Abstract
This report describes a new human B7-like gene designatedB7-H2. Cell surface expression of B7-H2 protein is detected in monocyte-derived immature dendritic cells. Soluble B7-H2 and immunoglobulin (Ig) fusion protein, B7-H2Ig, binds activated but not resting T cells and the binding is abrogated by inducible costimulator Ig (ICOSIg), but not CTLA4Ig. In addition, ICOSIg stains Chinese hamster ovary cells transfected with B7-H2 gene. By suboptimal cross-linking of CD3, costimulation of T-cell proliferation by B7-H2Ig is dose-dependent and correlates with secretion of interleukin (IL)-2, whereas optimal CD3 ligation preferentially stimulates IL-10 production. The results indicate that B7-H2 is a putative ligand for the ICOS T-cell molecule.
Introduction
Costimulatory interactions between the B7 family ligands and their receptors play critical roles in the growth, differentiation, and death of T cells. Engagement of the T-cell costimulator CD28 by either specific antibodies or its natural ligands B7-1 and B7-2 increases antigen-specific proliferation of CD4+ T cells, enhances production of cytokines, induces maturation of CD8+ effector T cells,1-3 and promotes T-cell survival.4 Signaling through homologous CTLA-4 receptor of B7-1 and B7-2 on activated T cells, however, is thought to deliver a negative signal that inhibits T-cell proliferation, interleukin (IL)-2 production, and cell cycle progression.5,6 Although B7-1 and B7-2 share only ∼20% homology in their amino acids, they have similar tertiary structures and costimulatory functions.7-10
Recent studies indicate that other members of the B7-CD28 family may also participate in the regulation of cellular and humoral immune responses. One of the new members is an inducible costimulator (ICOS), a CD28-like receptor.11 An F44 monoclonal antibody (mAb) against human ICOS costimulates T-cell growth and increases secretion of several cytokines including IL-10, interferon-γ, and IL-4, but not IL-2 in the presence of optimal doses of anti-CD3 antibody.11 Another new B7 family member is mouse B7h /B7RP-1.12,13 B7h/B7RP-1 does not bind to CD28 and CTLA-4 and can costimulate T-cell growth in the presence of antigenic signals. It has been shown that surface expression of B7h/B7RP-1 is up-regulated by tumor necrosis factor-α in the 3T3 fibroblast line and the increase of B7h/B7RP-1 messenger RNA (mRNA) is also observed in nonlymphoid tissues exposed to lipopolysaccharide (LPS).12Yoshinaga and associates13 demonstrated that B7h/B7RP-1 is a ligand for mouse CRP-1, a mouse homologue of human ICOS. Expression of a B7RP-1 fusion protein in transgenic mice leads to hyperplasia in several lymphoid organs and treatment of mice with B7h/B7RP-1 fusion protein enhanced oxazolone-induced contact hypersensitivity.13 We have recently reported a new member of the human B7 family, B7-H1.14 B7-H1 shares ∼20% identical amino acid sequence with B7-1 and B7-2 in the Ig V- and Ig C-like extracellular domains but differs more profoundly from B7-1 and B7-2 in the cytoplasmic domain. It is unlikely that B7-H1 is a human homologue of mouse B7h/B7RP-1 because identity of amino acids between them is less than 30%. Furthermore, B7-H1 does not bind to CD28, CTLA-4, and ICOS. Surface expression of B7-H1 can be detected in the majority of activated CD14+ macrophages and a fraction of activated T cells. B7-H1 costimulates T-cell responses in the presence of suboptimal doses of anti-CD3 mAb, enhances allogeneic mixed lymphocyte response, and preferentially induces IL-10 secretion from T cells.14
By searching molecules sharing homologies with the Ig V and Ig C domains of B7-1, B7-2, and B7-H1 in the NCBI database (http://www.ncbi.nlm.nih.gov) followed by subsequent cloning and sequencing, we identified a new B7-like gene designatedB7-H2 (B7homologue 2). In addition to an overall structure similarity to B7-1, B7-2, and B7-H1, B7-H2 binds ICOS and costimulates the proliferation and cytokine production of human T cells.
Materials and methods
Cells
Human embryonic kidney 293 and Chinese hamster ovary (CHO) cells are maintained in Dulbecco modified Eagle medium (DMEM; Gibco BRL, Gaithersburg, MD) containing 10% heat-inactivated fetal bovine serum (FBS) (HyClone, Logan, UT), 25 mmol/L HEPES, 100 U/mL penicillin G, and 100 μg/mL streptomycin sulfate. Dendritic cells (DCs) were generated from adherent peripheral blood mononuclear cells (PBMCs) by culturing in RPMI 1640 supplemented with 10% FBS in the presence of 80 U/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; Immunex, Seattle, WA) and 500 U/mL IL-4 (R & D Systems, Minneapolis, MN) for 7 days as described.15 The majority of cells in the culture at day 7 showed DC-like morphology with the veils and dendritic processes. FACS analysis demonstrates that 99% of these DC-like cells expresses HLA-DR and more than 65% of them expresses B7-1. Fewer than 5% of the cells stained positively with antibodies against CD14, CD16, and CD19. Approximately 50% of DC-like cells expressed CD4, but not CD3 or CD8 T-cell markers. The Internal Review Board of the Mayo Clinic has approved all studies using human PBMC.
Cloning of human B7-H2 cDNA and construction of B7-H2Ig fusion proteins
The NCBI database was screened for amino acid homology to the sequences of human B7-1, B7-2, and B7-H1 using the BLASTN algorithms. The complementary DNA (cDNA) sequence KIAA0653 in the database, which contained a large portion of B7-H2 sequence, was studied further. The full-length human B7-H2 cDNA was generated by polymerase chain reaction (PCR) from a human DC cDNA library prepared by SMART PCR cDNA synthesis kit (Clontech, Palo Alto, CA). The sequence of the forward primer is 5′-CCGCGGCCCAAGTTCT-3′ and the sequence of the reverse primer is 5′-GCCTCATTCCAGGATCACAG-3′. Both primers were derived from the KIAA0653 sequence. The resulting PCR product was sequenced using an ABI Prism 310 Genetic Analyzer. The B7-H2 cDNA was cloned into pcDNA3.1 vector (Invitrogen, Carlsbad, CA) as EcoRI-HindIII fragments.
The B7-H2 and Ig fusion gene was created by fusing the cDNA of the extracellular domain of B7-H2 in frame to the CH2-CH3 portion of mouse IgG2a or human IgG1.14 The resulting plasmids were transfected into 293 or CHO cells by Fugene 6 (Boehringer-Mannheim, Mannheim, Germany) according to the manufacturer's instruction. The B7-H2Ig fusion proteins were purified from the culture supernatants of transfected 293 cells grown in serum-free media (Gibco) by a protein G-Sepharose column (Pharmacia, Uppsala, Sweden). Human ICOSIg, B7-H1Ig, and CTLA4Ig were prepared as described previously.14
Flow cytometry analysis
To prepare antisera, BALB/c mice were immunized with purified B7-H2/mouse Ig fusion proteins (B7-H2mIg) in complete Freund adjuvant (Sigma, St Louis, MO) and boosted 3 times with B7-H2mIg in incomplete Freund adjuvant. Sera were collected 10 days after the last boost. The specificities of antisera were determined in enzyme-linked immunosorbent assay (ELISA) against B7-H2Ig and by FACS staining against 293 cells transfected with the pcDNA3.1 plasmid containing the B7-H2 full-length cDNA (phB7-H2). Prebleed mouse sera were used as controls. To detect human B7-H2 expression, DC at 1 × 106 were incubated with anti-B7-H2 antiserum (1:1000) or control serum (1:1000) in FACS buffer (phosphate-buffered saline [PBS], 3% fetal calf serum [FCS], 0.02% NaN3) at 4°C for 30 minutes. The cells were washed and further incubated with fluorescein isothiocyanate (FITC)-conjugated goat antimouse IgG (BioSource, Camarillo, CA) for 30 minutes at 4°C. Fluorescence was analyzed by a FACSCaliber flow cytometry (Becton Dickinson, Mountain View, CA) with Cell Quest software (Becton Dickinson).
To detect the expression of the counter-receptor of B7-H2 in T cells, nylon wool-purified T cells were cultured either unstimulated or stimulated with 5 μg/mL phytohemagglutinin (PHA; Sigma) for 1 to 3 days as indicated. The cells were then stained by B7-H2Ig and analyzed by FACS. CHO cell lines expressing surface B7-H2 were also established by selection of neomycin-resistant clones of cells transfected with the phB7-H2 plasmid. For indirect immunofluorescence staining, cells were first stained with anti-B7-H2 antiserum (1:1000) or ICOSIg for 30 minutes and further incubated with FITC-conjugated goat antimouse or antihuman IgG antibodies, respectively. Normal serum or purified human IgG1 were used as controls.
T-cell proliferation and cytokine assays
Enrichment of T cells was performed by passing nonadherent PBMCs of a healthy donor through nylon wool columns (Robbins Scientific Co, Sunnyvale, CA) as described previously.14 For the costimulation assay, flat-bottomed 96-well microplates were first coated with 100 μL of anti-CD3 mAb of indicated doses at 4°C overnight. After intensive washing with PBS, the plates were further coated with B7-H2Ig or control IgG at 37°C for 4 hours and then the purified T cells were added to the wells at 2 × 105cells/well in triplicate. The cells were cultured for 72 hours and3H-TdR at 1.0 μCi/well was added during the last 15 hours. The incorporation of 3H-TdR was counted by MicroBeta Trilux liquid scintillation counter (Wallac, Finland). To detect cytokines, supernatants were collected at 24, 48, and 72 hours of the cultures and the concentrations of IL-2 and IL-10 were determined by sandwich ELISA methods (PharMingen, San Diego, CA) according to manufacturer's instructions. B7-H1Ig in immobilized form and an anti-CD28 mAb (PharMingen) in soluble form were included for comparison. Polymyxin B at 10 μg/mL was also incorporated to completely neutralize potential endotoxin contamination.
Results
Molecular cloning and expression of human B7-H2 gene
Search of the NCBI database against amino acid sequences of human B7-1, B7-2, and B7-H1 revealed that the 5′-end of the KIAA0653 protein shared ∼20% homology to known B7 members. However, the KIAA0653 sequence encodes a putative protein with 558 amino acids and its 3′-end amino acid sequence does not have structure similarity to the members of the B7 family. Sequencing analysis of the cDNA isolated from human DCs by reverse transcriptase (RT)-PCR, however, revealed a different nucleic acid sequence from codon 1028. As a result, a stop codon TGA was identified. We have thus identified a new cDNA species encoding a putative protein with 302 amino acids, designated as B7-H2. B7-H2 encodes a glycosylated, type I protein consisting of Ig V-like domain, Ig C-like domain, hydrophobic transmembrane domain and cytoplasmic tail (Figure 1A). Four structural cysteines (as labeled by stars in Figure 1B), which are apparently involved in forming the disulfide bonds of the Ig V and Ig C domains, are well conserved in all B7 members (Figure 1B). B7-H2 shares an overall homology to B7-1 (24% amino acid identity), B7-2 (21%), and B7-H1 (21%) based on analysis using the multiple sequence alignment program of McVector 6.5 software (Figure 1B). Similar analysis shows that human B7-H2 and mouse B7h/B7RP-1 share ∼ 46% of homology, indicating that B7-H2 may be a human homologue of mouse B7h/B7RP-1.
Putative amino acid sequence of
B7-H2 gene and alignment of the B7 family members. (A) Predicted amino acid sequence of human B7-H2. The predicated signal peptide, Ig V-like domain, Ig C-like domain, transmembrane region, and the potential N-linked glycosylation site (*) are indicated. The nucleic acid and amino acid sequences of B7-H2 are available from GeneBank under accession number AF289028. (B) Alignment of the B7-H2 with B7-1, B7-2, and B7-H1. Identical amino acid residues are shaded in bold and conserved residues are boxed. Cysteine residues that may be important in the forming of disulfide bond inside Ig V or Ig C domains are indicated (*).
Putative amino acid sequence of
B7-H2 gene and alignment of the B7 family members. (A) Predicted amino acid sequence of human B7-H2. The predicated signal peptide, Ig V-like domain, Ig C-like domain, transmembrane region, and the potential N-linked glycosylation site (*) are indicated. The nucleic acid and amino acid sequences of B7-H2 are available from GeneBank under accession number AF289028. (B) Alignment of the B7-H2 with B7-1, B7-2, and B7-H1. Identical amino acid residues are shaded in bold and conserved residues are boxed. Cysteine residues that may be important in the forming of disulfide bond inside Ig V or Ig C domains are indicated (*).
To determine whether B7-H2 is expressed as a membrane-bound surface protein, we first constructed a plasmid containing the cDNA of the extracellular region of B7-H2 fused in frame with the Fc portion of the human IgG1. The resulting plasmid was transfected into 293 cells and the B7-H2Ig fusion protein was purified by protein G column from the culture supernatants. Antisera against B7-H2 were prepared by immunization of BALB/c mice with the purified B7-H2mIg. The antisera are specific for B7-H2 because these sera did not stain CHO cells transfected with B7-1, B7-2, or B7-H1 (data not shown) but stained B7-H2. FACS analysis indicates that although there is no substantial expression of unfractionated PBMCs (data not shown), DCs generated from adherent PBMCs in the presence of GM-CSF and IL-4 express B7-H2 on their surface (Figure 2; −LPS). Induction of DC maturation by stimulation with LPS led to a high level expression of B7-2 (Figure 2; +LPS), B7-1, and major histocompatibility class (MHC) II (data not shown). Surprisingly, treatment by LPS significantly down-regulated the expression of B7-H2 as shown by FACS analysis with anti-B7-H2 antibodies (Figure 2).
Expression of human B7-H2 on cytokine-induced dendritic cells.
DC-generated by GM-CSF and IL-4 were stained with antibodies to B7-2, B7-H2 (open), or control Ig (shaded). LPS in the lower panel was used in the DC cultures at a concentration of 1 μg/mL for 24 hours.
Expression of human B7-H2 on cytokine-induced dendritic cells.
DC-generated by GM-CSF and IL-4 were stained with antibodies to B7-2, B7-H2 (open), or control Ig (shaded). LPS in the lower panel was used in the DC cultures at a concentration of 1 μg/mL for 24 hours.
Binding of B7-H2 to ICOS molecule on activated T cells
We have examined the expression of the counter-receptor of B7-H2 by FACS analysis. Indirect immunoflurorescence staining using B7-H2Ig showed that the counter-receptor did not express on resting PBMCs. However, T cells that were stimulated by PHA expressed high levels of counter-receptor of B7-H2. The expression can be detected 24 hours after stimulation and is sustained up to 72 hours (Figure3A). Similar results were also obtained using T cells that were activated with anti-CD3 plus anti-CD28 mAbs. Therefore, the counter-receptor of B7-H2 appears to be inducible in T cells.
Binding of B7-H2 to ICOS on activated T cells.
(A) Expression of the counter-receptor of B7-H2 on activated T cells. Resting (0 hour) and activated T cells were stained with B7-H2Ig (open) or control Ig (shaded) and analyzed by flow cytometry. T cells were activated with PHA (5 μg/mL) for the indicated times (24, 48, and 72 hours). (B) ICOSIg abrogates the binding of B7-H2 to activated T cells. Purified T cells were stimulated with 5 μg/mL PHA for 48 hours. Activated T cells were stained with control Ig (shaded) or 1 μg of B7-H2Ig (open) in the presence of buffer, ICOSIg (10 μg), or CTLA4Ig (10 μg) and analyzed by flow cytometry. (C) Binding of ICOSIg to cell surface B7-H2. CHO cells transfected with phB7-H2 (open) or parental vector (shaded) were stained with ICOSIg or anti-B7-H2 antibody and analyzed by flow cytometry.
Binding of B7-H2 to ICOS on activated T cells.
(A) Expression of the counter-receptor of B7-H2 on activated T cells. Resting (0 hour) and activated T cells were stained with B7-H2Ig (open) or control Ig (shaded) and analyzed by flow cytometry. T cells were activated with PHA (5 μg/mL) for the indicated times (24, 48, and 72 hours). (B) ICOSIg abrogates the binding of B7-H2 to activated T cells. Purified T cells were stimulated with 5 μg/mL PHA for 48 hours. Activated T cells were stained with control Ig (shaded) or 1 μg of B7-H2Ig (open) in the presence of buffer, ICOSIg (10 μg), or CTLA4Ig (10 μg) and analyzed by flow cytometry. (C) Binding of ICOSIg to cell surface B7-H2. CHO cells transfected with phB7-H2 (open) or parental vector (shaded) were stained with ICOSIg or anti-B7-H2 antibody and analyzed by flow cytometry.
Two costimulatory receptors, CTLA-4 and ICOS, can be induced on activated T cells.2 11 We therefore examined whether soluble ICOSIg or CTLA4Ig can block the binding of B7-H2Ig to activated T cells. As shown in Figure 3B, the binding activity of B7-H2Ig to PHA-activated T cells can be completely abrogated by the inclusion of ICOSIg, but not CTLA4Ig, indicating that the competition of ICOSIg in our assay is specific. This result suggests that ICOS is a potential counter-receptor for B7-H2. To further confirm this finding, we exam-ined whether CHO cells, a B7-H2 negative cell line, could confer ICOSIg binding activity after transfection to express B7-H2gene. We constructed a plasmid, phB7-H2, in which the full-length cDNA of human B7-H2 was inserted into a pcDNA3.1 vector. As shown in Figure3C, CHO cells transfected with phB7-H2 (CHO/B7-H2) could be stained by ICOSIg. As a positive control, anti-B7-H2 can also bind to CHO/B7-H2 but not to CHO/mock cells. Our results thus indicate that B7-H2 binds ICOS on T cells.
B7-H2 costimulates T cell proliferation
To determine costimulatory function of B7-H2, we purified T cells from human PBMC of healthy donors and stimulated them with B7-H2Ig in the presence of suboptimal doses of an anti-CD3 mAb. T-cell proliferation was determined by incorporation of 3H-TdR after 3-day culture. B7-H2Ig, when immobilized on plastic plates, enhanced T-cell proliferation up to 5-fold compared to the control Ig in the presence of 40 ng/mL of immobilized anti-CD3 mAb (Figure4A). The costimulatory effects of the B7-H2-Ig were dose-dependent (Figure 4B). In the absence of anti-CD3, B7-H2Ig in concentrations of 5 μg/mL had no effect on T-cell proliferation (Figure 4A). Our results demonstrate that B7-H2 engagement costimulates T cell response.
Costimulation of T-cell proliferation by B7-H2Ig.
(A) Purified T cells were cultured in the presence of immobilized anti-CD3 mAb at indicated doses (horizontal axis) and immobilized B7-H2Ig (open circle) or control Ig (closed circle) at 5 μg/mL; cpm indicates counts per minute. (B) Purified T cells were cultured with indicated doses of B7-H2Ig (open circle) or control Ig (closed circle) in the presence of immobilized anti-CD3 mAb at 20 ng/mL.
Costimulation of T-cell proliferation by B7-H2Ig.
(A) Purified T cells were cultured in the presence of immobilized anti-CD3 mAb at indicated doses (horizontal axis) and immobilized B7-H2Ig (open circle) or control Ig (closed circle) at 5 μg/mL; cpm indicates counts per minute. (B) Purified T cells were cultured with indicated doses of B7-H2Ig (open circle) or control Ig (closed circle) in the presence of immobilized anti-CD3 mAb at 20 ng/mL.
IL-2 and IL-10 secretion by B7-H2 costimulation
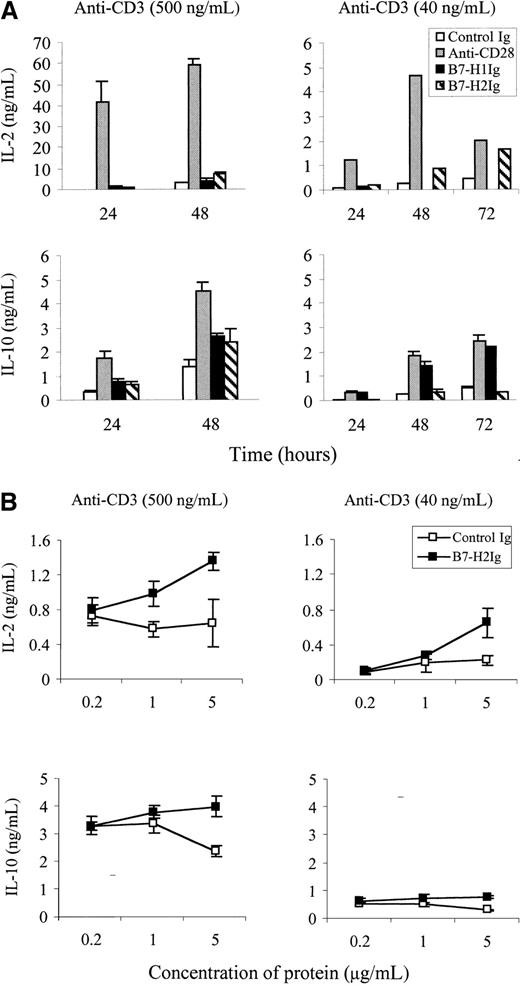
It has been reported that stimulation of T cells by optimal cross-linking of OKT3 in the presence of a F44 mAb to ICOS can preferentially induce IL-10 but not IL-2 production.11 To examine whether B7-H2Ig could do so, the levels of IL-2 and IL-10 in the T-cell culture supernatants by the stimulation of B7-H2Ig and an optimal dose of an anti-CD3 mAb (500 ng/mL) were determined by sandwich ELISA. Our preliminary experiments demonstrated that T cells proliferated vigorously at this dose of anti-CD3 mAb (data not shown). Immobilized B7-H2Ig moderately increases IL-10 secretion at the 48-hour point. In contrast, T cells costimulated by B7-H2Ig in the presence of a suboptimal dose (40 ng/mL) of anti-CD3 mAb had slightly increased levels of IL-2 but not IL-10. IL-10 did not increase throughout the 72 hours, whereas IL-2 was moderately elevated at 48 and 72 hours (Figure5). As expected, both B7-H1Ig and anti-CD28 mAb costimulated IL-10 production disregarding the doses of anti-CD3.14 Our results thus suggest that different strengths of CD3 engagement can affect the pattern of cytokine secretion by B7-H2 engagement.
Cytokine secretion by primary T cells in the presence of B7-H2Ig.
(A) Purified T cells were stimulated by precoated high (500 ng/mL) (left panels) or suboptimal doses (40 ng/mL) (right panels) of anti-CD3 mAb in the presence of immobilized B7-H2Ig, B7-H1Ig, or control Ig (5 μg/mL). Anti-CD28 mAb was used at 5 μg/mL in soluble form. Supernatants were collected at the indicated time points after stimulation and assayed for cytokines using sandwich ELISA. (B) Purified T cells were stimulated by precoated suboptimal (40 ng/mL) (right panels) or high doses (500 ng/mL) (left panels) of anti-CD3 mAb in the presence of the indicated concentration of immobilized control Ig or B7-H2Ig. Supernatants were collected at 48 hours after stimulation and assayed for IL-2 and IL-10 using sandwich ELISA.
Cytokine secretion by primary T cells in the presence of B7-H2Ig.
(A) Purified T cells were stimulated by precoated high (500 ng/mL) (left panels) or suboptimal doses (40 ng/mL) (right panels) of anti-CD3 mAb in the presence of immobilized B7-H2Ig, B7-H1Ig, or control Ig (5 μg/mL). Anti-CD28 mAb was used at 5 μg/mL in soluble form. Supernatants were collected at the indicated time points after stimulation and assayed for cytokines using sandwich ELISA. (B) Purified T cells were stimulated by precoated suboptimal (40 ng/mL) (right panels) or high doses (500 ng/mL) (left panels) of anti-CD3 mAb in the presence of the indicated concentration of immobilized control Ig or B7-H2Ig. Supernatants were collected at 48 hours after stimulation and assayed for IL-2 and IL-10 using sandwich ELISA.
Discussion
We have identified a human B7-like molecule and provide evidence that this molecule is a putative ligand for a recently described T-cell molecule ICOS. Similar to other B7 family members, the extracellular region of the B7-H2 protein has 4 conserved structural cysteines that are believed to form Ig V- and IgC-like domain.7-10 In addition, B7-H2 has a conserved tyrosine residue in the Ig V-like domain at the position of 80, which is identical to that of B7-1 at position 87, B7-2 at position 82, and B7-H1 at position 81 (Figure 1B). Despite a structural similarity, B7-H2 does not seem to bind the receptors of B7-1 and B7-2. B7-H2Ig binds strongly to activated but not resting T cells (Figure 3A). This observation precludes the possibility that B7-H2 is a ligand for CD28 because high levels of CD28 can be detected in the resting T cells.1 2 FACS analysis showed that ICOSIg, but not CTLA4Ig abrogated the binding of B7-H2 to activated T cells (Figure3B). In addition, ICOSIg stained CHO cells transfected with the B7-H2 cDNA (Figure 3C). Taken together, our results support that B7-H2 is a ligand for ICOS. Final confirmation of B7-H2 as the ligand for ICOS, however, awaits extensive studies including functional comparison of B7-H2Ig and anti-ICOS antibody.
By alignment analysis, B7-H2 shares ∼46% identity at amino acid level to B7h/B7RP-1, a recently described B7-like protein of mouse origin.12,13 This raises the possibility that B7h/B7RP-1 is a mouse homologue of B7-H2. It was shown that B7h binds weakly to activated, but not resting T cells,12 supporting this possibility. In mice, B7h/B7RP-1 can be detected on the surface of B cells, macrophages, and activated T cells but not in CD80+, Dec205+, and CD11c+ cells,13suggesting that B7h/B7RP-1 is not constitutively expressed on DCs. Our result, however, showed that B7-H2 was readily detected in monocyte-derived human DCs that were generated by GM-CSF and IL-4 (Figure 2). This result may be interpreted as the induction of B7-H2 by in vitro culture condition. Alternatively there may be a different expression pattern of B7-H2 in human and mouse tissues. In addition, the expression of B7-H2 can be down-regulated by exposure to LPS (Figure 2B) and the functional significance of this finding is unclear.
Costimulatory functions of B7-H2 were demonstrated by the ability that immobilized B7-H2Ig could amplify T-cell proliferation in the presence of suboptimal doses of anti-CD3 (Figure 4). It has been reported that stimulation of T cells with the anti-ICOS mAb F44 preferentially induces IL-10 production.11 We have found that B7-H2Ig is capable of inducing a moderate increase of IL-10 in the culture (Figure5A). However, this effect was only observed when a high dose, but not a suboptimal dose, of anti-CD3 mAb was used. In the presence of suboptimal doses of anti-CD3 mAb, B7-H2 costimulation slightly increases the production of IL-2 but not IL-10 (Figure 5B). Because suboptimal doses of anti-CD3 mAb mimic more closely to physiologic interaction between MHC-peptides and T-cell receptor-CD3 complexes, our results suggest that IL-10 production under antigen-specific interactions in vivo may not be a dominant event. Our study thus identifies a putative ligand for ICOS T-cell costimulatory molecule and suggests a regulatory function of B7-H2/ICOS interaction in the cell-mediated immune responses.
Acknowledgments
We thank Dallas Flies and Beth Martin for their excellent technical assistance and Kathy Jensen for editing the manuscript.
Supported in part by Mayo Foundation, and by National Institutes of Health (NIH) grants CA79915 and CA15083. G.Z. and K.T. are supported by NIH postdoctoral training grant CA09127 and US Army breast cancer research fellowship, respectively.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Note added in proof
During the review process of this manuscript, Ling and colleagues16 reported the sequence of a B7-like molecule called GL50 that is identical to human B7-H2.
Author notes
Lieping Chen, Department of Immunology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail:chen.lieping@mayo.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal