Abstract
Immunoglobulin secreted by myeloma cells contains a unique antigenic determinant (idiotype [Id]) that may serve as a tumor-specific antigen. Although Id-protein–specific T-cell responses have been reported in patients with myeloma, it is not known whether primary myeloma tumor cells can present naturally processed Id peptides on their surface as a target. We immunized 2 healthy human stem-cell donors with Id proteins from their recipients. T cells from the immunized donors released high levels of T-helper 1–type cytokines in response to stimulation with myeloma cells from their recipients. The T-cell–mediated cytokine response to tumor cells was blocked by a major histocompatibility complex (MHC) class I monoclonal antibody, whereas the response to soluble Id protein was dependent on MHC class II. To investigate whether Id-specific CD8+ T cells can recognize and kill autologous myeloma cells, we generated T cells from peripheral blood mononuclear cells from a third patient with myeloma by means of in vitro stimulation with autologous dendritic cells pulsed with Id protein. Tumor-specific lysis of myeloma cells was demonstrated by the lack of killing of autologous nonmalignant B cells or natural killer–sensitive K562 cells. Lysis of autologous myeloma targets was restricted by MHC class I molecules. These data represent the first report of class I–restricted T-cell recognition of fresh autologous myeloma targets and formally demonstrate that human myeloma cells can serve as targets of an Id-specific T-cell response.
Introduction
Multiple myeloma is a malignant disease characterized by clonal proliferation of plasma cells in the bone marrow. The monoclonal immunoglobulin (Ig) secreted by myeloma cells may serve as a tumor-specific antigen because of the unique antigenic structure in its variable regions (idiotype [Id]). In several experimental models, it was shown that active immunization with tumor-derived Ig induces protection against subsequent tumor challenge.1,2 A central role for T-cell immunity was suggested by the finding that the adoptive transfer of Id-specific CD4+ T-cell clones produced resistance to challenge with MOPC-315 plasmacytoma.3 In addition to anti-Id antibodies, both Id-specific CD4 and CD8 T-cell responses have been found in patients with myeloma.3-7 The feasibility of inducing Id-specific immune responses by vaccinating patients who have myeloma or B-cell lymphoma with Ig protein derived from their tumors has also been demonstrated.8,9 Furthermore, Kwak and colleagues10 reported that vaccination with Id conjugated to a carrier resulted in activation of CD4+ T cells in a bone marrow transplant donor and that such T-cell immunity could be adoptively transferred to an HLA-matched recipient with myeloma. However, there is no direct evidence indicating that Id-induced T cells can directly lyse autologous myeloma cells. The inability to demonstrate myeloma-specific lysis has been partly due to difficulty in generating protein-specific cytotoxic T cells (CTLs). This technical obstacle can now be overcome by using specialized antigen-presenting cells (APCs) called dendritic cells (DCs) to induce CTLs in vitro.
DCs have attracted attention for use as adjuvants in active immunotherapy of cancer. They are considered to be the most potent APC because they express not only a high level of major histocompatibility complex (MHC) class II but also a variety of costimulatory molecules, such as CD80, CD86, CD40, CD58, and intracellular adhesion molecule 1. Furthermore, DCs can take up protein antigens from the extracellular environment and present antigenic peptides by means of both class I and class II pathways, which leads to activation of both CD4 T-helper and CD8 CTLs.11 Taking advantage of the potency of DCs in promoting CTL responses, we used DCs pulsed with Id protein as a stimulator to establish a CTL line from the peripheral blood of a patient with myeloma.
We here demonstrate the recognition of freshly isolated myeloma targets by class I–restricted T cells obtained from 2 healthy HLA-matched donors vaccinated with their recipient's Id protein and granulocyte-macrophage colony-stimulating factor (GM-CSF) in vivo. We also report the lysis of myeloma tumor targets by an Id-specific CTL line generated in vitro in a fully autologous system.
Patients, materials, and methods
Donors and samples
SG, a healthy, fully HLA-matched, 38-year-old female sibling T-lymphocyte donor, was immunized against her recipient's myeloma Id protein. Specifically, after informed consent was obtained under protocol BB-IND 6269, she received 2 immunizations at a 2-week interval with 0.5 mg Ig protein purified from the plasma of her recipient and chemically conjugated to keyhole limpet hemocyanin (KLH).10 The protein was administered subcutaneously with GM-CSF (250 μg/m2) daily 4 times at the local injection site.10 Ten days after her second immunization, SG underwent leukapheresis for donor lymphocyte collection and infusion into the recipient. The leukapheresis sample and a sample of peripheral blood mononuclear cells (PBMC) collected before vaccination were the sources of T cells for the assay shown in Figure1. Tumor cells were isolated from a resected breast plasmacytoma in the recipient and used as fresh stimulators without any in vitro culturing.
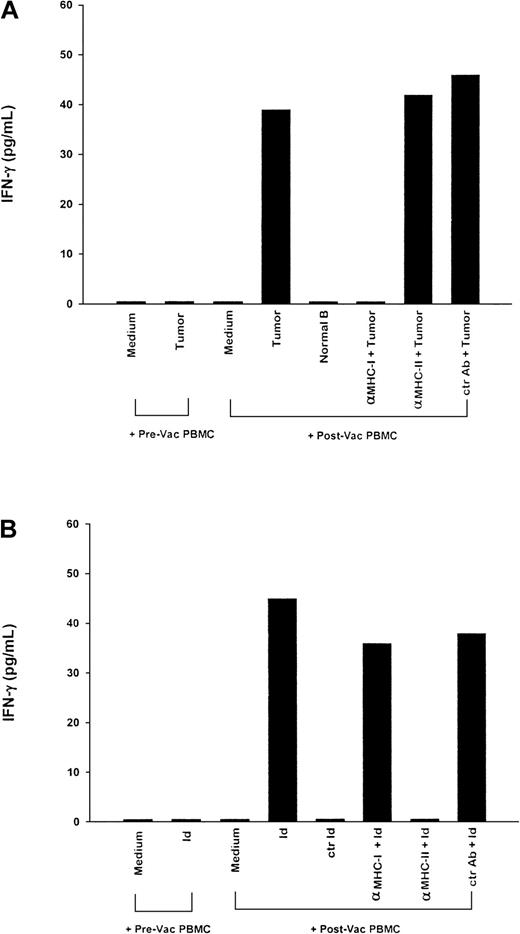
Tumor-specific, major histocompatibility complex (MHC) class I– restricted T-cell cytokine response induced in an HLA-matched donor (SG) vaccinated with myeloma idiotype (Id).
Peripheral blood mononuclear cells (PBMC) (2.5 × 106) obtained before and after vaccination from a healthy HLA-matched sibling donor who was vaccinated with the recipient's Id protein were cocultured with (A) 5 × 104 fresh plasmacytoma cells from the recipient, normal B cells from the recipient, or medium; and (B) the recipient's myeloma Id protein, isotype-matched control Id protein (50 μg/mL each), or medium. Anti-MHC class I or II or control monoclonal antibodies (MoAbs) were added as indicated. Five days later, supernatants were harvested and measured for cytokine production by enzyme-linked immunosorbent assay (ELISA). In an additional control preparation in which plasmacytoma cells from the recipient were cultured alone, none of the 3 cytokines tested (interferon γ [IFN-γ]), granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor α [TNF-α]) were detected in the culture supernatant (data not shown). The results are representative of 2 experiments.
Tumor-specific, major histocompatibility complex (MHC) class I– restricted T-cell cytokine response induced in an HLA-matched donor (SG) vaccinated with myeloma idiotype (Id).
Peripheral blood mononuclear cells (PBMC) (2.5 × 106) obtained before and after vaccination from a healthy HLA-matched sibling donor who was vaccinated with the recipient's Id protein were cocultured with (A) 5 × 104 fresh plasmacytoma cells from the recipient, normal B cells from the recipient, or medium; and (B) the recipient's myeloma Id protein, isotype-matched control Id protein (50 μg/mL each), or medium. Anti-MHC class I or II or control monoclonal antibodies (MoAbs) were added as indicated. Five days later, supernatants were harvested and measured for cytokine production by enzyme-linked immunosorbent assay (ELISA). In an additional control preparation in which plasmacytoma cells from the recipient were cultured alone, none of the 3 cytokines tested (interferon γ [IFN-γ]), granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor α [TNF-α]) were detected in the culture supernatant (data not shown). The results are representative of 2 experiments.
RE, a healthy, fully HLA-matched, 33-year-old male sibling T-lymphocyte donor, was immunized against the Id protein purified from his recipient's plasma after informed consent was obtained. During a 6-month period, he received a series of 4 immunizations with 0.5 mg purified Ig protein, either free or conjugated with KLH, administered with GM-CSF (250 μg/m2) daily 4 times. Whole blood collected before vaccination and 1 month after the final vaccination was the source of T cells for assays against the recipient's tumor cells. Tumor cells from the recipient were collected from a bone marrow aspirate obtained at relapse that consisted of 35% to 50% malignant plasma cells on morphologic evaluation.
Patients and samples
Patient HR (HLA A2, 30; B7, 13; Cw6, w7; DR 1, 4; DQ 1, 3; and DRw53) was a 53-year-old man with relapsed multiple myeloma stage III whose PBMC were collected by means of mobilization by granulocyte colony-stimulating factor and leukapheresis before high-dose chemotherapy. The leukapheresis product was the source of all T-cell, DC, and normal B-cell isolations described below. Autologous tumor cells used as targets were collected from a resected plasmacytoma, cryopreserved as a single suspension, and subjected to Ficoll-Hypaque gradient centrifugation immediately before use. Histologic evaluation of the tumor specimen before cryopreservation determined that it was plasma cell myeloma (Bartl grade 2, asynchronous type), and fluorescence-activated cell sorter (FACS) analysis revealed 48% plasma cells positive for both CD38 and CD45.
Myeloma Id proteins
Id proteins were purified from plasma by protein A–Sepharose chromatography with a low-pH elution. Protein purity (> 90%) was determined by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Plasma samples used as starting material were obtained from the patients during periods of active disease, and Id proteins represented more than 80% of the total Ig in these samples.
T-cell–mediated tumor-specific cytokine responses
PBMC (2.5 × 106) obtained from donors before or after vaccination were cultured for 5 days with either medium (RPMI 1640 with 10% National Cancer Tissue Culture 109, 10% fetal bovine serum, 2 mmol/L glutamine, 50 μmol/L 2-mercaptoethanol, 100 μmol/L nonessential amino acids, 1 mmol/L sodium pyruvate, 100 U/mL penicillin, and 100 mg/mL streptomycin), 5 × 104plasmacytoma/myeloma tumor cells from the recipient, normal B cells from the recipient isolated from PBMC by FACS using a fluoresceinated monoclonal antibody (MoAb) against the light chain not represented in the Id protein (Caltag, Burlingame, CA), myeloma Id protein from the recipient, or an isotype-matched control Id protein (50 μg/mL each)—each in the presence or absence of 50 μg/mL anti-MHC class I or II (G46-2.6 and Tu39, respectively; PharMingen, San Diego, CA) or control MoAb (MARG 2c-3; Sigma, St Louis, MO). Tumor cells were pretreated with the MoAbs for 16 hours before coculturing with PBMC. Supernatants were harvested, and cytokine release was measured by using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN).
Generation of DCs
DCs from patient HR were generated by following a protocol described previously.12 Briefly, PBMC were plated at 6 × 106 cells/mL in 15 mL serum-free AIM V medium in T75 flasks (Costar, Cambridge, MA). After 2 hours of incubation at 37°C, nonadherent cells were removed by gentle rinsing and cryopreserved for later use as responders in T-cell induction. The remaining adherent cells were cultured in AIM V medium with GM-CSF (800 U/mL) and interleukin (IL)-4 (500 U/mL) (Genzyme, Cambridge, MA) for 6 days at 37°C in 5% carbon dioxide. The resulting cells were harvested and confirmed to be DCs on the basis of their unique morphologic and phenotypic characteristics. The DCs were mainly large cells with cytoplasmic projections, and they often formed clumps. On FACS analysis, they brightly expressed CD11c, CD86, and HLA-DR but were negative for CD14 and other lineage markers.
Pulsing of DCs and induction of CTLs
DCs were washed twice in serum-free AIM V medium before being pulsed with Id protein from patient HR. DCs (1 × 106) were incubated with the Id protein (100 μg/mL) in 1 mL AIM V medium at 37°C for 6 hours or overnight. For induction of CTLs, DCs (2 × 105/well) pulsed with the protein were cocultured with autologous nonadherent PBMC (5 × 106/well) in complete RPMI 1640 medium containing 10% fetal bovine serum. The T-cell culture was restimulated every 7 to 10 days with the same number of pulsed DCs in the presence of IL-2 (5 U/mL) and IL-7 (10 ng/mL) (Genzyme).
T-cell–mediated autologous tumor cytotoxicity
Five days after restimulation with DCs pulsed with Id protein, the T cells derived from patient HR were recovered and used as effectors in either a 4-hour chromium 51 (51Cr) release assay or a 12-hour indium 111 (111In) release assay. Unmanipulated autologous plasmacytoma or normal B cells were labeled with either 51Cr (NEN, Boston, MA) or111In-oxine (MPI Pharmacy/Amersham, Arlington Heights, IL) and plated (5000 cells/well) in triplicate with effector cells in 96-well U-bottom microtiter plates. The plasmacytoma targets were also pretreated with 50 μg/mL anti-MHC class I (G46-2.6) or control MoAb (R2B-8; Sigma) for 2 hours, washed, and then used in the CTL assay described above at various ratios of effector to target (E/T). Data are reported as the mean (± SEM) percentage of specific lysis at each E/T ratio. All SEMs were under 5%. The percentage of specific lysis was calculated as follows: [(experimental release / maximum release) − (spontaneous release / maximum release)] × 100. The spontaneous release was 30% in the 51Cr release assay and 35% and 23%, respectively, in the first and second 111In release assays.
Analysis of intracellular cytokine and T-cell–phenotype by FastImmune assay
After 2 in vitro stimulations with DCs pulsed with Id protein, the antigen specificity and phenotype of the T-cell culture derived from patient HR were analyzed simultaneously by FastImmune assay.13 Briefly, effector cells were coincubated with stimulators at a ratio of 5 to 1 at 37°C. Autologous tumor cells were used as stimulators, and autologous PBMC were used as control stimulators. Two hours later, Brefeldin A (BFA) (Sigma) was added at the final concentration of 10 μg/mL to block secretion of cytokines. Cells were then incubated at 37°C for an additional 3 hours in the presence of BFA, washed, and fixed with 1% paraformaldehyde. Fixed cells were permeabilized and triple stained with conjugated antibodies of CD69-phosphatidylethanolamine, CD4–peridinin chlorophyll protein (PerCP) or CD8-PerCP, and interferon-γ (IFN-γ)–fluorescein isothiocyanate, conjugated (FITC) or tumor necrosis factor–α (TNF-α)–FITC (Becton Dickinson, Mountain View, CA). Cell samples were analyzed by a FACScalibur cytometer (Becton Dickinson). Both CD4+ and CD8+ T-cell populations were gated on and examined for their expression of CD69 and intracellular cytokine (IFN-γ).
Results
Tumor-specific T cells induced by immunization of HLA-matched donors with myeloma Id in vivo
We examined T-cell responses in PBMC isolated directly from healthy, HLA-matched, stem-cell transplant donors who had been vaccinated against their recipient's myeloma Id protein according to a clinical protocol designed to raise and transfer tumor-specific T-cell immunity from healthy donors.10 Figure 1 shows the results for donor SG. As expected, PBMC obtained before vaccination of this donor showed no significant response to stimulation with the recipient's myeloma cells (<15 ng/L IFN-γ, GM-CSF, and TNF-α). However, after vaccination, PBMC responded to tumor stimulation with markedly increased production of all 3 cytokines (IFN-γ, 49 ng/L; GM-CSF, 680 ng/L; and TNF-α, 150 ng/L) compared with the medium control (Figure 1A). Furthermore, PBMC did not respond to simulation by normal B cells from the recipient, suggesting that these responses were tumor specific. In addition, the tumor-specific cytokine responses were abrogated by anti-MHC class I MoAb (< 15 ng/L IFN-γ, GM-CSF, and TNF-α) but not by anti-MHC class II MoAb (IFN-γ, 52 ng/L; GM-CSF, 703 ng/L; and TNF-α, 210 ng/L), indicating that T-cell recognition of myeloma tumor cells was restricted by class I molecules. These results also suggest that the tumor-specific T-cell response was a direct effect of Id vaccination in vivo, since PBMC obtained before vaccination failed to produce any cytokine response.
In parallel, PBMC obtained from donor SG after vaccination were tested for response to the immunizing Id protein. The data shown in Figure 1B demonstrate the detection of Id-specific T cells, which responded to stimulation with myeloma Id protein from the recipient but not an isotype-matched myeloma Id protein (control Id). Furthermore, the response to soluble myeloma Id protein was abrogated when an anti-MHC class II MoAb, but not an anti-MHC class I MoAb, was added, suggesting that in contrast to the class I–restricted responses to intact myeloma tumor cells, the Id-specific response was restricted by class II molecules.
Comparable results were obtained with T cells derived from a second immunized donor (RE). Specifically, PBMC obtained after vaccination but not those obtained before vaccination produced a significant amount of IFN-γ in response to his recipient's myeloma cells (39 ng/L) (Figure2A). Lower amounts of GM-CSF and TNF were also produced in response to myeloma cells (29 and 30 ng/L, respectively; data not shown). Consistent with the results for donor SG, tumor-specific T-cell recognition of myeloma cells from RE's recipient was also blocked by an anti-MHC class I MoAb (<15 ng/L) but not by either an anti-MHC class II MoAb (42 ng/L) or an isotype-matched control MoAb (46 ng/L). Class II–restricted T cells specific for myeloma Id were also detected after stimulation with the immunizing Id protein after vaccination (Figure 2B).
Tumor-specific, MHC class I–restricted T-cell cytokine response induced in an HLA-matched donor (RE) vaccinated with myeloma Id.
The methods used were the same as those described in the legend for Figure 1. The results are representative of 2 experiments.
Tumor-specific, MHC class I–restricted T-cell cytokine response induced in an HLA-matched donor (RE) vaccinated with myeloma Id.
The methods used were the same as those described in the legend for Figure 1. The results are representative of 2 experiments.
Tumor-specific CTLs induced by DCs pulsed with myeloma Id in a fully autologous system
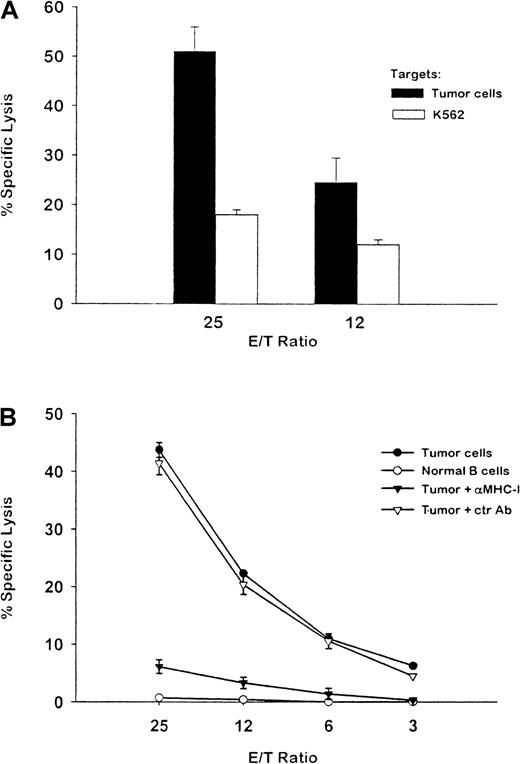
Nonadherent PBMC from patient HR were restimulated in vitro with autologous DCs pulsed with Id protein purified from his plasma. After repeated stimulation, a T-cell line (designated as HR) was established and subsequently tested for cytotoxicity against unmodified, cryopreserved autologous plasmacytoma cells by using either a51Cr release assay (Figure3A) or an 111In release assay (Figure 3B). FACS analysis showed that the T-cell line was composed of 74% CD3+ cells, 21% CD8+ cells, and 69% CD4+ cells (data not shown). The T cells effectively lysed autologous tumor cells (51% specific lysis at 25:1 E/T ratio). Natural killer–sensitive K562 cells were lysed only at low levels (Figure 3A). Furthermore, autologous normal B cells were not lysed by the T cells (5% at E/T ratio 25:1; Figure 3B), suggesting that the lysis was tumor specific. Moreover, the cytotoxicity of autologous tumor cells was almost completely blocked by pretreatment with an anti-MHC class I MoAb but not an isotype-matched control MoAb. These results indicate that the tumor-specific cytotoxic activity was mediated by class I–restricted CTLs.
Cytotoxic T-cell activity against fresh autologous myeloma cells.
A T-cell line was established by coculturing PBMC from patient HR with autologous dendritic cells (DCs) pulsed with myeloma Id. (A) The T-cell line was assayed at the indicated ratios of effector to target for lysis of chromium 51–labeled autologous plasmacytoma cells (solid bars) or natural killer–sensitive K562 cells (open bars) after repeated stimulation. (B) The T cells were assayed for lysis of indium 111–labeled autologous tumor cells. Tumor-specific cytotoxicity was blocked by pretreatment of tumor targets with an anti-MHC class I MoAb but not with a control antibody. Autologous normal B cells were used as a control target for specificity. The results shown are representative of 2 experiments.
Cytotoxic T-cell activity against fresh autologous myeloma cells.
A T-cell line was established by coculturing PBMC from patient HR with autologous dendritic cells (DCs) pulsed with myeloma Id. (A) The T-cell line was assayed at the indicated ratios of effector to target for lysis of chromium 51–labeled autologous plasmacytoma cells (solid bars) or natural killer–sensitive K562 cells (open bars) after repeated stimulation. (B) The T cells were assayed for lysis of indium 111–labeled autologous tumor cells. Tumor-specific cytotoxicity was blocked by pretreatment of tumor targets with an anti-MHC class I MoAb but not with a control antibody. Autologous normal B cells were used as a control target for specificity. The results shown are representative of 2 experiments.
T-cell–mediated autologous tumor–specific cytokine responses
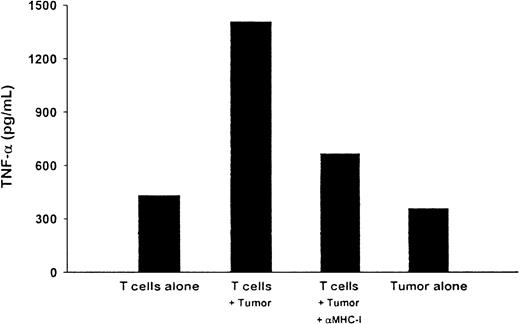
The HR T-cell line was also tested for its ability to recognize autologous tumor cells in a cytokine-release assay. TNF-α production was measured by ELISA in the supernatant from the T-cell line cocultured with autologous tumor cells for 6 days. For unknown reasons, relatively high levels of cytokine release were observed with both T cells alone and tumor cells alone. However, compared with T cells or tumor cells alone, T cells cocultured with the tumor cells produced significantly higher levels of TNF-α (Figure4). Moreover, this tumor cell–induced response was inhibited by the addition of an anti-MHC class I MoAb.
Tumor-specific, MHC class I–restricted release of cytokines by the T-cell line.
T cells derived from patient HR were assayed for release of TNF-α in response to stimulation by autologous plasmacytoma cells. The T cells (2.5 × 106) were cultured for 5 days with fresh autologous plasmacytoma cells (5 × 104) in either the absence or presence of an anti-MHC class I MoAb. T cells alone or tumor cells alone were used as controls. Supernatants from the cultures were harvested and assessed for TNF-α production by ELISA.
Tumor-specific, MHC class I–restricted release of cytokines by the T-cell line.
T cells derived from patient HR were assayed for release of TNF-α in response to stimulation by autologous plasmacytoma cells. The T cells (2.5 × 106) were cultured for 5 days with fresh autologous plasmacytoma cells (5 × 104) in either the absence or presence of an anti-MHC class I MoAb. T cells alone or tumor cells alone were used as controls. Supernatants from the cultures were harvested and assessed for TNF-α production by ELISA.
Analysis of intracellular cytokine and T-cell phenotype by FastImmune assay
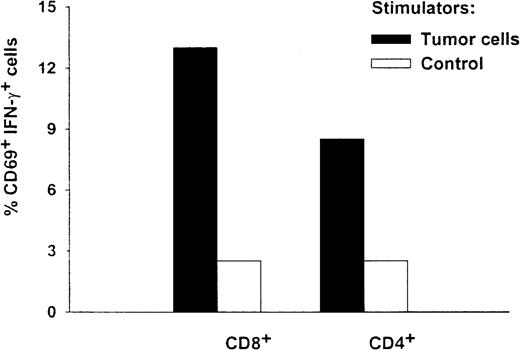
The antigen specificity and T-cell phenotype of the HR T cells were analyzed simultaneously by using a FastImmune assay. Figure5 shows that autologous tumor cells stimulated a significant percentage of both CD8 and CD4 T cells (13% of the CD8 and CD69 double-positive cell population and 9% of the CD4 and CD69 double-positive cell population, respectively) to secrete IFN-γ. In contrast, only background levels of CD8+ cells and CD4+ cells (3.7% and 3.3%, respectively) were activated by autologous PBMC controls. These results provide independent evidence that in vitro stimulation of PBMC by DCs pulsed with Id protein can induce CD8+ T cells that are specific for autologous myeloma cells.
Intracellular cytokine and phenotype analysis of T cells from patient HR.
After 2 in vitro stimulations by DCs pulsed with Id protein, the antigen specificity and T-cell phenotype of HR's T cells were analyzed simultaneously by FastImmune assay. Effector cells were coincubated with stimulators at a ratio of 5 to 1 at 37°C. Autologous tumor cells (solid bars) or autologous PBMC (open bars) were used as stimulators. After a 2-hour coincubation, Brefeldin A was added to block secretion of cytokines. Three hours later, cells were washed and fixed with 1% paraformaldehyde. Fixed cells were permeabilized and triple stained with direct conjugated antibodies of CD69-phosphatidylethanolamine, CD4– or CD8–peridinin chlorophyll protein, and IFN-γ–fluorescein isothiocyanate, conjugated. Cell samples were then acquired by a fluorescence-activated cell sorter (FACScalibur) cytometer. Either CD4+ (right panel) or CD8+ (left panel) T cells were gated on, and their expression of CD69 and intracellular cytokine was examined. Percentages of activated double-positive cells are shown.
Intracellular cytokine and phenotype analysis of T cells from patient HR.
After 2 in vitro stimulations by DCs pulsed with Id protein, the antigen specificity and T-cell phenotype of HR's T cells were analyzed simultaneously by FastImmune assay. Effector cells were coincubated with stimulators at a ratio of 5 to 1 at 37°C. Autologous tumor cells (solid bars) or autologous PBMC (open bars) were used as stimulators. After a 2-hour coincubation, Brefeldin A was added to block secretion of cytokines. Three hours later, cells were washed and fixed with 1% paraformaldehyde. Fixed cells were permeabilized and triple stained with direct conjugated antibodies of CD69-phosphatidylethanolamine, CD4– or CD8–peridinin chlorophyll protein, and IFN-γ–fluorescein isothiocyanate, conjugated. Cell samples were then acquired by a fluorescence-activated cell sorter (FACScalibur) cytometer. Either CD4+ (right panel) or CD8+ (left panel) T cells were gated on, and their expression of CD69 and intracellular cytokine was examined. Percentages of activated double-positive cells are shown.
Discussion
Successful development of a human tumor-specific antigen as a therapeutic T-cell vaccine requires at least 2 criteria. First, the candidate antigen must be shown to be immunogenic in human patients, either in vitro or in vivo. Equally important, however, is the demonstration that the tumor-cell target is capable of expressing the antigen in the form of peptide bound to MHC molecules at the cell surface. Id protein isolated from patients with myeloma may be considered an ideal antigen for immunotherapy because of the uniqueness of its idiotypic determinants and the fact that sufficient amounts of such proteins are easily obtainable from serum or urine. Previously, we and others showed that myeloma Id can be immunogenic for induction of a T-cell response against idiotypic determinants, either autologously in patients9 or in HLA-matched donors.10 The current study extends the observation of immunogenicity of this tumor antigen in vitro (patient HR) and in vivo (donors SG and RE) and, for the first time, establishes that autologous myeloma cells can serve as targets of a T-cell response directed against this antigen.
Specifically, strong tumor-specific and class I–restricted cytokine responses mediated by T cells were detected in PBMC from 2 healthy HLA-matched T-cell donors previously vaccinated in vivo with KLH-conjugated myeloma Id derived from the tumors of their respective recipient and GM-CSF (Figures 1A and 2A). As internal controls, these T cells were shown to recognize the idiotypic determinant of the myeloma Id protein, a response that was blocked by anti-MHC class II antibodies (Figures 1B and 2B). Such class II–restricted T-helper cells may be required for the generation and maintenance of the class I–restricted T-cell response. GM-CSF may be a critical component of this vaccine formulation for generation of CD8+ T-cell immunity. Previous studies in animal models demonstrated the ability of paracrine GM-CSF, either by gene delivery to tumor cells14 or as free cytokine with defined tumor antigens,15 to generate tumor-specific CD8+ T-cell responses. GM-CSF probably acts by recruiting professional APCs, including DCs, which may activate pathways of antigen processing that allow exogenous proteins to be presented by MHC class I molecules.
Alternatively, the ability of PBMC from patient HR to develop autologous tumor cytotoxicity was observed after stimulation with DCs pulsed with autologous myeloma Id protein ex vivo. Unlike the 2 donors, patient HR was not deliberately vaccinated with Id protein. Nevertheless, antigen-specific precursor T cells that could be expanded in vitro were presumably present. The resulting T cells showed strong cytotoxicity against autologous tumor cells but no cytotoxicity against autologous nonneoplastic B cells. The tumor-specific cytotoxicity was restricted by MHC class I molecules, since it was blocked by anti-MHC class I MoAbs (Figure 3). The T cells also released type 1 cytokines (IFN-γ, GM-CSF, and TNF-α) on tumor stimulation, in a tumor-specific and MHC class I– restricted manner (Figure 4). Finally, FastImmune studies indicated that both CD4 and CD8 T cells were involved in the tumor-specific cytokine response (Figure 5). To our knowledge, this is the first direct evidence of CTLs specific for human myeloma cells.
Although class I–restricted CD8+ T cells played a predominant effector role against tumor cells, CD4+T-helper cells were also observed in all 3 patients. Although the Id-specific T-helper cells were detected on stimulation with Id protein (Figures 1B and 2B), they were not directly reactive to tumor cells, since the cytokine response to tumor cells was not blocked by anti-MHC class II antibody (Figures 1A and 2A). One explanation for this finding is that Id-specific T-helper cells may play a role in the induction phase but not in the effector phase. For example, in the induction phase, CD4 T cells could facilitate in vivo induction of effector CD8+ T cells by the release of cytokines.16 17 It is also possible that the CD4+ T cells and CD8+ T cells recognize different antigenic epitopes on the Id protein. Similarly, CD4+ T cells that responded to secreted Id protein that was taken up, processed, and presented by APCs in the plasmacytoma tumor mass may have been detected (Figure 5).
For a study designed to ask the question of whether the tumor-cell target expresses the candidate tumor antigen at its surface, 2 potential sources of autologous myeloma cells must be considered. Homogeneous human myeloma cell lines can occasionally be established in immunodeficient mice by serial passage.16 However, fresh unmanipulated plasmacytoma cells are a better source for determining whether T cells elicited by vaccination in vivo or in vitro can recognize expression of the target antigen in its native form on autologous tumor cells. For example, the autologous HR tumor targets used were isolated from a solid plasmacytoma mass composed of at least 50% malignant plasma cells. The precise nature of the target antigen at the surface of these tumor cells recognized by Id-induced T cells remains to be elucidated. The neoplastic plasma cells in myeloma generally do not express intact Ig at the cell surface. Rather, the consistent observation of the blocking of T-cell recognition of myeloma cell targets by pretreatment with anti-MHC class I antibodies in all 3 patients studied strongly suggests T-cell recognition of an idiotypic peptide–MHC class I complex on the surface of the tumor cell. Consistent with this hypothesis, murine T-cell clones were found to be specific for a peptide comprising amino acids 91 to 101 of the λ–light chain variable region of MOPC-315 bound to an MHC class II molecule,3 a result suggesting that Id-specific T cells recognize determinants that are processed and presented rather than as intact protein. Mapping experiments based on Ig-sequence analysis are planned to determine the precise peptides recognized by human T cells from vaccinated donors.
CTLs are a critical component of antitumor immunity; therefore, a highly desirable goal of cancer vaccines is to develop novel approaches that will elicit potent, tumor-specific CTLs. DCs are considered to be the most potent APC, and both CD4+ and CD8+ T cells can be induced by antigen-loaded DCs.11 The requirement for T-cell immunity is highlighted in studies of multiple myeloma because any antibody response would be expected to be neutralized by the excess of circulating antigen in vivo. The finding of potent CTLs induced in vitro by stimulation with DCs pulsed with myeloma Id strongly supports the use of DCs as adjuvants in active in vivo immunotherapy with myeloma Id in patients with myeloma. Finally, the demonstration that myeloma tumor cells can express idiotypic determinants in such a way that they can be recognized as targets by Id-specific T cells raises the possibility of adoptive immunotherapy strategies using ex vivo expanded, antigen-specific CTLs from patients with myeloma (autologous CTLs) or from HLA-matched healthy T-cell donors.
Acknowledgments
We thank Dr Eli Gilboa for helpful discussions and Michelle St. Peter, Jong-Hee Nam, and Kevin Provost for technical assistance.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Larry W. Kwak, Building 567, Room 205, National Cancer Institute—FCRDC, Frederick, MD 21702-1201; e-mail:kwak@mail.ncifcrf.gov, or Yiwen Li, Department of Immunology, ImClone Systems Inc, 180 Varick Street, New York, NY 10014; e-mail:yiwen@imclone.com.

![Fig. 1. Tumor-specific, major histocompatibility complex (MHC) class I– restricted T-cell cytokine response induced in an HLA-matched donor (SG) vaccinated with myeloma idiotype (Id). / Peripheral blood mononuclear cells (PBMC) (2.5 × 106) obtained before and after vaccination from a healthy HLA-matched sibling donor who was vaccinated with the recipient's Id protein were cocultured with (A) 5 × 104 fresh plasmacytoma cells from the recipient, normal B cells from the recipient, or medium; and (B) the recipient's myeloma Id protein, isotype-matched control Id protein (50 μg/mL each), or medium. Anti-MHC class I or II or control monoclonal antibodies (MoAbs) were added as indicated. Five days later, supernatants were harvested and measured for cytokine production by enzyme-linked immunosorbent assay (ELISA). In an additional control preparation in which plasmacytoma cells from the recipient were cultured alone, none of the 3 cytokines tested (interferon γ [IFN-γ]), granulocyte-macrophage colony-stimulating factor, and tumor necrosis factor α [TNF-α]) were detected in the culture supernatant (data not shown). The results are representative of 2 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/8/10.1182_blood.v96.8.2828/5/m_h82000270001.jpeg?Expires=1769185950&Signature=E-SA9lbmuAMNlbB7M8GAxZ4eUsHBMLrfKDLQ2ZhkZDYiozrM4DQX3dyQGeI8LV44HJ1-9qmw~6tmZXyfqbsgehXChm6eWzkM~ESmggRKHQuK2pCPIdj-6g6TQLlmofesjsGq3rLOuhmHbDtteR1dKzmVdztvBYwu3uqXtoSMrvUyZkgnbDfbDQD8ArK-ENqNXMA1pfY5YPdmR7lbIV7O35IptZy7wJiOpAHfQ-lVp5hhM17ZvAHs-SmqPpc6rCWgZAXJf8HV3U7Rhn8bxP4d5x892dXvZmrhXrlS1vU66yMJaRErB5ky5Kxm9wE8lH5d9Va2bo15MI5WkUlqRsGLYw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal