Abstract
B-lymphocyte stimulator (BLyS) is a recently identified novel member of the tumor necrosis factor ligand superfamily shown to exist in a membrane-bound and soluble form. BLyS was found to be specifically expressed on cells of myeloid lineage and to selectively stimulate B-lymphocyte proliferation and immunoglobulin production. The expression of a cytokine involved in potentiation of humoral immune responses, such as BLyS, is expected to be strictly controlled. The goal of the present study was to examine regulation of BLyS levels in monocytic cells in response to cytokines and during their differentiation to macrophages and dendritic cells. The presence of BLyS on the cell surface and in the culture medium of both normal blood monocytes and on tumor cells of myelomonocytic origin was demonstrated. BLyS gene expression and levels of membrane-associated and soluble BLyS were found to be regulated by cytokines, in particular interferon (IFN)-γ and to a lesser extent interleukin-10 (IL-10). The expression of BLyS on monocyte membranes was retained following differentiation into macrophages, but detection on the surface of monocyte-derived dendritic cells required stimulation with IFN-γ. Both IFN-γ and IL-10 enhanced the release of soluble BLyS that was active in B-cell proliferation assays. Cells transfected with BLyS complementary DNA mutated in a predicted cleavage site failed to release BLyS into the culture medium, thereby suggesting that soluble BLyS was derived from the membrane form. These results provide further support for an important role for BLyS expressed in myeloid cells in B-cell expansion and antibody responses.
Introduction
The tumor necrosis factor (TNF) family of ligands encompasses an ever-growing group of proteins, characterized by homologous cysteine-rich domains, that participates in the regulation of diverse immune and inflammatory responses.1-4 All the members, with the exception of lymphotoxin-α, are type II membrane proteins. Their effects are mediated either by cell contact, through the interaction of the membrane-bound form of the ligand with its corresponding receptor, or by processing and shedding of the soluble form of the ligand.2,5-7 In addition, many of the proteins, including CD27L, CD30L, OX40L, CD40L, FasL, and 4-1BBL, have moderate-sized cytoplasmic regions that are capable of delivering signals when engaged by their receptors.7-10 The expression pattern of the family members is usually promiscuous, ranging from the broad cellular expression of TNF-α to a more restricted localization, such as that of CD40L expressed only on T cells. Moreover, the expression of the molecules is, in general, dependent on the activation state of the cells, being usually low or undetectable on resting cells.
Recently, we described a novel member of the TNF family of ligands, B-lymphocyte stimulator (BLyS), which was identified by searching an expressed sequence tag (EST) database for homology with known TNF-like molecules.11 The protein has been reported also as TALL-1 (TNF- and ApoL-related leukocyte-expressed ligand 1), BAFF (B-cell activator factor belonging to the TNF family), or THANK (TNF homologue that activates apoptosis, NF-κB, and JNK).12-14The human BLyS gene encodes for a 285 amino acid (aa) protein presenting a transmembrane region between aa 47 and 73 and lacking a putative signal peptide sequence. The recombinant soluble protein (aa 134-285) binds selectively to human primary B cells and tumor cell lines of the B lineage.11 BLyS was shown to induce B-cell proliferation in standard costimulation assays withStaphylococcus aureus Cowan I (SAC I) or antihuman immunoglobulin M (IgM). BLyS administration in mice resulted in a 5- and 2-fold increase in serum IgM and IgA, respectively.11In addition, mice transgenic for BAFF developed autoimmune disorders such as increased germinal center formation, production of autoantibodies, and Ig deposition in kidneys.15Collectively, these findings suggest that BLyS has a crucial role in the humoral immune response and that regulation of BLyS expression might consequently modulate B-cell function. Our aim was, therefore, to study synthesis and release of BLyS from cells of myeloid lineage and to investigate the regulation of BLyS expression in response to cytokines.
Materials and methods
Medium and reagents
The complete medium used for monocyte culture consisted of RPMI 1640 medium (Gibco BRL, Rockville, MD) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (FBS), 2 M L-glutamine, and 50 μg/mL gentamycin (Biofluids, Rockville, MD). The following recombinant human (rh) reagents were used: interferon-γ (rhIFN-γ), interleukin-10 (rhIL-10), rhIL-4, macrophage colony-stimulating factor (rhM-CSF), and granulocyte macrophage–stem cell factor (rhGM-SCF) (all from Peprotech, Rocky Hill, NJ). In addition, we used fluorescein isothiocyanate (FITC)– or phycoerythrin (PE)-conjugated monoclonal antibodies (mAbs): anti-CD3, anti-CD56, anti-CD20, anti-CD14 (Pharmingen, San Diego, CA), and PE-conjugated goat antimouse IgG F(ab′)2 (Southern Biotechnology, Birmingham, AL).
Cell cultures
Monocytes were obtained from human peripheral blood mononuclear cells (PBMNCs) by centrifugation of leukapheresis preparations (BRT, Baltimore, MD) through Histopaque gradients (Sigma Chemical, St Louis, MO) followed by counterflow centrifugal elutriation. Monocyte-derived macrophages were obtained by culturing the purified monocytes for 12-15 days in the presence of 20 ng/mL M-CSF.16 Monocyte-derived dendritic cells were obtained by culturing monocytes for 7-10 days in RPMI containing 10% FBS supplemented with 50 ng/mL GM-CSF and 20 ng/mL IL-4, according to the procedure described by Sallusto et al.17 CD34+-derived dendritic cells were generated from cord blood CD34+ progenitor cells isolated by a specific MACS kit (Miltenyi Biotech, Auburn, CA). CD34+ cells were cultured with 50 ng/mL SCF and 50 ng/mL GM-CSF for 7 days and then with GM-CSF and 10 ng/mL TNF-α for an additional 7 days. For the enzyme-linked immunosorbent assay (ELISA), cell-free supernatants from 3-day cultures in the presence or absence of cytokines were harvested and stored at −20°C.
Antibodies
Polyclonal antibodies (pAbs) were affinity-purified from antisera generated by immunizing rabbits with rhBLyS. We generated mAbs by the fusion of mouse myeloma cells P3 × 63Ag8.653 with splenocytes from BALB/C mice immunized with either histidine-tagged BLyS (mAbs 9B6 and 2E5) or soluble BLyS (mAb 15C10).
BLyS ELISA
BLyS-specific ELISA was established using 15C10 as capture mAb and rabbit affinity-purified pAb as detector. Culture supernatants were incubated overnight on 15C10-coated microplates at 4°C. Biotinylated pAb (200 ng/mL) and peroxidase streptavidin (Kirkergaard and Perry, Gaithersburg, MD) were added in sequential steps. Conversion of the substrate TMB (Kirkergaard and Perry) was measured at 450 nm. Each value was calculated as the mean ± SD of triplicate samples.
Flow cytometry
Cells were incubated with 2 μg/106 cells of mouse IgG1 or anti-BLyS mAb for 20 minutes on ice, washed, and then incubated with goat antimouse F(ab′)2 for a further 20 minutes on ice. Alternatively, cells were incubated with biotinylated 9B6 and stained with PE-conjugated streptavidin. Cells were washed, resuspended in propidium iodide solution, and analyzed using a flow cytometer and associated Cell Quest software (both from Becton Dickinson, San Jose, CA).
Quantitative polymerase chain reaction
BLyS messenger RNA (mRNA) levels were assessed in monocytes, macrophages, and dendritic cells using a 7700 Taqman Sequence Detector (Applied Biosystems, Foster City, CA); amplification primers 5′-ACCGCGGGACTGAAAATCT-3′ and 5′-CACGCTTATTTCTGCTGTTCTGA-3′; and a probe, 5′-CCACCAGCTCCAGGAGAAGGCAACTC-3′, that was designed to span the region from nucleotides 458-533 of the human BLyS sequence (GenBank accession No. AF132600). Total RNA was prepared from cells, and BLyS mRNA was detected by a one-step reverse transcriptase–polymerase chain reaction (RT-PCR) procedure. BLyS mRNA quantitation was conducted with the comparative Delta cycle threshold method using an 18S ribosomal RNA probe as endogenous reference.18 Expression levels in all the cell types tested are shown relative to expression levels in resting monocytes.
Generation of cells expressing mutant BLyS
Full-length BLyS1–285 was PCR-amplified and cloned as a BamHI/XbaI fragment into the pC4 mammalian expression vector. The mutations around the BLyS cleavage site, lysine132 to alanine (K132A) and arginine133 to alanine (R133A), were introduced by an overlapping 2-step PCR strategy with the subsequent amplicon subcloned as a BamHI/XbaI fragment into pC4. Expression constructs were then transfected (Lipofectamine; Life Technologies, Rockville, MD) into human 293T–embryonic-kidney cells. Supernatants were collected 36 hours after transfection and assayed in the B-cell proliferation assay.
B-cell proliferation
Human tonsillar B cells were purified by negative selection using the magnetic-activated cell-sorting (MACS) system. Purified cells were greater than 95% B cells as assessed by immunofluorescence staining for CD20 and CD19. We cultured 105 B cells per well in a 96-well plate for 4 days in the presence of 30 μg/mL anti-IgM (DA4-4 hybridoma; American Type Culture Collection, Manassas, VA) or 10−5 dilution SAC (Calbiochem, La Jolla, CA) and serial dilutions of the test-conditioned media. Proliferation was quantitated by pulsing the cells during the last 20 hours of culture with 0.0185 MBq (0.5 μCi) per well of [3H]thymidine (2.48 × 1011 Bq [6.7 Ci/mM]) (NEN Life Science Products, Boston, MA). The values are reported as the mean ± SD of triplicate wells.
IgG production
Purified B cells were cultured for 10 days at the cell density of 1 × 106 cells per mL in the presence of SAC and serial dilutions of the test-conditioned media. IgG levels in the culture supernatants were determined by ELISA using goat antihuman Ig pAb (Kirkergaard and Perry) as the capture antibody and biotinylated goat antihuman IgG (Southern Biotechnology Associates, Birmingham, AL) as the detector antibody.
Western blot analysis
Supernatants from 293T-cell cultures and 293T cells were harvested 36-48 hours after transfection. BLyS present in the supernatants was immunoprecipitated using 1 μg mAb 15C10 and protein A–agarose for 2 hours at 4°C. Immunoprecipitations were washed 4 times and boiled in 2 × SDS (sodium dodecyl sulfate) sample buffer, and the proteins were separated on 4%-20% gradient SDS-PAGE (polyacrylamide gel electrophoresis) gels. The 293T cells were washed once in ice-cold PBS and lysed in Brij buffer comprising 10 M Tris (tris[hydroxymethyl] aminomethane) (pH 7.5), 0.875% Brij, 0.125% NP-40, 2.0 mM ethylenediamine tetraacetic acid (EDTA), and 150 mM sodium chloride (NaCl) for 15 minutes on ice. We separated 60 μg of each lysate on 4%-20% gradient SDS-PAGE gels. Immunoblotting was performed using a rabbit pAb to BLyS, and the results were developed by Enhanced Chemiluminescence (Pierce).
Results
Expression and regulation of membrane-bound BLyS on PBMNCs
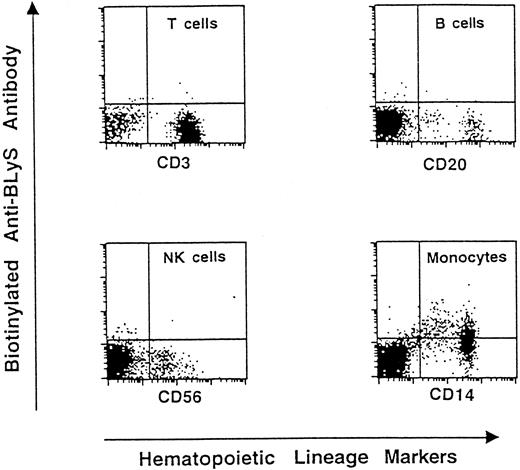
Our group has previously demonstrated that BLyS is preferentially expressed in hematopoietic tissues and is present on monocytes.11 In additional experiments we evaluated BLyS expression on freshly purified human PBMNCs. The cells were double-stained with PE-conjugated mAb 9B6 and with FITC-coupled antibodies specific for T, B, or NK cells or monocytes and then analyzed by flow cytometry. As shown in Figure1, monocytes are the only cells bound by the BLyS-specific mAb. The restricted expression of BLyS on cells of myeloid origin was further suggested by FACS analysis of a panel of hematopoietic tumor cell lines (Table 1). The cell lines HL-60, THP-1, and U937 displayed membrane-bound BLyS; in contrast, T cell lines (Jurkat and Sup-T1) and B cell lines (Namalwa, Reh, and IM-9) showed negative immunostaining.
Presence of membrane-bound BLyS on human PBMNCs.
We performed 2-color staining on freshly purified cells using FITC-coupled antibodies specific for CD3 (T lymphocytes), CD20 (B lymphocytes), CD56 (NK cells), and CD14 (monocytes). Presence of BLyS on the cell surface was detected by biotinylated 9B6 followed by PE-conjugated streptavidin. PBMNCs from 6 donors were analyzed for BLyS expression. A representative experiment is shown.
Presence of membrane-bound BLyS on human PBMNCs.
We performed 2-color staining on freshly purified cells using FITC-coupled antibodies specific for CD3 (T lymphocytes), CD20 (B lymphocytes), CD56 (NK cells), and CD14 (monocytes). Presence of BLyS on the cell surface was detected by biotinylated 9B6 followed by PE-conjugated streptavidin. PBMNCs from 6 donors were analyzed for BLyS expression. A representative experiment is shown.
Expression of BLyS on tumor cell lines
| Cell line . | Cellular morphology . | BLyS expression (mean fluorescence values) . |
|---|---|---|
| HL-60 | acute promyelocytic leukemia | 8.1 (3.3) |
| THP-1 | acute monocytic leukemia | 17.1 (3.5) |
| U937 | histiocytic lymphoma | 12.6 (4.5) |
| Jurkat | T-lymphocytic leukemia | 2.9 (3.1) |
| Sup-T1 | T-lymphoblastic lymphoma | 2.3 (1.8) |
| Namalwa | Burkitt's lymphoma | 2.7 (2.2) |
| Reh | acute lymphocytic leukemia | 3.8 (2.9) |
| IM-9 | multiple myeloma | 3.8 (2.8) |
| Cell line . | Cellular morphology . | BLyS expression (mean fluorescence values) . |
|---|---|---|
| HL-60 | acute promyelocytic leukemia | 8.1 (3.3) |
| THP-1 | acute monocytic leukemia | 17.1 (3.5) |
| U937 | histiocytic lymphoma | 12.6 (4.5) |
| Jurkat | T-lymphocytic leukemia | 2.9 (3.1) |
| Sup-T1 | T-lymphoblastic lymphoma | 2.3 (1.8) |
| Namalwa | Burkitt's lymphoma | 2.7 (2.2) |
| Reh | acute lymphocytic leukemia | 3.8 (2.9) |
| IM-9 | multiple myeloma | 3.8 (2.8) |
Levels of membrane-bound BLyS expression on myeloid cell lines were analyzed by flow cytometry. Cells were incubated with the mAb 2E5 or mouse IgG1 followed by PE-labeled goat antimouse F(ab′)2. Mean fluorescence values obtained with the isotype control are in parentheses.
The aim of the next series of experiments was to investigate whether cytokines known to induce B-cell activity modulate BLyS expression on monocytes. Cells from 9 donors were treated for 3 days with 100 ng/mL IL-10, 100 ng/mL IL-4, or 5 ng/mL IFN-γ. These cytokines have differential effects on monocytes: IFN-γ is the prototype of activating cytokines, whereas IL-10 and IL-4 have, in general, inhibitory effects. Results from the FACS analysis of 4 donors are shown in Table 2. In all the donors tested, the following was observed: a reduction in constitutive BLyS expression following IL-4 treatment; a marked increase of basal BLyS cell-bound expression following IFN-γ or IL-10 treatment; and a complete inhibition of IL-10–induced up-regulation by IL-4 treatment. However, IL-4 had a variable effect on IFN-γ–induced up-regulation. In the majority of donors (6 of 9), IFN-γ–induced BLyS expression was unchanged or slightly inhibited by IL-4 treatment, in 2 donors it was enhanced, and in a single donor it was completely inhibited.
Regulation of BLyS expression on monocytes by cytokine treatment
| Treatment . | BLyS expression (mean fluorescence values) . | |||
|---|---|---|---|---|
| Donor 1 . | Donor 2 . | Donor 3 . | Donor 4 . | |
| None | 4.2 (2.6) | 11.2 (5.3) | 2.0 (1.6) | 5.9 (2.7) |
| IFN-γ | 16.5 (2.4) | 62.0 (4.6) | 30.3 (1.5) | 30.6 (1.9) |
| IL-4 | 2.4 (2.2) | 3.6 (2.9) | 1.6 (1.1) | 2.1 (1.7) |
| IL-10 | 20.0 (3.6) | 43.6 (5.2) | 25.4 (1.6) | 58.8 (4.8) |
| IL-4 + IFN-γ | 3.7 (1.7) | 62.4 (3.1) | 72.8 (1.5) | 42.3 (1.6) |
| IL-4 + IL-10 | 3.6 (2.3) | 7.4 (2.6) | 2.0 (1.5) | 9.0 (2.0) |
| Treatment . | BLyS expression (mean fluorescence values) . | |||
|---|---|---|---|---|
| Donor 1 . | Donor 2 . | Donor 3 . | Donor 4 . | |
| None | 4.2 (2.6) | 11.2 (5.3) | 2.0 (1.6) | 5.9 (2.7) |
| IFN-γ | 16.5 (2.4) | 62.0 (4.6) | 30.3 (1.5) | 30.6 (1.9) |
| IL-4 | 2.4 (2.2) | 3.6 (2.9) | 1.6 (1.1) | 2.1 (1.7) |
| IL-10 | 20.0 (3.6) | 43.6 (5.2) | 25.4 (1.6) | 58.8 (4.8) |
| IL-4 + IFN-γ | 3.7 (1.7) | 62.4 (3.1) | 72.8 (1.5) | 42.3 (1.6) |
| IL-4 + IL-10 | 3.6 (2.3) | 7.4 (2.6) | 2.0 (1.5) | 9.0 (2.0) |
Following 3-day treatment with the cytokines, monocytes were incubated with anti-BLyS mAb 9B6 or mouse IgG1 and then with PE-labeled goat antimouse F(ab′)2. Cells were then analyzed using a FACScan. Mean fluorescence values obtained with the isotype control are in parentheses.
The enhancement of membrane-bound BLyS expression by IFN-γ and IL-10 treatment was time-dependent, reaching a maximal value after a 3-day exposure to the cytokines (data not shown).
Membrane-bound BLyS expression on macrophages and dendritic cells
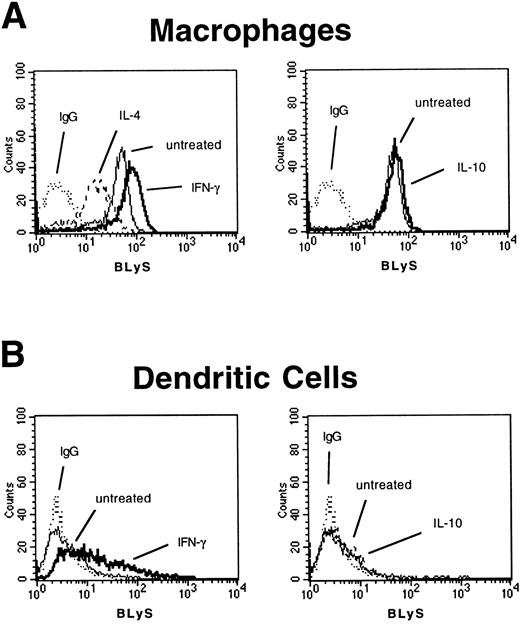
Monocytes are present in circulating blood, whereas in tissues and in secondary lymphoid organs, macrophages and dendritic cells represent the majority of the cells of monocytic origin. Peripheral blood monocytes are capable of differentiating in vitro into both macrophages and dendritic cells depending on the signals delivered to the precursor cells. To define BLyS expression on these monocyte-derived subpopulations, monocytes were treated with GM-CSF and IL-4, which allows for differentiation to dendritic cells (CD14− and CD1a+), or with M-CSF, which induces the differentiation to macrophages (CD68+). FACS analysis showed that in vitro–derived macrophages expressed membrane-bound BLyS (Figure 2A). Because cytokines modulated BLyS expression on monocytes, we tested their effect on the differentiated macrophages. BLyS-specific immunostaining was enhanced by IFN-γ treatment and was reduced by IL-4 (Figure 2A).
Membrane-bound BLyS expression on macrophages and dendritic cells.
The presence of membrane-bound BLyS on (A) macrophages and (B) dendritic cells was analyzed by FACS analysis after a 3-day incubation in the presence of IFN-γ, IL-10, or IL-4. A representative experiment is shown.
Membrane-bound BLyS expression on macrophages and dendritic cells.
The presence of membrane-bound BLyS on (A) macrophages and (B) dendritic cells was analyzed by FACS analysis after a 3-day incubation in the presence of IFN-γ, IL-10, or IL-4. A representative experiment is shown.
In contrast, membrane-bound BLyS was not detected by FACS analysis on monocyte-derived dendritic cells, although expression of cell surface BLyS was induced by a 3-day treatment with IFN-γ (Figure 2B). It remains possible that the failure to detect membrane-bound BLyS is due to the IL-4 used in the culture system to differentiate monocytes into dendritic cells. To address this issue, dendritic cells were obtained by differentiating CD34+ cord blood progenitor cells in the presence of GM-CSF, CSF, and TNF-α. The mean fluorescence value of the cells incubated with biotinylated 9B6 and PE-labeled streptavidin was similar to the mean fluorescence value of the cells incubated with biotinylated isotype control: 7.7 and 3.3, respectively. These results suggest that dendritic cells of myeloid origin have a very low level of membrane-bound BLyS, independent of the culture conditions used to generate the cells.
Regulation of BLyS gene expression
To determine whether the changes in cell surface expression of BLyS were regulated at the level of transcription, quantitative PCR was conducted with mRNA from monocytes, macrophages, and dendritic cells stimulated with the cytokines. Cells from 3 different donors were used, and a generally similar pattern of expression was seen in the various cell types. As shown in the representative experiment depicted in Figure 3, a low level of BLyS transcript was observed in unstimulated monocytes. In all donors tested, IFN-γ treatment caused over 10-fold increase in BLyS-specific mRNA. Interestingly, IL-10 treatment did not affect BLyS mRNA level. In macrophages, IFN-γ treatment produced a 6-fold increase of the level of BLyS transcripts, while IL-10 treatment resulted in less than a 4-fold increase. In dendritic cells, BLyS transcripts were increased by 8-fold following IFN-γ treatment, but they were not increased following IL-10 treatment. Induction of BLyS mRNA levels was also observed after LPS treatment in macrophages and dendritic cells. Kinetic analysis of BLyS mRNA in the various cell types showed a maximal level of induction after 1 day of IFN-γ treatment (data not shown). The level of BLyS mRNA found in B cells was minimal.
BLyS mRNA levels in cells of monocytic origin.
Monocytes, macrophages, dendritic cells, or B cells were cultured for 1 day with or without factors. Quantitative PCR was conducted as reported in “Materials and methods.” Expression levels in all the cell types tested are shown relative to the expression level in resting monocytes.
BLyS mRNA levels in cells of monocytic origin.
Monocytes, macrophages, dendritic cells, or B cells were cultured for 1 day with or without factors. Quantitative PCR was conducted as reported in “Materials and methods.” Expression levels in all the cell types tested are shown relative to the expression level in resting monocytes.
Soluble BLyS is released by myeloid cells and has functional activity
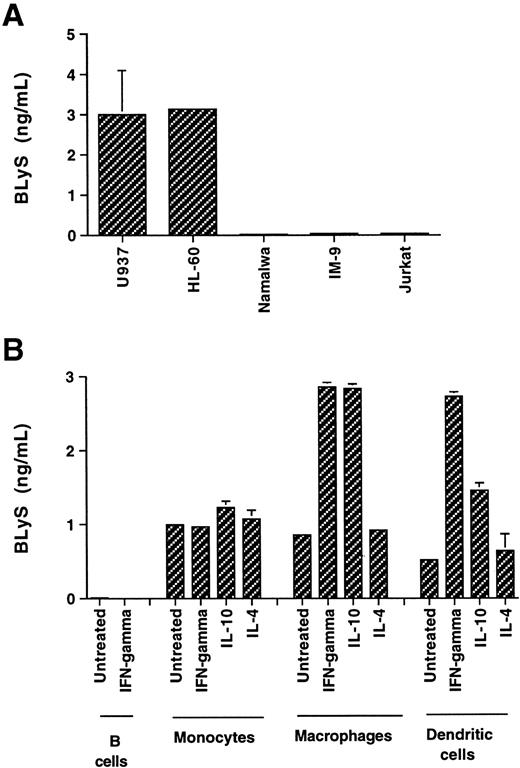
Because several members of the TNF family, among them TNF-α, CD40L, and FasL, can be produced as a soluble protein, we have investigated the possibility that soluble forms of BLyS are produced by monocytic cells. Using a specific 2-site ELISA, conditioned media from tumor cells of various origins were analyzed for BLyS content (Figure4A). BLyS was found in the media conditioned by the myeloid cell lines U937 and HL-60, but not in the media conditioned by the T and B tumor lines Jurkat, Namalwa, or IM-9 cells. The identity of the protein released in the culture medium of U937 cells was confirmed by capturing the soluble BLyS on an affinity column. Sequencing of the purified 17-kD protein revealed an NH2-terminal sequence of AVQGP (alanine valine glutamine glycine proline) (O. Galperina and D. Parmelee, unpublished results, January 2000), as it was previously found for the recombinant BLyS.11
Soluble BLyS is released from myeloid cells.
The amount of released BLyS was determined by ELISA in the medium, which was conditioned for 3 days, from the following cell cultures: (A) U937, HL-60, Namalwa, IM-9, and Jurkat (plated at the cell density of 1 × 106 cells per mL) and (B) 3 × 106 cells per mL B cells and monocytes and 1 × 106 cells per mL macrophages and dendritic cells.
Soluble BLyS is released from myeloid cells.
The amount of released BLyS was determined by ELISA in the medium, which was conditioned for 3 days, from the following cell cultures: (A) U937, HL-60, Namalwa, IM-9, and Jurkat (plated at the cell density of 1 × 106 cells per mL) and (B) 3 × 106 cells per mL B cells and monocytes and 1 × 106 cells per mL macrophages and dendritic cells.
To further characterize the release of the soluble protein, 3-day culture media from primary cells of different donors (3-4 donors, depending on the cell type) were tested in the ELISA (Figure 4B). Monocytes from various donors released BLyS in the range of 0.5-3 ng for 3 × 106 cells. Incubation of the cells with IFN-γ, IL-10, or IL-4 did not substantially change the amount of protein released in the majority of the donors tested. The cells for the representative donor depicted in Figure 4B released approximately 1 ng protein during the 3-day culture. Macrophages (1 × 106) produced 0.9 ng protein. Treatments with IFN-γ and IL-10 induced a 3-fold increase in BLyS release. IL-4 treatment did not change the BLyS level found in the culture media. In addition, we found that 1 × 106 dendritic cells were able to produce soluble BLyS. Cells from the donor shown in Figure 4B produced 0.7 ng protein. As observed in macrophages, the release in dendritic cells was increased 4-fold by IFN-γ and 2.5-fold by IL-10 in this donor. In summary, human cells of monocytic lineage produce soluble BLyS, and the amount released was donor- and cell type–dependent (range, 0.2-4 ng per million cells), with more protein released by differentiated cells on a per-cell base.
Having determined that BLyS is released by myeloid cells, we next investigated if soluble BLyS present in the conditioned medium is active in a standard B-cell costimulation assay (Figure5A). BLyS contained in a U937-conditioned medium, used at the final concentration of 5.6 ng/mL, induced a 3-fold increased of B-cell [3H]thymidine uptake compared to the cells stimulated with only anti-IgM. The proliferation was BLyS-specific because it was completely abrogated by the neutralizing mAb 15C10, but not by a control mAb. In addition, BLyS present in the U937-conditioned medium was able to strongly enhance SAC-induced IgG synthesis from tonsillar B cells (Figure 5B). Therefore, released BLyS is active at low concentrations, similarly to the recombinant protein.11
Released BLyS induces B-cell proliferation and IgG production.
(A) [3H]thymidine incorporation by B cells was assessed after a 4-day culture with serial dilutions of U937-conditioned medium (indicated by a closed circle) in presence of the neutralizing mAb 15C10 (indicated by an open circle) or an unrelated mAb of the same isotype (indicated by closed triangles). Anti-IgM was added to the culture as costimulus. [3H]thymidine incorporation of B cells in the presence of anti-IgM was 630 dpm. (B) Purified B cells were cultured for 10 days with SAC and serial dilutions of the U937-conditioned medium in the absence or presence of mAb 15C10. IgG production was evaluated by ELISA.
Released BLyS induces B-cell proliferation and IgG production.
(A) [3H]thymidine incorporation by B cells was assessed after a 4-day culture with serial dilutions of U937-conditioned medium (indicated by a closed circle) in presence of the neutralizing mAb 15C10 (indicated by an open circle) or an unrelated mAb of the same isotype (indicated by closed triangles). Anti-IgM was added to the culture as costimulus. [3H]thymidine incorporation of B cells in the presence of anti-IgM was 630 dpm. (B) Purified B cells were cultured for 10 days with SAC and serial dilutions of the U937-conditioned medium in the absence or presence of mAb 15C10. IgG production was evaluated by ELISA.
Soluble BLyS is produced by proteolytic cleavage
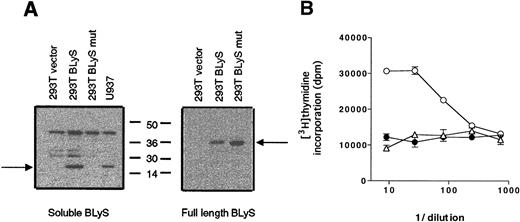
Recombinant BLyS is readily produced in the supernatants of various transfected cell systems, with an amino terminus sequence beginning with A134.11 13 Because the BLyS sequence does not contain a predicted signal peptide, a possible mechanism for the release of BLyS from the cells is the proteolytic cleavage of the membrane-bound protein. Our next aim was to determine whether a putative protein cleavage site was present in the polybasic sequence ofBLyS overlapping amino acids R133 and A134. A double mutation was introduced into full-length BLyS in the 2 basic amino acids that are located immediately upstream of A134: K132 was substituted by A132 and R133 by A133. The mutant cDNA (K132A and R133A) was then transfected into 293T cells, and supernatants from the transfected cells were tested for expression by Western blotting. Supernatants were also collected from 293T cells transfected with either the wild-type BLyS gene or the parental expression vector.
As shown in Figure 6A, both the wild-type and the mutant cDNA were efficiently expressed, as full-length BLyS was detected in the whole-cell extracts from the transfected cells (right panel). In contrast, soluble BLyS was found only in the supernatant of 293T cells transfected with the wild-type form of BLyS, but soluble BLyS was not in the supernatant of cells expressing the noncleavable form of BLyS (left panel). The supernatants were tested for biological activity in a B-cell proliferation assay in the presence of SAC (Figure 6B). In contrast to the wild-type BLyS supernatant, which was significantly active down to approximately a 100-fold dilution, the mutant BLyS supernatant did not increase B-cell proliferation, indicating that soluble functional protein was not cleaved and released into the culture medium. It therefore appears that aa 132 and/or aa 133 play a critical role in directing cleavage of surface-bound BLyS to a soluble form.
BLyS protein containing an altered cleavage site is not released.
(A) Supernatants (left panel) and whole-cell extracts (right panel) from 293T cells transfected with the indicated plasmids were analyzed for expression of BLyS. (B) We transfected 293T cells with cDNA ofBLyS mutant (K132A and R133A; indicated by a closed circle),BLyS full length (indicated by an open circle), or the expression vector (indicated by an open triangle). Culture supernatants from the transfected cells were then tested for biological activity in a standard B-cell proliferation assay in the presence of SAC.
BLyS protein containing an altered cleavage site is not released.
(A) Supernatants (left panel) and whole-cell extracts (right panel) from 293T cells transfected with the indicated plasmids were analyzed for expression of BLyS. (B) We transfected 293T cells with cDNA ofBLyS mutant (K132A and R133A; indicated by a closed circle),BLyS full length (indicated by an open circle), or the expression vector (indicated by an open triangle). Culture supernatants from the transfected cells were then tested for biological activity in a standard B-cell proliferation assay in the presence of SAC.
Discussion
In this report we have extended our earlier observation that BLyS is produced by monocytes with the finding that the protein is also synthesized by macrophages and by monocyte-derived dendritic cells. In addition, we have demonstrated that BLyS belongs to the category of the functionally active soluble ligands of the TNF superfamily. In the TNF ligand family, the efficacy of the membrane-bound and the released proteins varies greatly. Membrane-bound TNF-α is readily processed to the soluble form that is responsible for most of the immune and inflammatory TNF-related effects.19 FasL is also rapidly proteolytically cleaved from the surface of human cells. However, soluble FasL has greatly reduced apoptotic-inducing capacity, and its release in the circulation does not have pathological implications.20 In contrast, the activity of CD40L on B cells totally depends on the interaction of the membrane-bound protein with its receptor.21 Previous experiments from our group and other laboratories had suggested the possibility of BLyS being a soluble mediator. Mammalian cells transfected with BLyS cDNA secreted active protein,11,13 and overexpression of the protein in the liver of transgenic mice caused effects at distal sites such as the B-cell compartment in the secondary lymphoid organs.15 Using a BLyS-specific ELISA, we have shown that BLyS is released from cells of monocytic origin and thus may have systemic activity as a secreted cytokine.
Having established that released BLyS is a stimulatory factor for B cells, it remains to be fully investigated whether the membrane-bound form participates to BLyS biological activity. It should be pointed out that Schneider et al13 have shown that B cells proliferate when cocultured with paraformaldehyde-fixed 293 cells stable-transfected with BLyS, thus suggesting a role for the cell surface protein. Experiments are currently under way to further define the physiological function of membrane-bound BLyS in regard to B-cell stimulation.
The experiments conducted with cells transfected with a mutantBLyS cDNA strongly indicate that soluble BLyS is produced by enzymatic processing of the membrane-bound protein, as suggested previously.13 It appears, however, that there is no correlation between the levels of cell surface–associated BLyS and concentrations of soluble BLyS found in the cultures. The data suggest that there might be additional rate-limiting regulatory steps involved in the release process. It is known that cellular differentiation markedly alters the proteolytic enzymatic profile in monocytic cells,22-24 and therefore higher concentrations of enzyme(s) or more active proteolytic enzyme(s) might be present in dendritic cells and not in monocytes.
Expression of membrane-bound and soluble BLyS was investigated following cytokine treatment. We have used 3 cytokines, IL-10, IL-4, and IFN-γ, characterized by a strong activity on monocytes and B cells. While all of the cytokines have stimulating effects on B cells, IL-10 and IL-4 are considered to be inhibitory cytokines for monocyte activation. IFN-γ was confirmed to be a stimulator of both membrane-bound and soluble BLyS expression. Surprisingly, IL-10 was also able to increase BLyS expression on monocytes and BLyS release from macrophages and dendritic cells. Enhancement of protein levels by IL-10 is not unprecedented. Although this cytokine down-regulates the release of many inflammatory cytokines and inhibits the activities of monocytes and macrophages,25,26 it was also reported to up-regulate the expression of IL-1 antagonist protein and several membrane-bound proteins such as the chemokine receptor CCR5 and the formyl peptide receptor.27 28
Our experiments indicate that the enhancing effects of IFN-γ and IL-10 are differentially regulated. IFN-γ, but not IL-10 treatment, increased BLyS mRNA levels in monocytes and dendritic cells. In addition, in monocytes IL-4 completely inhibited IL-10–induced up-regulation of membrane-bound BLyS but not IFN-γ–induced up-regulation. These data suggest that IL-10 might increase BLyS protein levels through a translational or posttranslational mechanism that is sensitive to IL-4 treatment. Taken together, these results show that BLyS production in monocytic cells is regulated differently compared with TNF-α, the other member of the TNF family of soluble ligands expressed by this cell type. IFN-γ stimulation is not sufficient to induce TNF-α release from monocytes because it has only a priming effect.29 Moreover, IL-10 treatment inhibits TNF-α release,30 whereas IL-4 treatment enhances its secretion in macrophages.31
Many lines of evidence support the theory that in vivo–circulating monocytes, upon migration into tissues, undergo phenotypic and functional maturation into macrophages or dendritic cells.32-34 Differentiation of monocytes to macrophages is associated with the acquisition of a strong phagocytic and endocytic capacity and the enhancement of their degradative function.35-37 Dendritic-cell progenitors, once activated by antigens, migrate from the periphery to the secondary lymphoid organs where they become highly efficient antigen-presenting cells.33,38,39 Recent studies have underlined a critical function shared by these accessory cells: that is, the direct activation of B cells.40-42 In this context, Dubois et al43 have suggested a 2-step model of B-cell activation in which the first step, dendritic cell–induced proliferation of naive B cells, is regulated by unknown soluble factor(s). Our data suggest that BLyS may be one of these mediators, thereby contributing to the direct induction of B-cell proliferation.
The ability of monocytes, macrophages, and dendritic cells to regulate BLyS expression is consistent with their proposed roles in B-cell activation and Ig secretion. Antigen-primed B lymphocytes enter peripheral tissues in response to pro-inflammatory signals released from local sites of infection or tissue damage. At these sites, macrophages remove damaged tissues and potentiate local immunoresponsiveness by elaborating a variety of cytokines. In this setting, macrophage-derived BLyS may enhance B-cell function by increasing Ig secretion from antigen-reactive B cells. In contrast, naive B cells within secondary lymphoid tissues may use dendritic cell–derived BLyS as a proliferative factor that synergizes with antigen-specific signals and CD40/CD40L interactions. Thus, the ability of BLyS to influence both B-cell activation and effector function requires that BLyS be selectively available during specific stages of an immune response. The experiments presented in this manuscript identify potent regulators of BLyS expression and provide the basis for continued investigation of the mechanisms involved in cytokine-mediated immune regulation.
Acknowledgments
We thank O. Galperina and D. Parmelee for purification and N-terminal sequencing of the released BLyS; Y. Li and J. Zhang for purification of the recombinant BLyS; and D. Morahan, E. Cochrane, and P. Garcia for assistance with assays.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bernardetta Nardelli, Human Genome Sciences, Inc, 9410 Key West Ave, Rockville, MD 20850; e-mail:bernardetta_nardelli@hgsi.com.


![Fig. 5. Released BLyS induces B-cell proliferation and IgG production. / (A) [3H]thymidine incorporation by B cells was assessed after a 4-day culture with serial dilutions of U937-conditioned medium (indicated by a closed circle) in presence of the neutralizing mAb 15C10 (indicated by an open circle) or an unrelated mAb of the same isotype (indicated by closed triangles). Anti-IgM was added to the culture as costimulus. [3H]thymidine incorporation of B cells in the presence of anti-IgM was 630 dpm. (B) Purified B cells were cultured for 10 days with SAC and serial dilutions of the U937-conditioned medium in the absence or presence of mAb 15C10. IgG production was evaluated by ELISA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/1/10.1182_blood.v97.1.198/6/m_h80110564005.jpeg?Expires=1769171619&Signature=ONXtRuzv2pv18QAOdBbY-EE9Jihzf5mBU7HobOdDy0ETalomzKx7qOBDbh4esmouWggnIs2OifN~lBlKZ7Aw96tZQFXF-2pBGLiv1o~H2ax0YD7X3pb-LuA2vOpElwkzYEzZQcYo3~4DkAXtif0CUmFKQFbmYqNqJOAJSQNTVjzETm3633J4CXUMcG7Ct0X~A~BnJ4JuxVT9aMdG4E07HJbRslzESTQJPP9ona3X6Je7vYc-JkKMsCBDPEP5pVHaCf3-5AGjj2c24uik6uZbVIXNhMhiR6XllutbOdtrPQKX65ya5xPsYj1IFt7sxflpurO0yHhdsK2wCbVkLuoqaA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal