Abstract
Multiple myeloma (MM) is an invariably fatal disease that accounts for approximately 1% to 2% of all human cancers. Surprisingly little is known about the cellular pathways contributing to growth of these tumors. Although the cytokine interleukin-6 has been suggested to be the major stimulus for myeloma cell growth, the role of a second potential growth factor, insulin-like growth factor I (IGF-I), has been less clearly defined. The IGF-I signaling cascade in 8 MM cell lines was examined. In 7 of these, the IGF-I receptor (IGF-IR) was expressed and autophosphorylated in response to ligand. Downstream of IGF-IR, insulin receptor substrate 1 was phosphorylated, leading to the activation of phosphatidylinositol-3′-kinase (PI-3K). PI-3K, in turn, regulated 2 distinct pathways. The first included Akt and Bad, leading to an inhibition of apoptosis; the second included the mitogen-activated protein kinase (MAPK), resulting in proliferation. Biologic relevance of this pathway was demonstrated because in vitro IGF-I induced both an antiapoptotic and a proliferative effect. Importantly, in vivo administration of IGF-I in SCID mice inoculated with the OPM-2 line led to approximately twice the growth rate of tumor cells as in controls. These results suggest that IGF-I activates at least 2 pathways effecting myeloma cell growth and contributes significantly to expansion of these cells in vivo.
Introduction
Multiple myeloma (MM) is a progressive B-lineage neoplasia characterized by the accumulation of malignant plasma cells in the bone marrow and the occurrence of extensive osteolytic bone destruction.1,2 The disease is incurable and accounts for approximately 1% to 2% of total human cancers.3 In spite of extensive study, little is known about the molecular lesions or biochemical abnormalities contributing to the initiation or development of this disease. To date, the interleukin-6 (IL-6) pathway is the single signaling cascade most consistently implicated in MM. The role of IL-6 in this disease has been clearly demonstrated in model systems wherein IL-6 null mice normally fail to develop plasma cell tumors,4,5 though these tumors can be induced in an IL-6–independent manner by selected agents.6 In human myeloma a critical role for IL-6 is suggested based largely on associative observations. Some MM cell lines are IL-6 dependent,7-9 and IL-6 is a proliferative signal for primary MM explants.10 Furthermore, patient IL-6 levels have been suggested in some studies11,12 to be a prognosticator of disease severity, and attempts to interfere with IL-6 signaling in clinical trials have led to transient effects on tumor growth.13
It should be noted, however, that it is unclear whether all MM passes through an IL-6–dependent phase or whether a subset of tumors arises as IL-6 independent. It is also unknown whether IL-6–dependent tumors routinely become IL-6 independent during disease progression. Given these possibilities, it is critical to identify other pathways that may coordinately enhance MM growth or substitute as a growth stimulus in the absence or loss of IL-6 dependence. Herein, we describe studies of the insulin-like growth factor I (IGF-I) signaling cascade, which suggest that this pathway may play such a role in the development of MM.
Materials and methods
Cell lines
Eight human MM lines—ANBL-6, Brown, Delta-47, OPM-2, 8226, KMM1, H929, and MM-14414—were obtained from Dr Michael Kuehl. Five B-cell lymphoma lines (BL-30, BL-41, Daudi, Namalwa, and ST486)15,16 were kindly provided by Dr Kishor Bhatia. The T-cell leukemia lines Jurkat and Molt17 and the NK line YT18 were kindly provided by Dr Warren Leonard. ANBL-6 is IL-6 dependent, and the remaining 7 MM lines are IL-6 independent. Cells were cultured in RPMI 1640 (Biofluids, Rockville, MD) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 50 μg/mL streptomycin. IL-6 at 10 ng/mL was added to the medium for the growth of ANBL-6.
Immunoprecipitation and Western blot analysis
Cells (1 × 107) were grown in serum-free media for at least 18 hours, either stimulated with human IGF-I (100 ng/mL) for 10 minutes at 37°C or not stimulated. Then they were washed twice with ice-cold phosphate-buffered saline (PBS) containing 1 mmol/L Na3VO4 and 1 mmol/L NaF and placed in cold lysis buffer (50 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 1% NP-40, 2.5 mmol/L EDTA, 10 mmol/L NaPPi, 1 mmol/L NaF, 1 mmol/L Na3VO4, 1 mmol/L 4-(2-aminoethyl)–benzenesulfonyl fluoride hydrochloride, 20 μg/mL aprotinin, and 20 μg/mL leupeptin) for 30 minutes on ice. Cell debris was removed by centrifugation at 12 000g for 30 minutes at 4°C. Immunoprecipitations were performed on total cell lysate with either anti–IGF-I receptor (IGF-IR) (Santa Cruz Biotechnology, Santa Cruz, CA), anti-insulin response substrate (IRS)-1, or anti–IRS-2 antibodies (Upstate Biotechnology, Lake Placid, NY), followed by binding to protein-A coupled beads. Beads were washed 4 times in lysis buffer, and the bound proteins were eluted, fractionated by 8% to 10% SDS-PAGE, and transferred to polyvinylidene difluoride membrane (Millipore, Bedford, MA). Membranes were blotted with different antibodies, as indicated in Figures1, 2, and3, followed by a horseradish peroxidase–conjugated secondary antibody. Proteins were detected by enhanced chemiluminescence. For detection of Akt and Bad, direct Western blots were performed using antibodies from Upstate Biotechnology and New England Biolabs (Beverly, MA).
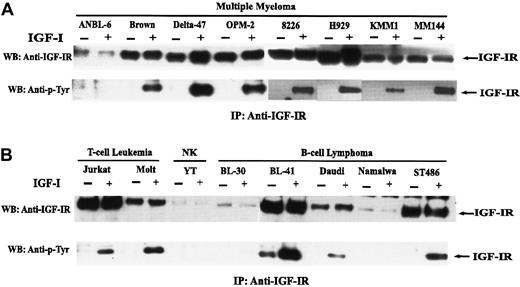
Expression and autophosphorylation of IGF-IR.
Cells were serum-starved for 18 hours and were untreated (−) or treated (+) with IGF-I. Lysates from 1 × 107 cells were incubated with anti–IGF-IR antibody, and immunoprecipitates were prepared and analyzed as described in “Materials and methods.” Blots were probed with antibody to the IGF-IR (A) or phosphotyrosine (B).
Expression and autophosphorylation of IGF-IR.
Cells were serum-starved for 18 hours and were untreated (−) or treated (+) with IGF-I. Lysates from 1 × 107 cells were incubated with anti–IGF-IR antibody, and immunoprecipitates were prepared and analyzed as described in “Materials and methods.” Blots were probed with antibody to the IGF-IR (A) or phosphotyrosine (B).
Effect of IGF-1 on tyrosine phosphorylation of IRS-2 or IRS-1 and their association with PI-3K.
Cells were unstimulated (−) or stimulated (+) with IGF-I for 10 minutes after 18 hours of serum starvation. Cell lysates were immunoprecipitated with anti–IRS-2 (A) or anti–IRS-1 (B), subjected to SDS-PAGE, and blotted with the indicated antibodies. Mouse plasmacytoma line 12.2 (A) and 32D cells transfected with IRS-1 (B) were used as positive controls for IRS-2 and IRS-1, respectively. Positions of IRS-2, IRS-1, and the p85 subunit of PI-3K are as indicated.
Effect of IGF-1 on tyrosine phosphorylation of IRS-2 or IRS-1 and their association with PI-3K.
Cells were unstimulated (−) or stimulated (+) with IGF-I for 10 minutes after 18 hours of serum starvation. Cell lysates were immunoprecipitated with anti–IRS-2 (A) or anti–IRS-1 (B), subjected to SDS-PAGE, and blotted with the indicated antibodies. Mouse plasmacytoma line 12.2 (A) and 32D cells transfected with IRS-1 (B) were used as positive controls for IRS-2 and IRS-1, respectively. Positions of IRS-2, IRS-1, and the p85 subunit of PI-3K are as indicated.
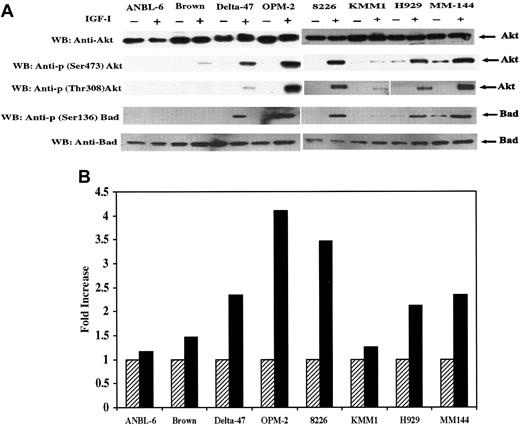
IGF-I–induced phosphorylation and kinase activity of Akt.
(A) Serum starved cells were untreated (−) or treated (+) with IGF-I. Total protein extracts (25 μg) from each cell line indicated were subjected to SDS-PAGE and blotted with anti-Akt, anti-p (Ser473) Akt, anti-p (Thr308) Akt, Anti-Bad, or anti-p (Ser136) Bad. (B) Cells were serum-starved for 18 hours and untreated (hatched bar) or treated (solid bar) with IGF-I. Lysates from 1 × 107 cells were immunoprecipitated with anti-Akt antibody and subjected to an in vitro kinase assay as described in “Materials and methods.” IGF-I–stimulated Akt activity is presented as fold increase, calculated by dividing the cpm of nonstimulated cells by the cpm of IGF-I–stimulated counterparts. Data represent average values of 2 independent assays.
IGF-I–induced phosphorylation and kinase activity of Akt.
(A) Serum starved cells were untreated (−) or treated (+) with IGF-I. Total protein extracts (25 μg) from each cell line indicated were subjected to SDS-PAGE and blotted with anti-Akt, anti-p (Ser473) Akt, anti-p (Thr308) Akt, Anti-Bad, or anti-p (Ser136) Bad. (B) Cells were serum-starved for 18 hours and untreated (hatched bar) or treated (solid bar) with IGF-I. Lysates from 1 × 107 cells were immunoprecipitated with anti-Akt antibody and subjected to an in vitro kinase assay as described in “Materials and methods.” IGF-I–stimulated Akt activity is presented as fold increase, calculated by dividing the cpm of nonstimulated cells by the cpm of IGF-I–stimulated counterparts. Data represent average values of 2 independent assays.
Akt immunoprecipitation kinase assay
Cells were serum-starved for 18 hours and treated as described above. Cell lysates from 1 × 107 cells were immunoprecipitated with anti-Akt antibody and subjected to an in vitro Akt activity assay using the Akt Immunoprecipitation Kinase Assay Kit (Upstate Biotechnology). Briefly, the assay uses a synthesized peptide (RPRAATF) related to the sequence surrounding the phosphorylation site of GSK3 as an Akt-specific substrate. Reactions were carried out in 40 μL of reaction buffer containing 10 μCi γ-32P–ATP and 100 μmol/L substrate for 10 minutes. The supernatant was removed, mixed with 20 μL 40% TCA, and transferred to P81 phosphocellulose paper for washing and counting. The increase in Akt kinase activity was calculated as a fold increase of 32P incorporation by dividing the mean value of non-IGF-I–stimulated cells by that of the corresponding IGF-I–stimulated cells.
MAPK assay
Cells (1 × 106) were serum starved for 48 hours and either untreated or treated with IGF-I for 10 minutes before lysis. Immunoprecipitations were performed by incubating lysates with immobilized anti-p-p44/42 MAPK (New England BioLabs). Immunoprecipitates were incubated with Elk-2 fusion protein in the presence of adenosine triphosphate and phosphorylated product detected with anti-p-Elk-2 antibody. Phosphorylated Elk-2 protein was quantitated by densitometry tracing.
3H thymidine incorporation assay
Exponentially growing cells were washed with PBS, resuspended in serum-free media, and cultured in 24-well plates with or without 100 ng/mL of IGF-I at a density of 1 × 105/well for 72 hours. To measure DNA synthesis, 1 μCi 3H-thymidine (Amersham International, Arlington Heights, IL) was added during the final 4 hours of culture. Uptake of 3H-thymidine was determined in triplicate samples by liquid scintillation counting. Mitogenic activity was represented by a fold increase of3H-thymidine uptake calculated in the same manner as described above for Akt kinase activity.
In vivo tumorigenesis studies
The OPM-2 line was used for in vivo inoculation into SCID mice. Cells were washed with PBS 3 times before inoculation. Ten mice were injected with 1 × 107 OPM-2 cells intraperitoneally, of which 4 mice (IGF-I group) received 20 μg IGF-I in 0.5 mL PBS 3 times per week for 4 weeks. The remaining 6 mice (control, no IGF-I group) received only PBS. Animals were monitored 2 times per week for tumor growth after inoculation. The control group was killed at day 30 because of the presence of large, palpable tumors. Animals in the IGF-I group were killed at day 60. Tumor volume was determined by measurement of the largest diameter and the diameter at a right angle.
Results
Expression and autophosphorylation of IGF-I receptor in response to IGF-I stimulation
To evaluate a possible role for IGF-I in MM, levels of IGF-IR were first analyzed in a series of MM and other lymphoid cell lines. Receptor was immunoprecipitated from the indicated lines and blotted with either antibody to the receptor or phosphotyrosine. As can be seen (Figure 1), 7 IL-6–independent lines—Brown, Delta-47, OPM-2, 8226, H929, KMM1, and MM144—expressed readily detectable amounts of IGF-IR, whereas the IL-6–dependent line ANBL-6 evidenced a lower level of receptor. The functional status of IGF-IR in terms of autophosphorylation was examined in response to IGF-I stimulation. As seen, autophosphorylation was observed in 7 of 8 lines with no detectable phosphorylation observed in ANBL-6, consistent with the low level of receptor protein. Two T-cell leukemia lines expressed IGF-IR, which was autophosphorylated in an IGF-I–dependent manner, whereas the NK line YT exhibited only minimal expression of IGF-IR. Among B-cell lymphomas, BL-41 and ST486 expressed levels of IGF-IR comparable to that seen in the IL-6–dependent MM lines, and somewhat lower levels were observed in Daudi. IGF-I–dependent autophosphorylation was observed in each of these 3. The downstream effect of IGF-IR activation in terms of subsequent 3H-thymidine incorporation was clearly distinct among these differing cell types (see below).
IGF-I–induced phosphorylation of insulin receptor substrate proteins and their association with phosphatidylinositol-3′-kinase
To establish whether a functional signaling pathway was activated downstream of the IGF-IR, we next examined IRS proteins previously shown to be phosphorylated by both the insulin and the IGF-I receptors.19 20 IRS-2 (Figure 2A) and IRS-1 (Figure 2B) expression and tyrosine phosphorylation were examined in nonstimulated and stimulated cells. IRS-2 expression was observed only in Brown but not in the other 3 or 4 additional lines examined (not shown). No detectable level of tyrosine phosphorylation of IRS-2 was observed in Brown on IGF-I stimulation. In contrast, IRS-I was expressed in all 4 lines with the highest expression level in ANBL-6, in which IRS-1 was constitutively phosphorylated. In the other 3 lines, tyrosine phosphorylation of IRS-1 was strongly IGF-I–dependent in Delta-47 and OPM-2 and weakly so in Brown. Similar results, as seen in the IRS-1–expressing lines, were also obtained in 3 of 4 additional lines (8226, H929, and MM144) analyzed (Figure 1), in which IRS-1 phosphorylation was, again, IGF-I dependent (not shown).
One of the mechanisms by which phosphorylated IRS proteins transduce downstream signals is interaction with the SH2 homology domains of other proteins.21 PI-3K has been identified as one such target that associates with IRS-1 or IRS-2 through the SH2 domain of its p85 regulatory subunit.22 Coimmunoprecipitation was, therefore, used to evaluate this interaction. Tyrosine-phosphorylated IRS-1 protein was observed to associate with the p85 subunit of PI-3K in 3 lines (Figure 2B) in an IGF–dependent manner, whereas interaction in ANBL-6 was constitutive. Although phosphorylated IRS-1 or IRS-2 was not detected in Brown, association with the p85 subunit of PI-3K was still observed, most likely because of greater sensitivity of anti-p85 antibody. Again, a similar association of PI-3K with IRS-I was observed in 3 additional lines, and weak, but detectable, interaction was observed in a fourth (not shown).
IGF-I induces Akt phosphorylation and its kinase activity
A search for further downstream members in this pathway led to analysis of Akt/PKB. Akt has been shown to be a target of PI-3K–generated signals23-25 and to participate in growth factor maintenance of cell survival26 after activation by phosphorylation of residues Thr 308 and Ser 473. Therefore, we investigated Akt activation using specific antibodies that detect phosphorylation at these sites. Akt was expressed in all 8 lines and, with the exception of ANBL-6, phosphorylated at both positions in response to IGF-I (Figure 3A). Time-course analysis of Akt phosphorylation in lines H929 and MM144 revealed maximal phosphorylation at 3 minutes in H929 and between 3 and 10 minutes in MM144. To assess the functional consequences of Akt phosphorylation, an in vitro kinase assay was performed in which a synthetic peptide (RPRAATF) related to the sequence surrounding the phosphorylation site of GSK-3 was used as substrate. As shown in Figure 3B, the kinase activity of IGF-I–stimulated cells was increased approximately 2- to 4-fold in Delta-47, OPM-2, H929, 8226, and MM144. Only minimal increases were found in ANBL-6, Brown, and KMM1 lines. Thus, in response to IGF-I stimulation, kinase activity correlated directly with the phosphorylation levels of Akt at Ser 473 and Thr 308.
Additional downstream signaling in the IGF-I pathway
Targets further downstream in this pathway are the subjects of considerable investigation. Growth factor and cytokine signaling through Akt has recently been suggested to play a role in protecting cells from apoptosis, at least in part, by phosphorylation and inactivation of the proapoptotic Bcl-2 family member, Bad.27 28 Thus, the status of Bad was examined using an antibody specific for phosphorylation on Ser 136. IGF-I–dependent Bad phosphorylation was observed in 5 of 8 lines (Figure 3A), and the level of phosphorylation correlated with Akt phosphorylation and kinase activity described in Figure 3. To confirm the antiapoptotic effect of Bad phosphorylation, a caspase activity assay was performed (not shown) that revealed a decrease in caspase 3 activity after IGF-I stimulation that, again, correlated with Bad phosphorylation levels.
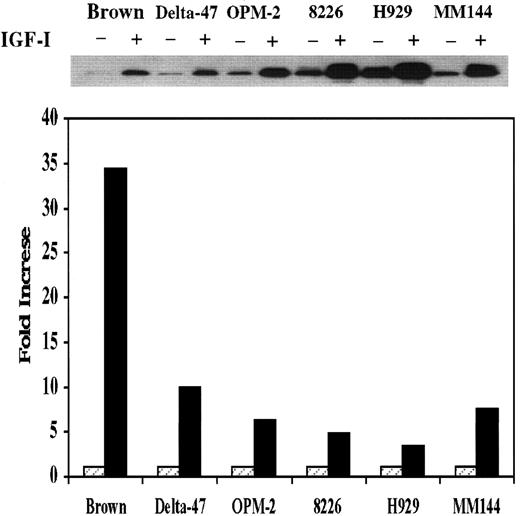
Activation of the MAPK pathway by IGF-I
The above data indicate that IGF-I activates at least one pathway that inhibits apoptosis. To explore the possibility that additional pathway(s) might be activated that promote proliferation, we next examined the status of the MAPK cascade. For these studies, an in vitro kinase assay was used after immunoprecipitation of MAPK from 6 MM lines. As seen in Figure 4, kinase activity was increased in Delta-47, OPM-2, 8226, and MM144 by approximately 5- to 10-fold and 35-fold in Brown. An increase in H929 was lowest at 3.5-fold. Time-course analysis revealed maximal activation of MAPK by 3 minutes. Thus, an additional pathway associated with mitogenesis as opposed to antiapoptosis has also been activated in these lines.
MAPK activity in MM lines.
An in vitro MAPK assay was performed as described in “Materials and methods.” Fold increase was calculated by dividing the density of bands obtained from nonstimulated cells by that of IGF-I–stimulated counterparts. Data represent average values of 2 independent assays.
MAPK activity in MM lines.
An in vitro MAPK assay was performed as described in “Materials and methods.” Fold increase was calculated by dividing the density of bands obtained from nonstimulated cells by that of IGF-I–stimulated counterparts. Data represent average values of 2 independent assays.
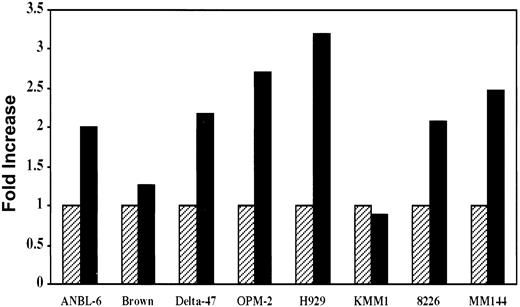
Biologic relevance of IGF-I pathway activation
The data presented above clearly demonstrate activation of the IGF-I signaling pathway in most MM cell lines. It was, therefore, important to determine whether this activation was biologically significant in terms of tumor cell growth. To address this question, we first analyzed the mitogenic effect of IGF-I on in vitro growth of MM cells. Cells were serum starved for 68 hours, then pulsed with3H-thymidine either in the presence or absence of IGF-I. Compared to controls, IGF-I stimulated approximately 2- to 3-fold increases in 3H-thymidine incorporation in 6 of 8 lines (Figure 5). This effect was completely inhibited in the presence of antibody to the IGF-I receptor. In contrast to the MM lines, the 2 T-cell leukemias, Jurkat and Molt, that expressed IGF-IR and exhibited IGF-I–dependent autophosphorylation (Figure 1) showed no increase in 3H-thymidine incorporation on IGF-I stimulation. Among the B lymphomas expressing IGF-IR (Figure1), BL-41, with the highest level of expression, exhibited a 2-fold increase in thymidine uptake whereas increases in Daudi and ST486 were minimal at 1.3 and 1.2 fold, respectively (not shown).
Effect of IGF-I on in vitro proliferation of MM cells.
Cells from indicated lines were seeded at 105/well in 24-well plates and incubated in the presence (solid bar) or absence (hatched bar) of 100 ng/mL of IGF-I for 68 hours in the absence of serum, followed by a 4-hour pulse with 1 μCi of 3H-thymidine. IGF-I–induced mitogenesis is presented as fold increase calculated by dividing the mean value of the untreated wells by that of IGF-I–stimulated counterparts. Data represent average values of triplicate samples from 3 separate experiments.
Effect of IGF-I on in vitro proliferation of MM cells.
Cells from indicated lines were seeded at 105/well in 24-well plates and incubated in the presence (solid bar) or absence (hatched bar) of 100 ng/mL of IGF-I for 68 hours in the absence of serum, followed by a 4-hour pulse with 1 μCi of 3H-thymidine. IGF-I–induced mitogenesis is presented as fold increase calculated by dividing the mean value of the untreated wells by that of IGF-I–stimulated counterparts. Data represent average values of triplicate samples from 3 separate experiments.
To evaluate whether IGF-I has an effect on tumor growth in vivo, we used OPM-2 because this line exhibited one of the highest mitogenic responses to IGF-I and was found to grow in SCID mice. Two groups of animals were injected with OPM-2 cells. The control group was not treated, whereas IGF-I was given to the experimental group 3 times/wk for 4 weeks (Table 1). As can be seen, tumors in the IGF-I treatment group grew to approximately twice the size in about half the time as in the control group. Thus, IGF-I produced a marked effect on tumor growth in vivo.
Effect of IGF on in vivo tumor growth
| . | Control . | IGF-I . |
|---|---|---|
| No. mice with tumors | 4 of 6 | 3 of 4 |
| Tumor volume | 1.325 ± 0.18 | 2.914 ± 0.20 |
| Days after inoculation | 50 | 30 |
| . | Control . | IGF-I . |
|---|---|---|
| No. mice with tumors | 4 of 6 | 3 of 4 |
| Tumor volume | 1.325 ± 0.18 | 2.914 ± 0.20 |
| Days after inoculation | 50 | 30 |
The IGF-I group received 20 μg IGF-I in PBS 3 times/wk for 4 weeks, and the control group received PBS alone. Tumor volume was calculated at the time of sacrifice by measurement of the largest diameter and the diameter at a right angle.
Discussion
Considerable attention has been focused in the past several years on the role of cytokines, particularly IL-6, in the development of MM. IL-6 has been suggested as a major growth factor in this disease based on a number of associative findings such as the IL-6 dependence of some myeloma lines,7,8 the proliferative response of primary explants to this cytokine,10 correlation of disease severity with circulating IL-6 levels,11,12 and the transient effects on tumor burden on patients treated with antibodies to IL-6 or IL-6R.13 Given that it is unknown whether all MM evolves through an IL-6–dependent phase and that the frequency with which IL-6–dependent MM progresses to IL-6 independence remains undetermined, it is surprising how little is known about other cytokines or growth factors that may either augment or replace IL-6 responsiveness. One such candidate growth factor is IGF-I. Early studies involving a limited number of IL-6–independent cell lines revealed that most responded to this growth factor with modest increases in proliferation.29,30 More recently, Jelinek et al31 demonstrated that though IGF-I could, again, induce modest proliferation in 4 IL-6–dependent lines, simultaneous treatment with both factors resulted in a markedly enhanced effect. These results, together with studies suggesting a role for IGF-I in a variety of malignancies,32 indicate that the IGF-I signaling pathway may play an important role in MM development. To date, little is certain regarding the biochemical cascade constituting this pathway in myeloma cells and regarding whether IGF-mediated proliferation observed in vitro reflects an in vivo role for this growth factor. The current studies were, therefore, performed to delineate IGF-I signaling in MM cells and to address the question of in vivo relevance.
IGF-IR expression was observed in all 8 MM lines investigated. In 7 of 8, receptor autophosphorylation was ligand dependent. Among other lymphoid tumors examined, similar results were obtained with 2 of 2 T-cell leukemias and 3 of 5 B-cell lymphomas. The effect of IGF-I on MM cells is likely not to represent a promiscuous and minimal response to growth factors in general because most lines also express c-met, the receptor for hepatocyte growth factor, but do not respond to this ligand (N.-L.G. and S.R., unpublished data, March 1999). Receptor phosphorylation resulted in IRS-1 activation and subsequent association with, and activation of, PI-3K. IRS-2 was only expressed in a single line (Brown). Activation of PI-3K led to phosphorylation of Akt at Ser 473 and Thr 308, and the level of phosphorylation at these sites correlated with Akt kinase activity.
Further downstream, Bad was found to be clearly phosphorylated in 5 of 8 lines and weakly phosphorylated in a sixth. The levels of Bad phosphorylation correlated with Akt activity, indicating that Akt phosphorylates Bad, as has been reported in BALB/c 3T3 and PC 12 cells.27 Bad phosphorylation is functionally important in cell death because this molecule forms heteromeric complexes with 2 antiapoptotic factors, Bcl-2 and Bcl-XL, thus inhibiting their function in cell survival.33 Phosphorylation of Bad results in dissociation of these complexes and binding to 14-3-3 protein. This interaction releases Bcl-2 and Bcl-XL, resulting in cell survival.34 The functional consequence of Bad phosphorylation was confirmed by the assessment of caspase 3 as an indicator of apoptosis. Caspase 3 activity was reduced in all lines in direct relation to the degree of Bad phosphorylation. The above results clearly define a functional IGF signaling pathway in most MM lines that can be traced to elements inhibiting apoptosis and likely represents the same pathway involved in IGF-I–mediated inhibition of dexamethasone-induced apoptosis reported in the 8226 cell line.35
In addition to inhibiting apoptosis, IGF-I was found to activate the MAPK pathway in all MM lines tested (Figure 4). It has been suggested that this pathway accounts for the proliferative response induced by IL-6 in IL-6–dependent myeloma.36,37 It should be cautioned that this suggestion is based on limited data, including a murine IL-6–dependent hybridoma and 1 of 2 explants from patients with MM. Interestingly, Anderson et al37 subsequently demonstrated that the MAPK pathway could not be activated in MM lines that were IL-6 independent because of the failure to phosphorylate Sos 1. Consistent with this is our observation that IL-6 fails to enhance the proliferation of any of the IL-6–independent lines in the current study. The current data indicate that even though MM lines may lose the ability to activate this pathway in response to IL-6, they may still proliferate in response to IGF-I, which effectively activates the same MAPK pathway. IGF-I stimulation fails to induce phosphorylation of Stat3 in the IL-6 pathway, indicating no interaction between IGF-I and the IL-6 cascade. Thus, IGF-I may act not only to enhance IL-6–mediated proliferation in IL-6–dependent myeloma but to provide sufficient antiapoptotic and proliferative stimuli to cells that have lost the ability to respond to IL-6 to promote their survival and outgrowth.
The biologic relevance of the IGF-I pathway in terms of tumor cell growth was further demonstrated in vitro and in vivo. In vitro proliferation assays revealed that 6 of 8 lines exhibited 2- to 3-fold increases in mitogenesis, as has been reported30 for other MM lines. Interestingly, the 2 T-cell leukemias that expressed IGF-IR at levels similar to those seen among the myelomas failed to demonstrate 3H-thymidine incorporation in response to IGF-I, even though receptor activation was clearly observed. This result suggests the possibility of a fundamental difference between B- and T-lineage neoplasias in response to IGF-I. Among 5 B-cell lymphomas examined, 2 expressed IGF-IR at levels comparable to those seen in the IL-6–independent MM lines, but only one evidenced3H-thymidine incorporation in the same range as the myelomas. Thus, the IGF-I pathway may, for reasons not understood, become more capable of delivering a biologically detectable signal in the latter stages of B-lineage development than in less mature cell types.38 More important from a biologic standpoint, when the OPM-2 line was inoculated into SCID mice, tumors grew to approximately twice the size in half the time in IGF-treated animals as in controls. This finding indicates that the modest IGF-I proliferative effect, likely coupled with the inhibition of apoptosis, is reflected in a marked promotion of in vivo tumor growth. We have not detected IGF-I production by myeloma cells using an ELISA assay, and thus suggest that the IGF-I to which myeloma cells respond is produced in a paracrine fashion as it is present in virtually all body fluids. It is further suggested that IGF-I acts synergistically with IL-6 in the growth of early-stage myeloma, consistent with its effect on IL-6–dependent lines in vitro.31 Assuming that MM may become IL-6 independent at later stages, the current results with IL-6–independent lines indicate that, in the absence of IL-6 responsiveness, IGF-I may alone play a significant role in the maintenance and progression of this disease. Thus, it is possible that therapeutic approaches that modulate both the IL-6 and the IGF-I pathways may prove to be important approaches to the treatment of MM.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stuart Rudikoff, Laboratory of Cellular and Molecular Biology, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892; e-mail: rudikoff@helix.nih.gov.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal