Abstract
Glycoprotein (GP) IIb-IIIa plays a critical role in platelet aggregation and platelet-mediated clot retraction. This study examined the intramolecular relationship between GPIIb-IIIa activation and fibrinogen binding, platelet aggregation, and platelet-mediated clot retraction. To distinguish between different high-affinity activation states of GPIIb-IIIa, the properties of an antibody (D3) specific for GPIIIa that induces GPIIb-IIIa binding to adhesive protein molecules and yet completely inhibits clot retraction were used. Clot retraction inhibition by D3 was not due to altered platelet-fibrin interaction; however, combination treatments of D3 and adenosine diphosphate (ADP) inhibited full-scale aggregation and decreased the amounts of GPIIb-IIIa and talin incorporated into the core cytoskeletons. Morphologic evaluation of the D3/ADP aggregates showed platelets that were activated but to a lesser extent when compared to ADP only. ADP addition to platelets caused an increase in the number of D3 binding sites indicating that ligand had bound to the GPIIb-IIIa receptor. These data suggest that high-affinity GPIIb-IIIa– mediated ligand binding can be separated mechanistically from GPIIb-IIIa–mediated clot retraction and that clot retraction requires additional signaling through GPIIb-IIIa after ligand binding. The conformation recognized by D3 represents the expression of a GPIIb-IIIa activation state that participates in full-scale platelet aggregation, cytoskeletal reorganization, and clot retraction.
Introduction
The primary function of platelets is to initiate repair to damaged vessels beginning with platelet adherence to the exposed subendothelium and culminating in fibrin clot retraction. When the endothelium is damaged, platelets adhere to adhesive proteins in the subendothelial matrix, spread on the surface, become activated, and secrete their granular contents. Components released from the platelets may activate additional platelets that aggregate and form a growing thrombus.1-3 The recruitment of additional platelets into a thrombus is a result of fibrinogen binding to its activated receptor, glycoprotein (GP) IIb-IIIa. The bound ligand cross-links receptors on adjacent platelets through its multivalent structure.4
Once a thrombus has formed, wound healing is promoted by plugging the damaged vessel wall, arresting bleeding, and retracting the resultant clot to facilitate wound closure. Platelets mediate clot retraction as ligand-bound GPIIb-IIIa on extended pseudopodia interact with the cytoskeleton and generate tensile forces mainly associated with platelet-fibrin interactions.5-7
A member of the integrin family of proteins, GPIIb-IIIa is a heterodimeric glycoprotein complex found on the platelet surface and in the α granules.4,8,9 When platelets are activated, GPIIb-IIIa is also activated and binds fibrinogen.10-12The initial binding of fibrinogen is reversible but undergoes a time-dependent stabilization.13 The GPIIb-IIIa complex can also be converted to an active form that binds fibrinogen without conventional platelet agonists. This GPIIb-IIIa activation occurs through the binding of unique complex activating monoclonal antibodies (mAbs). D3, an anti-GPIIIa mAb, causes fibrinogen binding to GPIIb-IIIa through the propagation of a conformational change in the receptor molecule.14 D3 also preferentially binds to ligand-induced binding sites (LIBS) on GPIIb-IIIa. LIBS are regions on the receptor that become exposed after ligand binding and modulate secondary platelet function by shifting the conformational equilibrium in the presence of ligand.15Some of the affected functions include the secondary wave of platelet aggregation (during which secretion was observed),15platelet adhesion to collagen,16 and clot retraction.17
These studies were designed to explore the involvement of the LIBS recognized by D3 in clot retraction and related events such as platelet binding to fibrin(ogen), full-scale, irreversible platelet aggregation, and as a result of this aggregation, alterations in platelet cytoskeletal composition. D3 strongly inhibited clot retraction, and although D3 enhanced platelet binding to fibrinogen, it did not either inhibit or enhance platelet binding to immobilized fibrin. The addition of D3 to adenosine diphosphate (ADP)-treated platelets decreased their aggregation response and altered cytoskeletal reorganization by decreasing the amount of talin in the isolated cytoskeletal cores. This addition also reduced the amount of GPIIb-IIIa incorporated into the cytoskeleton. These results suggest that the ligation of a GPIIb-IIIa LIBS has critical involvement in coordinating irreversible postreceptor occupancy events.
Materials and methods
D3 monoclonal antibody
D3 is a GPIIIa-specific IgG1k antibody that activates the GPIIb-IIIa complex and induces fibrinogen binding to platelets in a manner independent of platelet activation.18 In addition, D3 binding is increased by occupying GPIIb-IIIa with either fibrinogen, von Willebrand factor or ligand recognition sequence (RGDS) peptides.19 D3 was purified from culture supernatant or ascites by MAPS II chromatography (BioRad, Richmond, CA) that uses immobilized protein A to purify IgG1 antibodies. Purity was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Clot retraction studies
Whole blood (5 mL) was placed in glass tubes along with D3 or control mouse IgG and incubated at 37°C for 24 hours. The clot was removed and residual serum and red blood cells were centrifuged at 1500g for 10 minutes. The serum volume was recorded and the percent fluid volume in the clot (%FVC) was calculated as follows: [(total volume − serum volume )/ total volume ) × 100] − hematocrit = % FVC.
Platelet binding to fibrin(ogen)
Six parts blood were drawn into 1 part ACD (0.05 mol/L sodium citrate, 0.1 mol/L dextrose, and 0.07 mol/L citric acid) and centrifuged at 135g for 20 minutes. Platelets were separated from plasma by centrifugation at 850g, washed twice with CGS (10 mmol/L trisodium citrate, 30 mmol/L dextrose, and 120 mmol/L NaCl, pH 6.5) and resuspended at 2.5 × 108/mL in modified Tyrode buffer (138 mmol/L NaCl, 2.9 mmol/L KCl, 12 mmol/L NaHCO3, 0.36 mmol/L NaH2PO4, 1.8 mmol/L CaCl2, 0.4 mmol/L MgCl2, 0.1% bovine serum albumin [BSA], and 5.5 mmol/L dextrose, pH 7.4). Platelet surface proteins were 125I-labeled using Na125I (New England Nuclear, Boston, MA) and the lactoperoxidase catalyzed iodination procedure as previously described.20 Multiwell culture plates (Becton Dickinson, Lincoln Park, NJ) were coated with 20 μg/well fibrinogen (prepared in our laboratory with modification of Weathersby and colleagues21) for 2 hours at 37°C. Some of the wells were then treated with 200 ng/well thrombin (generously donated by Dr John Fenton II, Albany, NY) for 10 minutes at 37°C and then 400 ng/well hirudin (Sigma Chemical, St. Louis, MO) for 10 minutes at 37°C. All wells were washed with CM-PBS (phosphate-buffered saline with 1 mmol/L CaCl2 and 0.5 mmol/L MgCl2) and blocked overnight using 2.5 mg/mL BSA in CM-PBS. Platelets (1 × 108/well) labeled with 125I were added to plates in the presence of D3 or mouse IgG (100 μg/well) and incubated for 10 minutes at 37°C. After 3 washes with CM-PBS, adherent platelets were solubilized with 2% SDS and counted using an LKB Wallac 1282 Compugamma counter (LKB Instruments, Inc, Gaithersberg, MD).
D3/ADP aggregation studies
Blood was drawn using the anticoagulant 0.109 mol/L buffered citrate or 3.0 mmol/L phenylalanine-proline-arginine chloromethyl ketone (PPACK, Calbiochem, San Diego, CA) at a ratio of 9:1. Platelet-rich plasma (PRP) was obtained by centrifugation at 135g for 20 minutes. The platelet count was adjusted to 2.5 × 108/mL with autologous platelet-poor plasma (PPP) obtained by centrifugation of residual red cells at 1500gfor 20 minutes. Aliquots of 0.45 mL PRP were incubated in a Payton aggregometer (Buffalo, NY) with various combinations of saline (50 μL), control mouse IgG (50 μg), or D3 (50 μg) and ADP or arachidonic acid (AA, 500 μg/mL, Helena Laboratories, Beaumont, TX). Platelets were either untreated or pretreated with aspirin (ASA, 0.2 mmol/L) or its carrier ethanol (EtOH). The ADP concentration was chosen as one that just gave a biphasic aggregation response in the buffered citrate anticoagulant. This concentration was subject to donor variability but ranged from 1 to 3 μmol/L. In some experiments, the platelets were preincubated with antibody or saline for 2 minutes unstirred before the addition of ADP or AA; in others, D3 and ADP were preincubated together before stirring was initiated. Another course of treatment included the simultaneous addition of saline or antibody with ADP. These components were added to the stirring aliquot and the light transmittance through the stirring platelets was recorded by a chart recorder. The maximal percentage of light transmittance was calculated within 6 minutes.
Lumiaggregometry
Lumiaggregometry was performed according to the method of White and coworkers.22 Adenosine triphosphate (ATP) assay mix (firefly luciferin/luciferase reagent [FFE]) and ATP standard were obtained from Sigma Chemical. The aggregometer was standardized for 100% light transmittance using PPP. PRP was pipetted into a reaction cuvette and prewarmed to 37°C for at least 2 minutes. FFE was added to the PRP and 0% light transmission for aggregation was set. To run the complete standard curve, 3 concentrations of ATP were assayed in duplicate. The maximum number of pen deflections registered for each standard was recorded. Duplicate values were averaged and a standard curve constructed on a linear scale with the ordinate representing pen deflections, and the abscissa, nanomoles ATP. To measure platelet release, PRP was mixed with FFE, the peak number of pen deflections obtained with various agonists was determined, and nanomoles ATP released was read directly from the standard curve.
Radiolabeled D3 binding studies
D3 was radiolabeled with 125I (New England Nuclear) for binding studies. Iodo-beads iodination reagent (Pierce Chemical Co, Rockford, IL) was used to label the antibody. Labeled antibody was separated from free iodine by gel filtration through a Sephadex G-25M column (Pharmacia, Uppsala, Sweden). The concentration of labeled antibody was determined by measuring absorption at 280 nm using an extinction coefficient of 1.5. The PRP was added such that the platelet count was 2 × 108/mL. Radiolabeled D3 or mouse IgG was added (final concentrations: 0.025-0.5 μmol/L) and the mixture incubated for 5 minutes at 37°C. ADP (10 μmol/L) was added with the antibody and the platelets immediately isolated, or ADP was added with the antibody for a 2-minute incubation. In another set of tubes, D3 or IgG was preincubated with the platelets for 2 minutes, and the ADP was added and incubated for 3 minutes. Three aliquots of 100 μL each were layered onto 1.0 mL of modified Tyrode buffer containing 20% sucrose and 0.1% BSA in microfuge tubes; an additional 100 μL aliquot was used for determining total counts. The microfuge tubes were centrifuged at 11 600g for 4 minutes; the tips were amputated and transferred to tubes for counting. The γ emissions from the pellets were counted using an LKB Wallac 1282 Compugamma counter (LKB Instruments). Specific binding was quantitated by subtracting nonspecific binding (IgG) from D3 binding. The specific counts were converted into D3 antibody molecules bound and plotted versus the moles of antibody added. The maximum number of D3 binding sites was determined from the binding curves. The mean ± SD was determined for each treatment.
Cytoskeleton isolation
After a 6-minute reaction time, the stirred PRP was taken directly from the aggregometer cuvette and rapidly lysed with an equal volume of ice-cold 2% Triton X-100 extraction buffer (100 mmol/L Tris, 10 mmol/L EGTA, 2 mmol/L 2-mercaptoethanol, 2 μmol/L leupeptin, 2 mmol/L phenylmethyl-sulfonyl-fluoride [PMSF], and 100 mmol/L benzamidine, pH 7.4). The samples were then vortexed for 10 seconds and centrifuged at 10 000g for 5 minutes. The pellet was washed 2 times with extraction buffer containing only 1% Triton X-100 and solubilized with reducing sample buffer (0.5 mol/L Tris, 40% glycerol, 8% SDS, and 8% bromphenol blue). Whole platelet lysates were prepared by adding 2 drops of Sequester-Sol to 0.5 mL of citrated PRP and centrifuging at 1200g for 10 minutes.23 Pellets were washed with ETS buffer (10 mmol/L Tris, 150 mmol/L NaCl, and 5 mmol/L EDTA) and solubilized with reducing sample buffer. All samples were electrophoresed through 5% to 20% exponential gradient polyacrylamide gels overnight at a constant voltage of 25 V, and gels were stained with Coomassie brilliant blue.24 Western blotting was performed as previously described.25Following transfer at 700 mA for 1.5 hours, the nitrocellulose was incubated in a 3% BSA blocking buffer containing 10 mmol/L Tris, 150 mmol/L NaCl, and 0.02% NaN3, pH 7.4. After blocking for 2 hours, the primary antibody was added and incubated overnight. The primary antibody was a polyclonal antibody specific for GPIIb-IIIa and was generously donated by Dr David Phillips of COR Therapeutics, South San Francisco, CA. Blots were washed in a buffer with the same ingredients as the blocking buffer containing only 1% BSA. The blots were then incubated with an affinity purified goat antirabbit antibody conjugated to horseradish peroxidase (HRP; BioRad, Richmond, CA) and developed with 4-chloro-1-napthol and hydrogen peroxide. GPIIb-IIIa incorporation was compared to the percent aggregation response by calculating the percent change in aggregation and GPIIb-IIIa incorporation when the ADP treatment data were normalized to 100%. For some treatments, quantitative Western blotting was also performed. For GPIIb-IIIa detection, polyclonal antibodies SEW8 (anti-GPIIb) and Fire and Ice (anti-GIIIa) were used in conjunction with an affinity purified goat antirabbit HRP conjugate. The anti-GPIIb and anti-GPIIIa antibodies were generously provided by Dr Peter Newman (Blood Center of SE Wisconsin, Milwaukee WI). For talin detection, gels were either stained by Coomassie brilliant blue or quantitative Western blotting was performed using a goat antihuman talin antibody (generously provided by Dr Joan Fox, Cleveland Clinic, Cleveland, OH) followed by an antigoat/sheep IgG peroxidase conjugate. Binding was detected by chemiluminescence using the Super Signal West Dura Extended Duration Substrate (Pierce Chemical).
Scanning electron microscopy
Platelets were fixed as previously described.17 26Briefly, PRP was diluted 1:5 with 150 mmol/L NaCl and fixed by addition of an equal volume of 2% glutaraldehyde in 150 mmol/L NaCl for 60 minutes. The fixed platelets were suction filtered onto Nucleopore polycarbonate filters (0.45 μm) that had been preincubated with 100 mmol/L polylysine buffer. The filters were washed once for 10 minutes with 150 mmol/L NaCl and once with distilled water. The fixed platelets were dehydrated by successive 10-minute incubations in 30%, 50%, 70%, and 90% ethyl alcohol and 2 incubations in 100% ethyl alcohol. Critical point drying was performed in a Tousimis SamDri-79 critical point drying apparatus. The filters were gold-coated in a Technics Hummer II sputter coating device and imaged on a JSM-840A scanning electron microscope (Tousimis, Rockville, MD) at 20 KU accelerating voltage.
Results
Clot retraction studies
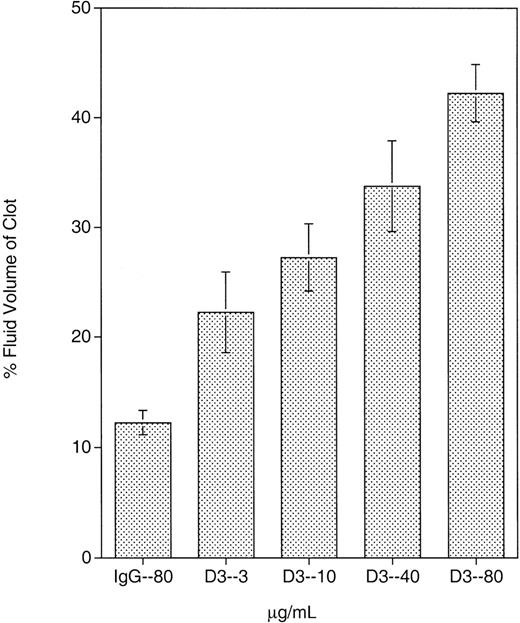
Previous data demonstrated that D3 induced fibrinogen binding to GPIIb-IIIa; however, the contribution of this LIBS site in other events mediated by GPIIb-IIIa, such as clot retraction, has not been well characterized. The effect of D3 on clot retraction in whole blood is presented in Figure 1. As described in “Materials and methods,” the extent of clot retraction is determined by the percent fluid volume of the clot. Thus, the higher the percent volume of fluid in the clot, the greater the inhibition of clot retraction. The clots were also examined visually on removal from the glass tubes. Normal retracted clots are smooth and firm; poorly retracted clots are leaky and friable. At the 4 D3 concentrations tested, clot retraction was inhibited over that in the presence of control mouse IgG. Clots retrieved from D3-treated blood were leaky and friable. At 80 μg/mL, D3 produced a clot that was approximately 40% fluid volume. At 40, 10, and 3 μg/mL, D3 also inhibited clot retraction and produced clots with 34%, 27%, and 22% fluid volume, respectively. Although the SD was large for these D3 concentrations, there was a significant increase in percent fluid volume at 40 and 80 μg/mL compared to that obtained with mouse IgG (P < .02). D3 also inhibited clot retraction in PRP (data not shown) when compared to mouse IgG or other GPIIb-IIIa mAbs.
Whole blood clot retraction inhibition by D3.
D3 or mouse IgG was incubated with whole blood for 24 hours during which clotting and clot retraction took place. Mouse IgG was present at 80 μg/mL, and D3 was present at 4 different concentrations: 3, 10, 40, and 80 μg/mL. At all concentrations tested, D3 exhibited some extent of clot retraction. Results shown are the mean of 4 separate experiments.
Whole blood clot retraction inhibition by D3.
D3 or mouse IgG was incubated with whole blood for 24 hours during which clotting and clot retraction took place. Mouse IgG was present at 80 μg/mL, and D3 was present at 4 different concentrations: 3, 10, 40, and 80 μg/mL. At all concentrations tested, D3 exhibited some extent of clot retraction. Results shown are the mean of 4 separate experiments.
Platelet binding to fibrinogen and fibrin in the presence or absence of D3
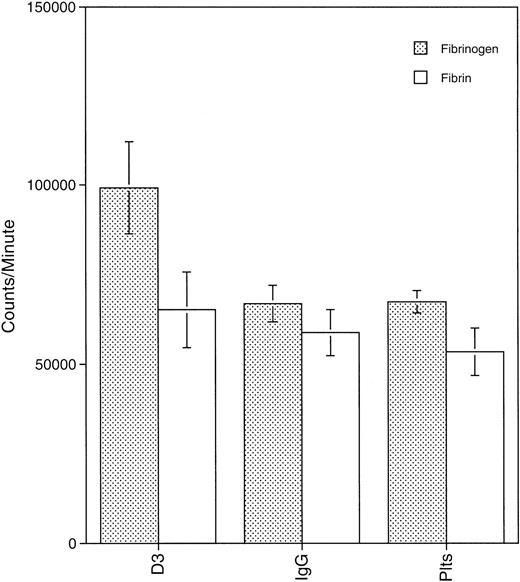
Clot retraction is a dynamic process that involves the interaction of GPIIb-IIIa with fibrin on the platelet surface and the reorganization of the platelet cytoskeleton.27 28 To determine if mAb binding to a GPIIIa LIBS altered platelet-fibrinogen or platelet-fibrin interactions, we measured binding of platelets in the presence or absence of D3 to fibrin and fibrinogen coated onto multiwell plates. Figure 2 is a graph of the mean results of 3 experiments. D3 enhanced platelet binding to fibrinogen over that of IgG-treated platelets or control platelets (P < .02). Platelet binding to fibrin in the presence or absence of D3 was not significantly different as D3 did not alter platelet adherence to fibrin over that observed with IgG-treated platelets (P > .02). Thus, although D3 enhanced platelet-fibrinogen interaction, the mAb did not alter platelet-fibrin interaction even though data demonstrated that D3 completely inhibited clot retraction.
Platelets binding to fibrin(ogen) in the presence or absence of D3.
Platelets (1 × 108/well) labeled with 125I were added to plates coated with fibrin(ogen) (20 μg/well) and incubated for 10 minutes at 37°C. After 3 washes with CM-PBS, adherent platelets were solubilized with 2% SDS and counted. Results shown are the mean of 3 experiments.
Platelets binding to fibrin(ogen) in the presence or absence of D3.
Platelets (1 × 108/well) labeled with 125I were added to plates coated with fibrin(ogen) (20 μg/well) and incubated for 10 minutes at 37°C. After 3 washes with CM-PBS, adherent platelets were solubilized with 2% SDS and counted. Results shown are the mean of 3 experiments.
Aggregation studies with D3/ADP
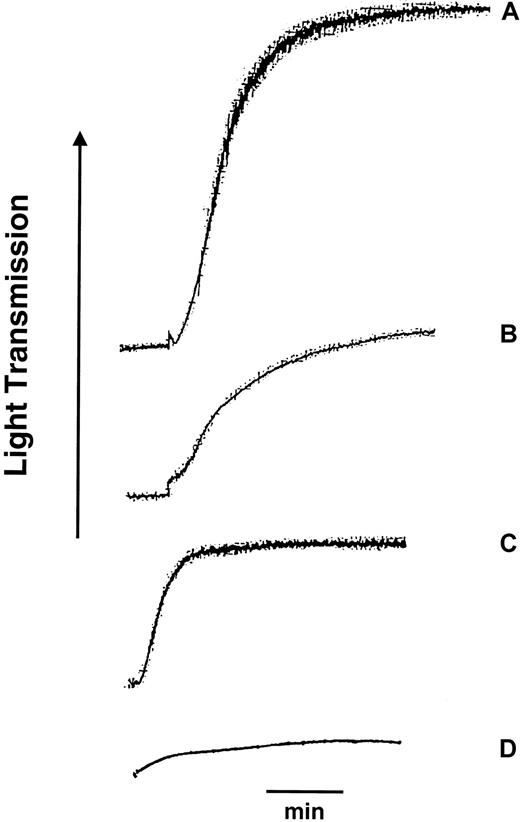
To further examine the role of the D3 LIBS site in events mediated by fibrinogen binding, various combinations of saline, control mouse IgG, or D3 with ADP or AA were used to examine platelet aggregation response. In some studies platelets were treated either with ASA or its carrier, EtOH. When 1 to 3 μmol/L ADP was added with saline, mouse IgG, or alone, the aggregation response was approximately 70% (Table1, Figure3). When PRP was preincubated with D3 for 2 minutes before addition of ADP or if added simultaneously with ADP, the aggregation response was lowered to 40%, a response typically seen with D3 alone and significantly less than that observed with ADP alone. Unexpectedly, when D3 and ADP were preincubated in PRP for 2 minutes before stirring was initiated, the final aggregation response after stirring was only 7% (Table 1, Figure 3). These data suggested that D3 binding prevented full-scale aggregation response to ADP and that preincubation of platelets with ADP and D3 before stirring prevented significant platelet cross-linking and platelet aggregation.
Mean percent aggregation for D3 and IgG in the presence of ADP
| Treatment . | Mean % aggregation . | SEM . | No. . |
|---|---|---|---|
| D3 alone | 37 | 1.1 | 9 |
| D3 preincubated 2 min/ADP | 32 | 1.8 | 9 |
| D3/ADP added simultaneously | 40 | 3.4 | 8 |
| D3/ADP 2 min incubation | 7 | 0.6 | 4 |
| ADP/mouse IgG | 81 | 2.0 | 6 |
| ADP/saline | 78 | 3.3 | 6 |
| ADP alone | 79 | 2.6 | 7 |
| Treatment . | Mean % aggregation . | SEM . | No. . |
|---|---|---|---|
| D3 alone | 37 | 1.1 | 9 |
| D3 preincubated 2 min/ADP | 32 | 1.8 | 9 |
| D3/ADP added simultaneously | 40 | 3.4 | 8 |
| D3/ADP 2 min incubation | 7 | 0.6 | 4 |
| ADP/mouse IgG | 81 | 2.0 | 6 |
| ADP/saline | 78 | 3.3 | 6 |
| ADP alone | 79 | 2.6 | 7 |
Platelets (2.5 × 108/mL) were stirred in an aggregometer cuvette and the described treatments were added. Percent aggregation was calculated at 6 minutes.
Platelet aggregation response to ADP, D3, and combination treatments with D3 and ADP.
Platelets in plasma (PRP) anticoagulated with citrate were treated either with 2 μmol/L ADP (A), 50 μg D3 (B), 50 μg D3 for 2 minutes prior to stirring then 2 μmol/L ADP (C), or 50 μg D3 and 2 μmol/L ADP for 2 minutes prior to stirring (D). Tracings shown are representative of 3 normal donors. Bar indicates 1 minute.
Platelet aggregation response to ADP, D3, and combination treatments with D3 and ADP.
Platelets in plasma (PRP) anticoagulated with citrate were treated either with 2 μmol/L ADP (A), 50 μg D3 (B), 50 μg D3 for 2 minutes prior to stirring then 2 μmol/L ADP (C), or 50 μg D3 and 2 μmol/L ADP for 2 minutes prior to stirring (D). Tracings shown are representative of 3 normal donors. Bar indicates 1 minute.
To explore whether the plasma-free calcium concentrations affected our results, blood was collected into the PPACK anticoagulant, which, unlike buffered citrate, maintains the physiologic level of free calcium in the plasma while still providing anticoagulation. No significant difference in aggregation response was observed with the 2 anticoagulants in the extent of ADP response when added singly (76% ± 5% in citrate versus 71% ± 2% in PPACK) or in combination with D3 (28% ± 4% in citrate versus 28% ± 6% in PPACK; P = .4 and P=.9, respectively). Thus, the D3-mediated effects on GPIIb-IIIa are not sensitive to plasma free calcium levels.
Lumiaggregometry studies
Because the aggregation response of ADP-treated platelets in the presence of D3 was dramatically inhibited, we measured platelet secretion to determine whether D3 directly inhibited secretion or if D3 inhibited aggregation through an effect on GPIIb-IIIa. Released ATP as a result of platelet secretion can be measured using lumiaggregometry, which uses the luciferin/luciferase reaction to measure ATP levels.22 Table 2 presents values of released ATP (nanomoles) during the aggregation treatments listed in Table 1. These values are representative of 3 experiments. When ADP was added alone, or with saline or mouse IgG, secretion occurred with approximately 0.5 nmoles ATP released. In the presence of D3 alone, no secretion occurred. D3 added in any combination with ADP, whether simultaneously or preincubated, inhibited secretion. However, when thrombin was used as an agonist, the presence of D3 caused a decrease in platelet aggregation response (77% versus 56%, n = 2) but did not inhibit secretion (0.44 versus 0.51 nmoles, n = 2). These data suggested that D3 directly exerted its effect on platelet aggregation and that D3 inhibition of secretion with ADP, typically considered a weak agonist, was due solely to the inhibition of GPIIb-IIIa–mediated aggregation and not the direct inhibition of the platelet release reaction. Studies carried out in PPACK anticoagulant support these observations because platelet aggregation via ADP activation in PPACK is not dependent on the release reaction. We also compared the platelet aggregation response to AA on aspirin pretreatment or on D3 binding. If D3 were inhibiting the cyclooxygenase pathway in platelets, then the level of aggregation inhibition by ASA with D3 should be similar to ASA-treated platelets when stimulated by AA. Because D3 blocked platelet aggregation to AA similar to that observed with thrombin (25% versus 27% inhibition, respectively) and ASA significantly blocked AA (91% inhibition, n = 3), our data would suggest that D3 is not exhibiting its profound effect through inhibition of cyclooxygenase.
Luminaggregometry results for D3 and ADP-induced aggregation
| Treatment . | % Aggregation . | Nanomoles ATP released . |
|---|---|---|
| D3 alone | 32 | 0 |
| D3 preincubated 2 min/ADP | 19 | 0 |
| D3/ADP added simultaneously | 28 | 0.03 |
| D3/ADP 2 min incubation | 2 | 0 |
| ADP/mouse IgG | 73 | 0.47 |
| ADP/saline | 65 | 0.46 |
| ADP alone | 70 | 0.44 |
| Treatment . | % Aggregation . | Nanomoles ATP released . |
|---|---|---|
| D3 alone | 32 | 0 |
| D3 preincubated 2 min/ADP | 19 | 0 |
| D3/ADP added simultaneously | 28 | 0.03 |
| D3/ADP 2 min incubation | 2 | 0 |
| ADP/mouse IgG | 73 | 0.47 |
| ADP/saline | 65 | 0.46 |
| ADP alone | 70 | 0.44 |
Platelets (2 × 108/mL) were stirred in an aggregometer cuvette and the described treatments were added. Percent aggregation was calculated at 6 minutes. ATP release was measured from a standard curve as described in “Materials and methods.”
Radiolabeled D3 binding studies
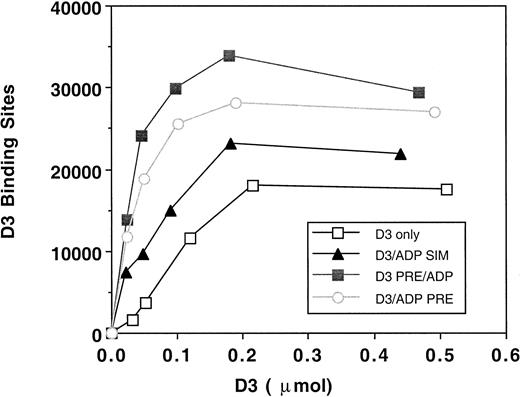
Because the effect of D3 on platelet aggregation was due to its inhibition of GPIIb-IIIa function, we examined D3 epitope expression in the presence of ADP, by radiolabeled D3 binding studies. Treatments that mimicked platelet aggregation assays included incubation of D3 for 5 minutes, D3 and ADP incubated for 2 minutes only, D3 and ADP added and the platelets immediately isolated, or D3 preincubated for 2 minutes and then ADP added for 3 minutes. Figure4 is a representative saturation isotherm of D3 binding to platelets under the treatment conditions. The maximal number of D3 binding sites on unstimulated platelets was 14 523 ± 3912 (n = 5). Previous studies under equilibrium conditions19 have demonstrated 2 classes of D3 binding sites on resting platelets: (1) a high-affinity site with a Bmax of 2110 and a Kd of 2 nmol/L and (2) a low-affinity site with a Bmax of 7850 and a Kdof 87 nmol/L. When platelets were treated by a simultaneous addition of D3 and ADP and immediately isolated, the maximal number of D3 binding sites was 20 900 ± 7905 (n = 4). Both incubation of D3 and ADP for 2 minutes and the preincubation of D3 for 2 minutes then addition of ADP for 3 minutes resulted in maximal D3 binding sites of 33 844 ± 12 866 and of 28 346 ± 6692, respectively. Thus, our binding studies suggest that even though aggregation was inhibited, GPIIb-IIIa was sufficiently occupied by ligand to mediate a platelet aggregation response.
D3 binding isotherms to platelets.
Platelets (2.5 × 108/mL) were incubated with125I-D3 (■, n = 4) or mouse IgG (n = 4), final concentrations of 0.025, 0.05, 0.1, 0.2, and 5 μmol/L, for 5 minutes at 37°C. ADP (10 μmol/L) was added either simultaneously with the antibody (▴, n = 4) or was preincubated along with the antibody for 2 minutes (○, n = 5). In one set of tubes, D3 or IgG was preincubated with the platelets for 2 minutes, and the ADP was added for 3 minutes (▪, n = 4). Gamma emissions were counted. Specific binding was quantitated by subtracting the nonspecific binding (IgG) from the D3 binding. Binding isotherms were generated by plotting the number of antibody binding sites versus moles of antibody added. A representative graph is shown.
D3 binding isotherms to platelets.
Platelets (2.5 × 108/mL) were incubated with125I-D3 (■, n = 4) or mouse IgG (n = 4), final concentrations of 0.025, 0.05, 0.1, 0.2, and 5 μmol/L, for 5 minutes at 37°C. ADP (10 μmol/L) was added either simultaneously with the antibody (▴, n = 4) or was preincubated along with the antibody for 2 minutes (○, n = 5). In one set of tubes, D3 or IgG was preincubated with the platelets for 2 minutes, and the ADP was added for 3 minutes (▪, n = 4). Gamma emissions were counted. Specific binding was quantitated by subtracting the nonspecific binding (IgG) from the D3 binding. Binding isotherms were generated by plotting the number of antibody binding sites versus moles of antibody added. A representative graph is shown.
Cytoskeletal alterations induced by D3
Our data showed that D3 did not alter GPIIb-IIIa–fibrin interaction. Therefore, it was possible that the inhibition of full-scale aggregation and of clot retraction could be, in part, cytoskeletal driven through altered GPIIb-IIIa transmembrane signaling. Low-speed cytoskeletal cores were isolated after platelet aggregation was induced by various combinations of D3 and ADP (Table 1). Figure5A is a representative gel of 3 experiments. Lane 1 contains the cytoskeletal core of stirred platelets that had no agonist added. As previously reported,29 some actin was incorporated, but there was very little actin-binding protein (ABP) or myosin present. Talin was not detected. Lane 3 contains cytoskeletons isolated from platelets aggregated by ADP. Incorporation of ABP, talin, myosin, and actin was increased when compared to platelets stirred without agonist (lane 1). Cytoskeletons from platelets aggregated by D3 addition only are in lane 2. There was an increase in the amounts of ABP, myosin, and actin as compared to stirred but untreated platelets, but these levels were decreased compared to the ADP treatment. In addition, talin was not present in either stirred or D3-treated samples. When D3 and ADP were added to platelets, either simultaneously or with a 2-minute D3 preincubation, the amount of cytoskeletal protein incorporation was intermediate to that observed when either ADP or D3 was added alone (lanes 4 and 5 versus lane 3 and 2). The biggest difference was the absence of talin incorporation in the D3/ADP cytoskeletons compared to ADP only. Quantitative Western blots using an antitalin antibody demonstrated that the cytoskeletal cores from ADP/D3-treated platelets contained 5% or less of the levels of talin incorporated in the cores isolated from the ADP-treated platelets (Figure 5B, lane 3). Interestingly, D3 binding studies of platelets treated by the combination of ADP/D3 indicated that GPIIb-IIIa had bound fibrinogen (28 346 D3 binding sites; Figure 4). Platelets that were preincubated with D3 and ADP for 2 minutes before stirring was initiated had decreased amounts of incorporated cytoskeletal proteins compared to ADP alone (lane 6 versus lane 3, respectively). Although a large number of GPIIb-IIIa complexes were in a ligand-bound conformation (33 844; Figure 4), the aggregation response was greatly decreased as a result of this treatment (7%, Table 1). Therefore, if GPIIb-IIIa is activated such that fibrinogen binds, it is possible that a relatively static milieu may result in a ligand bound GPIIb-IIIa that is unable to participate in platelet aggregate formation.
The effect of D3 and ADP on the composition of the cytoskeleton of platelet aggregates.
After 6 minutes in the aggregometer, platelet samples were lysed, and the low-speed cytoskeletal pellet was isolated. Pellets were solubilized with reducing sample buffer and electrophoresed through a 5% to 20% exponential gradient polyacrylamide gel. (A) Coomassie blue-stained gel. The gel shown is representative of 3 experiments. Lane 1 contains spontaneous aggregation; lane 2, D3 aggregated platelets; lane 3, ADP aggregated platelets; lane 4, D3/ADP added simultaneously; lane 5, D3 2 minutes preincubation and ADP; lane 6, D3 and ADP preincubation 2 minutes; lane 7, whole platelets; lane 8, molecular weight standards. (B) Quantitative Western blot. Cytoskeletal cores isolated from D3 aggregated platelets (lanes 1, 4, 7, and 10), ADP aggregated platelets (lanes 2, 5, 8, and 11) and D3 2 minutes of preincubation and ADP (lanes 3, 6, 9 and 12) were electrophoresed at protein gel loads of 100%, 75%, 50%, and 25% and transferred to nitrocellulose. The blots were probed with a goat antihuman talin antibody and binding was detected using an HRP conjugated secondary antibody and chemiluminescence. Shown is a representative blot of 3 independent experiments. WP indicates whole platelets.
The effect of D3 and ADP on the composition of the cytoskeleton of platelet aggregates.
After 6 minutes in the aggregometer, platelet samples were lysed, and the low-speed cytoskeletal pellet was isolated. Pellets were solubilized with reducing sample buffer and electrophoresed through a 5% to 20% exponential gradient polyacrylamide gel. (A) Coomassie blue-stained gel. The gel shown is representative of 3 experiments. Lane 1 contains spontaneous aggregation; lane 2, D3 aggregated platelets; lane 3, ADP aggregated platelets; lane 4, D3/ADP added simultaneously; lane 5, D3 2 minutes preincubation and ADP; lane 6, D3 and ADP preincubation 2 minutes; lane 7, whole platelets; lane 8, molecular weight standards. (B) Quantitative Western blot. Cytoskeletal cores isolated from D3 aggregated platelets (lanes 1, 4, 7, and 10), ADP aggregated platelets (lanes 2, 5, 8, and 11) and D3 2 minutes of preincubation and ADP (lanes 3, 6, 9 and 12) were electrophoresed at protein gel loads of 100%, 75%, 50%, and 25% and transferred to nitrocellulose. The blots were probed with a goat antihuman talin antibody and binding was detected using an HRP conjugated secondary antibody and chemiluminescence. Shown is a representative blot of 3 independent experiments. WP indicates whole platelets.
GPIIb-IIIa incorporation into cytoskeleton cores
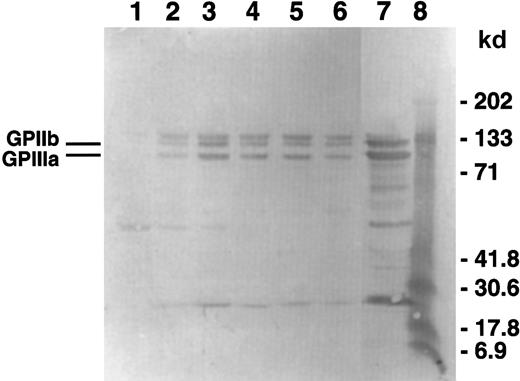
To measure GPIIb-IIIa incorporation in the cytoskeletal cores, Western blot analysis of the isolated cytoskeletons was carried out using an anti-GPIIb-IIIa polyclonal antibody. Figure6 is a blot of the same samples shown in the gel in Figure 5A that is representative of 3 experiments. The cytoskeletons isolated from platelets aggregated with ADP only have the highest relative amount of GPIIb-IIIa incorporation (lane 3). Regardless of the amount of time that D3 is present, either with or without ADP, the amount of GPIIb-IIIa incorporation was decreased (lanes 2 and 4-6). The appearance of the doublet band corresponding to the apparent molecular weight range of GPIIb is probably due to nonspecific binding of the antibodies used in this detection system because the quantitative Western blots using another antibody combination did not contain the doublet (data not shown). Using densitometric analysis of quantitative Western blots, we determined that the incorporation of GPIIb-IIIa into the cytoskeletal cores corresponded to the extent of platelet aggregation induced by the various treatments (Figure 7).
GPIIb-IIIa incorporation into the cytoskeleton.
Using the samples from the gel in Figure 3, Western blots were performed and probed using a polyclonal antibody against GPIIb-IIIa. The gel shown is representative of 3 experiments. Lane 1 contains spontaneous aggregation; lane 2, D3 aggregated platelets; lane 3, ADP aggregated platelets; lane 4, D3/ADP added simultaneously; lane 5, D3 2 minutes of preincubation and ADP; lane 6, D3 and ADP preincubation for 2 minutes; lane 7, whole platelets; lane 8, molecular weight standards.
GPIIb-IIIa incorporation into the cytoskeleton.
Using the samples from the gel in Figure 3, Western blots were performed and probed using a polyclonal antibody against GPIIb-IIIa. The gel shown is representative of 3 experiments. Lane 1 contains spontaneous aggregation; lane 2, D3 aggregated platelets; lane 3, ADP aggregated platelets; lane 4, D3/ADP added simultaneously; lane 5, D3 2 minutes of preincubation and ADP; lane 6, D3 and ADP preincubation for 2 minutes; lane 7, whole platelets; lane 8, molecular weight standards.
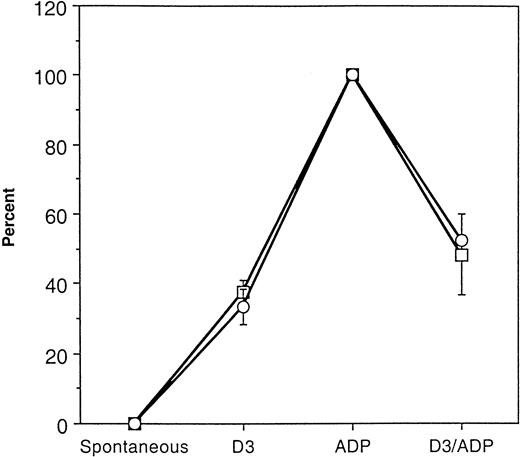
Comparison of GPIIb-IIIa incorporation into the cytoskeletal cores with platelet aggregation response.
Aggregation response and GPIIb-IIIa incorporation in response to ADP was normalized to 100% in 3 separate treatment groups. The percent maximum aggregation response (■) and percent maximum GPIIb-IIIa incorporation (○) was determined for spontaneous, D3, and D3 then ADP treatments. The data are the mean ± SD (n = 3).
Comparison of GPIIb-IIIa incorporation into the cytoskeletal cores with platelet aggregation response.
Aggregation response and GPIIb-IIIa incorporation in response to ADP was normalized to 100% in 3 separate treatment groups. The percent maximum aggregation response (■) and percent maximum GPIIb-IIIa incorporation (○) was determined for spontaneous, D3, and D3 then ADP treatments. The data are the mean ± SD (n = 3).
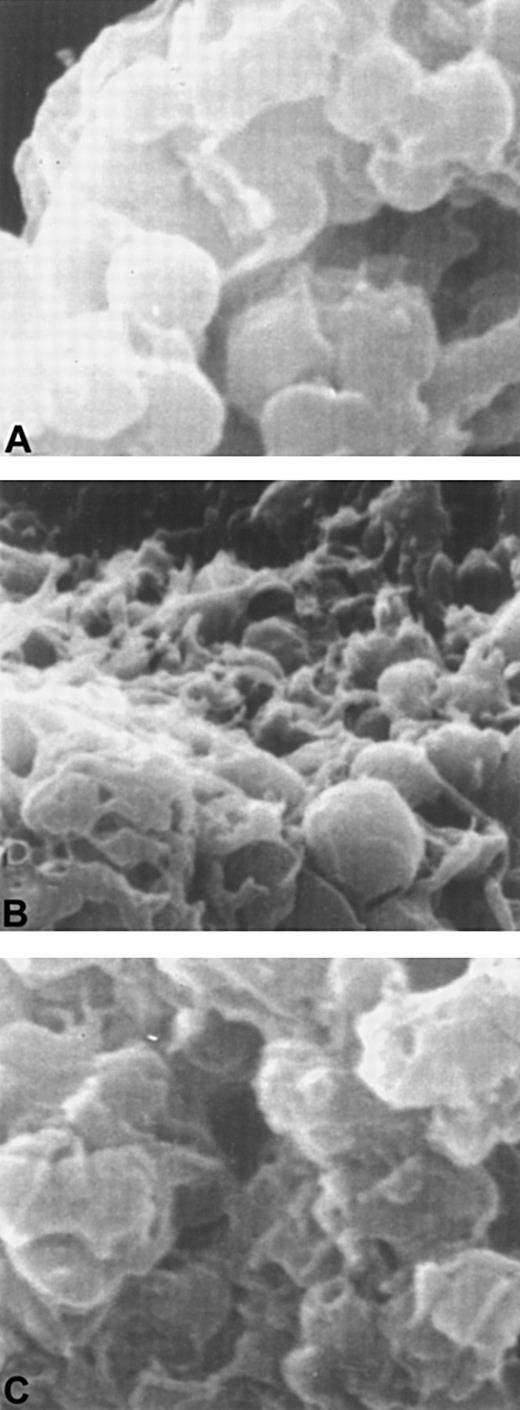
Scanning electron microscopy of platelets treated with D3 and/or ADP
To examine changes in platelet morphology induced by different aggregation responses, scanning electron microscopy was performed (Figure 8). Platelets aggregated by D3 were smooth and discoid yet had adhered into an aggregate. ADP-treated platelets had a spiny, amorphous appearance and had projected pseudopodia. The platelets aggregated by D3 and ADP appeared to be an intermediate of the 2 aggregates formed when either agonist or D3 was added alone. Platelets from the combination treatment appeared to have fewer pseudopodia and retained a more discoid appearance.
Scanning electron microscopy of platelets treated with D3 or ADP or both D3 and ADP.
Panel A shows platelets treated with D3. Platelets incubated with ADP are shown in panel B, and platelets with D3 and ADP are shown in panel C.
Scanning electron microscopy of platelets treated with D3 or ADP or both D3 and ADP.
Panel A shows platelets treated with D3. Platelets incubated with ADP are shown in panel B, and platelets with D3 and ADP are shown in panel C.
Discussion
The major findings of this study are (1) full-scale platelet aggregation may require the engagement of talin and the incorporation of GPIIb-IIIa into the platelet cytoskeletal core, (2) fibrinogen or fibrin binding to high affinity GPIIb-IIIa is not sufficient for platelet aggregation or clot retraction, and (3) ligation of a specific neoepitope plays a direct role in the suppression of platelet aggregation and integrin signaling that led to cytoskeletal assembly and clot retraction. These data suggest that the exposure of this GPIIIa LIBS as recognized by the D3 antibody is important in mediating subsequent transmembrane signaling mechanisms associated with full-scale platelet aggregation and clot retraction.
Clot retraction is the result of forces generated as GPIIb-IIIa interacts with fibrin. Clot retraction is absent in some patients with Glanzmann thrombasthenia, typically those with no expression of GPIIb-IIIa.3 Transmission electron microscopy and microfluorimetry also demonstrate a lack of fibrin binding to platelets from these patients. The molecular basis of clot retraction has been suggested to be the clustering of GPIIb-IIIa complexes that provide a location of fibrin anchorage to the platelet.27 It has been suggested that different domains on GPIIb-IIIa are responsible for fibrinogen binding and fibrin binding. Although D3 enhanced platelet binding to fibrinogen, it did not affect platelet binding to fibrin. The results of Cohen and colleagues28 where the effects of mAbs and fibrinogen-derived peptides on clot tension were studied support this theory. A possibility is that D3 places a constraint on the conformation of GPIIb-IIIa such that the cytoskeleton does not efficiently interact with the complexes in the membrane. Consequently, typical retraction of the clot does not occur. Interestingly, we recently reported data on a patient with type II Glanzmann thrombasthenia who had approximately 30% of the normal levels of GPIIb-IIIa and absent platelet aggregation but normal clot retraction.29 Even though the addition of RGDS peptides did not induce increased binding of the anti-LIBS D3, the antibody inhibited clot retraction. These data taken together with our studies would support the hypothesis that the contact sites on the GPIIIa subunit differ for fibrin and fibrinogen.
D3 induces platelet aggregation independently of platelet activation. This aggregation usually gives approximately a 40% increase in light transmittance in the aggregometer and exhibits no secretion as measured by lumiaggregometry. When an agonist such as ADP is added at a sufficient concentration to platelets, ATP is secreted from platelet granules.30 Based on our results, these amounts vary from 0.30 to 0.55 nmol/L ATP when aggregation was induced by 1 to 3 μmol/L ADP. When D3 is added to platelets along with ADP, there is no secretion, regardless of the amount of time that D3 is present. The aggregation tracings reflect this lack of a full-scale response as a lowered percentage of aggregation in the presence of D3. Interestingly, when D3 and ADP are preincubated together before stirring is initiated, the aggregation response is inhibited dramatically, and there is no secretion. This lack of platelet cross-linking is possibly due to increased intraplatelet fibrinogen binding that inhibited fibrinogen cross-linking to other receptors on adjacent platelets. It is possible that D3 places a constraint on the conformation of GPIIb-IIIa such that, even in the presence of ADP, a full-scale response could not occur. If higher concentrations of ADP such as 10 μmol/L were added in addition to D3, the aggregation response was still decreased, but not to the same extent as when lower ADP concentrations were added. Secretion did occur, but it was more of a slow steady “trickle” as opposed to the “burst” seen with high doses of ADP alone.
Cytoskeletal protein analysis of D3-aggregated platelets demonstrated that although these platelets had bound fibrinogen, they failed to show increased ABP, talin, and F-actin seen in the isolated cytoskeletons of ADP-aggregated platelets. A possible scenario is that although ADP is driving the platelets to an activated state where GPIIb-IIIa is incorporated into the cytoskeleton and full-scale aggregation occurs, the activation of GPIIb-IIIa by D3 allows fibrinogen binding to occur but prevents cytoskeletal reorganization and its linkage to GPIIb-IIIa. This is a plausible explanation because D3 can induce fibrinogen binding without platelet activation.18 An equilibrium is reached as evidenced by the scanning electron micrographs where the D3/ADP aggregates have an appearance that is intermediate of the aggregates induced by either agent alone. This effect is also seen in the measurements of aggregation responses. When Western blots of the cytoskeletal cores were probed with an antibody against GPIIb-IIIa, the presence of D3 caused a reduction in the amount of GPIIb-IIIa incorporated into the cytoskeleton when compared to that of ADP alone. Interestingly, the extent of GPIIb-IIIa incorporation into the cytoskeletal cores corresponded exactly to the aggregation response induced by ADP, D3, or the combination treatment.
In the presence of ADP, there was an increase in D3 binding to platelets in plasma due to bound ligand.14 When ADP and D3 were added simultaneously, and the bound antibody immediately separated from the free antibody, the number of binding sites was increased, but not to the extent as when ADP was present for 3 or 5 minutes. A 3-minute incubation with ADP was sufficient to convert a significant number of GPIIb-IIIa molecules to the ligand-occupied form (28 346 D3 binding sites) because there are on average 60 000 to 80 000 GPIIb-IIIa molecules per platelet.31 The on rate for D3 binding to platelets is 6.7 × 105 mol/L per minute. In the presence of 1 mmol/L RGDS, the rate increased about 70-fold to 4.5 × 107 mol/L per minute (T.H.M., M.M.W., and L.K.J., unpublished observations). Another mAb that inhibits clot retraction is 7E3. It is GPIIb-IIIa complex specific and has an on rate that falls between the values of the D3 on rates (2.03 × 106 mol/L per minute for resting platelets and 6.5 × 106 mol/L per minute for ADP-activated platelets).32 The platelet activation resulting from ADP addition may provide the additional fibrinogen binding required to increase the number of D3 binding sites.
The conformation of GPIIb-IIIa that expresses the D3 LIBS appears to be important in receptor–ligand-mediated interactions because 2 important receptor functions, aggregation and clot retraction, are affected by antibody binding to this region. In support of these findings, earlier studies by Pelletier and associates33 used activating anti-LIBS antibodies to demonstrate that outside-in integrin signals represent a mechanism for regulating integrin-mediated adhesion. They also surmised that the conformation state (ie, activation state) of the integrin is the basis for the formation of subpopulations that may modulate cellular responses. Fox and coworkers34 concluded that the association of GPIIb-IIIa with cytoplasmic actin results from the binding of fibrinogen to GPIIb-IIIa. These results were further extended to assert that the movement of the occupied receptor into the open canalicular system allows retraction to occur by the inward movement of the externally bound fibrin clot. D3 may inhibit the movement of GPIIb-IIIa-fibrin(ogen) complexes by interfering with the GPIIb-IIIa molecules that associate with the cytoskeleton.
A study comparing the inhibitory effects of a GPIIb-IIIa antagonist that mimics the RGD binding domain and a purinoreceptor antagonist demonstrates that there is early reversible incorporation of myosin, actin, and α-actinin in response to platelet activation by ADP.35 These changes occur parallel with GPIIb-IIIa activation and before platelet aggregation. Further evidence in this study suggests that cytoskeletal assembly is not complete until platelets are physically aggregated. It is tempting to speculate that D3 binding to GPIIb-IIIa reverses or prevents the early incorporation of proteins into the cytoskeleton in response to ligand binding. These data suggest that ligation of a specific LIBS site affects transmembrane signaling through GPIIb-IIIa such that organization of the platelet cytoskeleton is altered. Further activation-induced changes do not occur because GPIIb-IIIa has been locked into a conformation that may not allow for movement into the open canalicular system (for clot retraction) but is still able to cross-link receptors on adjacent platelets to form an aggregate.
These observations are supported by the studies of Rooney and coworkers36 who proposed a 2-step model for clot retraction whereby platelets are recruited into the clot by interaction with fibrinogen in the first step. In the second step, the transmission of the contractile force is generated from the platelets and the fibrin strands. Another hypothesis is that the binding of an anti-LIBS such as D3 may affect the phosphorylation state of tyrosine residues 747 and 759 on GPIIIa that were recently shown to be critical in platelet aggregation and clot retraction.37 Preliminary data, however, suggest that the phosphorylation state of GPIIIa relative to GPIIb-IIIa incorporated in the cytoskeletal cores was not affected by the binding of D3 (L.K.J. et al, unpublished observations).
In contrast to an earlier report,38 our data would suggest that a critical linkage of GPIIb-IIIa to the cytoskeleton requires association of the integrin to the contractile protein talin rather than myosin for the initiation of clot retraction. It is possible that myosin may play a more important role in the generation of tensile strength once a sufficient linkage has been established. In support of our conclusions, we observed that the myosin levels in the cytoskeletal cores were not significantly different among the various treatment groups even though platelet aggregation and clot retraction differences were observed. Studies by Knezevic and coworkers39demonstrated direct binding of GPIIb-IIIa to talin and suggested a role of the cytoplasmic tails of both receptor subunits in this interaction. Moreover, the lack of talin incorporation in the cytoskeletons from D3-treated platelets suggests that talin is a critical component of the facilitating full-scale platelet aggregation and clot retraction on ligand binding to GPIIb-IIIa. A recent study by Calderwood and coworkers40 supports this hypothesis because they showed specific binding of the head domain of talin to the integrin β-subunit cytoplasmic tails and modulated integrin function. Further studies to understand the role of GPIIb-IIIa outside-in signaling in regulating platelet function and its effect on assembly of cytoskeletal complexes are in progress.
Supported by American Heart Association Grant-in-Aid and an Established Investigatorship of the American Heart Association (L.K.J.) and by the National Institutes of Health grant HL53514 (L.K.J.)
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Lisa K. Jennings, Vascular Biology Program, University of Tennessee-Memphis, Rm H300, Coleman Bldg, 956 Court Ave, Memphis, TN 38163; e-mail: ljennings@utmem.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal