Abstract
A subset of blood cells from patients with B-cell chronic lymphocytic leukemia (CLL) spontaneously differentiates in vitro into large, round, or fibroblast-like adherent cells that display stromal cell markers, namely vimentin and STRO-1. These cells also express stromal cell–derived factor-1 (SDF-1), a CXC chemokine that ordinarily is secreted by marrow stromal cells. Leukemia B cells attach to these blood-derived adherent cells, down-modulate their receptors for SDF-1 (CXCR4), and are protected from undergoing spontaneous apoptosis in vitro. Neutralizing antibodies to SDF-1 inhibit this effect. Moreover, the rapid deterioration in the survival of CLL B cells, when separated from such cells, is mitigated by exogenous SDF-1. This chemokine also results in the rapid down-modulation of CXCR4 and activation of p44/42 mitogen-activated protein-kinase (ERK 1/2) by CLL B cells in vitro. It is concluded that the blood of patients with CLL contains cells that can differentiate into adherent nurse-like cells that protect leukemia cells from undergoing spontaneous apoptosis through an SDF-1–dependent mechanism. In addition to its recently recognized role in CLL B-cell migration, SDF-1–mediated CLL B-cell activation has to be considered a new mechanism involved in the microenvironmental regulation of CLL B-cell survival.
Introduction
B-cell chronic lymphocytic leukemia (CLL), the most common adult leukemia in the Western hemisphere, is characterized by the relentless accumulation of long-lived, mature, monoclonal B cells in the blood, secondary lymphoid tissues, and marrow.1Circulating leukemia cells primarily are arrested in the G0/G1 phase of the cell cycle and are resistant to undergoing programmed cell death.2,3 This is hypothesized to contribute to the noted resistance of CLL cells to standard chemotherapy.4 Understanding the mechanism(s) that contribute to the resistance of CLL cells to apoptosis could lead to new and more effective therapeutic strategies for patients with this disease.
Despite their longevity in vivo, CLL cells often undergo spontaneous apoptosis under conditions that support the growth of human B-cell lines in vitro.2,5 This implies that such ex vivo conditions lack essential survival factors and that the resistance to apoptosis is not intrinsic to the CLL cell.5-8 It recently has been shown that CLL B cells, but not normal CD5+ B cells, may be rescued from spontaneous or corticosteroid-induced apoptosis when cultured with human marrow stromal cells.6,9 In patients with CLL, the marrow invariably is infiltrated with leukemia cells. Furthermore, the extent of marrow infiltration correlates with clinical stage and prognosis.10 11 These observations indicate that regulatory signals in the marrow microenvironment, particularly contact with accessory cells, such as marrow stromal cells, may be important for the prolonged survival of CLL cells in vivo.
The marrow is a complex tissue containing hematopoietic progenitor cells and their progeny in close contact with a connective tissue network of mesenchymal-derived cells collectively referred to as stroma.12 During B-cell development in the marrow, programmed cell death is a physiologic regulator of homeostasis, diverting a large fraction of B-lineage cells into an apoptotic death pathway to eliminate functionless or potentially harmful cells.13,14 Critical factors for the survival of selected B cells are interactions with stromal cells in the marrow microenvironment, expression of surface immunoglobulin molecules, and expression of apoptosis-regulatory proteins, such as bcl-2.15 16
A major advance for studies on the regulation of B lymphopoiesis by stromal cells was the development of the long-term B-cell culture system by Whitlock and Witte.17 In these cultures, B-cell lymphopoiesis is supported by adherent stromal cells that develop into a layer in long-term marrow cultures. B cells adhere to or migrate under this layer of marrow stromal cells,18 which is similar to the spontaneous migration of CLL B cells beneath marrow stromal cells (pseudo-emperipolesis) that we recently characterized.19 Based on these observations it has been proposed that stromal cell contact and short-range growth factors are critical determinants for B lymphopoiesis.12 20
The chemokine stromal cell–derived factor-1 (SDF-1) plays an important role in B-cell development. High levels of SDF-1 are produced by stromal cells within the marrow, the primary site of early B-cell differentiation.21,22 SDF-1–deficient, or SDF-1–receptor-deficient, mice display severe defects in the generation of B cells but not of T cells.23-26 SDF-1 regulates B lymphopoiesis by retaining B-cell precursors in close contact with stromal cells within the supportive hematopoietic microenvironment,27 preventing their premature release into the circulation. Moreover, SDF-1 may also function as a B-cell growth factor. Initially, SDF-1 was designated pre–B-cell growth-stimulating factor (PBSF), because recombinant SDF-1 supported the proliferation of a stroma cell-dependent B-cell line (DW34).21 More recent studies also indicated that SDF-1 can have a direct effect on the growth of B-lineage cells.28,29 Most recently, we found that CLL B cells also express functional receptors for SDF-1 and migrate to stromal cells that secrete this chemokine.19
To gain insight into the regulation of CLL cell survival by stromal cells and their products, in particular SDF-1, we characterized the conditions necessary for the survival of CLL cells in long-term cultures of peripheral blood mononuclear cells (PBMC) from patients with CLL.
Materials and methods
Cell purification, cell lines
After obtaining informed consent, blood samples were collected from patients fulfilling diagnostic and immunophenotypic criteria for common B-cell CLL at the University of California at San Diego Medical Center.1 The patients were not previously treated and had not received recombinant growth factors or exogenous cytokines. Peripheral blood mononuclear cells were isolated by density-gradient centrifugation over Ficoll-Hypaque (Pharmacia, Uppsala, Sweden). Cells were used fresh or viably frozen in fetal calf serum (FCS) containing 5% dimethyl sulfoxide for storage in liquid nitrogen. The viability of the CLL cells was always greater than 85% at the time of initiation of the CLL PBMC cultures, as determined by staining the cells with 5 μg/mL propidium iodide (PI; Molecular Probes, Eugene, OR) for 15 minutes at 37°C. All CLL PBMC samples examined contained more than 90% CLL B cells, as determined by FACS analysis with anti-CD19, anti-CD5, and anti-CD3 monoclonal antibodies (mAb). The murine marrow stromal cell line M2-10B4 was purchased from the American Type Culture Collection (Rockville, MD). CLL cells and cell lines were cultured at 37°C, 5% CO2 in RPMI 1640 supplemented with 10% FCS and penicillin–streptomycin–glutamine (Gibco BRL, Rockville, MD; complete RPMI medium).
Chemokine, antibodies, flow cytometry
Synthetic human SDF-1α (1-67) was purchased from Upstate Biotechnology (Lake Placid, NY). Monoclonal antibodies used for flow cytometry that were specific for CXCR4 (12G5), CD3, CD19, or isotype controls were purchased from PharMingen (San Diego, CA). The mAb STRO-130 was purchased from the Developmental Studies Hybridoma Bank, The University of Iowa (Iowa City, IA). The mAbs specific for Vimentin (V9) or CD68 mAb (EBM11) were purchased from DAKO A/S (Glostrup, Denmark), and those used for immunohistochemistry that were specific for CD14 or CD106 and isotype controls were purchased from PharMingen. For inhibition of the SDF-1–mediated “nursing” function of nurse-like cells (NLC), anti–SDF-1 polyclonal goat IgG was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). To remove sodium azide, we used the Ultrafree-0.5 Centrifugal Filter Device (Millipore, Bedford, MA). For flow cytometry, the cells were adjusted to a concentration of 5 × 106 cells/mL in RPMI 1640 with 0.5% bovine serum albumin (FACS buffer). 5 × 105 cells were stained with saturating antibody concentrations for 30 minutes at 4°C, washed 2 times, and analyzed on a FACSCalibur (Becton Dickinson, Mountain View, CA). Flow cytometry data were analyzed using the FlowJo 2.7.4 software (Tree Star, San Carlos, CA).
Long-term cultures of PBMC from patients with CLL
To examine the survival of CLL cells in long-term cultures with or without marrow stromal cells, PBMC from patients with CLL were suspended in RPMI 1640 supplemented with 10% FCS and penicillin–streptomycin–glutamine (Gibco BRL) to a final concentration of 1.5 × 107 cells/mL. These cells were assessed for viability and expression of CD19, CD3, and CXCR4 before they were plated in tissue culture-treated 6-well plates with or without stromal cells (4 mL/well). For cocultures with marrow stromal cells, M2-10B4 stromal cells were seeded the day before initiation of CLL cultures into 6-well plates at a concentration 2 × 105 cells per well. The CLL cells then were cultured at 37°C in 5% CO2 in air for 14 days, during which 300-μL aliquots were removed at the indicated time points from alternative wells for viability assays, as described below. At the same time, cultures were examined by phase-contrast microscopy for the outgrowth of adherent NLC, and the outgrowth of these cells was measured by counting the adherent cells in 3 different visual fields per patient sample at 200× magnification.
After 14 days, the nonadherent CLL cells were harvested by vigorously pipetting the contents of the well and subsequently rinsing the plates with complete RPMI medium. Harvested cells were washed and then suspended to a concentration of 2.5 × 106 cells/mL in complete RPMI medium. Cells then were plated onto 125-cm2tissue culture plates and incubated for 2 hours at 37°C in 5% CO2 in air to remove any adherent cells by plastic adherence. In the meantime, an aliquot of the harvested cells was examined for expression of CD19, CD3, and CXCR4 and for viability. Afterward the CLL cells again were harvested and suspended in complete RPMI medium to a concentration of 1 × 107 cells/mL. Then CLL cells from each patient sample were divided in half and either plated back onto the adherent NLC or plated onto a fresh 6-well plate without NLC. Aliquots were removed at the indicated time points for viability assays from both culture conditions. The supernatants from CLL PBMC long-term cultures were harvested on day 14 and used as conditioned medium from NLC. Conditioned medium from M2-10B4 cells was generated as described.19 To determine effects of these conditioned media on CLL cell survival, the CLL cells that were separated from NLC on day 14 were cultured without NLC either in fresh complete RPMI medium or complete RPMI medium mixed 1:1 with conditioned medium.
Fluorescence in situ hybridization
We cultured PBMC from a patient with leukemia cells noted to have trisomy 12 in chambered slides for 14 days. Cultures were rinsed with sterile saline, fixed twice in 3:1 methanol:acetic acid, and air-dried. Pretreatment and hybridization were performed by a modification of a previously described protocol.31Briefly, slides were pretreated in 0.75% pepsin solution, dehydrated in ethanol series, and denatured in 70% formamide. Hybridization was performed with directly labeled CEP 12 spectrum orange alpha satellite DNA probes (Vysis, Downers Grove, IL) for 15 to 18 hours, washed, and counterstained with 4′,6-diamine 2′phenylindole dihydrochloride (DAPI II) (Vysis). Nurse-like cells (large nuclei) and CLL cells (small nuclei) were scored for number of fluorescent signals per nucleus for 500 interphase nuclei.
SDF-1 expression of NLC from the blood of patients with CLL
PBMC from patients with CLL were isolated and suspended in complete RPMI medium to a concentration of 1.5 × 107cells/mL and incubated in 75-cm2 tissue culture flasks (Falcon) for 14 to 21 days. After this time, nonadherent lymphoid cells were vigorously washed off; this was followed by 2 washing steps. The complete removal of lymphocytes from the layer of NLC was verified by phase-contrast microscopy.
NLC were lysed in the culture flask, and this was followed by RNA extraction with the Qiagen RNeasy kit as described by the manufacturer (Qiagen, Santa Clarita, CA). RNA then was used for first-strand cDNA synthesis with the SuperScript preamplification system (Gibco BRL, Rockville, MD), according to the manufacturer's instructions. The following human SDF-1β–specific primers were used: 5′ primer, GAG AAT TCA TGA ACG CCA AGG TCG TG; 3′ primer, GAT CTA GAT CAC ATC TTG AAC CTC TTG. A sequenced plasmid containing the human SDF-1β cDNA was used as a positive control. The annealing temperature was 58°C, and the reaction proceeded for 35 cycles. To normalize for the amount of RNA, we performed RT-PCR for human glyceraldehyde-3-phosphate dehydrogenase (GA3PD), as described.32 To exclude that cDNA from residual CLL B cells contributed to the amplification signal, CLL B cells from 4 representative patients were purified with CD19-Dynabeads according to the manufacturer's instructions (Dynal AS, Oslo, Norway); then followed RNA extraction, cDNA synthesis, and RT-PCR for human SDF-1, as described above.
Rescue of CLL cell viability by synthetic SDF-1α
To determine the effect of exogenous SDF-1 on the survival of CLL B cells, we replaced NLC in long-term cultures of CLL B cells 14 days after initiation of the cultures with 500 ng/mL synthetic SDF-1α (Upstate Biotechnology). Subsequently, the viability was monitored and compared to that of the same CLL B cells in wells with or without NLC, as described below.
Measurement of cell death
Determination of CLL cell viability in this study is based on the analysis of mitochondrial transmembrane potential (Δψm) by 3,3′ dihexyloxacarbocyanine iodine (DiOC6) and cell membrane permeability to PI, as described.33 34 For viability assays, 300 μL CLL cell suspension was collected at the indicated time points and transferred to FACS tubes containing 300 μL of 60 nmol/L DiOC6(Molecular Probes) and 10 μg/mL PI (Molecular Probes) in FACS buffer. Cells then were incubated at 37°C for 15 minutes and analyzed within 30 minutes by flow cytometry using a FACSCalibur (Becton Dickinson). Fluorescence was recorded at 525 nm (FL-1) for DiOC6 and at 600 nm (FL-3) for PI. For comparison, CLL cell viability was also examined using the different relative size and granularity (forward scatter and side scatter) characteristics of vital and dead cells.
Immunophenotyping of nurse-like cells
CLL PBMC isolated by density-gradient centrifugation were cultured at 37°C and 5% CO2 for 14 days in sterile 4-well tissue culture-treated microchamber slides (Falcon). For controls, PBMC from healthy donors were purified from buffy coat cells obtained from The San Diego Blood Bank (San Diego, CA). The cells were seeded at a concentration of 1.5 to 2 × 107 cells/mL in RPMI 1640 supplemented with 10% FCS and penicillin–streptomycin–glutamine (1-mL cell suspension per well). After 14 days, the slides were washed with phosphate-buffered saline (PBS) to remove nonadherent cells, and the adherent cells were fixed in ice-cold 4% paraformaldehyde solution for 20 minutes. Immunohistochemical staining was performed according to the manufacturer's instructions using the VECTASTAIN Elite ABC kit (Vector Laboratories, Burlingame, CA). Briefly, after quenching of endogenous peroxidase activity with 0.3% H2O2, the slides were incubated with diluted normal blocking serum from the same species as the secondary antibody. Then the slides were incubated with the primary mAb at 4°C overnight, washed 3 times in PBS, and incubated at room temperature for 1 hour with biotinylated secondary antibody (antimouse IgG or IgM mAb from Vector Laboratories or PharMingen, respectively). The antibody–biotin conjugates were detected with an avidin–biotin–peroxidase complex, applied for 30 minutes at room temperature. A color reaction was developed using 3,3′-diaminobenzidine (DAB), and, finally, specimens were lightly counterstained with hematoxylin (Vector). For controls, slides were incubated with an isotype control Ig of irrelevant specificity, eg, MOPC21 (PharMingen). The slides were examined on a Zeiss Axiophot microscope (Carl Zeiss, Thornwood, NY), and digital images were captured with a Hamamatsu cooled CCD color camera (Hamazatsu, Bridgewater, NJ).
Phospho-p44/42 mitogen-activated protein kinase assay
The p44/42 mitogen-activated protein kinase (MAPK) assays were performed as described.35 Briefly, CLL cells were serum-starved for 2 hours, and then lysates from 1 × 107CLL cells per sample were prepared after stimulation with 200 ng/mL SDF-1α at the indicated time points. Protein content was determined using the Pierce (Rockford, IL) Coomassie Protein Assay Reagent. Equal amounts of protein were separated by polyacrylamide gel electrophoresis (PAGE) and transferred onto nitrocellulose membranes (Bio-Rad Laboratories, Richmond, CA). Western blot analysis was performed using the phospho-p44/42 MAPK rabbit polyclonal antibody Thr202/Tyr204 (New England BioLabs, Beverly, MA) that specifically recognizes the phosphorylated (active), but not the nonphosphorylated, form of p44/42 MAPK protein. Immunoreactive bands were visualized using horseradish peroxidase–conjugated goat-antirabbit secondary antibody (New England BioLabs) and the enhanced chemiluminescence system (ECL; Amersham).
Data analysis, statistics
Results are shown as mean ± SD or SEM of at least 3 experiments each. For statistical comparison between groups, the Student paired t test or the Bonferroni t test was used. Analyses were performed using the Biostatistics software developed by Stanton A. Glantz (University of California at San Francisco, CA). Flow cytometry data were analyzed using the FlowJo software (Tree Star).
Results
Viability of CLL cells on marrow stromal cells and outgrowth of adherent cells from the blood of patients with CLL
To study the effect of marrow stromal cells on spontaneous apoptosis of CLL cells in vitro, we examined the viability of CLL cells in cultures with or without murine marrow stromal cells (M2-10B4) over time. CLL cells plated onto mouse stromal cells retained their initial viability throughout the 14 days of the study (Figure1A). In comparison, CLL cells cultured in flasks without murine stromal cells had an initial decrease (18% ± 9%) in viability over the first 24 hours. Any further reduction in viability over time was minimal (1%-2% decrease; Figure1A). Concomitantly, we noted the outgrowth of adherent cells in such cultures, to which many of the CLL cells were attached. These adherent cells were observed after 3 days in culture, and their numbers increased in the first 5 days (Figure 1B). Thereafter, the number of adherent cells did not significantly increase. However, the cells increased in size and then formed a layer of large, round, adherent, or fibroblast-like cells after 14 days.
Survival of CLL B cells and outgrowth of NLC in vitro.
(A) Marrow stromal cells protect CLL B cells from spontaneous apoptosis in vitro. Presented is the mean relative viability (±SEM) of CLL B cells from 6 patients cultured in the presence (boxes) or absence (diamonds) of the murine marrow stromal cell line M2-10B4. The viability of the CLL cells was determined at the time points indicated. This is displayed as the normalized percentage viability relative to that noted at the initiation of the culture (day 0). (B) Outgrowth of adherent NLC from PBMC of patients with CLL. Cultures of PBMC from 4 representative patients with CLL were examined microscopically for the number of adherent cells at the indicated time points. Lines connect the mean number (±SEM) of adherent cells from each patient counted at 200× magnification in 3 different visual fields at each time point.
Survival of CLL B cells and outgrowth of NLC in vitro.
(A) Marrow stromal cells protect CLL B cells from spontaneous apoptosis in vitro. Presented is the mean relative viability (±SEM) of CLL B cells from 6 patients cultured in the presence (boxes) or absence (diamonds) of the murine marrow stromal cell line M2-10B4. The viability of the CLL cells was determined at the time points indicated. This is displayed as the normalized percentage viability relative to that noted at the initiation of the culture (day 0). (B) Outgrowth of adherent NLC from PBMC of patients with CLL. Cultures of PBMC from 4 representative patients with CLL were examined microscopically for the number of adherent cells at the indicated time points. Lines connect the mean number (±SEM) of adherent cells from each patient counted at 200× magnification in 3 different visual fields at each time point.
Nurse-like cells from the blood of patients protect CLL cells from in vitro apoptosis
Separation of CLL cells from NLC 14 days after the initiation of CLL PBMC long-term cultures resulted in a subsequent continuous decline in the viability of CLL cells from each of 12 patients. These CLL cells showed a reduction in mitochondrial membrane potential (Δψm), which is a characteristic early event during apoptosis.34 However, a reduction in Δψmalone only can be observed for a short time during cell death; therefore, most of the CLL cells undergoing apoptosis had a decreased Δψm and an increased cell membrane permeability to PI, whereas vital cells exclude PI and have a high Δψm(Figure 2A). For comparison, the fraction of CLL cells that had a size and granularity characteristic of vital cells was determined. This was found to be similar to the fraction of vital cells, as determined by DiOC6/PI staining in all cases (Figure 2B).
Blood NLC protect CLL B cells from spontaneous apoptosis in vitro.
(A) Determination of CLL B-cell viability by staining with DiOC6 and PI. Presented are contour maps of CLL B cells from a representative patient defining the relative green (DiOC6) and red (PI) fluorescence intensities of the leukemia cells on the horizontal and vertical axes, respectively. The vital cell population (DiOC6bright, PI exclusion) was determined for CLL cells cultured in the presence (left box) or absence (right box) of NLC. Vital cells were gated as indicated by the polygons with the broken lines. Relative percentage numbers of vital cells are displayed above each of these gates. (B) Determination of CLL B-cell viability as assessed by light scatter. These contour plots show the relative forward-light scatter (or FSC) or granularity (side-light scatter, or SSC) of CLL cells from the same patient sample as displayed in A, cultured with (left box) or without (right box) NLC. Vital cells were gated as indicated by the circles with the broken lines, and the relative percentage numbers of vital cells are indicated above each of these gates. (C) Viability of CLL cells in the presence or absence of NLC. CLL cells from 6 patients were removed from NLC in long-term CLL B-cell cultures 14 days after seeding, and the leukemia cells were plated in either wells with NLC (squares) or without NLC (diamonds). Displayed are the mean percentage viability values (±SEM) of leukemia cells assessed at the indicated time points relative to those noted at initiation of culture on day 0.
Blood NLC protect CLL B cells from spontaneous apoptosis in vitro.
(A) Determination of CLL B-cell viability by staining with DiOC6 and PI. Presented are contour maps of CLL B cells from a representative patient defining the relative green (DiOC6) and red (PI) fluorescence intensities of the leukemia cells on the horizontal and vertical axes, respectively. The vital cell population (DiOC6bright, PI exclusion) was determined for CLL cells cultured in the presence (left box) or absence (right box) of NLC. Vital cells were gated as indicated by the polygons with the broken lines. Relative percentage numbers of vital cells are displayed above each of these gates. (B) Determination of CLL B-cell viability as assessed by light scatter. These contour plots show the relative forward-light scatter (or FSC) or granularity (side-light scatter, or SSC) of CLL cells from the same patient sample as displayed in A, cultured with (left box) or without (right box) NLC. Vital cells were gated as indicated by the circles with the broken lines, and the relative percentage numbers of vital cells are indicated above each of these gates. (C) Viability of CLL cells in the presence or absence of NLC. CLL cells from 6 patients were removed from NLC in long-term CLL B-cell cultures 14 days after seeding, and the leukemia cells were plated in either wells with NLC (squares) or without NLC (diamonds). Displayed are the mean percentage viability values (±SEM) of leukemia cells assessed at the indicated time points relative to those noted at initiation of culture on day 0.
Before the assessment of CLL cell viability, the immunophenotype of the cells was determined using anti-CD19 and anti-CD3 mAbs on the initial day of the long-term cultures (d0), and 2 weeks later (d14), when CLL cells were removed from the adherent NLC. Figure 2C displays the viability of CLL cells from a representative experiment, in which CLL cell viability was monitored for 12 days either after their separation from the NLC (Figure 2C, diamonds; n = 6) or after they were replated onto NLC (Figure 2C, boxes; n = 6). Cells recovered from long-term cultures were predominantly CLL B cells (97.4% ± 1.8%; n = 6), with only a few detectable T cells (1.7% ± 1.7%; n = 6). Therefore, it can be assumed that the viability data presented in Figure 2C reflect the viability of the CLL B cells. The viability of CLL cells replated onto NLC remained stable over time (Figure 2C, boxes), whereas the same CLL cells without NLC had a continuous decrease in viability. Mean relative viability (±SEM) by day 12 in cultures without NLC decreased to 21% ± 7%, compared to the viability of the CLL cells at the beginning of the culture, whereas the mean relative viability (105% ± 6%) in cocultures with NLC did not decrease during this time. Conditioned media from CLL PBMC cultures did not improve the viability of CLL cells separated from NLC (n = 6) at any time point examined (data not shown).
Immunophenotyping of nurse-like cells from the blood of patients with CLL
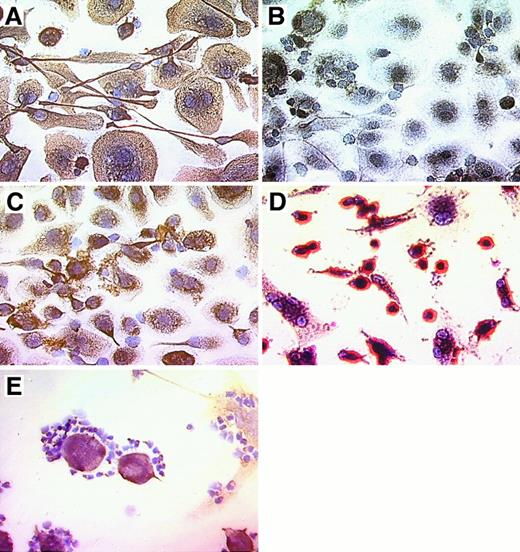
Nurse-like cells derived from the blood of patients with CLL uniformly bound mAb specific for the stromal cell marker vimentin (Figure 3A,E), whereas no staining was observed for samples incubated with a control mAb of irrelevant specificity, MOPC-21 (Figure 3B). Moreover, the NLC weakly stained with the STRO-1 IgM mAb. This mAb recognizes stromal cells that have the capacity to recapitulate the hematopoietic microenvironment in vitro30 (Figure 3C). In contrast, NLC were negative for CD3, CD19, CD83, and VCAM-1 (CD106) (data not shown). Nurse-like cells express CD68, a member of a family of acidic, highly glycosylated, lysosomal-associated membrane proteins (data not shown). The morphology and immunophenotype of adherent cells from the blood of healthy donors were different from the adherent NLC from CLL blood samples. Here, a population of smaller cells that were strongly positive for CD14 accounted for most of the adherent cells, whereas cells with the morphology and phenotype of NLC were infrequent (Figure 3D). Figure 3E demonstrates the attachment of vimentin-negative CLL cells to vimentin-positive NLC.
Phenotype of NLC derived from the blood of patients with CLL.
CLL B cells from representative patients were cultured for 14 to 21 days, and nonadherent cells were vigorously (A-D) or carefully (E) removed before immunohistochemical examination. Cells were stained with antibody–biotin conjugates that subsequently were developed with an avidin–biotin–peroxidase complex and DAB, resulting in a brown-red stain for positive cells. Specimens were lightly counterstained with hematoxylin (Vector) and were photographed at 400× magnification. Displayed are pictures of NLC derived from the blood of patients with CLL (A-C, E) or adherent cells derived from the blood of a normal adult donor (D). Adherent NLC from the blood of patients with CLL expressed stromal cell markers vimentin (A, E) and STRO-1 (C). CLL B cells, on the other hand, did not express vimentin (E). Control staining with the MOPC-21 mAb of irrelevant specificity was negative (B). In contrast, most adherent cells derived from the blood of normal adult donors (D) were smaller and strongly positive for CD14. The close physical interaction of NLC and CLL B cells is demonstrated in (E), where vimentin-negative CLL cells are attached to vimentin-positive NLC. This is typically seen in long-term cultures of CLL B cells, suggesting a close, symbiotic relationship of these 2 cell types.
Phenotype of NLC derived from the blood of patients with CLL.
CLL B cells from representative patients were cultured for 14 to 21 days, and nonadherent cells were vigorously (A-D) or carefully (E) removed before immunohistochemical examination. Cells were stained with antibody–biotin conjugates that subsequently were developed with an avidin–biotin–peroxidase complex and DAB, resulting in a brown-red stain for positive cells. Specimens were lightly counterstained with hematoxylin (Vector) and were photographed at 400× magnification. Displayed are pictures of NLC derived from the blood of patients with CLL (A-C, E) or adherent cells derived from the blood of a normal adult donor (D). Adherent NLC from the blood of patients with CLL expressed stromal cell markers vimentin (A, E) and STRO-1 (C). CLL B cells, on the other hand, did not express vimentin (E). Control staining with the MOPC-21 mAb of irrelevant specificity was negative (B). In contrast, most adherent cells derived from the blood of normal adult donors (D) were smaller and strongly positive for CD14. The close physical interaction of NLC and CLL B cells is demonstrated in (E), where vimentin-negative CLL cells are attached to vimentin-positive NLC. This is typically seen in long-term cultures of CLL B cells, suggesting a close, symbiotic relationship of these 2 cell types.
Fluorescence in situ hybridization for trisomy 12
Leukemia cells of one patient had trisomy 12, allowing us to examine whether this genetic abnormality also was present in the population of NLC. NLC and CLL cells from this patient were examined by fluorescence in situ hybridization (FISH) analysis, using chromosome 12–specific alpha satellite DNA probes on the nuclei of cells in long-term culture. CLL and NLC cells could be distinguished from each other by nuclear size and morphology, the NLC displaying a 2-fold larger nuclear diameter and a more oblong appearance (Figure4). Trisomy 12 was clearly detectable in the CLL population by FISH analysis and was not observed in the overwhelming majority of NLC from the same patient (Figure 4). Evaluation of the signal distribution in 500 small nuclei revealed 75.6% had 3 fluorescent signals, 18.8% had 2 signals, 3.8% had 1 signal, and 1.4% had no observable signal. Evaluation of 500 larger nuclei revealed 4.6% had 3 signals, 89.4% had 2 signals, 4.2% had 1 signal, and 0.6% had no signal. These findings show that the NLC and CLL populations do not share identical chromosomal complements, indicating that the NLC are not part of the CLL clone.
Fluorescence in situ hybridization for trisomy 12.
Depicted are the nuclei of NLC (large ovals) and CLL cells (small circles) examined for trisomy 12 by FISH. The 2 large NLC nuclei have only 2 bright fluorescence signal spots, whereas the 4 CLL cell nuclei each have 3 bright signal spots, reflecting the presence of trisomy 12.
Fluorescence in situ hybridization for trisomy 12.
Depicted are the nuclei of NLC (large ovals) and CLL cells (small circles) examined for trisomy 12 by FISH. The 2 large NLC nuclei have only 2 bright fluorescence signal spots, whereas the 4 CLL cell nuclei each have 3 bright signal spots, reflecting the presence of trisomy 12.
Nurse-like cells induce down-modulation of CXCR4 on CLL cells in vitro
We evaluated the surface immunophenotype of CLL B cells before and after 14 days of coculture with NLC. Leukemia cells were noted to retain the expression of B-cell surface antigens such as CD19. However, there was marked reduction in the staining of CLL B cells for CXCR4 after 14 days in culture. The mean fluorescence intensity ratio (MFIR) of CLL B cells in long-term culture with NLC was significantly lower (MFIR, 18.6 ± 4; n = 8) than that of CLL B cells at the time of initiation of the cultures (MFIR, 303 ± 49; n = 8). Figure5 displays anti-CXCR4 and isotype control stains of CLL B cells from 2 representative patients before and after coculture with NLC. However, the removal of CLL cells from NLC allowed the return of CXCR4 expression to levels comparable to pretreatment levels.
Down-regulation of CXCR4 on CLL B cells cultured with NLC.
Histograms of leukemia B cells from 2 representative patients with CLL, indicating the relative red (anti-CXCR4) fluorescence intensity of CLL cells before (shaded) or after coculture with NLC (bold lines). Thin lines represent the isotype control staining. MFIR values are displayed next to each histogram.
Down-regulation of CXCR4 on CLL B cells cultured with NLC.
Histograms of leukemia B cells from 2 representative patients with CLL, indicating the relative red (anti-CXCR4) fluorescence intensity of CLL cells before (shaded) or after coculture with NLC (bold lines). Thin lines represent the isotype control staining. MFIR values are displayed next to each histogram.
Nurse-like cells express mRNA for stromal cell–derived factor-1
SDF-1 is a potent chemoattractant for CLL B cells and an important mediator in cellular interaction of CLL cells with marrow stromal cells.19 CXCR4 is the only receptor for this chemokine. SDF-1 is expressed and secreted by marrow stromal cells19,21,22 but not by blood leukocytes.36 37 We found that NLC isolated from the cells of each of 4 different patients with CLL expressed SDF-1 mRNA (Figure6A). In contrast, we did not detect SDF-1 expression in cDNA from purified CLL B cells from any of 4 patients.
SDF-1 mRNA expression by NLC and p44/42 mitogen-activated protein kinase (Erk 1/2) activation in CLL B cells by SDF-1α.
(A) cDNA from purified NLC from 4 patients with CLL was examined for the expression of SDF-1 mRNA by RT-PCR. The specific 230-bp PCR fragment is visible in each of the 4 NLC samples (lanes 2-5), and in the positive control, namely an SDF-1–containing plasmid (lane 6). Lanes 7-11 display PCR fragments of the expected size using GA3PD primers for the NLC samples (lanes 7-10) but not for the SDF-1 plasmid control. Lanes 1 and 12 display the separation of the 100-bp marker DNA, and the 200, 600, and 1000 bp bands are marked on the left side. (B) p44/42 MAPK activation in CLL B cells treated with SDF-1α at 200 ng/mL. CLL cell lysates were obtained at the time points indicated on the horizontal axis and examined for phospho-p44/42 MAPK protein by Western blot analysis. Protein bands of the expected sizes of 42 and 44 kd, as indicated on the left, were prominent after the stimulation of CLL cells with SDF-1α, whereas they were only weakly apparent before activation by SDF-1 (time 0).
SDF-1 mRNA expression by NLC and p44/42 mitogen-activated protein kinase (Erk 1/2) activation in CLL B cells by SDF-1α.
(A) cDNA from purified NLC from 4 patients with CLL was examined for the expression of SDF-1 mRNA by RT-PCR. The specific 230-bp PCR fragment is visible in each of the 4 NLC samples (lanes 2-5), and in the positive control, namely an SDF-1–containing plasmid (lane 6). Lanes 7-11 display PCR fragments of the expected size using GA3PD primers for the NLC samples (lanes 7-10) but not for the SDF-1 plasmid control. Lanes 1 and 12 display the separation of the 100-bp marker DNA, and the 200, 600, and 1000 bp bands are marked on the left side. (B) p44/42 MAPK activation in CLL B cells treated with SDF-1α at 200 ng/mL. CLL cell lysates were obtained at the time points indicated on the horizontal axis and examined for phospho-p44/42 MAPK protein by Western blot analysis. Protein bands of the expected sizes of 42 and 44 kd, as indicated on the left, were prominent after the stimulation of CLL cells with SDF-1α, whereas they were only weakly apparent before activation by SDF-1 (time 0).
SDF-1α induces activation of the p44/42 MAPK signal transduction pathway
Engagement of CXCR4 by SDF-1α induces the rapid transient activation of the p44/42 MAPK signaling pathway in CXCR4-expressing cell lines.38 39 A rapid and robust activation of p44/42 MAPK was observed on the addition of SDF-1α to CLL cells from each of 6 patients. Figure 6, panel B shows a representative time course of p44/42 MAPK activation after stimulation with 100 ng/mL SDF-1α.
Synthetic SDF-1α rescues CLL B cells from apoptosis in vitro
We examined whether SDF-1 participates in mediating survival signals from NLC to CLL B cells. Experiments were performed in which the NLC were replaced with synthetic SDF-1α. We initiated the long-term cultures of CLL PBMC, as described above. On day 14, nonadherent CLL cells were removed from the adherent NLC. The CLL cells were divided into equal parts and then incubated under 3 different conditions. They were either plated back onto the NLC, plated into wells without NLC, or plated into wells without NLC but supplemented with 500 ng/mL synthetic human SDF-1α. Cell viability was observed thereafter at the times indicated.
The viability of CLL cells cultured without NLC was significantly greater in cultures supplemented with SDF-1α than in cultures without this chemokine (Figure 7A). The mean relative viability of CLL cells without NLC 1 day after separation from the NLC (d1) was 71.1% ± 2.7% (n = 6), whereas the mean viability of the same samples in cultures supplemented with SDF-1α was 84.5% ± 3.1% (n = 6; P = .009). However, the addition of SDF-1α to cultures without NLC did not completely protect CLL cells from apoptosis, compared to CLL cells plated back onto the NLC (93.5% ± 2.9% at d1;P = .06).
Protection of CLL B cells from spontaneous apoptosis in vitro is partially mediated by SDF-1.
(A) Synthetic SDF-1α could protect CLL cells from spontaneous apoptosis. Displayed is the mean relative viability of CLL cells from each of 6 representative CLL patients cultured with (squares) or without (circles and diamonds) NLC. Cultures without NLC were supplemented with SDF-1α at 500 ng/mL on day 0 (circles) or were cultured in medium alone (diamonds). Viability was assessed at the times indicated on the horizontal axis. Without NLC, leukemia cells cultured with SDF-1α had significantly higher viability than did leukemia cells cultured without SDF-1 (P < .005; Student t test). Nonetheless, the viability of CLL cells cultured without NLC and SDF-1α (circles) was less than that of CLL cells cultured with NLC (boxes). (B) Anti–SDF-1 antibody inhibits the survival of CLL B cells in cultures with NLC. CLL cells were separated from NLC as described and were replated into wells with (solid symbols) or without (open symbols) NLC. To wells with or without NLC, anti–SDF-1 antibody at 10 μg/mL (circles) or 1 μg/mL (triangles), or a control IgG (squares), was added at day 0. Viability was subsequently determined for each of these conditions at the time points indicated on the horizontal axis. Displayed are the mean (±SD) viability values of samples from each of 3 representative patients.
Protection of CLL B cells from spontaneous apoptosis in vitro is partially mediated by SDF-1.
(A) Synthetic SDF-1α could protect CLL cells from spontaneous apoptosis. Displayed is the mean relative viability of CLL cells from each of 6 representative CLL patients cultured with (squares) or without (circles and diamonds) NLC. Cultures without NLC were supplemented with SDF-1α at 500 ng/mL on day 0 (circles) or were cultured in medium alone (diamonds). Viability was assessed at the times indicated on the horizontal axis. Without NLC, leukemia cells cultured with SDF-1α had significantly higher viability than did leukemia cells cultured without SDF-1 (P < .005; Student t test). Nonetheless, the viability of CLL cells cultured without NLC and SDF-1α (circles) was less than that of CLL cells cultured with NLC (boxes). (B) Anti–SDF-1 antibody inhibits the survival of CLL B cells in cultures with NLC. CLL cells were separated from NLC as described and were replated into wells with (solid symbols) or without (open symbols) NLC. To wells with or without NLC, anti–SDF-1 antibody at 10 μg/mL (circles) or 1 μg/mL (triangles), or a control IgG (squares), was added at day 0. Viability was subsequently determined for each of these conditions at the time points indicated on the horizontal axis. Displayed are the mean (±SD) viability values of samples from each of 3 representative patients.
Antibody to SDF-1 inhibits the protection of CLL B cells from spontaneous apoptosis by NLC
We examined whether antibodies to SDF-1 could inhibit the effect of NLC on the survival of CLL B cells in vitro. CLL B cells from long-term cultures were separated from NLC, as described above, and replated into multiple wells that did or did not contain NLC. At the same time, anti–SDF-1 antibody or control immunoglobulin was added to separate wells, and viability was examined at the time points indicated (Figure 7B).
The viability of CLL cells cultured on NLC was significantly reduced by the addition of anti–SDF-1 but not the control antibody in all cases. In contrast, the addition of anti–SDF-1 antibody did not significantly change the viability of CLL cells in samples without NLC (Figure 7B).
Discussion
The initial aim of this study was to determine the factors involved in regulating the survival of CLL cells in vitro when cultured on marrow stromal cells. CLL cells cultured on murine marrow stromal cells retain their high viability for 14 days (Figure 1A). This is similar to the findings of previous studies using human marrow stromal cells to protect CLL cells from spontaneous apoptosis.6 9Our observation that murine stromal cells protect CLL cells from apoptosis indicates that factors responsible for this protective effect can function across species barriers. This excludes several hematopoietic factors, such as interleukin-4 and granulocyte–macrophage colony-stimulating factor, which have species-restricted activities, from being essential to the protective effects of marrow stromal cells for leukemia B cells in vitro.
When mononuclear cells from the blood of patients with CLL were cultured without stromal cells, we were surprised to observe a relatively stable viability after an initial decrease in the first 24 to 48 hours (Figure 2C). This was associated with an outgrowth of adherent cells to which CLL B cells became attached and that protected the leukemia cells from spontaneous apoptosis in vitro. When the CLL cells were removed from these cells, the CLL B cells experienced a rapid decline in viability, whereas the same CLL B cells retained their initial viability when replated onto the NLC. Because the adherent cells supported the survival of CLL cells in vitro and CLL B cells became attached to them, we called them nurse-like cells, or NLC.
Nurse cells were first recognized in situ in the thymus, where they form characteristic complexes with immature T lymphocytes and play an important role in thymocyte maturation and differentiation.40 This cellular interaction is characterized by the active invasion into thymic nurse cells by thymocytes (emperipolesis). In vitro, it has been recognized that T- or B-lineage cells can spontaneously migrate beneath adherent cells derived from long-term marrow cultures41,42 or dermal tissue43 or from the synovium of patients with rheumatoid arthritis.44 Although lymphocytes crawl under these cells, they do not become internalized. As such, this process is called pseudo-emperipolesis, and the supporting cells are termed nurse-like cells. The close physical interaction and the capacity to support the survival and differentiation of lymphocytes are the main characteristics of NLC. These 2 features were also noted for the interaction between CLL B cells and the NLC described in this study. However, whereas many of the CLL B cells became attached to the NLC, they did not display the characteristic appearance of pseudo-emperipolesis by phase-contrast microscopy. This may be owing to the observed lack of CD106 (VCAM-1) on NLC derived from the blood of patients with CLL because interaction between CD106 and its respective ligand on lymphocytes (VLA-4 or CD49d) plays an important role in mediating pseudo-emperipolesis.41 44
When cultured, the PBMC from patients with CLL developed abundant numbers of NLC that became the predominant population of adherent cells. In contrast, PBMC of healthy donors rarely generated such NLC when cultured under identical conditions. This may be because of a difference between the blood of patients with CLL and that of healthy donors in the relative proportion of cells that can give rise to such NLC. Patients with CLL may have greater numbers of circulating NLC progenitor cells, possibly secondary to the infiltration of the marrow by leukemia B cells. Alternatively, CLL cells may elaborate factors, such as transforming growth factor-beta (TGF-β), that could support the outgrowth of NLC in vitro. TGF-β is secreted by CLL cells at high concentrations45 and is known to be a potent differentiation factor for stromal cells.46 47 As such, the CLL cells may contribute to the distinct phenotype of the adherent cells in CLL long-term cultures as a result of a symbiotic relationship between NLC and CLL cells in vitro. In any case, we cannot explain the abundance of NLC in CLL blood cultures with a model proposing that such cells are clonally related to the CLL cells or are derived from a common transformed precursor cell. In addition to the finding that NLC do not express B-cell differentiation antigens, NLC do not share cytogenetic abnormalities with the CLL B-cell clone, as demonstrated by our studies on the patient with leukemia cells that had trisomy 12 (Figure 4).
Phenotypic characterization of CLL NLC suggests they are related to marrow stromal cells. These cells lack expression of B-cell or T-cell differentiation antigens and do not express CD83, a marker of mature dendritic cells.48 On the other hand, NLC are noted to express stromal cell markers, such as vimentin and STRO-1. Moreover, NLC express mRNA for SDF-1 and support CLL B-cell survival through the action of this chemokine. Marrow stromal cells are known to be an important source of SDF-1,21,26 whereas PBMC do not constitutively express this chemokine.36,37 Moreover, it also is recognized that stromal cells derived from the marrow17,18,49-51 or extramedullary sites44 can support B-cell survival and differentiation. In view of these reports, it is tempting to speculate that the blood-derived NLC described in this study are derived from circulating immature stromal cells. However, further studies are required to establish this relationship.
The initial decrease in CLL cell viability in the first 24 hours and the lack of microscopically identifiable NLC during the initial 2 to 3 days of culture suggest that NLC are functionally immature in the bloodstream and gain their capacity to “nurse” CLL cells during in vitro differentiation. Therefore, mature counterparts of NLC are likely to play a role in protecting CLL B cells from apoptosis in distinct lymphoid (and nonlymphoid) tissue microenvironments rather than in the bloodstream. Trafficking of CLL B cells between the blood and the marrow or the lymphoid tissues is a new concept that now is receiving increased attention. The finding that CLL B cells express functional chemokine receptors19,52,53 implies that CLL B cells can actively migrate to the marrow and to secondary lymphoid tissues, where an interaction with NLC may occur. In such microenvironments, CLL B cells are likely to encounter surface-bound SDF-1 on marrow stroma21 or reticulum cells in secondary lymphoid tissues.54 CLL cells would be expected to down-regulate the expression of CXCR4 receptors within these tissue microenvironments rather than when they are circulating freely in the blood.19 55
We recently characterized the chemokine SDF-1 as a potent chemoattractant for CLL cells that is required for the spontaneous migration of CLL cells beneath marrow stromal cells in vitro.19 The attachment of CLL B cells to the surface of NLC, the expression of SDF-1 mRNA by NLC, and the down-modulation of CXCR4 on CLL cells cultured on NLC suggest that SDF-1, made by NLC, plays a role in the interaction between these cell types. From our earlier studies, it appears that the role of SDF-1 lies in attracting CLL cells, leading to the characteristic picture of NLC surrounded by CLL cells (Figures 1C, 3E). However, the current study demonstrates that SDF-1 also can function as a CLL B-cell survival factor that may play a role in the microenvironmental regulation of resistance to apoptosis.
SDF-1 is a highly conserved chemokine that has 99% homology between mouse and human, which allows for its action across species barriers. Because it also is a growth factor for stromal cell–dependent B-lineage cells,21,29 we hypothesized that SDF-1 may play a dual role in the interaction between NLC and CLL B cells, functioning not only as a chemoattractant but also as a “nursing” factor. Lagneaux et al6 earlier noted that CLL cell survival on marrow stromal cells was dependent on close contact because CLL B cells underwent apoptosis when separated from stromal cells by a micropore filter. These authors reasoned that hematopoietic cytokines might not play a major role in protecting CLL cells from apoptosis in such cultures. However, soluble factors were not rigorously excluded in these experiments, because cytokines can be retained on the surfaces of stromal cells and thus may be effective only within a short range. Growth factors bound to heparan sulfate on the surfaces of marrow stromal cells are the biologically relevant forms of hematopoietic cytokines in the marrow microenvironment.56 SDF-1 is a highly basic protein that binds through a cluster of basic amino acids in the first β-strand to cell membrane heparan sulfates, leaving the N-terminal signaling domain exposed for interaction with its receptor, CXCR4.57 As such, a proteoglycan-bound SDF-1, immobilized on the surface of cells, appears to be the functional form of this chemokine in vivo.57 This may explain why conditioned medium from NLC cells did not significantly improve the survival of CLL cells in vitro, as also noted earlier for stromal cell–conditioned medium.6 Nevertheless, the observations that synthetic SDF-1α could inhibit the spontaneous apoptosis of CLL cells that were separated from NLC in vitro and that antibodies against SDF-1α inhibited the protective effect of NLC for CLL cells indicate that SDF-1 can function as a survival factor for CLL cells in vitro.
Consistent with this, we found that the stimulation of CLL cells with synthetic SDF-1α induced rapid, transient activation of p44/42 MAPK (ERK1/2), a key signaling pathway for promoting cell survival through transcription-dependent and -independent mechanisms.58,59Through MAPK-activated effector molecules (Rsks), activation of the MAPK promotes cells survival directly by inactivating the pro-apoptotic BAD protein, and indirectly by activating the transcription factor CREB, which is important for the transcriptional up-regulation of the anti-apoptotic Bcl-2 gene.60 These observations suggest that SDF-1 on stromal cells or NLC engages CLL B cells through CXCR4 and thereby affects components of the cell death machinery, leading to the noted resistance of CLL cells to apoptosis.3,4 16
Future studies will have to define whether additional factors, such as integrin receptors, have a role in mediating adhesion or survival signals between B lymphocytes and respective stromal cell ligands, such as fibronectin and VCAM-1 (CD106). Signals from integrin receptors can synergize with those induced by cytokines, such as SDF-1, in regulating the organization of the cytoskeleton, transcriptional activation, and cell survival or proliferation.61
In summary, this study demonstrates that CLL cell survival in vitro can be regulated by blood-derived NLC that protect CLL B cells from apoptosis through an SDF-1–dependent mechanism. In this symbiotic system, the chemokine SDF-1 functions not only as a CLL cell chemoattractant but also as a survival factor for CLL cells. As such, this study provides a new mechanism by which accessory cells can regulate the survival of neoplastic B cells even outside the marrow microenvironment. Future studies may define whether substances that inhibit interactions between NLC and CLL B cells affect the survival of CLL cells in vitro and in vivo. Such approaches could lead to new therapeutic avenues for patients with B-cell CLL.
Acknowledgments
We thank Dr James R. Feramisco for assistance with the preparation of the photomicrographs. We also thank Diane A. Nguyen and T. A. Johnson for their excellent technical assistance.
Supported in part by Deutsche Krebshilfe grant D-96-17136 (J.A.B.), Deutsche Forschungsgemeinschaft grant SA 623/2-1 (M.B.), and National Institutes of Health grants 5R37-CA49870-11 and PO1-CA81534 (T.J.K.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Thomas J. Kipps, Division of Hematology/Oncology, School of Medicine, University of California at San Diego, 9500 Gilman Drive, La Jolla, CA 92093-0663; e-mail: tkipps@ucsd.edu.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal