Abstract
Recent data demonstrate that the introduction into skeletal muscle of an adeno-associated viral (AAV) vector expressing blood coagulation factor IX (F.IX) can result in long-term expression of the transgene product and amelioration of the bleeding diathesis in animals with hemophilia B. These data suggest that biologically active F.IX can be synthesized in skeletal muscle. Factor IX undergoes extensive posttranslational modifications in the liver, the normal site of synthesis. In addition to affecting specific activity, these posttranslational modifications can also affect recovery, half-life in the circulation, and the immunogenicity of the protein. Before initiating a human trial of an AAV-mediated, muscle-directed approach for treating hemophilia B, a detailed biochemical analysis of F.IX synthesized in skeletal muscle was carried out. As a model system, human myotubes transduced with an AAV vector expressing F.IX was used. F.IX was purified from conditioned medium using a novel strategy designed to purify material representative of all species of rF.IX in the medium. Purified F.IX was analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), N-terminal sequence analysis, chemical γ-carboxyglutamyl analysis, carbohydrate analysis, assays for tyrosine sulfation, and serine phosphorylation, and for specific activity. Results show that myotube-synthesized F.IX has specific activity similar to that of liver-synthesized F.IX. Posttranslational modifications critical for specific activity, including removal of the signal sequence and propeptide, and γ-carboxylation of the N-terminal glutamic acid residues, are also similar, but carbohydrate analysis and assessment of tyrosine sulfation and serine phosphorylation disclose differences. In vivo experiments in mice showed that these differences affect recovery but not half-life of muscle-synthesized F.IX.
Introduction
Hemophilia B is an X-linked hemorrhagic disorder characterized by a functional deficiency of clotting factor IX (F.IX). The current treatment for hemophilia B is based on replacement using plasma-derived or recombinant F.IX, either in response to bleeding episodes or prophylactically. The short half-life of the protein, requiring multiple intravenous injections, the expense of these products, and, in the case of plasma-derived concentrates, the risk for blood-borne disease, are all disadvantages of the current protein-based therapy.1-8
The development of somatic gene therapy for F.IX deficiency is an attractive therapeutic option. The continuous presence in the bloodstream of amounts of F.IX as low as 1% of normal has been shown to be clinically relevant in terms of preventing or substantially reducing the frequency of spontaneous bleeds into the joints, soft tissues, or intracranial space.9,10 Factor IX is normally synthesized in the liver, but from a gene therapy standpoint, there is a strong motivation for evaluating alternative target tissues in this population because of the high prevalence of viral hepatitis (approximately 90%) among adults with severe hemophilia.11-13
Skeletal muscle has been extensively tested as a target tissue for gene delivery.14,15 The transfer of foreign DNA into muscle cells by viral or nonviral vectors results in efficient and stable expression of a variety of transgenes.16-20
Adeno-associated virus 2 (AAV) is a single-stranded DNA virus of 4.7 kb, which was developed as a transducing vector capable of long-term expression in nondividing cells such as liver and skeletal muscle.16,21-23 Previous reports showed long-term expression of therapeutic levels of F.IX in small and large animal models of hemophilia B treated with intramuscular injection at multiple sites of an AAV vector encoding human or canine F.IX, respectively.24,25 The canine hemophilia B model26 allowed us to determine that the transgene product (canine factor IX) is biologically active based on shortening of the whole blood clotting time and of the activated partial thromboplastin time.
Factor IX is a vitamin K–dependent glycoprotein synthesized as a precursor molecule of 461 amino acids. It undergoes extensive posttranslational modification, including cleavage and removal of the pre-pro leader sequence of 46 amino acids; γ-carboxylation of the first 12 glutamic acid residues; partial β-hydroxylation of Asp 64; N-linked glycosylation at Asn 157 and Asn 167; O-linked glycosylation at Ser 63, Ser 61, and Thr 159, 169, 172, and 179; sulfation of Tyr 155; and phosphorylation at Ser 158.27 28 Whether the modifications critical for biologic activity are carried out efficiently in human muscle has not been determined.
As part of the preclinical studies designed to assess the potential efficacy of an AAV-mediated, muscle-directed approach, we sought to analyze posttranslational modifications of F.IX synthesized in human muscle. In this study, we have used a model system consisting of human myotubes in culture, transduced with a recombinant AAV vector expressing human F.IX. The myotube-synthesized protein has been isolated from conditioned medium and characterized functionally and biochemically. These data indicate that the material synthesized in human myotubes is similar to plasma-derived (liver-synthesized) F.IX.
Materials and methods
Production and purification of recombinant adeno-associated virus
Transduction of human myotubes
With informed consent from subjects, human myoblasts were isolated from muscle biopsy samples, induced to proliferate in media containing 20% fetal calf serum for 3 days, and then differentiated into mature myotubes in media containing 10% horse serum.31 Myotubes were then transduced in vitro with AAV-CMV-hF.IX vector at multiplicity of infection (MOI) ranging from 5000 to 80 000 in serum-free media (Opti-MEMI; Life Technologies, Gaithersburg, MD) for 6 to 8 hours. Subsequently, the media were changed to DMEM (3 parts): media 199 (1 part) (Life Technologies) with 10% horse serum treated with barium sulfate (to remove residual F.IX), 1% penicillin-streptomycin, and 15 μg/mL vitamin K1. Culture medium was collected from each dish daily and filtered through an HV filter (Millipore, Bedford, MA), benzamidine was added to 5 mM, and media were stored at −80°C.
Immunofluorescence staining of primary culture myotube cells
Characterization of human myotubes was carried out using a rabbit anti-desmin monoclonal antibody (Sigma Chemical, St Louis, MO) and, for detection, an anti-rabbit IgG fluorescein-conjugated antibody (DAKO, Carpinteria, CA). Human factor IX was stained using a goat anti-human FIX antibody and, for detection, an anti-goat IgG rhodamine-conjugated antibody (Chemicon International, Temecula, CA).
Purification of myotube-expressed recombinant F.IX from conditioned media
Culture medium conditioned by AAV-hF.IX transduced myotubes was thawed at 37°C and mixed with benzamidine (5 mM) and soybean trypsin inhibitor (10 μg/mL media) and loaded onto a Q-Sepharose column (Amersham-Pharmacia, Piscataway, NJ) equilibrated in 20 mM Tris, pH 7.4–150 mM NaCl–2 mM benzamidine. Q-Sepharose was washed in 20 mM Tris, pH 7.4–150 mM NaCl–2 mM benzamidine and then with 20 mM Tris, pH 7.4–150 mM NaCl buffer before elution with 20 mM Tris, pH 7.4–750 mM NaCl.
Eluted protein was applied to an immunoaffinity column prepared using a monoclonal antibody to the heavy chain of human F.IX (9EA; Enzyme Research Laboratories, South Bend, IN). All the bound protein was eluted with 150 mM NaCl–50 mM glycine pH 2.5. Fractions containing F.IX were pooled, dialyzed against distilled water or 20 mM HEPES, pH 7.4–150 mM NaCl, and concentrated by ultrafiltration on an Amicon apparatus (Amicon, Danvers, MA).
SDS-PAGE and immunoblotting of myotube-synthesized F.IX
Purified F.IX was separated by 7.5% SDS-PAGE and detected using silver staining (Bio-Rad, Hercules, CA). For immunoblotting, purified F.IX was separated by 7.5% SDS-PAGE and transferred to a nitrocellulose membrane (Amersham, United Kingdom) using an electroblot system (Bio-Rad, Hercules, CA) at 100 mA for 1 hour at 4°C. The membrane was incubated with a 2000 dilution mouse anti-human F.IX monoclonal antibody FXC008 (Boehringer Mannheim, Indianapolis, IN) as primary antibody and anti-mouse IgG peroxidase conjugate using a chemiluminescent substrate (Pierce, Rockford, IN) as a detecting antibody.
Determination of specific activity of recombinant F.IX
F.IX concentration was determined using a previously described enzyme-linked immunosorbent assay (ELISA)32 (capture antibody, polyclonal rabbit anti-human F.IX [DAKO]; detecting antibody, peroxidase-conjugated polyclonal goat anti-human F.IX [Affinity Biologicals, Hamilton, Ontario, Canada]). The functional activity was determined by a clotting assay using a modified one-stage factor assay incubating 50 μL human F.IX-deficient plasma (Organon Teknika, Durham, NC) with 50 μL automated activated partial thromboplastin time (aPTT) reagent (Organon Teknika, Durham, NC), and 50 μL test sample for 3 minutes at 37°C. Fifty μL of 25 mM CaCl2 was added, and time to clot formation was measured using a fibrometer. Lyophilized human plasma (Verify, Organon Teknika) was used as a reference standard. Purified F.IX was added to imidazole buffer in a series of dilutions calculated to achieve aPTTs in the midrange of the standard curve (50-70 seconds). Three samples, each purified from a separate transduction experiment, were analyzed by ELISA and clotting assay, and the results were used to calculate specific activity.
To determine F.IX specific activity on conditioned media from transduced myotubes, lyophilized human plasma-derived F.IX (Mononine; Armour, Kankakee, IL) was added to conditioned media in the myotube culture to a final concentration of 1 IU/mL (5 μg/mL). A series of dilutions was calculated to achieve aPTTs in the midrange of the curve (50-70 seconds). Factor IX clotting activity was calculated after the exclusion of nonspecific clotting activity determined on the conditioned media from nontransduced myotubes. These results were used to determine specific activity obtained at different MOIs. In other experiments to establish the contribution of F.IX to the aPTT measurements on conditioned medium from transduced myotubes, the aPTT assay was performed before and after incubation at 37°C for 30 minutes with 2.5% anti-human IX monoclonal antibody FXC008 (Boehringer Mannheim, Indianapolis, IN).
N-terminal sequence analysis
Purified F.IX was eluted from the immunoaffinity column and dialyzed against water. The protein solution was subjected to amino acid microsequencing using an automated sequencer (173/A Microblotter; Applied Microsystems, Galveston, TX). However, γ-carboxyglutamic acid is not detected on automated Edman degradation sequencing (ie, it appears as a blank); thus efficiency of γ-carboxylation can be estimated by comparing yields at these cycles to yields (in picomoles) in previous and subsequent cycles. Uncarboxylated glutamic acid will be evident as glutamic acid residues in the sequence.
γ-Carboxyglutamic acid analysis
Five micrograms purified myotube-produced recombinant F.IX and a standard (plasma-derived F.IX) were subjected to alkaline hydrolysis, and free amino acids were separated and quantitated by high-performance liquid chromatography using a reverse-phase method (Commonwealth Biotechnologies, Richmond, VA). The area under the combined asparagine and aspartic acid peak was used to calculate total moles of protein in each sample. Gla content of the test material was calculated using the plasma-derived sample as a standard.
Carbohydrate analysis
Two types of analysis were performed on purified protein. First, enzymatic cleavage of N-linked glycans was carried out using recombinant N-glycosidase F (Boehringer Mannheim). Eight micrograms purified F.IX was incubated with 2.5 mU recombinant N-glycosidase F for 18 hours at 30°C in a mixture of 20 mM buffered sodium phosphate (pH 7.2)–10 mM sodium azide–50 mM EDTA–0.5% Nonidet P-40. Samples before and after N-glycosidase F treatment were analyzed by 7.5% SDS-PAGE; a change in apparent molecular weight suggested that glycan-linked residues were present and had been removed. In a second, more detailed analysis, N-linked oligosaccharide profiling was performed by fluorophore-assisted carbohydrate electrophoresis FACE (Glyko, Novato, CA). Each sample digestion was prepared with 100 μg purified protein. The samples were dried, resuspended in 25 μL of 50 mM phosphate buffer, pH 7.5, containing 0.1% SDS and β-mercaptoethanol, and heated at 100°C for 5 minutes. Then 5 μL of 7.5% NP-40 was added. The samples were subjected to digestion with 5 mU N-glycosidase F (Glyko) for 16 hours at 37°C to release N-linked carbohydrate. Sugars were then labeled with a fluorophore 8-aminonaphthalene 1,3,6-trisulfonate, disodium salt by reductive amination and separated by electrophoresis on a 21% polyacrylamide gel at a constant current of 20 mA at 5°C for 1 hour. Visualized bands were imaged and quantified using a FACE Imager (λ excitation = 368 nm, λ emission = 470 nm)33 and compared to a purified plasma-derived F.IX control. In a similar digestion reaction, 100μg purified protein was digested for 24 hours at 37°C with 2 mU neuraminidase (Glyko, Novato, CA).
Tyrosine sulfation
Tyrosine sulfation was analyzed using 35S-sulfate biosynthetic labeling of human myotubes or of Hep G2 cells transduced with AAV-hF.IX.34 35 Plates with comparable levels of F.IX production were selected, and medium was replaced with either sulfate-free medium or methionine-free medium (Cell & Molecular Technologies, Lavallette, NJ). After 30 minutes (sulfate) or 2 hours (methionine), media were replaced with media containing35S-labeled H2SO4 or35S-labeled methionine (NEN Life Science Products, Boston, MA). Sixteen hours later, conditioned medium was harvested, and F.IX was immunoprecipitated using a polyclonal antibody (A0300; DAKO). Samples were then split into 2 equal aliquots, and 1 aliquot was digested with recombinant N-glycosidase F. Both samples were then subjected to 7.5% SDS-PAGE. Proteins were transferred to a nitrocellulose membrane and visualized by autoradiography. Results were normalized based on 35S-methionine content determined by densitometry of F.IX bands. In another experiment, the same membrane was used for confirmation of these identified bands by immunoblotting as described above.
Serine phosphorylation
The extent of serine phosphorylation was analyzed using an anti-phosphoserine antibody. Purified F.IX samples, either plasma-derived or myotube-synthesized, were first treated with N-glycosidase F (Boehringer Mannheim) for 18 hours at 37°C in 20 mM sodium phosphate (pH 7.2)–10 mM sodium azide–50 mM EDTA–0.5% Nonidet P-40. Two ELISA determinations were then carried out side by side, one using as capture antibody a monoclonal anti-phosphoserine (PSR-45; Sigma), the other using a polyclonal rabbit anti-hF.IX (DAKO). Samples were applied in duplicate to plates, and a peroxidase-conjugated polyclonal goat anti-hF.IX (Affinity Biologicals, Hamilton, Ontario, Canada) was used as the detecting antibody.
In vivo functional activity of myotube-synthesized F.IX
To determine the in vivo functional activity of myotube-synthesized F.IX, we carried out 2 experiments in mice. Two micrograms commercially available plasma-derived (Mononine; Armour, Kankakee, IL) Chinese hamster ovary (CHO) cell-synthesized F.IX (Benefix; Genetic Institute, Cambridge, MA) and myotube-synthesized F.IX was injected intravenously by the tail vein in 10 mice (strain C57Bl/6; Jackson Laboratories, Bar Harbor, ME). Blood samples were collected by retro-orbital bleeding before injection and after 30 minutes, 6, 12, and 24 hours. In another group of 12 hemophilia B mice (strains C57Bl/6 and 129), the experiment was repeated using 10 μg each protein, and citrated blood samples were collected by tail transection after 30 minutes and 24 hours. Pharmacokinetics parameters including half-life and peak plasma concentration were determined using the graphing program Prism (Graphpad, San Diego, CA). In the hemophilia B mice, the clotting activity of infused F.IX was determined by one-stage modified aPTT assay.36
Results
Purification of myotube-expressed recombinant F.IX from conditioned media
Transduction of human myotubes with AAV-hF.IX at an MOI of 10 000 resulted in the secretion of 200 to 300 ng F.IX/mL per 24 hours. Note that at this MOI, nearly all cells in culture are transduced (Figure1). Purification of recombinant F.IX from conditioned media is often achieved using a conformation-specific antibody37 that allows recovery of only the fully carboxylated species. In this case, however, we wanted to recover all species secreted from the myotubes, without regard to the efficiency with which posttranslational modifications had been completed. We therefore used as an initial step binding to and elution from a Q-Sepharose column. Ion exchange chromatography can be used to purify fully modified F.IX if a calcium gradient is used for the elution step,38 but our method used sodium chloride elution without a calcium gradient. Using plasma-derived F.IX, we showed that this step allows recovery of 89% of plasma-derived F.IX. Using the same strategy, 83% of myotube-synthesized factor IX was recovered from the starting material. Moreover, 10% of the starting material of both proteins was recovered when the flow-through fraction from the first binding step was reloaded onto a new Q-Sepharose column. Thus, almost 90% of myotube-synthesized F.IX and plasma-derived F.IX in the crude culture media can be recovered from the first column step.
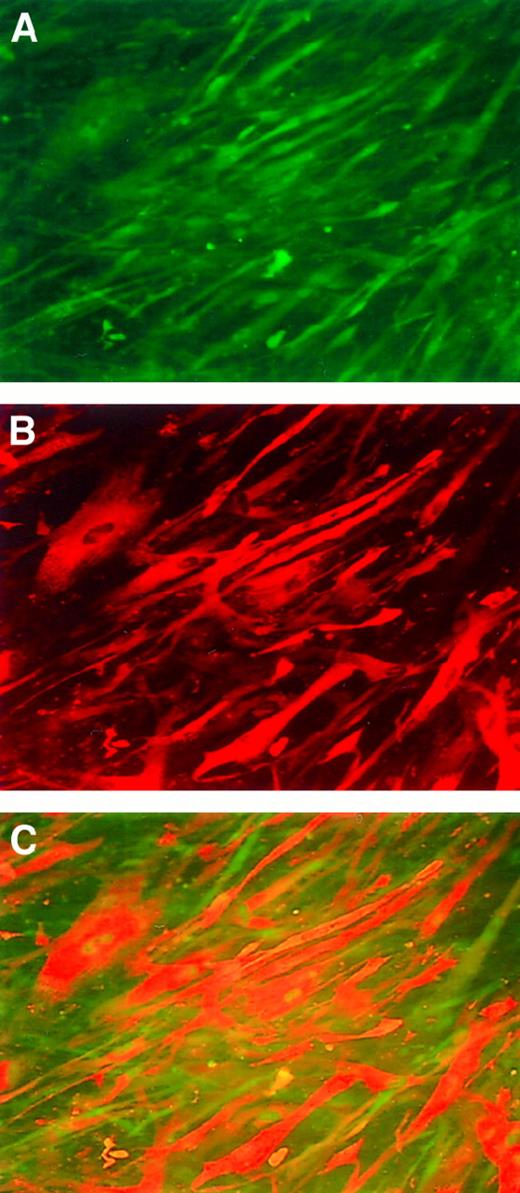
Immunofluorescence staining of human myotubes transduced with AAV-hF.IX.
(A) Cells are stained with an anti-desmin antibody (detecting antibody is FITC labeled). (B) Anti-factor IX antibody (detecting antibody rhodamine labeled). (C) Co-localization experiment.
Immunofluorescence staining of human myotubes transduced with AAV-hF.IX.
(A) Cells are stained with an anti-desmin antibody (detecting antibody is FITC labeled). (B) Anti-factor IX antibody (detecting antibody rhodamine labeled). (C) Co-localization experiment.
The next step in the purification makes use of a monoclonal antibody to the heavy chain of human F.IX (9EA). This monoclonal antibody was chosen specifically because the heavy chain has relatively few post-translational modifications. This strategy permits the recovery of 48% of F.IX from conditioned medium (Table1).
Yield from purification of myotube-synthesized F.IX
| . | Total F.IX:Ag protein (μg) . | % . |
|---|---|---|
| Conditioned medium (volume of 2L) | 790 | 100 |
| Q-Sepharose | 683 | 86.5 |
| Immunoaffinity | 380 | 48 |
| . | Total F.IX:Ag protein (μg) . | % . |
|---|---|---|
| Conditioned medium (volume of 2L) | 790 | 100 |
| Q-Sepharose | 683 | 86.5 |
| Immunoaffinity | 380 | 48 |
The myotube-synthesized F.IX purified from conditioned medium migrates as a single band with an apparent molecular weight (Mol wt) of 55 000 (Figure 2A). It migrates in a fashion similar to HepG2 cell-synthesized F.IX (lane 3), and to Mononine (Armour), a commercially available plasma-derived F.IX (lane 4). Immunoblotting identifies these proteins as human F.IX (Figure 2B).
Purification of myotube-synthesized hF.IX .
(A) 7.5% SDS-PAGE of purified human F.IX. Lane 1, myotube-synthesized hF.IX. Lane 2, HepG2 cell-synthesized F.IX. Lane 3, plasma-derived hF.IX. (B) Western blot of the same gel using a monoclonal antibody to the heavy chain of hF.IX. Lane 1, myotube-synthesized hF.IX. Lane 2, HepG2 cell-synthesized hF.IX. Lane 3, plasma-derived hF.IX (Mononine).
Purification of myotube-synthesized hF.IX .
(A) 7.5% SDS-PAGE of purified human F.IX. Lane 1, myotube-synthesized hF.IX. Lane 2, HepG2 cell-synthesized F.IX. Lane 3, plasma-derived hF.IX. (B) Western blot of the same gel using a monoclonal antibody to the heavy chain of hF.IX. Lane 1, myotube-synthesized hF.IX. Lane 2, HepG2 cell-synthesized hF.IX. Lane 3, plasma-derived hF.IX (Mononine).
Specific activity of the purified myotube-synthesized F.IX
Specific activity was determined on myotube-synthesized F.IX purified from 3 separate transduction experiments using an MOI of 10 000. These all showed specific activity approximately equal to that of plasma-derived F.IX (Table 2). The average of the 3 experiments yielded a specific activity of 180 U/mg,46 similar to the value of 200 U/mg, the published value for plasma-derived F.IX.39
Specific activity of myotube-synthesized F.IX
| . | [FIX] ELISA (mg/mL) . | Activity (U/mL) . | Specific activity (U/mg) . | % Normal . |
|---|---|---|---|---|
| PD-F.IX | 0.0222 | 3.74 | 167 | 84 |
| MS-F.IX sample 1 | 0.0037 | 0.515 | 136 | 68 |
| MS-F.IX sample 2 | 0.0097 | 1.72 | 177 | 89 |
| MS-F.IX sample 3 | 0.0005 | 0.0116 | 231 | 115 |
| MS-F.IX sample 4 | 0.0075 | 1.41 | 188 | 112 |
| Mean | 91 ± 12 |
| . | [FIX] ELISA (mg/mL) . | Activity (U/mL) . | Specific activity (U/mg) . | % Normal . |
|---|---|---|---|---|
| PD-F.IX | 0.0222 | 3.74 | 167 | 84 |
| MS-F.IX sample 1 | 0.0037 | 0.515 | 136 | 68 |
| MS-F.IX sample 2 | 0.0097 | 1.72 | 177 | 89 |
| MS-F.IX sample 3 | 0.0005 | 0.0116 | 231 | 115 |
| MS-F.IX sample 4 | 0.0075 | 1.41 | 188 | 112 |
| Mean | 91 ± 12 |
PD-F.IX, plasma-derived F.IX; MS-F.IX, myotube-synthesized F.IX.
Specific activity of myotube-synthesized F.IX in conditioned medium at different MOIs
Triplicate plates of human myotubes were transduced with AAV-hF.IX at a range of MOIs from 5000 to 80 000. The median specific activity was 177 ± 45 U/mg, with a range from 108 to 233 U/mg). A progressive reduction in the specific activity was observed at MOIs greater than 20 000 (Table 3), suggesting that the capacity for some critical posttranslational modification is exceeded at high MOI.
Specific activity of myotube-synthesized F.IX in the conditioned medium from myotubes transduced with AAV-CMV-hF.IX at a range of MOI
| MOI . | [FIX] ELISA (mg/mL) . | Activity (U/mL) . | Specific activity (U/mg) . |
|---|---|---|---|
| 5000 | 0.071 | 0.0135 | 188.5 |
| 10 000 | 0.265 | 0.062 | 233 |
| 20 000 | 0.221 | 0.047 | 212 |
| 40 000 | 0.396 | 0.058 | 146 |
| 80 000 | 0.656 | 0.0712 | 108 |
| mock | 0 | 0.001 | — |
| MOI . | [FIX] ELISA (mg/mL) . | Activity (U/mL) . | Specific activity (U/mg) . |
|---|---|---|---|
| 5000 | 0.071 | 0.0135 | 188.5 |
| 10 000 | 0.265 | 0.062 | 233 |
| 20 000 | 0.221 | 0.047 | 212 |
| 40 000 | 0.396 | 0.058 | 146 |
| 80 000 | 0.656 | 0.0712 | 108 |
| mock | 0 | 0.001 | — |
Additional evidence of the activity of the myotube-synthesized F.IX was obtained by determining the inhibitory effect of the monoclonal antibody FXOO8. This antibody binds to the heavy chain of factor IXa and impairs its interaction with factor VIIIa, resulting in a reduced activation of factor X.40 Factor IX clotting activity measured using the aPTT on myotube-conditioned media ranged from 6.4% to 10% of normal plasma but decreased to values less than 1% after the incubation of conditioned media with an anti-human factor IX monoclonal antibody (data not shown).
N-terminal sequence analysis
N-terminal amino acid sequence analysis of the single band shown in Figure 2A (myotube-synthesized F.IX) demonstrates that the band contains a single species with a sequence identical to that of mature plasma-derived F.IX (Table 4). Thus the signal sequence and the propeptide are accurately and efficiently removed.
NH2 terminal amino acid sequence analysis of the myotube-synthesized factor IX
| Cycle . | Amino acid . | pmol . | Cycle . | Amino acid . | pmol . |
|---|---|---|---|---|---|
| 1 | Tyr | 76.2 | 20 | (Glu) | —4-150 |
| 2 | Asn | 66.5 | 21 | (Glu) | —4-150 |
| 3 | Ser | 34.1 | 22 | Lys | 49.7 |
| 4 | Gly | 56.1 | 23 | (Cys) | —4-150 |
| 5 | Lys | 76.6 | 24 | Ser | 12.6 |
| 6 | Leu | 68.8 | 25 | Phe | 28.3 |
| 7 | (Glu) | —4-150 | 26 | (Glu) | —4-150 |
| 8 | (Glu) | —4-150 | 27 | (Glu) | —4-150 |
| 9 | Phe | 64.4 | 28 | Ala | 33.9 |
| 10 | Val | 72.6 | 29 | Arg | 32.3 |
| 11 | Gln | 54.7 | 30 | (Glu) | —4-150 |
| 12 | Gly | 44.7 | 31 | Val | 10.5 |
| 13 | Asn | 39.4 | 32 | Phe | 12.8 |
| 14 | Leu | 59.4 | 33 | (Glu) | —4-150 |
| 15 | (Glu) | —4-150 | 34 | Asn | 8.9 |
| 16 | Arg | 82.4 | 35 | Thr | 11.5 |
| 17 | (Glu) | —4-150 | 36 | (Glu) | —4-150 |
| 18 | (Cys) | —4-150 | 37 | Arg | 12.1 |
| 19 | Met | 64.0 |
| Cycle . | Amino acid . | pmol . | Cycle . | Amino acid . | pmol . |
|---|---|---|---|---|---|
| 1 | Tyr | 76.2 | 20 | (Glu) | —4-150 |
| 2 | Asn | 66.5 | 21 | (Glu) | —4-150 |
| 3 | Ser | 34.1 | 22 | Lys | 49.7 |
| 4 | Gly | 56.1 | 23 | (Cys) | —4-150 |
| 5 | Lys | 76.6 | 24 | Ser | 12.6 |
| 6 | Leu | 68.8 | 25 | Phe | 28.3 |
| 7 | (Glu) | —4-150 | 26 | (Glu) | —4-150 |
| 8 | (Glu) | —4-150 | 27 | (Glu) | —4-150 |
| 9 | Phe | 64.4 | 28 | Ala | 33.9 |
| 10 | Val | 72.6 | 29 | Arg | 32.3 |
| 11 | Gln | 54.7 | 30 | (Glu) | —4-150 |
| 12 | Gly | 44.7 | 31 | Val | 10.5 |
| 13 | Asn | 39.4 | 32 | Phe | 12.8 |
| 14 | Leu | 59.4 | 33 | (Glu) | —4-150 |
| 15 | (Glu) | —4-150 | 34 | Asn | 8.9 |
| 16 | Arg | 82.4 | 35 | Thr | 11.5 |
| 17 | (Glu) | —4-150 | 36 | (Glu) | —4-150 |
| 18 | (Cys) | —4-150 | 37 | Arg | 12.1 |
| 19 | Met | 64.0 |
Picomole amounts of residual amino acids were at or below background levels.
γ-Carboxyglutamic acid analysis
The Gla domain of F.IX contains 12 potential sites for γ-carboxylated glutamic acid residues. Using chemical Gla analysis, myotube-synthesized F.IX has 10.5 Gla residues/mol F.IX, which is slightly lower than 12 Gla residues/mol detected in 2 plasma-derived F.IX samples analyzed as controls. These data are consistent with those obtained on N-terminal sequence analysis, in which the automated sequencing through residue 37 shows the low yields typically seen for γ-carboxylated glutamic acid (Gla) residues (Table 4). Glu 7, 8, 15, 17, 20, 21, 26, 27, 30, 33, and 36 are each less than 10% of the yield at the previous and subsequent cycles. Only a single γ-carboxylated residue, Gla 40, was not assessed by N-terminal sequencing. The results are in agreement with those reported for plasma-derived F.IX and suggest that these Gla residues are more than 90% carboxylated.
Carbohydrate analysis
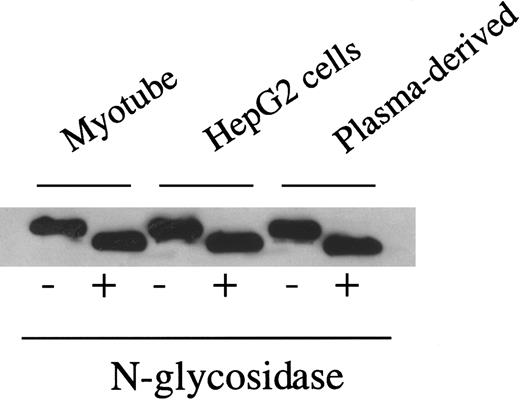
As noted earlier, F.IX contains both N- and O-linked oligosaccharide residues. Sites of O-linked glycosylation within liver-synthesized F.IX have been identified at residues Ser 53, Ser 61, Thr 159, Thr 169, Thr 172, and Thr 179,28 41 but the function of these residues remains unknown. The O-linked sites are not easily analyzed by enzymatic release because O-glycanase digestion requires an α-GalNAc linkage, nor are they readily examined using oligosaccharide profiling because enzymatic removal and fluorescent labeling of O-linked residues are inefficient processes that require large amounts of starting material to yield detectable signal. Given the absence of a known function and the technical difficulties of O-linked analysis, we have confined our analysis to the N-linked residues. Enzymatic digestion of myotube-synthesized F.IX by N-glycosidase F results in a decrease in apparent molecular weight (Figure 3), consistent with the removal of N-linked carbohydrate. This result is identical to that seen with plasma-derived F.IX or F.IX purified from AAV-hF.IX-transduced HepG2 cells.
Western blot of myotube-synthesized and hepatocyte-derived F.IX after enzymatic cleavage by N-glycosidase F.
Western blot of 7.5% SDS-PAGE gel of purified F.IX after digestion with N-glycosidase F using a monoclonal antibody to the heavy chain of F.IX. Myotube-synthesized hF.IX undigested (lane 1), digested (lane 2), HepG2-synthesized F.IX undigested (lane 3), digested (lane 4), and plasma-derived F.IX undigested (lane 5), digested (lane 6).
Western blot of myotube-synthesized and hepatocyte-derived F.IX after enzymatic cleavage by N-glycosidase F.
Western blot of 7.5% SDS-PAGE gel of purified F.IX after digestion with N-glycosidase F using a monoclonal antibody to the heavy chain of F.IX. Myotube-synthesized hF.IX undigested (lane 1), digested (lane 2), HepG2-synthesized F.IX undigested (lane 3), digested (lane 4), and plasma-derived F.IX undigested (lane 5), digested (lane 6).
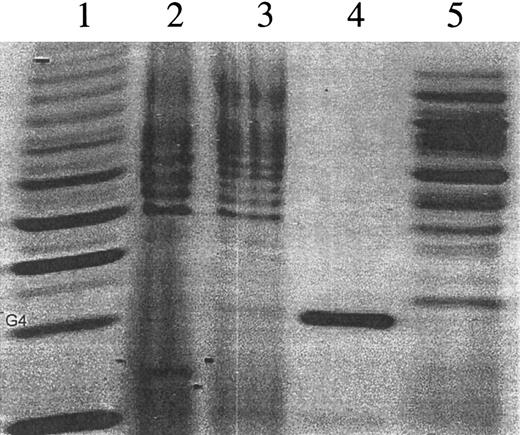
A more detailed analysis of N-linked carbohydrate residues was carried out using oligosaccharide profiling, in which oligosaccharide residues are cleaved off, labeled with a fluorophore, and analyzed electrophoretically33,42 (Figure4). Positions of the oligosaccharides in each lane of Figure 4 are determined relative to the glucose ladder in lane 1, and the quantification of oligosaccharides is based on the relative fluorescent intensity compared to that of 100 pmol maltotetrose standard band (G4) shown in lane 4.42 A well-characterized control glycoprotein, trypsin inhibitor, was treated identically to the control for enzymatic activity and fluorescent labeling (Figure 4, lane 5). Oligosaccharide profiling indicates that, though a number of the labeled oligosaccharide bands are similar in abundance and in position in a comparison of profiles for myotube-synthesized versus plasma-derived F.IX, a number of differences are detected (Figure 4). As an additional profiling step, labeled oligosaccharides were incubated with neuraminidase to release sialic acid. In carbohydrate electrophoresis, separation of complex oligosaccharides is influenced both by negative charges contributed by sialic acid and by the size (hydrodynamic volume) of the oligosaccharide. Therefore, an oligosaccharide containing sialic acids can migrate faster than a smaller oligosaccharide without sialic acids.33 42 Thus, removal of sialic acid from individual oligosaccharide bands results in a net upward shift of bands due to a combination of the loss of charge and mass (Figure5). Again, this suggests that many, but not all, bands are identical because each band represents a unique oligosaccharide. Thus, though both proteins exhibit a complex pattern of N-glycosylation with many similarities, the patterns are not identical.
Fluorophore-assisted carbohydrate electrophoresis (FACE) profiling of the N-linked oligosaccharide.
Oligosaccharides were released by enzymatic digestion with N-glycosidase F from myotube-synthesized F.IX (lane 2) and plasma-derived F.IX (lane 3). Lane 1 contains a ladder of glucose polymers. The position of Glucose 4 (G4) is indicated, and N-linked oligosaccharides migrate in the gel above position G4. N-glycosidase F digestion of trypsin inhibitor serves as a control for enzyme activity (lane 5). A maltotetrose quantitative standard equal to 100 pmol Glucose 4 oligosaccharides was used as control for quantitation (lane 4).
Fluorophore-assisted carbohydrate electrophoresis (FACE) profiling of the N-linked oligosaccharide.
Oligosaccharides were released by enzymatic digestion with N-glycosidase F from myotube-synthesized F.IX (lane 2) and plasma-derived F.IX (lane 3). Lane 1 contains a ladder of glucose polymers. The position of Glucose 4 (G4) is indicated, and N-linked oligosaccharides migrate in the gel above position G4. N-glycosidase F digestion of trypsin inhibitor serves as a control for enzyme activity (lane 5). A maltotetrose quantitative standard equal to 100 pmol Glucose 4 oligosaccharides was used as control for quantitation (lane 4).
Fluorophore-assisted carbohydrate electrophoresis (FACE) profiling of sialic acid residues.
(Lane 1) Wheat starch digest control yielding glucose polymers ranging from G3 (lowest band in lane 1) to approximately G15. In lanes 2 to 5, equal amounts of labeled oligosaccharide profile mixtures are loaded after a 24-hour incubation either with (lanes 3, 4) or without (lanes 2, 5) neuraminidase. (Lanes 2, 3) Myotube-synthesized F.IX. (Lanes 4, 5) Human plasma-derived F.IX.
Fluorophore-assisted carbohydrate electrophoresis (FACE) profiling of sialic acid residues.
(Lane 1) Wheat starch digest control yielding glucose polymers ranging from G3 (lowest band in lane 1) to approximately G15. In lanes 2 to 5, equal amounts of labeled oligosaccharide profile mixtures are loaded after a 24-hour incubation either with (lanes 3, 4) or without (lanes 2, 5) neuraminidase. (Lanes 2, 3) Myotube-synthesized F.IX. (Lanes 4, 5) Human plasma-derived F.IX.
Tyrosine sulfation
The tyrosine residue at position 155 is sulfated in plasma-derived F.IX. Using in vitro incorporation of labeled 35S-sulfate in HepG2 cells (to model liver synthesis) and myotubes, we sought to determine whether this residue was also sulfated in myotube-synthesized F.IX. Labeling of cells with 35S-methionine allows normalization for total protein synthesis from one experiment to another (myotube vs HepG2 cell synthesized); the35S-H2SO4 should reflect sulfation of the tyrosine at 155 after the contribution from sulfation in N-linked carbohydrate residues is removed34,43 44 by N-glycosidase F treatment (Figure 6). Assuming that HepG2 cells model hepatocyte synthesis and that the sulfate label in O-linked carbohydrate residues is identical in myotube- and HepG2 cell-synthesized F.IX, the results show that myotube-synthesized F.IX is minimally sulfated (1.2%) at Tyr 155 compared to HepG2 cell-synthesized F.IX.
Tyrosine 155 sulfation of myotube-synthesized and Hep G2 cell-synthesized F.IX.
(A) 35S-H2SO4 labeling. (B)35S-methionine labeling of synthesized recombinant FIX. Lanes 1 and 3 are myotube-synthesized F.IX. Lanes 2 and 4, HepG2 synthesized F.IX. Lanes 3 and 4, F.IX after digestion with N-glycosidase F. Lanes 1 and 2, undigested proteins. (C) Western blot of the same gel using a monoclonal antibody to heavy chain of hF.IX demonstrated that the band shown in panel A is human F.IX.
Tyrosine 155 sulfation of myotube-synthesized and Hep G2 cell-synthesized F.IX.
(A) 35S-H2SO4 labeling. (B)35S-methionine labeling of synthesized recombinant FIX. Lanes 1 and 3 are myotube-synthesized F.IX. Lanes 2 and 4, HepG2 synthesized F.IX. Lanes 3 and 4, F.IX after digestion with N-glycosidase F. Lanes 1 and 2, undigested proteins. (C) Western blot of the same gel using a monoclonal antibody to heavy chain of hF.IX demonstrated that the band shown in panel A is human F.IX.
Serine phosphorylation
A monoclonal antibody against phosphoserine residues permits relative quantitation of serine phosphorylation once carbohydrate groups (located in close proximity to Ser 158 resulting in steric hindrance of the antibody) have been removed. Analysis was performed on both purified plasma-derived F.IX and purified myotube-synthesized F.IX. The results show that 15 ± 3 ng/mL of 230 ± 80 ng/mL myotube-synthesized F.IX is phosphorylated, whereas 195 ± 6 ng/mL of 495 ± 15 ng/mL of plasma-derived F.IX is phosphorylated. Note that the differing affinities of the 2 antibodies preclude calculation of the percentage of F.IX that is phosphorylated but allow determination of relative levels of serine phosphorylation of myotube-synthesized F.IX (16%) compared to plasma-derived F.IX.
Myotube-synthesized F.IX is biologically active in vivo but has a lower recovery than plasma-derived F.IX
We measured recovery, half-life, and biologic activity of myotube-synthesized F.IX by conducting the appropriate pharmacokinetic studies in hemophilic and normal mice. To determine recovery and half-life, normal mice were infused with 2 μg plasma-derived (n = 4 mice), CHO cell-synthesized (n = 3 mice), or myotube-synthesized (n = 3 mice) F.IX. In humans and animals, F.IX disappearance from plasma can be described in terms of a 2-component model, with a rapid disappearance (termed α), corresponding to distribution into the tissues, followed by a much slower decay (termed β), thought to correspond to F.IX catabolism.45 The t1/2 for plasma-derived F.IX was 2.19 hours, and for myotube-synthesized F.IX it was 2.58 hours. The CHO-synthesized F.IX (Benefix; Genetic Institute) showed the longest half-life, 4.2 hours (n = 3 mice) (Figure7). These values are similar to those obtained in dogs after the infusion of either CHO cell-synthesized or plasma-derived F.IX46—that is, the 40% recovery after the injection of protein was higher for plasma-derived F.IX (Figure 7) than the 18% myotube- or CHO cell-synthesized F.IX. Factor IX clotting activity assayed by modified aPTT was carried out in 12 hemophilic animals after the infusion of F.IX preparations (10 μg/animal). In all animals the aPTT shortened into the normal range (22-39 seconds) after F.IX injection. Before and 30 minutes after infusion, aPTTs were 70.1 ± 11 seconds to 32.7 ± 1.3 seconds for plasma-derived F.IX, 69 ± 17 seconds to 32.0 ± 2.4 seconds for CHO cell-synthesized F.IX, and 68.55 ± 4.5 to 33.1 ± 1.0 seconds for myotube-synthesized F.IX. Values were similar at 24 hours, within the normal range of 22 to 39 seconds, reflecting the high initial dose.
Plasma concentration of F.IX after intravenous infusion in mice.
Plasma concentration of plasma-derived mononine (PD), CHO-synthesized, Benefix (BF), and myotube-synthesized F.IX (MD) after the intravenous injection of 2 μg protein in mice. Values are showed as mean ± SD of 4 (PD) or 3 (BF, MD) animals per group.
Plasma concentration of F.IX after intravenous infusion in mice.
Plasma concentration of plasma-derived mononine (PD), CHO-synthesized, Benefix (BF), and myotube-synthesized F.IX (MD) after the intravenous injection of 2 μg protein in mice. Values are showed as mean ± SD of 4 (PD) or 3 (BF, MD) animals per group.
Discussion
Previous work has shown that biologically active F.IX can be synthesized in a variety of tissues, including skeletal muscle cell.47-49 In addition to demonstrating the biologic activity of muscle-synthesized protein, however, we wanted to conduct detailed biochemical characterization because the nature of the posttranslational modifications can influence the recovery of the protein28 and, theoretically, the immunogenicity.50
Short of isolating muscle-synthesized F.IX from the plasma of treated human subjects, there is no straightforward method for obtaining F.IX synthesized in skeletal muscle because it is not possible to maintain mature myocytes in culture. However, myoblasts can be differentiated to myotubes in tissue culture, and these faithfully model many of the characteristics of mature skeletal muscle. In our experimental system, human F.IX was purified from conditioned medium using an ion exchange step followed by an immuno-affinity column with a monoclonal antibody to the heavy chain. Usual procedures for recovering recombinant F.IX from conditioned medium make use of antibodies to the light chain.37 We wanted to avoid this strategy because our goal was to recover material representative of all species of recombinant F.IX in the conditioned medium, not simply that fraction that was correctly posttranslationally modified. Based on a determination of F.IX content in conditioned medium and total amounts of purified protein isolated, our recovery of F.IX from conditioned medium was approximately 50%.
The purified myotube-synthesized F.IX yielded a single band on SDS-PAGE that migrated in a fashion identical to that of plasma-derived F.IX, and N-terminal sequence analysis of the recombinant F.IX was identical to that of mature plasma-derived F.IX. The requirement for cleavage of the propeptide of the F.IX is critical for functional activity.51 Although the enzyme responsible for the proteolytic cleavage is not identified with certainty, a candidate enzyme, PACE/Furin, can process the pro-FIX precursor when F.IX is overexpressed in a CHO cell system.52 The level of expression of PACE/Furin in skeletal muscle is unknown, but within the limits of F.IX expression in this study (range, 200-300 ng/mL per 24 hours), no abnormality was found in propeptide cleavage based on N-terminal sequence analysis of purified myotube-synthesized F.IX. These data suggest that the signal sequence and propeptide of precursor F.IX are accurately and efficiently removed in human myotubes.
The direct γ-carboxyglutamic acid analysis and the N-terminal sequence analysis of the myotube-synthesized F.IX demonstrate efficient carboxylation at 11 of 12 γ-carboxyglutamic acid residues. Carboxylation at residue 40 was not evaluated in the N-terminal sequence; however, it has been demonstrated39 53 that the absence of carboxylation at residues 36 and 40 does not reduce the specific activity of factor IX. In fact, the specific activity of the purified protein is comparable to that of plasma-derived F.IX. In addition, the correction of clotting assays in hemophilic mice and dogs suggests that myotube-synthesized F.IX is biologically active.
In previous work54 we have demonstrated that the γ-glutamyl carboxylase is present in skeletal muscle, but at a level only 5% to 10% of that found in the liver. This level of enzyme appears to be sufficient to provide full carboxylation of F.IX synthesized in myotubes in culture at the MOIs evaluated here. It is perhaps important to recognize that though the hepatocyte has at least 6 substrates for the γ-glutamylcarboxylase, skeletal muscle transduced with AAV-F.IX presumably has only one, so it may be that the reduced levels of enzyme in skeletal muscle are indeed adequate to produce fully carboxylated F.IX in vivo.
Addition of N-linked oligosaccharides often is an obligatory event for the folding and assembly of polypeptides.50N-glycosylation can be required for biologic activity and for the efficient transport of glycoproteins through the secretory pathway. In addition, it may influence half-life in the circulation.28,50 Sixty percent of the carbohydrate in factor IX is at N-linked sites located within the activation peptide.55 We analyzed the glycosylation of the myotube-synthesized factor IX using enzymatic cleavage of N-linked glycans and oligosaccharide profiling. Band patterns after enzymatic removal of N-linked oligosaccharides or of sialic acid residues suggest a similar amount of carbohydrate in myotube-synthesized F.IX and plasma-derived F.IX, though the patterns are not identical. In general, oligosaccharide profiling of plasma-derived F.IX is more heterogeneous because the product is derived from many donors. Some of the differences in glycosylation between myotube- and hepatocyte-synthesized F.IX likely reflect simply differences in cell type, just as the differences in glycosylation between plasma-derived and recombinant F.IX reflect differences in both the species (human vs hamster) and the cell type.8,15 It is theoretically possible that a gene therapy approach could, through overexpression of the transgene, result in an abnormal glycosylation pattern, but there is no evidence that the cellular machinery for N-glycosylation can be saturated.28
In plasma-derived F.IX, tyrosine at position 155 is sulfated; in contrast, recombinant F.IX expressed in CHO cells8 and, as shown here, myotube-synthesized F.IX are inefficiently sulfated at Tyr 155. Whether these cells have the capacity for tyrosine sulfation is unknown. Recently, the enzymes that catalyze tyrosine sulfation, tyrosyl protein sulfotransferase (TPST), have been identified. Northern blot analysis demonstrates that levels of expression of both TPST 1 and TPST 2 transcripts in skeletal muscle are similar to those seen in liver, but the functional activity of these proteins is unknown.56-59 Reduced tyrosine sulfation is likely to decrease the recovery of F.IX in vivo.28 60 In agreement with these findings, we have shown that the recovery of myotube-synthesized F.IX and CHO-cell synthesized F.IX is substantially reduced compared to that of plasma-derived F.IX.
Finally, serine phosphorylation at position 158 was also evaluated. The levels of serine phosphorylation in myotube-synthesized F.IX are only 16% of those of plasma-derived F.IX, which reflects a limited capacity of the muscle cells to perform this modification. According to published data, CHO-derived recombinant F.IX contains no phosphorylation at this position, but no effect on the specific activity was observed.8 Thus, myotube-synthesized F.IX, like CHO-derived recombinant F.IX, is poorly phosphorylated at this residue, and, as has been observed with CHO-derived recombinant F.IX, this does not appear to affect specific activity.
In summary, F.IX undergoes a series of complex post-translational modifications that are required for efficient secretion, normal recovery and half-life, and biologic activity. We have used a human myotube culture system to demonstrate that functional hF.IX can be produced in cells derived from skeletal muscle. Moreover, the detailed biochemical characterization of recombinant hFIX produced in myotubes demonstrates that these cells are able to perform the most important of the posttranslational modifications required for functional activity. Studies of recovery and half-life of myotube-synthesized F.IX in mice demonstrate reduced recovery but similar half-life compared to plasma-derived F.IX. Conclusions from these biochemical studies are also supported by studies in hemophilic dogs, in which shortening of clotting assays was demonstrated after the introduction of an AAV-F.IX vectors into skeletal muscle.25
Together the data presented here permit us to state with a reasonable degree of certainty that F.IX synthesized in myotubes has a biochemical profile and a biologic activity comparable to hepatocyte-synthesized F.IX and should thus result in correction of the hemostatic defect in human hemophilia B.
Supported by National Institutes of Health grants RO1 HL 53668 and RO1 HL 48322.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Katherine A. High, Division of Hematology, The Children's Hospital of Philadelphia, 310A Abramson Research Center, 34th Street and Civic Center Boulevard, Philadelphia, PA 19104.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal