Abstract
Leukocyte adhesion deficiency type II (LADII) is a rare inherited disorder of fucose metabolism. Patients with LADII lack fucosylated glycoconjugates, including the carbohydrate ligands of the selectins, leading to an immunodeficiency caused by the lack of selectin-mediated leukocyte-endothelial interactions. A simple and effective therapy has recently been described for LADII, based on the administration of oral fucose. Parallel to this treatment the lack of E- and P-selectin ligands on neutrophils was corrected, and high peripheral neutrophil counts were reduced to normal levels. This study reports that discontinuation of this therapy leads to the complete loss of E-selectin ligands within 3 days and of P-selectin ligands within 7 days. Peripheral neutrophil counts increased parallel to the decrease of selectin ligands. Selectin ligands reappeared promptly after resumption of the fucose therapy, demonstrating a causal relationship between fucose treatment and selectin ligand expression and peripheral neutrophil counts.
Introduction
Selectins initiate the contact formation between leukocytes and endothelial cells and thereby the extravasation of leukocytes.1 They form a family of 3 cell adhesion molecules of which 2 are inducible on the surface of endothelial cells (E- and P-selectin), and one is constitutively expressed on most leukocytes (L-selectin). Although the precise carbohydrate structure of selectin ligands has not yet been determined definitively, ample evidence suggests that they resemble or are derivatives of the tetrasaccharide sialyl Lewis X (sLex) (NeuAcα2,3-Galβ1,4[Fucα1,3] GlcNAc). Fucose is an essential structural element of all known selectin ligands as has been demonstrated in mice deficient for the gene for fucosyltransferase VII.2 Thus, a defect in fucose metabolism would be expected to severely hamper leukocyte entry into tissue.
Leukocyte adhesion deficiency type II (LADII) is a still-undefined genetic defect that results in the lack of fucosylated glycoconjugates, including sialyl Lewis X. Patients suffer from recurrent episodes of infections, persistent leukocytosis, and severe mental and growth retardation.3-5 Leukocyte rolling in postcapillary venules of such patients is markedly reduced, and selectin ligands on leukocytes are missing.6,7 The genetic defect seems to affect intracellular GDP-fucose supply because culturing of fibroblasts of different LADII patients in the presence of fucose can rescue the expression of fucosylated glycoconjugates.8,9 We have recently described a new case of LADII10 and have established a successful therapy based on the administration of oral fucose.9 Since the onset of therapy, neutrophil counts were reduced to normal levels, no episodes of fever were observed, and nearly normal expression levels of first P- and later of E-selectin ligands were reached while fucose doses were gradually increased. However, we could not demonstrate whether fucose treatment did indeed cause the changes or whether they merely occurred coincidentally. This question was now addressed by discontinuing therapy for 9 days, during which time leukocyte counts, expression of selectin ligands on the patient's neutrophils, and other parameters were closely followed.
Study design
A detailed description of the patient has been given elsewhere.10 Fucose therapy on the boy was started at 14 months of age and conducted for 16 months before it was interrupted for 9 days. Permission for the trial on this patient was obtained from the Human Subject Committee of the University Clinic of Münster, Germany. Body weight at this time was 7900 g (3rd percentile, 10 400 g) and body length 76 cm (3rd percentile, 84 cm). Isolation and fluorescence-activated cell sorter (FACS) analysis of peripheral blood leukocytes as well as determination of serum fucose concentrations were performed as described.9 The selectin-immunoglobulin G (IgG) chimeras (used at 25 μg/mL) contained the lectin, epidermal growth factor, and first 2 consensus repeats of mouse E- or P-selectin, respectively, fused to the Fc-part of human IgG1.11Fc-receptors were blocked as described.10
Results and discussion
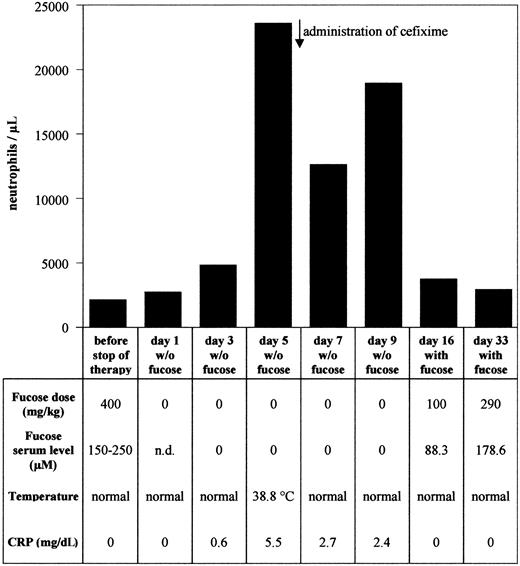
The first 280 days of fucose therapy have been documented.9 Since then, the therapy has been continued for another 190 days with 5 daily doses of 400 mg fucose/kg (15 g per day). During the whole period of 470 days, neutrophil counts stayed in the normal range [<8.5 × 109/L (<8500/μL)], and no fever episodes were observed. Serum fucose concentrations determined 60 to 90 minutes after fucose ingestion were in the range of 150 to 250 μM. Following these 470 days of therapy, treatment with fucose was discontinued for 9 days. Serum fucose concentration levels dropped below detection limit (≤ 5 μM) as was determined on day 3, 5, 7, and 9 after onset of discontinuation of therapy (Figure 1).
Peripheral neutrophil counts and other therapy parameters during discontinuation and resumption of fucose therapy.
Peripheral neutrophil counts, fucose doses, serum fucose levels, body temperature, and C reactive protein (CRP) were recorded for each time point as indicated.
Peripheral neutrophil counts and other therapy parameters during discontinuation and resumption of fucose therapy.
Peripheral neutrophil counts, fucose doses, serum fucose levels, body temperature, and C reactive protein (CRP) were recorded for each time point as indicated.
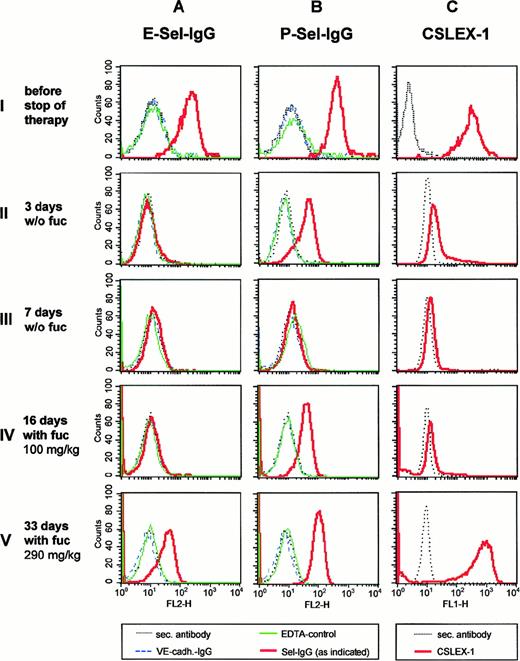
Expression of E- and P-selectin ligands on neutrophils was determined by FACS analysis, using selectin-IgG fusion proteins as probes that were detected by fluorescence-labeled secondary antibodies (Figure2). At the third day without fucose, E-selectin ligands were already undetectable, sLex levels were strongly reduced, whereas expression levels of P-selectin ligands were partially reduced (Figure 2, row II). P-selectin ligands and sLex were almost undetectable on day 7 after onset of discontinuation of fucose therapy (Figure 2, row III). We conclude that fucose in the patient's diet was necessary for the generation of selectin ligands.
Expression of selectin ligands and sLex during discontinuation and resumption of fucose therapy.
Neutrophils were isolated at the time points before (row I) and during discontinuation (rows II and III) and during resumption of therapy (rows IV and V) as indicated on the left. Expression levels were analyzed by flow cytometry, using the following reagents: (A and B) E-selectin–IgG (E-Sel–IgG) or P-selectin–IgG (P-Sel–IgG) in the presence of Ca++ (red, bold line), or in the presence of EDTA (green, thin line), VE-cadherin–IgG (blue, dashed line); (C) anti-sLex monoclonal antibody CSLEX-1 (red, bold line). In each case the fluorescence-labeled secondary antibody alone (negative control) was depicted in black (dotted line).
Expression of selectin ligands and sLex during discontinuation and resumption of fucose therapy.
Neutrophils were isolated at the time points before (row I) and during discontinuation (rows II and III) and during resumption of therapy (rows IV and V) as indicated on the left. Expression levels were analyzed by flow cytometry, using the following reagents: (A and B) E-selectin–IgG (E-Sel–IgG) or P-selectin–IgG (P-Sel–IgG) in the presence of Ca++ (red, bold line), or in the presence of EDTA (green, thin line), VE-cadherin–IgG (blue, dashed line); (C) anti-sLex monoclonal antibody CSLEX-1 (red, bold line). In each case the fluorescence-labeled secondary antibody alone (negative control) was depicted in black (dotted line).
Peripheral neutrophil counts were determined on day 1, 3, 5, 7, and 9 after discontinuing therapy. As shown in Figure 1, neutrophil counts increased 11-fold at day 5. At the same time, C reactive protein (CRP) was elevated from undetectable levels to 5.5 mg/dL, and body temperature increased to 38.8°C. Cefixime was administered, resulting in normal body temperature and 2-fold reduction of CRP levels. Neutrophil counts were partially reduced but stayed at elevated levels during the course of fucose therapy discontinuation. Our results establish that interruption of fucose substitution therapy for only a few days led to the loss of selectin ligands, accompanied by leukocytosis and elevated body temperature.
Fucose therapy was resumed after 9 days of discontinuation, starting with 5 daily doses of 100 mg/kg for the first 16 days, followed by 200 mg/kg doses for the next 10 days and a further increase to 290 mg/kg later. At 16 days after restarting therapy, P-selectin ligand expression levels had been partially reconstituted, whereas sLex was almost and E-selectin ligands were still completely undetectable (Figure 2, row IV). At 33 days after restarting therapy, E-selectin ligands were reexpressed, whereas P-selectin ligands and sLex had reached near normal levels. Thus, similar to the original start of therapy, our results demonstrate that higher fucose levels are necessary for the expression of E-selectin ligands than for the expression of P-selectin ligands. This might indicate that P-selectin ligands require lower levels of fucosylation for selectin-binding than E-selectin ligands. Indeed, a study on mouse- activated T cells indirectly suggested that lower activation and fucosylation levels were necessary for the binding to P-selectin than for the binding to E-selectin.12 PSGL-1, the major ligand of P-selectin, has only very few fucosylated glycan side chains, and only one side chain can be sufficient for high affinity binding to P-selectin.13 14
Parallel to the reexpression of selectin ligands, peripheral neutrophil counts dropped to normal levels when therapy was resumed (Figure 1). Our results establish a causal relationship between fucose treatment and selectin ligand expression. Furthermore, restoration of P-selectin ligands alone was already sufficient to restore normal neutrophil counts, whereas E-selectin ligands, as detectable by FACS analysis, were not required for this effect. In this context it is interesting that the analysis of mice with multiple targeted deficiencies in selectin genes revealed a predominant role for P-selectin in regulating leukocyte behavior in mice.15
A major concern from the onset of therapy had been that the H-antigen, the α1,2-fucosylated core structure of the blood group antigens, could be expressed on fucose therapy, possibly causing problems with autoimmune antibodies. Surprisingly, this structure has not yet appeared during more then 1.5 years of therapy. It is possible that higher levels of fucose are necessary for the expression of the H-antigen than for the expression of selectin ligands. Alternatively, erythrocyte progenitors might have a quantitatively insignificant or inefficient salvage pathway for GDP-fucose synthesis.
The genetic defect that leads to LADII has not yet been identified. For one of the first patients a possible defect indirectly affecting the activity of GDP-D-mannose-4,6-dehydratase was reported.16An attempt to treat the patient with low doses of fucose did not yield a positive therapeutic response. This could either be based on defects in a different gene or on defects of different parts of the same gene, resulting in different sensitivity to the rescue by externally added fucose.17 Cell extracts of our patient displayed normal activity levels of the dehydratase and the FX protein.18Instead, decreased import of GDP-fucose into the Golgi of these cells was observed, indicating that the basis for LADII might be a defect in the transport rather than the synthesis of GDP-fucose.19
Acknowledgment
K. Holtmann is gratefully acknowledged for help with the FACS analysis.
Supported in part by the Deutsche Forschungsgemeinschaft, SFB 293 (K.L. and D.V.).
K.L. and T.M. contributed equally to this report.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dietmar Vestweber, Institute of Cell Biology, ZMBE, University of Münster, Von-Esmarch-Str. 56, 48149 Münster, Germany; e-mail: vestweb@uni-muenster.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal