Abstract
The tissue homing of malignant hematic cells has both diagnostic and pathogenetic importance. Although such homing is incompletely understood, it generally involves cell adhesion and migration mediated by a number of adhesion receptors and cytokines. In this article, the potential importance of hyaluronan (HA) is examined for the tissue homing of hairy cells (HCs) in hairy cell leukemia (HCL). It is shown that HCs readily adhere to, and spontaneously move on, HA-coated surfaces using CD44. This indicates that activated CD44 and spontaneous movement on HA form part of the intrinsically activated phenotype of HCs. Interleukin-8 (IL-8) inhibited HC movement on HA, and this cell arrest was accompanied by increased actin polymerization and a more pronounced association of CD44 with the cytoskeleton. All of these findings are in sharp contrast to our previous observations with chronic lymphocytic leukemia cells, which are nonmotile on HA, but in response to IL-8 become polarized and motile using the receptor for HA-mediated motility rather than their apparently inactive CD44. Immunohistochemical examination of HCL tissues showed the ubiquitous presence of IL-8 and the prominence of HA in bone marrow stroma and hepatic portal tracts. This suggests that CD44-HA interactions are important in HC homing to these sites, but not to splenic red pulp or hepatic sinusoids, where HA is largely absent. Moreover, engagement of CD44 on HCs stimulates fibronectin synthesis, an observation that is likely to be relevant to the restriction of fibrosis in the disease to HC-infiltrated areas containing HA.
Introduction
Cell homing to bone marrow (BM) and lymphoreticular tissues is of crucial importance not only for normal hematopoiesis, but also for the dissemination of many hematic and nonhematic malignancies. Such homing in general involves specific adhesion/motility receptors and ligands and cytokines present in the tissue environment. Identification of the particular receptors, ligands, and stimuli involved in cell homing in different diseases is a subject of great current interest because it provides important pathogenetic insights that may suggest ways of interfering with cell homing that could be therapeutically beneficial.
We have been interested in the mechanisms of tissue homing of the malignant cells of chronic lymphoid malignancies, especially chronic lymphocytic leukemia (CLL) and hairy cell leukemia (HCL).1-7 Previously, we focused on integrins and their protein ligands1-6 and, more recently, on the cellular receptors for the ubiquitous tissue matrix glycosaminoglycan, hyaluronan (HA).7 The present work is concerned with the potential role of CD44 and its principal ligand HA in hairy cell (HC) homing and in fibronectin (FN) synthesis at different tissue sites.
HCL is a chronic B-cell disorder with a number of highly distinctive features.8 Prominent among these are the homing properties of the pathognomonic HCs. For example, HCs have a predilection for the BM and splenic and hepatic sinusoids but show little tendency to accumulate in lymph nodes.9-11 Furthermore, in the involved organs, the HCs adopt a distinctive tissue distribution. Thus, in the spleen, the HCs home to the red pulp where they often line the sinusoids and may replace the endothelial cells.12 In the liver, the HCs also line the sinusoids but, in addition, are found in large numbers in the portal tracts.10 In the BM, HCs synthesize FN and form a close association with it in a fine reticulin meshwork that is so distinctive that it has diagnostic importance.1 11
We have previously shown that some of these tissue interactions are probably attributable to HC integrins. Thus, HCs express a consistent and distinctive pattern of activated integrin receptors.3For example, the cells possess α4β1 and α5β1, through which they interact with FN in the BM1,3 and with vascular cell adhesion molecule (VCAM) in splenic red pulp and BM stroma.6 The HCs also possess αvβ3 receptors, which mediate spontaneous movement on vitronectin (VN); such an interaction may be important for migration within the splenic red pulp, an area specifically rich in VN.3
HCs also express CD44,13 a well-recognized nonintegrin receptor for cell homing, especially to BM.14,15 For example, hematopoietic-progenitor homing to BM is known to depend on both the interaction of β1 integrins with VCAM and the interaction of CD44 with HA.15 Because the role of CD44 in the homing behavior of HCs has not been explored previously, we here report on the in vitro behavior of HCs on HA with and without stimulation with interleukin-8 (IL-8). IL-8 is the best characterized member of the C-X-C chemokine family and is produced by, and acts on, a wide variety of cells,16 including normal and malignant B cells.7,17,18 The choice of IL-8 as a potentially relevant chemokine for HC homing was based on our recent studies of CLL cell behavior on HA.7 The latter cells become motile on HA in the presence of IL-8 and, despite expressing CD44, use an alternative HA receptor, the receptor for HA-mediated motility (RHAMM),19 for this movement.7
Here we demonstrate that HCs, in contrast to CLL cells, are spontaneously motile on HA, that the receptor involved is apparently constitutively active CD44, and that IL-8 inhibits, rather than enhances, this CD44-HA–dependent cell movement. Furthermore, our examination of the distribution of HA and IL-8 in HC-infiltrated tissues suggests a role for the HA-CD44 interaction in homing to BM and perhaps to hepatic portal tracts, but not to splenic red pulp, where HA was completely absent. Because HA was confined to tissue sites with prominent fibrosis, we examined the effect of CD44 engagement on FN synthesis by HCs. This engagement greatly enhanced FN production and may account for the presence in the disease of fibrosis in BM, but not spleen.
Materials and methods
Diagnosis of HCL
All patients had typical disease as determined by clinical presentation, morphology, tartrate-resistant acid phosphatase staining, and immunophenotype.
HC isolation and purification
Peripheral blood HCs were isolated by Ficoll-Hypaque density gradient centrifugation (Lymphoprep; Nycomed, Oslo, Norway). Samples were further depleted of T cells and monocytes using magnetic beads (Dyna-Bead; Dynal A.S., Oslo, Norway) coated with antibodies against CD3 and CD14. After this depletion, contaminating cells were less than 1% as measured by monoclonal antibody (MoAb) staining and flow cytometry.
Monoclonal antibodies
The following MoAbs were used: anti-CD44 (50B4, a blocking MoAb; a gift from Dr M. Letarte, Hospital for Sick Children, Toronto); Leu-44 and 2C5 nonblocking reagents (Becton Dickinson, Oxford, UK and R & D Systems, Abingdon, UK); Hermes-1 (Endogen, Woburn, MA); anti-RHAMM (3T3.5, a blocking antibody; a gift from Dr E. Turley, Hospital for Sick Children); anti-CD54 (a blocking antibody; R & D Systems) and Leu-54 (a nonblocking MoAb; Becton Dickinson); anti-CD38 (leu-17, a nonblocking MoAb; Becton Dickinson); antichemokine receptors IL-8RA, IL-8RB, and macrophage inflammatory protein-1α (MIP-1α) (all from Pharmingen, Oxford, UK); anti–IL-8 (a blocking MoAb; R&D Systems); and anti–FN-3E2 (Sigma, Poole, UK). All of these MoAbs are of the IgG1 isotype with the exception of FN-3E2 (IgG2a). To control for nonspecific effects of the MoAbs, we used the corresponding isotypes of nonimmune Igs.
Flow cytometry
The surface receptor expression of cells in suspension was measured by an indirect technique using goat anti-mouse (GAM) Ig–fluorescein isothiocyanate (FITC) (Becton Dickinson) detection of bound specific MoAbs. Both first- and second-layer antibodies were used at saturating concentrations. For detection of cytoplasmic F-actin, the cells were fixed and permeabilized with a solution of paraformaldehyde (3.7%) and lysophosphatidylcholine (5 μg/mL) before staining with rhodamine-labeled phalloidin.
Time-lapse video microscopy
Petri dishes were coated overnight with 100 μg/mL HA (Pharmacia, Uppsala, Sweden) or, in certain experiments, with VN (20 μg/mL) or FN (50 μg/mL). Dishes were washed and HCs were added (2 × 106/mL) with or without IL-8 (10 ng/mL) or MIP-1α (100 pg/mL). In preliminary experiments, ranges of concentrations of IL-8 (1, 10, and 100 ng/mL) and MIP-1α (5, 100, and 500 pg/mL) were tested; the effect of IL-8 was maximal at 10 ng/mL, whereas MIP-1α had no effect at any of these concentrations. The dishes were then placed on a heated (37°C) microscope stage and filmed for 2 hours using TLVM. Cell movement was determined by sequential tracing on the video screen. A cell was considered to have moved when its position had changed by more than one diameter. This method allows the percentage and speed of motile cells to be calculated. For the calculations of speed, the HCs were assumed to be 12 μm in diameter.
To establish the receptors responsible for cell motility, we incubated the HCs with blocking MoAbs to CD44 (20 μg/mL), RHAMM (10 μg/mL), and CD54 (40 μg/mL), and an IgG1 control (40 μg/mL), for 30 minutes on ice before addition to Petri dishes. Also, to ensure that the observed effect was due to HA, we preincubated HA-coated Petri dishes with hyaluronidase (50 TRU; Calbiochem, Nottingham, UK) for 30 minutes at 37°C before washing and addition of HCs.
Adhesion assay
Cell adhesion was measured as described previously using35S-methionine–labeled HCs.6 Briefly, HCs were incubated overnight in methionine-free RPMI medium (Gibco BRL, Paisley, Scotland) containing 30 μCi 35S-methionine (Amersham, Bucks, UK). After extensive washing, 2 × 105cells in 100 μL RPMI with or without IL-8 (10 ng/mL) were incubated for 40 minutes at 37°C in microtiter plates that had been precoated overnight with HA (100 μg/mL). Following incubation, the wells were washed extensively to remove nonadherent cells. Scintillant was added and radioactivity was measured by a Microbeta plate counter (Wallac 1450 liquid scintillation counter; Wallac, Milton Keynes, UK). Adhesion was calculated as a percentage of total counts produced by 2 × 105 labeled cells. All measurements were made in triplicate.
Confocal microscopy
Microscope slides were coated with 100 μg/mL HA overnight. After washing, HCs were added to these slides with or without IL-8 (10 ng/mL) and incubated at 37°C for 40 minutes. Slides were then washed and the adherent cells were fixed with 3.8% paraformaldehyde, washed again, and allowed to dry. The cells were then stained with anti-CD44 MoAb, followed by GAM-FITC as the second layer. For detection of F-actin, the cells were stained with rhodamine-phalloidin (Molecular Probes, Leiden, Holland). Fluorescence was analyzed using a Microradiance confocal microscope (Bio-Rad, Hemel Hempstead, Herts, UK).
Immunoblotting of CD44
For the preparation of cell lysates, HCs were first incubated on HA-coated wells for 30 minutes at 37°C. Adherent cells were then scraped off and lysed with lysis buffer (10 mmol/L Tris, pH 7.4, 150 mmol/L NaC1, 1% Triton X-100, and phenylmethylsulfonyl fluoride 4 mmol/L) for 30 minutes at 4°C. After centrifugation, Triton-insoluble fractions were dissolved with sodium dodecyl sulfate (SDS) buffer to solubilize cytoskeleton-bound CD44.
SDS-polyacrylamide gel electrophoresis (SDS-PAGE) was performed under reducing conditions using 5% to 15% gradient gels. After SDS-PAGE, proteins were transferred to Immobilon-P membranes using a Trans-Blot electrophoretic transfer cell (Bio-Rad). Membranes were blocked for 1 hour at 37°C with 100 mmol/L NaC1, 0.1% Tween-20, and 10 mmol/L Tris, pH 7.5 (TBST) containing 5% (wt/vol) nonfat dry milk and then overlaid with the 2C5 anti-CD44 MoAb at a concentration of 2 μg/mL in TBST containing 1% milk. After overnight incubation on a rocker platform at 4°C, the membrane was washed 3 times with TBST and overlaid for 30 minutes at 37°C with horseradish peroxidase (HRP)-conjugated GAM Ig at a concentration of 0.2 μg/mL in TBST containing 5% milk. After 3 washes with TBST, MoAb-labeled proteins were detected using enhanced chemiluminescence reagent (Amersham).
Tissue staining
Paraffin-embedded, formaldehyde-fixed tissues were used. HA was detected with HRP-conjugated HA-binding protein (HABP; a gift from Chugai Biopharmaceuticals, San Diego, CA) using the method of Ichida et al.20 After clearing with xylene, we treated the slides with H2O2 and blocked them with 10 mg/mL bovine serum albumin (BSA). The slides were then incubated for 30 minutes with HABP-HRP (40 μg/mL; maximal staining obtained at a concentration of greater than 20 μg/mL) and then with diaminobenzidine substrate (DAB, 0.5 mg/mL; H2O2, 1 μg/mL; Sigma) for a further 20 minutes. The slides were finally counterstained with hematoxylin. In addition, to ensure the specificity of staining, sections of each of the tissues studied were digested for 30 minutes at 37°C with hyaluronidase (50 TRU; Calbiochem) before staining for HA.
IL-8 was detected by the method of Brew et al.21 Briefly, after clearing and rehydration, the slides were boiled in 10 mmol/L sodium citrate buffer (pH 6) for 10 minutes and blocked with 10 mg/mL BSA before overnight incubation with anti–IL-8 MoAb (25 mg/mL). Sections were then incubated with GAM-biotin (Zymed, San Francisco, CA) and then with ExtrAvidin alkaline phosphate (Sigma) before exposure to substrate (Fast Red/ Naphthol AS MX phosphatase and levamisole; Sigma). The slides were counterstained with hematoxylin.
Measurements of FN synthesis by HCs
FN was measured by a triple-layer immunocytochemical method and by a modified enzyme-linked immunosorbent assay (ELISA) technique. For the immunocytochemical method, coverslips were coated overnight with BSA (1 mg/mL), with HA (100 μg/mL), with a rat anti-CD44 MoAb (7 μg/mL), or with control IgG2a (7 μg/mL). HCs in serum-free RPMI medium were cultured on these coverslips in 24-well plates for 24 hours at 37°C in 5% CO2. Cells were then fixed and stained with an FN-3E2 MoAb or isotype control, and FN was detected by the ExtrAvidin-peroxidase staining kit (Sigma).
For the modified ELISA method, HCs were cultured in 96-well plates. Coating and FN detection were performed as described above. Color change of the peroxidase substrate DAB was read by a multiscan plate reader at 450 nm. Five wells were used for each sample.
To ensure that equal numbers of cells were examined on each surface, we lysed the cells and estimated protein by a commercial assay (Bio-Rad). The levels of cellular protein measured on each surface were comparable.
Results
HCs are spontaneously motile on HA
TLVM showed that a proportion (approximately 40%) of HCs were spontaneously motile on HA-coated plastic dishes (Table1), and throughout the observation period (2 hours), this number remained constant. The motile cells exhibited a characteristic polar appearance with a blunt leading edge and a trailing tail. Most of the remaining cells were seen to adhere and lose their rounded appearance, but were considered nonmotile because minimal positional change (less than one cell diameter) was observed.
Movement of HCs on HA- or VN-coated plastic surfaces
| Surface and treatment . | n* . | Speed of moving cells (μm/min) . | % . |
|---|---|---|---|
| HA alone | 12 | 7.6 ± 0.4 | 39 ± 3 |
| HA + IL-8 | 12 | 3.5 ± 0.2 | 9 ± 1 |
| HA + IL-8 + anti-IL-8 MoAb | 3 | 8.7 ± 1.2 | 35 ± 4 |
| HA + IgG1 + IL-8 | 3 | 3.2 ± 0.2 | 10 ± 1 |
| HA + MIP-1α | 4 | 6.6 ± 0.5 | 38 ± 3 |
| VN alone | 6 | 3.9 ± 1.6 | 8 ± 2 |
| VN + IL-8 | 6 | 6.4 ± 1.2 | 22 ± 3 |
| Surface and treatment . | n* . | Speed of moving cells (μm/min) . | % . |
|---|---|---|---|
| HA alone | 12 | 7.6 ± 0.4 | 39 ± 3 |
| HA + IL-8 | 12 | 3.5 ± 0.2 | 9 ± 1 |
| HA + IL-8 + anti-IL-8 MoAb | 3 | 8.7 ± 1.2 | 35 ± 4 |
| HA + IgG1 + IL-8 | 3 | 3.2 ± 0.2 | 10 ± 1 |
| HA + MIP-1α | 4 | 6.6 ± 0.5 | 38 ± 3 |
| VN alone | 6 | 3.9 ± 1.6 | 8 ± 2 |
| VN + IL-8 | 6 | 6.4 ± 1.2 | 22 ± 3 |
Movement was recorded by time-lapse video microscopy (TLVM) over 2 hours. Results are means ± 1 SEM. HC indicates hairy cell; HA, hyaluronan; VN, vitronectin; IL, interleukin; MoAb, monoclonal antibody; MIP-1α, macrophage inflammatory protein-1α.
Number of separate experiments carried out; where n ≤ 4, the experiments involved different HCL patients; where n = 6 or 12, 6 different patients were studied.
On uncoated plastic surfaces (4 experiments using cells from 4 different patients), no HC movement occurred, and the cells became adherent and more spread than on HA. Hyaluronidase treatment of the HA-coated surfaces abolished HC movement (3 experiments in 2 patients), and the cell morphology resembled that on uncoated plastic.
Receptors mediating HC motility on HA
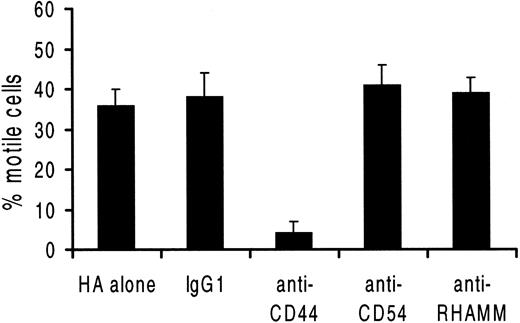
Of the 4 receptors for HA (CD38, CD44, CD54, and RHAMM) potentially expressed by HCs,22 only CD44 and CD54 were detected (Table 2). CD44 staining showed a single population of uniformly and strongly positive cells. A blocking anti-CD44 MoAb abrogated movement on HA, whereas blocking anti-CD54 and anti-RHAMM antibodies (and a class-specific IgG1 control MoAb) had no effect on the number of motile cells or their speed of movement (Figure 1). Taken together, these data indicate that HCs move spontaneously on HA (but not on uncoated plastic) and that this movement is dependent on CD44.
Expression of HA and chemokine receptors by HCs
| MoAb . | Reactive cells . | |
|---|---|---|
| % . | MFI . | |
| CD44 | 94 ± 1 | 511 ± 76 |
| CD54 | 77 ± 2 | 80 ± 15 |
| CD38 | 3 ± 1 | 70 ± 22 |
| RHAMM | 2 ± 1 | 8 ± 3 |
| IL-8RA | 57 ± 3 | 9 ± 1 |
| IL-8RB | 50 ± 3 | 7 ± 1 |
| MIP-1α R | 48 ± 2 | 12 ± 1 |
| MoAb . | Reactive cells . | |
|---|---|---|
| % . | MFI . | |
| CD44 | 94 ± 1 | 511 ± 76 |
| CD54 | 77 ± 2 | 80 ± 15 |
| CD38 | 3 ± 1 | 70 ± 22 |
| RHAMM | 2 ± 1 | 8 ± 3 |
| IL-8RA | 57 ± 3 | 9 ± 1 |
| IL-8RB | 50 ± 3 | 7 ± 1 |
| MIP-1α R | 48 ± 2 | 12 ± 1 |
Staining was performed by a 2-layer technique, and fluorescence was detected by flow cytometry in monocyte- and T-cell -depleted peripheral blood mononuclear cells from 6 patients. The results are means ± 1 SEM. MFI indicates mean fluorescence intensity; RHAMM, receptor for HA-mediated motility; other abbreviations as in Table 1.
Blocking of HC movement on HA-coated surfaces.
Cells were preincubated with the relevant MoAb before placing on HA-coated plates. The percentage of motile cells and their speed of movement (not shown) were recorded by TLVM (n = 3 experiments involving 3 different patients; results are means ± 1 SEM).
Blocking of HC movement on HA-coated surfaces.
Cells were preincubated with the relevant MoAb before placing on HA-coated plates. The percentage of motile cells and their speed of movement (not shown) were recorded by TLVM (n = 3 experiments involving 3 different patients; results are means ± 1 SEM).
Because IL-8 induces CLL cell movement on HA,7 we next tested the effect of the chemokine on HC movement on this substrate.
IL-8 inhibits spontaneous HC movement on HA
IL-8 markedly reduced the movement of HCs on HA (Table 1); an anti–IL-8 MoAb restored movement, whereas a class-specific control MoAb did not affect the IL-8–induced reduction of motility. The C-C chemokine MIP-1α, known to attract normal B cells at picomolar concentrations,23 had no effect on HC movement on HA (Table 1) even though HCs were shown to possess MIP-1α receptors as well as receptors for IL-8 (Table 2).
In view of the chemokine nature of IL-8 and the induction of CLL cell movement by the cytokine, this abrogation of CD44-HA–mediated movement was unexpected. We therefore tested the effect of IL-8 on the previously demonstrated3 αvβ3-mediated HC movement on VN. In contrast to its inhibiting effect on cell movement on HA, IL-8 potentiated HC movement on VN-coated surfaces (Table 1). However, HCs strongly adherent to FN via α4/5β1 integrins remained nonmotile in the presence of IL-8 (data not shown). Therefore, the arrest by IL-8 of cell movement on HA seemed to be a particular effect of IL-8 stimulation on motility mediated by the CD44-HA interaction.
Because it is known that cell movement and adhesion involve changes in polymerization and redistribution of F-actin,24 we next examined F-actin in HCs on HA by confocal microscopy and fluorescence-activated cell sorter (FACS) analysis.
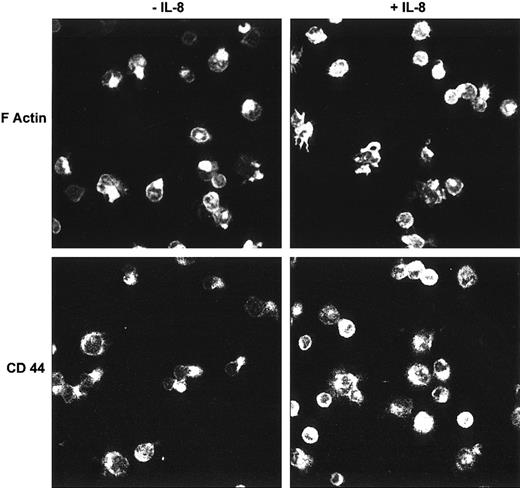
Distribution of F-actin and CD44 in HCs on HA with or without IL-8
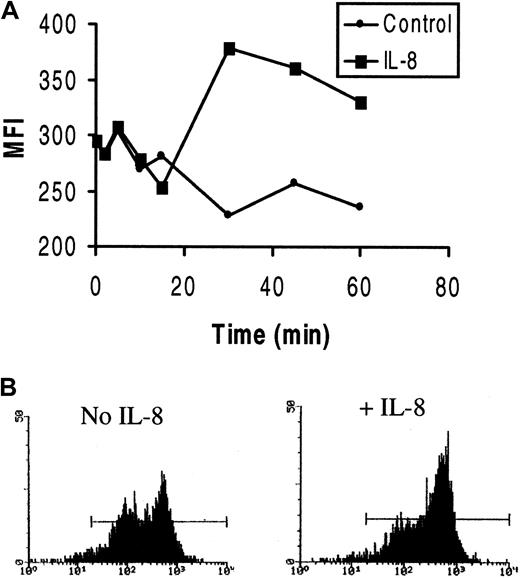
In HCs that had become polarized on HA, confocal microscopy demonstrated that most F-actin was located in the tail area of the cell, although some was also seen at the leading edge (Figure2). In contrast, in the presence of IL-8, few polarized cells were observed and the F-actin content of the cells appeared increased. Moreover, in these IL-8–treated cells, the F-actin was mainly distributed in distinct focal areas, especially in irregularly shaped, spread cells. The enhancement of F-actin polymerization by IL-8 was confirmed by FACS analysis of permeabilized cells (Figure 3A). Furthermore, the FACS profiles (Figure 3B) demonstrated 2 distinct cell populations with different staining intensities. These 2 populations presumably correspond to the motile (less bright) and nonmotile (more bright) populations found in the motility assays. IL-8 stimulation clearly caused the redistribution of cells from the less bright to the bright population.
Analysis by confocal microscopy of F-actin and CD44 in HCs on HA with or without IL-8.
HCs adherent to HA were stained with rhodamine-phalloidin (for F-actin) or with an anti-CD44 MoAb. In the absence of IL-8, both F-actin and HA showed a polarized distribution in many cells, whereas few or no such polarized cells were observed in the presence of IL-8 (representative examples of 6 separate experiments).
Analysis by confocal microscopy of F-actin and CD44 in HCs on HA with or without IL-8.
HCs adherent to HA were stained with rhodamine-phalloidin (for F-actin) or with an anti-CD44 MoAb. In the absence of IL-8, both F-actin and HA showed a polarized distribution in many cells, whereas few or no such polarized cells were observed in the presence of IL-8 (representative examples of 6 separate experiments).
Effect of IL-8 on F-actin polymerization in HCs.
HCs were incubated with or without IL-8, and polymerized F-actin was measured by FACS analysis of fixed and permeabilized cells stained with rhodamine-phalloidin. (A) Representative example of 3 experiments with cells from 3 patients. The intensity of staining varied but was similarly enhanced by IL-8 in all instances. (B) Two representative FACS histograms of F-actin in unstimulated or IL-8–stimulated cells on HA after 30 minutes of incubation. Note the presence of 2 distinct populations and the recruitment of cells to the F-actin–bright population after IL-8 stimulation.
Effect of IL-8 on F-actin polymerization in HCs.
HCs were incubated with or without IL-8, and polymerized F-actin was measured by FACS analysis of fixed and permeabilized cells stained with rhodamine-phalloidin. (A) Representative example of 3 experiments with cells from 3 patients. The intensity of staining varied but was similarly enhanced by IL-8 in all instances. (B) Two representative FACS histograms of F-actin in unstimulated or IL-8–stimulated cells on HA after 30 minutes of incubation. Note the presence of 2 distinct populations and the recruitment of cells to the F-actin–bright population after IL-8 stimulation.
We next looked at the effect of IL-8 on the distribution of CD44. Before addition of the cytokine, CD44, like F-actin, was located mainly at the tails of HCs that were polarized on HA (Figure 2), but was more uniformly distributed in nonpolarized cells. In the presence of IL-8, the distribution of CD44 again resembled that of F-actin in being found mainly in focal areas of flattened cells (Figure 2).
The confocal data of HCs examined in the absence of chemokine, by showing polarization of both F-actin and CD44, therefore support our TLVM evidence of spontaneous HC motility on HA involving CD44. Furthermore, taken together with the FACS findings, our data indicate that IL-8 increases the overall F-actin polymerization of the HCs and causes F-actin and CD44 to form focal accumulations in areas of adhesion to HA. However, an adhesion assay showed that this IL-8–induced change in the distribution of F-actin and CD44 was not accompanied by a change in the strength of adhesion (77% ± 3% cells adherent in the absence of IL-8; 79% ± 2% cells adherent in the presence of cytokine; n = 3). Therefore, the IL-8–induced arrest of cell movement involved changes in cytoskeletal dynamics that are not directly translated into enhanced adhesion.
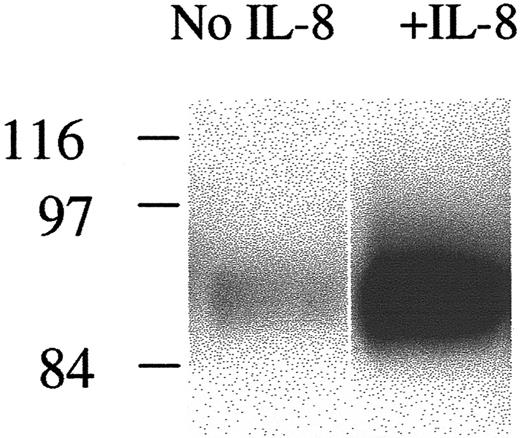
To examine biochemically the association between CD44 and F-actin, suggested by their colocalization in confocal images, we next looked at CD44 association with the Triton-insoluble cytoskeleton in HCs on HA before and after stimulation by IL-8. Figure4 shows that, in parallel with the IL-8–induced increase in F-actin formation demonstrated in Figures 2and 3, there was also an increase in CD44 in the Triton-insoluble cell fraction.
Effect of IL-8 on the association of CD44 with the cytoskeleton.
HCs were incubated on HA with or without IL-8. Cells were then lysed with Triton, and the lysates were centrifuged. The Triton-insoluble fractions were resuspended in SDS buffer and then run on SDS-PAGE, and protein was transferred to a nitrocellulose membrane and immunoblotted with anti-CD44.
Effect of IL-8 on the association of CD44 with the cytoskeleton.
HCs were incubated on HA with or without IL-8. Cells were then lysed with Triton, and the lysates were centrifuged. The Triton-insoluble fractions were resuspended in SDS buffer and then run on SDS-PAGE, and protein was transferred to a nitrocellulose membrane and immunoblotted with anti-CD44.
To investigate how these observations might relate to the behavior of HCs within tissues, we next examined the distribution of HA and IL-8 in the relevant HCL and normal lymphoreticular tissues.
The distribution of HA and IL-8 in HCL and normal lymphoreticular tissues
In HCL, the malignant HCs are predominantly found in the red pulp of the spleen and in the BM and liver; peripheral lymph nodes are largely spared. We therefore examined HCL spleen, BM, and liver as compared with the corresponding normal tissues.
Spleen.
Apart from strong staining around blood vessels, the infiltrated red pulp was completely unreactive for HA (Figure5); residual white pulp contained strong staining. An approximately similar distribution of HA staining was observed in normal spleen (ie, red pulp lacked reactivity but the white pulp showed moderate reticular staining). Regarding IL-8, moderate staining was present throughout the infiltrated HCL red pulp; the chemokine was detected throughout normal spleen (not shown).
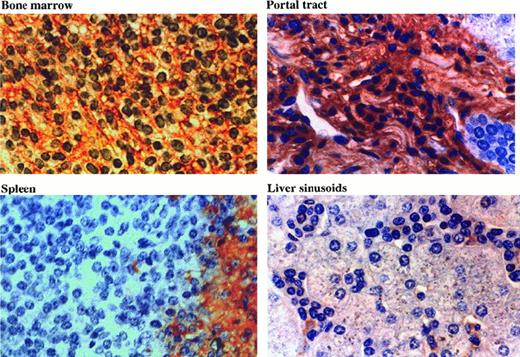
Distribution of HA in HCL tissues.
Tissue sections were stained with HRP-conjugated HA binding protein (see “Materials and methods”). In bone marrow, a reticular network of HA is clearly seen. In portal tracts, the HA is associated with the dense matrix. Splenic red pulp lacks HA, whereas the residual white pulp is positive. In the hepatic sinuses, only the macrophages are stained.
Distribution of HA in HCL tissues.
Tissue sections were stained with HRP-conjugated HA binding protein (see “Materials and methods”). In bone marrow, a reticular network of HA is clearly seen. In portal tracts, the HA is associated with the dense matrix. Splenic red pulp lacks HA, whereas the residual white pulp is positive. In the hepatic sinuses, only the macrophages are stained.
Bone marrow.
In areas of extensive HC involvement, prominent HA staining was observed in the form of a reticular network (Figure 5). In noninvaded parts of the BM, minor reticular staining was present, and here reactivity resembled that of normal BM. IL-8 was readily detectable in areas of HC infiltration; in normal BM, weak to moderate staining was observed in most areas (not shown).
Liver.
In both normal and HCL tissue, HA staining was largely confined to portal tracts, whereas the sinuses were mainly unreactive (Figure 5). In the portal tracts, the infiltrating HCs appeared flattened and surrounded by HA-rich matrix. In contrast, HCs in the sinuses were round, and often in mutual contact without any interposed matrix. The only cells in the sinuses containing HA were resident macrophages, and HCs were consistently found in close association with these cells. IL-8 was ubiquitously present throughout both HCL and normal liver and was found in both portal tracts and hepatic parenchyma (not shown).
HA-CD44 interaction stimulates FN production by HCs
In the above histologic studies, we were struck by the clear correlation between the distribution of HA and the previously reported localization of the FN matrix produced by HCs.1 Thus, FN matrix is found in HC-infiltrated BM and portal tracts,1,11,25 where HA is abundant. In contrast, both FN and HA are absent from the splenic red pulp, a major site of HC infiltration. This suggests that interaction with HA may be involved in the stimulation of FN synthesis by HCs. This notion is supported by the previous demonstration that CD44-mediated adhesion of monocytes is important in the pathogenesis of myelofibrosis.26
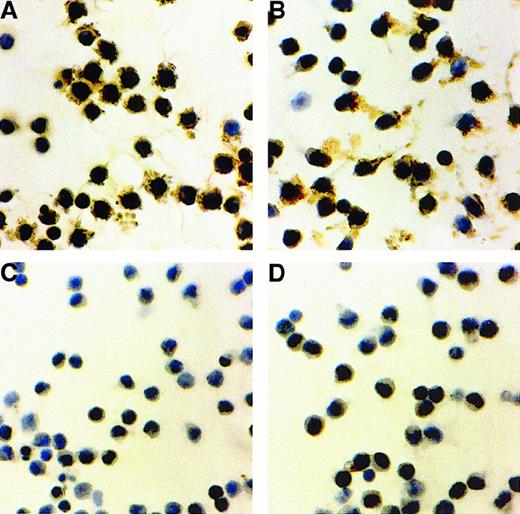
We therefore cultured HCs on HA- and anti-CD44 MoAb-coated plastic and looked at the effect of these 2 forms of CD44 engagement on FN synthesis by HCs. Figure 6 shows that HCs cultured on both CD44 ligands produced much greater amounts of FN than did the cells cultured on uncoated plastic (not shown) or on plastic coated with albumin or isotypic (IgG2a) control antibody. These immunocytochemical data were quantitatively confirmed by a modified ELISA method (Figure 7).
Fibronectin synthesis by HCs stimulated with surface-bound HA or anti-CD44.
HCs were cultured in serum-free medium for 24 hours on plastic coated with HA (A) or anti-CD44 (B). Plastic coated with albumin (C) or with IgG2a (D) was used as control. Fibronectin (FN) was detected by indirect staining with an anti-FN MoAb. Representative examples of 5 experiments with cells from 3 HCL patients are shown.
Fibronectin synthesis by HCs stimulated with surface-bound HA or anti-CD44.
HCs were cultured in serum-free medium for 24 hours on plastic coated with HA (A) or anti-CD44 (B). Plastic coated with albumin (C) or with IgG2a (D) was used as control. Fibronectin (FN) was detected by indirect staining with an anti-FN MoAb. Representative examples of 5 experiments with cells from 3 HCL patients are shown.
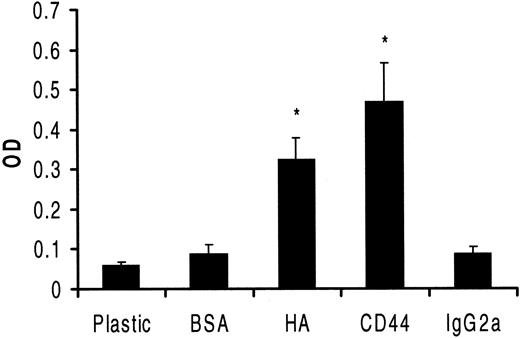
Quantitative estimation of HC FN production on differently coated surfaces.
HCs were cultured in serum-free medium for 24 hours in 96-well plates coated as indicated. FN production was quantified by a modified ELISA involving an anti-FN MoAb as the first layer, a biotin-conjugated goat anti-mouse antibody as second layer, and streptavidin-peroxidase as third layer. Peroxidase substrate was then added to each well for 15 minutes, and the product of the reaction was measured in a plate reader. The results expressed in optical density units show relative differences in FN on different surfaces (3 experiments involving cells from 3 patients; each measurement performed 5 times). The increased production rates, as compared with the controls, of FN on HA or anti-CD44 were both significant (P < .01).
Quantitative estimation of HC FN production on differently coated surfaces.
HCs were cultured in serum-free medium for 24 hours in 96-well plates coated as indicated. FN production was quantified by a modified ELISA involving an anti-FN MoAb as the first layer, a biotin-conjugated goat anti-mouse antibody as second layer, and streptavidin-peroxidase as third layer. Peroxidase substrate was then added to each well for 15 minutes, and the product of the reaction was measured in a plate reader. The results expressed in optical density units show relative differences in FN on different surfaces (3 experiments involving cells from 3 patients; each measurement performed 5 times). The increased production rates, as compared with the controls, of FN on HA or anti-CD44 were both significant (P < .01).
Discussion
The interaction of cells with HA is of great importance for the homing of cells to specific tissue microenvironments and for cell behavior within these microenvironments. This interaction is part of a complex process involving a number of other cellular and extracellular components, including integrins and their ligands and a variety of cytokines.
In our studies of chronic lymphoid malignancies, we have already ascribed a possible role to different integrins in the tissue homing behavior of the malignant cells of both CLL and HCL.1-6More recently, we have explored the behavior of CLL cells on HA and demonstrated that, by using RHAMM, a proportion of these cells become motile when stimulated by IL-8. We have proposed that these interactions may be important for CLL cell migration through HA-rich tissues such as lymph nodes and splenic white pulp.7 These observations prompted us to examine the behavior of HCs on HA in the presence or absence of IL-8.
Here we show that, in contrast to CLL cells, HCs are spontaneously motile on HA and that CD44, rather than RHAMM, is involved in this process. Although all HCs uniformly expressed high levels of CD44, only a proportion (approximately 40%) of cells displayed this motility. The reason for this is unclear, but functional heterogeneity within both HC and CLL clones has been observed repeatedly by us2,3,7 and others.27 28 Possible explanations include senescence, maturation, or functional changes related to recirculation between blood and different tissues. In the latter case, the subpopulation of cells that has recently exited from tissues would be expected to be transiently refractory to tissue homing stimuli.
Because the CD44 on normal B cells requires activation for HA binding,29 the spontaneous movement of HCs on HA indicates that in the malignant cells, the receptor is constitutively active. HCs display many features of activation, including a dynamic pattern of surface ruffling3,11 and activated integrins.8It is therefore likely that both CD44 activation and the spontaneous cell movement on HA are manifestations of this intrinsic cell activation. Regarding the activation of CD44 on HCs, this might arise either from activation-dependent expression of particular variant isoforms with high affinity for HA30 or from inside-out activation of CD44 through conformational change.31 In this context, it should be noted that although CLL cells express CD44, the lack of involvement of this receptor in CLL cell movement on HA indicates that the CLL receptor is expressed in an inactive form.
In the light of our previous report that IL-8 stimulates CLL cell movement on HA, our demonstration here that the chemokine inhibits HC movement on this substratum was unexpected. This suggests that either the activated state of HCs or the engagement of CD44 rather than RHAMM modifies the stimulatory effect of IL-8. In both CLL cells and HCs, IL-8 caused actin polymerization, but the pattern of F-actin distribution within the 2 cell types was very different. In CLL cells on HA, IL-8 induced a polar distribution of F-actin,7whereas in the present study, the constitutively polar distribution of the protein in already motile HCs was converted into distinct focal adhesions and cell arrest.
Different forms of cytoskeletal organization of F-actin are known to be linked to differences in cell motility and are regulated by different members of Rho-family GTPases.32-34 Thus, active Rac is associated with lamellipodia formation and motility,35whereas Rho activation is associated with pronounced focal adhesion formation and arrest of motility.36 Our recent investigations of CLL cells suggested that IL-8 stimulation together with RHAMM engagement by HA lead to Rac activation in these cells. In contrast, our present studies suggest that in HCs, Rac is already constitutively active and that IL-8 promotes Rho activation in these cells, with consequent inhibition of their motility. Moreover, in HCs both before and after IL-8 stimulation, CD44 seemed to colocalize with F-actin, suggesting that the 2 proteins are in some way associated. However, after IL-8 stimulation, far more CD44 was found associated with the Triton-insoluble cytoskeleton, suggesting either that this association becomes enhanced or that the cytoskeleton and associated proteins in the IL-8–treated cells become less readily detergent soluble. The constitutive signals in HCs and their effects on the Rho GTPases, actin polymerization, and CD44 association with different forms of actin are currently under investigation in this laboratory.
Whatever its precise mechanism, this behavior of HCs on HA in the presence and absence of IL-8 could play an important role in their tissue homing in HCL. For this reason, we examined relevant tissues and showed that HA was differentially expressed at different tissue sites, whereas IL-8 was ubiquitously present. Thus, HA was abundant in infiltrated BM and portal tracts, but was completely absent from splenic red pulp and largely absent from hepatic sinusoids. This suggests a role for the CD44-HA interaction in HC homing to BM and portal tracts, but not to splenic red pulp or hepatic sinusoids.
These conclusions should be viewed in the light of previous work regarding normal and malignant hematic cell homing. Three types of receptors have been shown to be important, namely integrins, selectins, and CD44. For example, homing to BM generally involves both β1-integrin–VCAM and CD44-HA interactions,10 whereas the transmigration of lymphocytes across the high endothelial venules of lymph nodes is heavily dependent on L-selectin and β2-integrins.37 Within particular areas of lymphoreticular tissues, migration patterns are complex and still not well understood. During this tissue migration, normal lymphocytes receive signals for maturation and differentiation. In contrast, malignant cells, which are arrested at a particular differentiation stage, often show a predilection for accumulation at particular tissue sites. Thus, CLL cells often accumulate in the splenic white pulp and lymph nodes,38 whereas HCs home to splenic red pulp and generally spare peripheral lymph nodes.11 Both cell types are consistently found in BM, but in HCL the infiltrating malignant cells are associated with distinctive tissue fibrosis not found in CLL BM.11 Previous work from this department has suggested that HC homing to splenic red pulp involves both α4β1-VCAM and αvβ3-VN interactions.3 In contrast, the absence of CLL cell accumulation in this area of the spleen probably reflects the absence of αvβ3 integrin expression5 and a lack of constitutive activation of other integrins which, in HCs, are already activated by constitutive oncogenic event(s).8 The differences in lymph node involvement in the 2 diseases may well reflect L-selectin expression, which we have found to be largely absent from HCs. The present work, together with our recent studies of CLL,7 suggests that interaction with HA is not important for HC homing to spleen, but may be important for CLL cell migration within splenic white pulp and lymph node stroma.
As regards the CD44 of HCs and CLL cells, previous work has concentrated on the expression of this receptor without investigation of its functional state.13 39 Our previous and present work show that differences in the activation state of CD44 in the 2 cell types, and the presence on CLL cells, but not HCs, of an alternative HA receptor in the form of RHAMM, contribute to the markedly different tissue distributions of the 2 diseases. Furthermore, the movement of CLL cells on HA, and the arrest of HCs on this substratum after IL-8 stimulation, emphasize the importance for cell homing of the activation state of the cells and of the particular receptor used for interaction with HA.
It should be noted that at sites where HCs accumulate in the presence of HA and IL-8 (BM and portal tracts), there is histologic evidence of associated fibrosis. We have already shown that HCs have the capacity to synthesize and assemble FN.1 Others have demonstrated that CD44-HA engagement on monocytes and macrophages stimulates fibrosis.26 Taken together, these observations suggest a role for the CD44-HA interaction in the distinctive fibrosis of HCL. Our direct demonstration that CD44 engagement stimulates HCs to synthesize large amounts of FN strongly supports such a role and provides an explanation for the presence of fibrosis in BM, but not in spleen, in HCL.
Supported by the Leukaemia Research Fund (United Kingdom) and the North West Cancer Research Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
K. A. Aziz, Department of Haematology, 3rd Fl, Duncan Bldg, Royal Liverpool University Hospital, Daulby St, Liverpool, L69 3GA, United Kingdom.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal