SH2 domain is not required for activation of PI 3-kinase in primary myeloid cells from mice with CML-like disease
We assayed PI 3-kinase activity in extracts from spleens and peripheral blood leukocytes from mice with CML-like disease induced by p210 Bcr/Abl wild-type and p210 ΔSH2, populations that consisted predominantly of maturing myeloid cells. Relative to control bone marrow from normal Balb/c mice, we found elevated levels of PI 3-kinase activity in antiphosphotyrosine immunoprecipitates from recipients of p210 wild-type–transduced marrow and equivalent activity in extracts from recipients of p210 ΔSH2-transduced marrow (Figure9). Similar results were obtained with assay of whole cell lysates (data not shown). These results demonstrate that the SH2 domain is not absolutely required for activation of PI 3-kinase in primary myeloid cells by Bcr/Abl.
The SH2 domain of p210 Bcr/Abl is not required for activation of PI 3-kinase in malignant myeloid cells from mice with CML-like disease.
Lysates from spleen cells or peripheral blood leukocytes from mice with CML-like disease induced by wild-type p210 and the p210 ΔSH2 mutant were assayed for PI 3-kinase activity in antiphosphotyrosine immunoprecipitates. Wright-Giemsa staining of cytospin preparations indicated that all populations were more than 90% maturing myeloid cells (neutrophils, macrophages, and erythroid cells for spleen, and neutrophils, metamyelocytes, and myelocytes for peripheral blood) (data not shown). Results represent the mean and standard error of PI 3-kinase activity in cell lysates from several independent animals for each genotype, with the number of samples analyzed indicated. As controls, Ba/F3 parental cells or a population transformed with wild-type p210 BCR/ABL (Ba/F3-210) and untransduced bone marrow from Balb/c mice (more than 70% maturing myeloid and erythroid cells by cytospin analysis) were used. The difference in mean PI 3-kinase activity between p210 WT and p210 ΔSH2 was not significant (P = .41, Student t test).
The SH2 domain of p210 Bcr/Abl is not required for activation of PI 3-kinase in malignant myeloid cells from mice with CML-like disease.
Lysates from spleen cells or peripheral blood leukocytes from mice with CML-like disease induced by wild-type p210 and the p210 ΔSH2 mutant were assayed for PI 3-kinase activity in antiphosphotyrosine immunoprecipitates. Wright-Giemsa staining of cytospin preparations indicated that all populations were more than 90% maturing myeloid cells (neutrophils, macrophages, and erythroid cells for spleen, and neutrophils, metamyelocytes, and myelocytes for peripheral blood) (data not shown). Results represent the mean and standard error of PI 3-kinase activity in cell lysates from several independent animals for each genotype, with the number of samples analyzed indicated. As controls, Ba/F3 parental cells or a population transformed with wild-type p210 BCR/ABL (Ba/F3-210) and untransduced bone marrow from Balb/c mice (more than 70% maturing myeloid and erythroid cells by cytospin analysis) were used. The difference in mean PI 3-kinase activity between p210 WT and p210 ΔSH2 was not significant (P = .41, Student t test).
There is no qualitative defect in activation of several signaling pathways in myeloid cells by p210 SH2
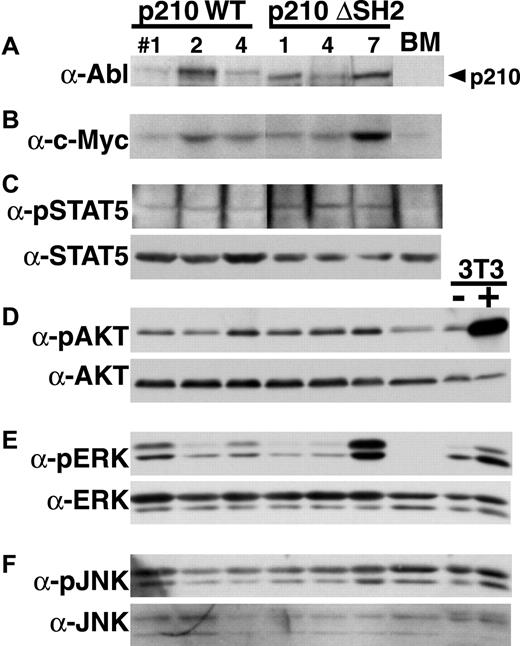
To determine whether there were defects in activation of specific signaling pathways in myeloid cells from mice with p210 ΔSH2-induced CML-like disease, we performed Western blotting with phosphorylation-specific antibodies for STAT5, Akt, ERK, and JNK on extracts from myeloid cells from mice with CML-like disease induced by p210 wild-type and p210 ΔSH2 (Figure10). We found moderate but equal tyrosine phosphorylation of STAT5 in both p210 wild-type and SH2-expressing cells. There was some variability in the level of phosphorylation of Akt, ERK, and JNK between individual samples, but no consistent and significant decrease in activation of these kinases in p210 ΔSH2-expressing cells. Similarly, levels of c-Myc were somewhat variable in different samples, but were not consistently lower in p210 ΔSH2-transformed cells.
No qualitative defect in activation of signaling pathways in primary myeloid cells expressing p210 ΔSH2.
Western blotting with the indicated antibodies was performed on extracts from myeloid cells isolated from mice with p210 wild-type–induced (left side) or p210 ΔSH2-induced (right side) CML-like disease. As controls, extract from untransduced Balb/c bone marrow (BM) and from NIH 3T3 cells without (−) and with (+) stimulation with PDGF were included at the far right. Equivalent amounts of total protein were present in each lane as judged by Ponceau S staining of the membrane. (A) Anti–C-terminal Abl antibody. (B) Anti-c-Myc antibody. (C) Antiphospho-STAT5 (top) and anti-STAT5A (bottom). (D) Anti-phospho-Akt (top) and anti-pan-Akt (bottom). (E) Anti-phospho-ERK (top) and anti-pan-ERK (bottom). Both p42 and p44 ERK are detected. (F) Anti-phospho-JNK (top) and anti-pan-JNK (bottom). Both p46 and p54 JNK are detected.
No qualitative defect in activation of signaling pathways in primary myeloid cells expressing p210 ΔSH2.
Western blotting with the indicated antibodies was performed on extracts from myeloid cells isolated from mice with p210 wild-type–induced (left side) or p210 ΔSH2-induced (right side) CML-like disease. As controls, extract from untransduced Balb/c bone marrow (BM) and from NIH 3T3 cells without (−) and with (+) stimulation with PDGF were included at the far right. Equivalent amounts of total protein were present in each lane as judged by Ponceau S staining of the membrane. (A) Anti–C-terminal Abl antibody. (B) Anti-c-Myc antibody. (C) Antiphospho-STAT5 (top) and anti-STAT5A (bottom). (D) Anti-phospho-Akt (top) and anti-pan-Akt (bottom). (E) Anti-phospho-ERK (top) and anti-pan-ERK (bottom). Both p42 and p44 ERK are detected. (F) Anti-phospho-JNK (top) and anti-pan-JNK (bottom). Both p46 and p54 JNK are detected.
Discussion
The Philadelphia chromosome is predominantly found in 2 distinct forms of human leukemia: the chronic myeloproliferative disease CML and the acute disease B-lymphoid or lymphoblastic leukemia. These leukemias are very different in their effect on the hematopoietic system, cell of origin, and response to therapy.1 CML is typified by overproduction of maturing myeloid cells with essentially normal differentiation, arises in a hematopoietic stem cell, and in chronic phase is very responsive to low-dose myelosuppressive agents like hydroxyurea and busulfan, and to interferon-alpha. In contrast, Philadelphia-positive B-lymphoblastic leukemia is characterized by a profound arrest in lymphoid differentiation, at least some cases appear to arise in a lymphoid progenitor, and the disease is only responsive to high-dose combinations of cytotoxic chemotherapeutic agents. Although both leukemias are directly caused by Bcr/Abl, the profound difference in the biology of these malignancies suggests that the critical signaling pathways for leukemogenesis by Bcr/Abl in the 2 disorders may differ as well. Our results provide the first direct evidence of a difference in Bcr/Abl signaling between these 2 diseases in a mouse model of Philadelphia-positive leukemogenesis. We found that the phosphotyrosine-binding function of the Abl SH2 domain is dispensable for induction of B-lymphoid leukemia by Bcr/Abl, but is required for efficient induction of CML-like disease by p210 Bcr/Abl. This difference is of potential clinical relevance, as small molecules designed to disrupt the interaction of the Abl SH2 domain with phosphotyrosine-containing ligands might have therapeutic activity in chronic phase CML, but would be predicted to be ineffective in treatment of Philadelphia-positive B-lymphoid leukemia.
We found that mutation of the Abl SH2 domain impairs the intrinsic tyrosine kinase activity of Bcr/Abl proteins. The R to K mutation in the FLVRES motif decreases kinase activity of p190 and p210 Bcr/Abl by about half, whereas complete deletion of SH2 profoundly lowers the activity of p190 and p210 to a level near or below that of c-Abl, respectively. A physical interaction between the Abl SH2 domain and Bcr first exon sequences has been postulated to contribute to activation of Abl kinase activity,30 and it is possible that the SH2 mutations interfere with this. Despite the negative effect on in vitro and in vivo Bcr/Abl tyrosine kinase activity, elimination of Abl SH2 domain binding to phosphotyrosine has no discernible impact on the ability of Bcr/Abl to transform primary bone marrow B-lymphoid progenitors in vitro and to induce B-lymphoid leukemia in vivo. Previous studies also found no defect in transformation of B-lymphoid cells in vitro by the p190 R552L mutant, but when these cells were adoptively transferred by subcutaneous injection into unirradiated SCID mice, there was a 75% decrease in efficiency of leukemia induction relative to p190 wild-type and an increase in disease latency from 2 weeks to 10 to12 weeks.27 The reason for the difference with our results is not known, but might reflect decreased survival or altered vascular migration of p190 R552L-transformed lymphoid cells after subcutaneous injection in SCID mice. In our studies, a moderate increase in disease latency was observed for p190 with a complete deletion of the SH2 domain. The increased latency with this mutant appears to reflect increased time required to develop the leukemia rather than the aggressiveness of the established disease, because the survival (4 to 7 weeks) of mice inoculated with p190 ΔSH2-transformed lymphoblasts in a secondary transplant was similar to secondary mice that received p190 wild-type-transduced cells (data not shown).
In contrast to lymphoid leukemogenesis, our results suggest a significant role for phosphotyrosine-binding by SH2 in the induction of CML-like myeloproliferative disease by p210 BCR/ABL. With a p210 mutant lacking SH2, we observed a decrease in the efficiency of induction of CML-like illness, with a delay in development of disease in some recipients and development of B-lymphoid leukemia in others. The demonstration of a qualitative and quantitative defect in leukemogenesis by the p210 ΔSH2 mutant is consistent with a previous descriptive study of myeloid leukemogenesis by this mutant in SCID mouse recipients.28 B-lymphoid leukemia is never observed in recipients of p210 wild-type–transduced bone marrow from 5-FU–treated donors,5 most likely because all mice die of CML-like disease within 4 weeks after transplant. Because the bone marrow target cells for these 2 diseases are distinct, this suggests that there is competition between the 2 forms of leukemia after transduction with the p210 ΔSH2 mutant. This model is supported by several observations: (1) Some recipients of p210 SH2-transduced marrow developed simultaneous CML-like disease and B-lymphoid leukemia, with distinct proviral clones in lymphoblasts and myeloid cells (Figure 8); (2) with the p210 R1053K mutant, there was evidence of myeloproliferative disease between 3 and 5 weeks after transplantation, with excess peripheral blood neutrophils and gross splenomegaly (Table 1), but the majority of these mice ultimately succumbed to B-lymphoid leukemia several weeks later, as increasing replacement of bone marrow with lymphoblasts caused progressive impairment of myelopoiesis; and (3) removal of B220-positive cells from p210 R1053K-transduced marrow results in predominantly CML-like disease in recipients (Figure 7C). Formal proof of this model will require purification and selective transplantation of transduced target cells for the CML-like disease and B-lymphoid leukemia.
We found that the SH2 domain was not required for efficient induction of CML-like disease by the p190 form of Bcr/Abl: p190 R552L was indistinguishable from wild-type p190, whereas p190 ΔSH2 induced CML-like disease in all recipients with only a slight delay. Therefore, although there is very little difference in the ability of wild-type p190 and p210 to induce CML-like disease,5 a significant difference between these closely related oncoproteins is revealed in the absence of phosphotyrosine binding by the SH2 domain. Differences between p190 and p210 have also been demonstrated for transformation of B-lymphoid cells in vitro4 and B-lymphoid leukemogenesis in vivo.5 This could reflect the higher intrinsic tyrosine kinase activity of p190,2 3 but this cannot be the sole explanation, because the in vitro kinase activity of p210 R1053K, which induces CML inefficiently and with a delay, is greater than that of p190 ΔSH2 (Figure 1). Alternatively, the presence of additional Bcr sequences in p210 Bcr/Abl (including the Dbl homology domain) may decrease the myeloid and/or lymphoid leukemogenicity of p210. The latter hypothesis can now be directly tested.
It is somewhat surprising that the defect in induction of CML-like disease by the p210 ΔSH2 mutant is not more severe, given the drastic nature of the mutation. By comparison, a p210 Bcr/Abl mutant lacking the Grb2 binding site in Bcr (Y177F) is completely unable to induce CML-like leukemia in this model system.31 The specific cause of the myeloid leukemogenesis defect of the p210 Bcr/Abl SH2 mutants is not known. Mice with p210 ΔSH2-induced CML-like disease have the same average number of proviral clones contributing to the disease process as recipients of p210 wild-type–transduced marrow. This suggests that equivalent numbers of stem/progenitor target cells are transduced with p210 wild-type and ΔSH2 BCR/ABL, but there is decreased activation by p210 ΔSH2 of one or more downstream signaling pathways that are critical for stimulation of myelopoiesis. We observed no consistent or significant decrease in PI 3-kinase activity in primary lymphoid and myeloid cells and in cell lines transformed by the Bcr/Abl SH2 mutants, despite decreased levels of cellular phosphotyrosine. In contrast, others have reported that p210 SH2 point and deletion mutants are completely defective for activation of PI 3-kinase in 32D and Ba/F3 cells.28 The reason for this discrepancy is unclear, but the use of PI rather than PIP2 as the lipid substrate in the previous study raises the possibility that contaminating PI 4-kinase activity accounted for some or most of the observed phosphorylation.19,32 Our results suggest that PI 3-kinase may be fully activated by submaximal levels of phosphorylation of PI 3-kinase binding partners such as c-Cbl19 and Gab233 and clearly demonstrate that direct or indirect binding of the p85 or p110 subunits of PI 3-kinase by the Abl SH2 domain is not required for activation. In addition, PI 3-kinase may be activated by Ras in cells expressing the Bcr/Abl SH2 mutants.
We also found no consistent or qualitative defects in activation of ERK, JNK, Akt, or STAT5 in p210 SH2-transduced myeloid cells. Further, although the Abl SH2 domain is required for transcriptional induction of the c-MYC gene after transient expression of v-abl and BCR/ABL in fibroblasts,34we observed similar levels of c-Myc protein in myeloid cells expressing the Bcr/Abl ΔSH2 mutant, suggesting that regulation of c-MYC by Bcr/Abl in proliferating hematopoietic cells is not SH2-dependent. These results argue against a solitary lack of activation of PI 3-kinase or another pathway by the p210 SH2 mutants, although a defect in an as yet unidentified signaling pathway cannot be excluded. It seems more plausible that the myeloid leukemogenesis defect of the p210 SH2 mutants reflects slightly dampened signaling in many downstream pathways as a consequence of the decreased in vitro and in vivo kinase activity of these mutants. This would explain why the defect in transformation by Bcr/Abl SH2 mutants can be complemented by coexpression of so many seemingly disparate proteins, including c-Myc,24 cyclin D1,35 constitutively active Akt,28 Shc,36 and p38 MAPK.36
In summary, we have shown here that the structural requirements for the Bcr/Abl fusion protein to induce B-lymphoid leukemia or CML are different. The phosphotyrosine-binding function of the Abl SH2 domain is not required for B-lymphoid leukemogenesis by Bcr/Abl but is necessary for efficient induction of CML-like myeloproliferative disease by p210 Bcr/Abl. Our results demonstrate the complexity of studying leukemogenesis in animal models by oncogenes likeBCR/ABL, which can transform multiple distinct hematopoietic target cells with different consequences. It is obvious that any model system of BCR/ABL leukemogenesis must be very well characterized to allow useful insights into the pathophysiology of human Philadelphia-positive leukemia. Further application of this bone marrow transduction/transplantation model system should illuminate the critical signaling pathways used by Bcr/Abl to stimulate myelopoiesis, and aid in directing drug development for treatment of CML.
Acknowledgments
We thank Dr Ann-Marie Pendergast for the p190 R552L cDNA, Dr Tomasz Skorski for the gift of 32D cells, and Dr David Frank for the generous gift of antiphospho-STAT5 antibodies. R.A.V. is a Scholar of the Leukemia and Lymphoma Society of America and the Carl and Margaret Walter Scholar in Blood Research at Harvard Medical School.
Supported by NIH grants HL03310 (R.L.I.), CA94536 (L.V.), and CA57593 (R.A.V.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Richard A. Van Etten, Center for Blood Research, 200 Longwood Ave, Boston, MA 02115; email:vanetten@cbr.med.harvard.edu.

![Fig. 1. SH2 mutation decreases Bcr/Abl tyrosine kinase activity in vitro. / Bcr/Abl proteins were expressed by transfection of 293T cells and labeled in vivo with 35[S]-l-methionine. Lysates were immunoprecipitated with anti-Abl antisera and immune complexes incubated with γ-32[P]-ATP and GST-Crk substrate, as described.5 (A) 35[S] label, indicating relative levels of expression of the different Abl proteins. (B) 32[P] label. The position of the GST-Crk substrate is indicated by the arrowhead. The kinase activity of the different Bcr/Abl proteins relative to c-Abl after correction for levels of expression is shown at the top. (C) Coomassie blue stain demonstrating equal amounts of the GST-Crk substrate in all reactions.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/1/10.1182_blood.v97.1.4/6/m_h80110554001.jpeg?Expires=1767695824&Signature=27utxBHeoSDlxqxHSQ~Dy1X8qVnGLwmBlDzsPApPeoQZX3Q4UQ8Oxsi~V10K6k~0B3ydY3ELR-RKNZ0yCmXHtVBSc4a1dzxvRFwTwEApJLGwxLaJRok0oAqxVjKhQqv5ePx-78p9E7kh9Q0xkCJL4gM8fj4c2ROrNuZE7Ow1FiILfR-FgVPM5KRJSo808oa5UuxRWBIOchc393IDe7D1R4ePtiQTUVLb6ggJh3IVx7cm3L0gE3VxG8QVuPRxr6gbuarmsmexb3aQRPGrsf0ueD263i0ymyv6w5s6t8YRnlG6rHdXkLcQEwHJSXa4VHI~BDyKLGGHKChxpRToI5SahQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal