Abstract
The adhesive mechanisms leading to the mobilization of hematopoietic progenitor cells (HPCs) from the bone marrow into the blood are poorly understood. We report on a role for selectins and fucoidan in progenitor mobilization. Baseline levels of circulating HPCs are increased in endothelial selectin-deficient (P/E−/−) mice. Similar levels are observed when E-selectin null (E−/−) mice are treated with anti-P-selectin antibody or with fucoidan (which inhibits P- and L-selectin function). In particular, administration of 2 doses of fucoidan (25 mg/kg) over 6 hours produces profound mobilization of progenitors in wild-type mice and the response is greatly enhanced in E−/− and P/E−/− mice. Competitive reconstitution experiments reveal that fucoidan also elicits long-term (more than 6 months) repopulating stem cells. Mobilization assays using chimeric mice harboring L-selectin–deficient progenitors and wild-type progenitors expressing the green fluorescence protein suggest that L-selectin expression is not required but confers an advantage for fucoidan-induced mobilization. Sulfation is critical as desulfated fucoidan is ineffective. In addition, sulphogalactosylceramide (sulfatide) but not heparin can induce HPC mobilization. Our results indicate that administration of sulfated glycans, especially with concurrent inhibition of E-selectin function, represents a powerful novel method for rapid mobilization of long-term–repopulating stem cells. These findings may help elucidate the mechanisms of HPC trafficking during development and adult life.
Introduction
Circulating hematopoietic progenitor cells (HPCs) were first observed several decades ago,1,2 and it was subsequently shown that the number of circulating HPCs could be augmented by chemotherapy, endotoxin administration, or stress.3-5 It is now recognized that multiple agents from various families of cytokines, including hematopoietic growth factors, inflammatory cytokines, and chemokines, are capable of increasing circulating progenitor counts in vivo.6,7 These mobilized HPCs are routinely used in the clinic as a source of hematopoietic stem cells for bone marrow transplantation. Mobilization is achieved with variable kinetics; for example, granulocyte colony-stimulating factor (G-CSF) requires days to achieve peak circulating HPC numbers,8 whereas interleukin 8 (IL-8) acts within minutes and its effect is short-lived.9
The adhesive mechanisms leading to the extravasation of mature leukocytes to areas of inflammation have been well characterized in the past decade. The initial tethering and rolling steps are largely mediated by selectins and their ligands, whereas the β2 integrins and immunoglobulin superfamily members mediate the subsequent firm adhesion and diapedesis.10 11
Distinct among the families of molecules mediating adhesion events within the vasculature, the selectin family consists of 3 members that contain a calcium-binding lectin domain.12-14 E-selectin expression by endothelial cells is induced by inflammatory cytokines, whereas P-selectin is stored in granules of endothelial cells and platelets, and can be rapidly transported to the cell surface after stimulation. L-selectin is constitutively present on the microvilli of mature leukocytes as well as on hematopoietic progenitors.15 16
Specific carbohydrate or polypeptide modifications have been defined as critical for selectin binding.17 These include sialylation, fucosylation, and sulfation. Certain sulfated glycans have been shown to interact with P- and L-selectin but not E-selectin.18,19 A prototypic example is fucoidan, a sulfated polysaccharide extracted from the brown seaweed Fucus vesiculosus, which can inhibit leukocyte rolling and inflammatory responses in vivo.20-22
Insight into the function of adhesion molecules in vivo was provided by gene-targeted null mutations.23,24 Mice lacking both endothelial selectins, for example, display impaired leukocyte extravasation to inflammatory sites leading to spontaneous skin infections.25,26 Although all 3 selectins appear to participate in leukocyte recruitment to inflammatory sites, analyses of mice harboring all possible combinations of selectin null mutations suggest distinct roles for each selectin member in leukocyte homeostasis and trafficking.27,28 In addition to abnormalities in leukocyte rolling and extravasation, endothelial selectin-deficient mice also exhibit alterations in hematopoiesis, including severe leukocytosis, elevated levels of hematopoietic cytokines, splenomegaly, and increased splenic hematopoietic progenitors.25 These findings suggested that endothelial selectins expressed in the bone marrow play a role in hematopoiesis.
It is interesting that the bone marrow constitutively expresses E-selectin and VCAM-1.25,29,30 We recently showed that endothelial selectins and VCAM-1 were both required for progenitor-bone marrow (BM) endothelium interactions31 and for optimal recruitment of progenitors to the bone marrow after transplantation.32 Recent studies also indicate that immature (CD34+ CD38−) human progenitor cells express a functional P-selectin ligand.33CD34+ cells can roll on endothelial selectins in a flow chamber and their integrin-mediated arrest may be triggered by the chemokine SDF-1.34 However, it is unclear whether a similar cascade of adhesion events are operative during progenitor mobilization from the bone marrow. Papayannoupoulou and colleagues35 have shown that blocking either the α4 integrin or VCAM-1 increased circulating blood progenitors and that this process required an intact c-kit ligand.36 CD44 has also been suggested to be involved in this process because an anti-CD44 antibody increased circulating colony-forming units-spleen (CFU-S; Vermeulen et al37).
Here, we evaluated the role of selectins and sulfated glycans in hematopoietic progenitor mobilization. We show that endothelial selectin deficiency or blockade increases circulating HPCs. Sulfated selectin inhibitors—fucoidan in particular—induce a rapid and powerful mobilization of hematopoietic progenitors. While enhanced in the absence of endothelial selectins, the effect of fucoidan appears to be also mediated through selectin-independent mechanisms.
Materials and methods
Animals and reagents for in vivo studies
E−/− and P/E−/− mice were generated by gene targeting25 and were backcrossed at least 7 times into the C57BL/6 background. Wild-type C57BL/6 and C57BL/6-Ly5.2 congenic mice were purchased from Charles River laboratories (Frederick Cancer Research Center, Frederick, MD). Breeding stocks of wild-type and L−/− mice, also generated by gene targeting27 were kindly provided by Drs S. Robinson and R. O. Hynes (M.I.T., Cambridge, MA). Both wild-type and L−/− animals were descendants of F2 intercrosses between C57BL/6 and 129Sv strains. Littermates from the F2 generation of this intercross were genotyped by polymerase chain reaction (PCR) to establish wild-type and L−/− matings. Breeding pairs of transgenic mice expressing the “enhanced” green fluorescent protein (EGFP) under the chicken beta-actin promoter,38 backcrossed into the C57BL/6 background, were a kind gift from Dr M. Okabe (Osaka University, Japan). Mice heterozygous for the EGFP transgene were bred with wild-type C57BL/6 mice and were identified on weaning by observing the green fluorescence of the ears and tails using a portable ultraviolet lamp. All animals used in this study were matched for sex and age (6-12 weeks). Mice were housed at Mount Sinai School of Medicine in the East Building barrier facility. Experimental procedures performed on the animals were approved by the Animal Care and Use Committee of Mount Sinai.
Rat antimouse P-selectin MAb RB40.34 (IgG1), rat IgG1, and rat IgG2a control antibodies were obtained from Pharmingen (San Diego, CA). Commercially obtained antibodies were endotoxin-tested (endotoxin level less than or equal to 0.01 ng/μg of protein byLimulus amebocyte lysate assay [LAL]). Rat anti-L-selectin (IgG2a), purified from supernatant of a MEL-14–producing hybridoma cell line (American Type Culture Collection, Rockville, MD), was a kind gift from Dr J. Frenette (Laval University, Quebec, Canada). Fucoidan (Fluka Lot No 385468/1) was resuspended in endotoxin-free phosphate-buffered saline (PBS). Endotoxin levels in antibody and fucoidan preparations were tested by LAL assay (sensitivity 0.06 EU/mL; BioWhittaker, Walkersville, MD), and if detectable, contaminating endotoxin was removed using a polymixin B column (Detoxi-Gel, Pierce, Rockford, IL).
Desulfation, carbohydrate, and sulfate analyses
Desulfation of sulfated polysaccharides was accomplished by solvolysis as described by Vieira et al.39 Briefly, fucoidan (100 mg) was dissolved in 10 mL of water and mixed with 1 g of Dowex 50-W H+ (Sigma). The solution was then neutralized with pyridine (EM Science, Gibbstown, NJ) and lyophilized. The resulting pyridium salt was dissolved in 10 mL of 9:1 (v/v) dimethyl sulfoxide:methanol and incubated at 80°C for 4 hours. Desulfated sugars were then dialysed extensively against PBS. The extent of desulfation was determined by barium chloride precipitation as described.40 Potential endotoxin contamination of the desulfated material was removed using a polymixin B column and carbohydrate content was assessed by the method of Dubois et al.41
Isolation of cells and colony-forming units in culture assays
Blood was harvested by retro-orbital sampling of mice anesthetized with tribromoethanol and collected in polypropylene tubes containing EDTA. Blood counts were obtained using an automated cell counter (Serono-Baker Diagnostics, Allentown, PA) and differential counts were determined from Wright-stained smears. Mononuclear cells were isolated by underlaying 400 μL of blood diluted in 3 volumes of PBS with lympholyte M (Cedarlane Laboratories, Hornby, Ontario, Canada) and by centrifugation at room temperature at 280g for 30 minutes. Contaminating erythrocytes (RBCs) were lysed in 0.8% NH4Cl and the remaining nucleated cells were washed thrice in RPMI. Bone marrow cells were harvested by aseptically flushing both femora of each animal in RPMI using a 21-gauge needle. A single cell suspension was obtained by gently aspirating several times with the same needle and syringe. Cells of both femora were pooled and the volume of each cell suspension was determined with a graduated pipette.
For CFU-C assays, one volume of hematopoietic cells was added to 9 volumes of methocult M3434 media (Stemcell Technologies, Vancouver, BC, Canada). Cells were plated in triplicate assays. Burst-forming units-erythroid (BFU-E) and granulocyte-macrophage colony-forming units (GM-CFUs) were scored on days 7-8. GM-CFUs and BFU-E showed similar changes in mobilization studies, therefore only the total numbers of CFU-C are reported. For mobilization experiments in chimeric mice (see below), CFU-Cs were grown in 0.8% methylcellulose (methocult M3100; Stemcell Technologies) containing 30% fetal bovine serum (FBS) (Intergen, Westchester), 1% bovine serum albumin (BSA), 10−4 mol/L 2-mercaptoethanol and conditioned medium (20% v/v) from WEHI3 cells (containing IL-3), HM-5 cell line (containing GM-CSF) and BHK/MKL (Baby Hamster Kidney cell line stably transfected with an expression vector containing the cDNA encoding for the secreted form of murine stem cell factor); cell lines were a kind gift from Dr S. Tsai, Mount Sinai School of Medicine.
Progenitor mobilization
Progenitor mobilization using antiadhesion antibody was accomplished by administering to wild-type and E−/− mice anti-P-selectin antibody or isotype-matched IgG control at a dose of 1 mg/kg, via the tail vein, daily for 3 days. Blood and bone marrow cells were collected 24 hours after the last dose and assayed for progenitor content as above. Fucoidan-induced progenitor mobilization was induced in wild-type or selectin-deficient mice by administering fucoidan at 25 mg/kg intraperitoneally (ip). Our preliminary studies showed that fucoidan is systemically absorbed via this route because this dose can inhibit leukocyte rolling in the cremaster muscle (L.W. and P.S.F., unpublished observations). In the first protocol, fucoidan or vehicle (PBS) were administered at 25 mg/kg intraperitoneally for 6 doses. The first 2 doses were given on day 0 (approximately12:00 and 20:00), 3 doses on day 1 (approximately 8:00, 12:00, and 20:00) and the final dose on day 2 ( approximately 8:00). In the second protocol, only 2 doses (25 mg/kg ip) were given over 6 hours. In both protocols, blood and femoral bone marrow cells were harvested 2 hours after the last dose.
Mobilization in chimeric mice
We developed an assay to compare directly the ability of L−/− and L+/+ progenitors to be mobilized after fucoidan treatment. Mice chimeric for L-selectin and EGFP expression were generated by adoptive bone marrow transfer. P/E−/− animals were used as recipients and were lethally irradiated (12.0 Gy) in 2 split doses, 3 hours apart, from a cesium source (Model I-68A, J.L. Shepherd, San Fernando, CA). Irradiated P/E−/− mice were then injected via the lateral tail vein with a mixture of nucleated bone marrow cells obtained from L−/− or wild-type control mice combined with bone marrow cells from EGFP trangenic mice (2 × 106 EGFP bone marrow nucleated cells mixed with 2 × 106 nonfluorescent bone marrow cells [L+/+ or L−/−]). After transplantation, mice were kept in a microisolator unit and fed ad libitum with sterile chow food and water. To avoid any contribution to circulating progenitors from the spleen, mice were splenectomized 2 weeks after BM transplantation as described by Frenette et al32 and progenitor mobilization was induced as described above after an additional recovery period of at least 1 month.
Long-term competitive reconstitution
To assess the ability of mobilized progenitors to competitively repopulate the bone marrow of a lethally irradiated host, progenitors were mobilized by treating E−/− mice (whose leukocytes bear the CD45.2 antigen) using 2 doses of fucoidan or vehicle as above (see scheme in Figure 4A). Blood from 5 to 6 animals per group was harvested and pooled 2 hours after the last dose. Erythrocytes were removed by 2 rounds of 0.8% NH4Cl lysis (1 part blood: 9 parts buffer) and washed thrice in RPMI. Fresh bone marrow competitor cells were obtained from femora of Ly 5.2 congenic mice (whose leukocytes harbor the CD45.1 antigen). CD45.1 bone marrow nucleated cells (1 × 105 per recipient mouse) were mixed with blood nucleated cells (1 mL per recipient mouse) from fucoidan or vehicle-treated E−/− mice (CD45.2) and injected into the tail vein of lethally irradiated CD45.1 recipient mice. Mice were housed in a microisolator and fed sterile food and sterile water containing antibiotics (during the first week only; trimethoprim, 24 mg/dL and sulfamethoxazole, 120 mg/dL). The proportion of leukocytes bearing CD45.1 and CD45.2 antigens was determined monthly after transplantation by flow cytometry. Competitive repopulating units (CRUs) were calculated as follows: CRU = % (C) / (100-%) where % is the measured percentage of donor cells and C is the number of fresh competitor marrow cells per 105.42
Flow cytometry
To stain the leukocyte CD45 antigen, whole blood (150 μL) was incubated, after blockade of Fc receptors with rat antimouse CD16/CD32 monoclonal antibody 1:50, with fluorescein (FITC)-conjugated mouse anti-CD45.2 and biotinylated mouse anti-CD45.1 antibodies 1:50 (all from Pharmingen). Blood cells were washed once in PBS/BSA 0.06% and stained with phycoerythrin (PE)-conjugated steptavidin (Jackson Immunoresearch, West Grove, PA). Erythrocytes were lysed in NH4Cl and the remaining leukocytes were washed thrice in PBS/BSA. To assess L-selectin expression in transplanted P/E−/− mice, 50 μL of whole blood was incubated with MEL-14 antibody (1:50 of 2 mg/mL), washed with PBS/BSA and stained with FITC-conjugated goat antirat IgG 1:250 (Cappel, Durham, NC). RBCs were then lysed and leukocytes were washed. In some experiments, day 8 CFU-Cs were individually picked under microscopy in PBS/BSA containing 5 mmol/L EDTA. Cells were dissociated, washed once, resuspended in PBS containing 10% mouse serum, and stained for L-selectin expression as above. Analysis of 10 000 events was performed on a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA).
Statistical analysis
All values are reported as mean ± standard error of the mean. Statistical significance for 2 unpaired groups was assessed by the Student t test. Multiple comparisons were analyzed using 1-way analysis of variance (ANOVA), Bonferroni's test.
Results
Absence or inhibition of endothelial selectins increases circulating hematopoietic progenitors
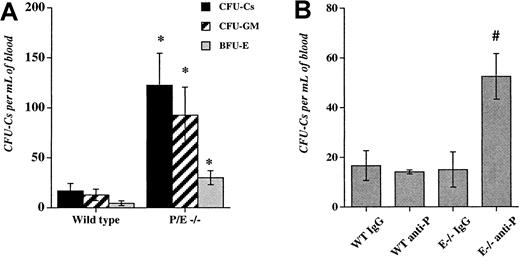
We previously observed an increase in the number of residual circulating progenitors in P/E−/− mice after bone marrow transplantation.32 To evaluate the influence of endothelial selectins on circulating progenitors during steady-state conditions, we assayed HPCs in the blood of wild-type and P/E−/− mice. As shown in Figure 1A, circulating HPCs were increased approximately 7-fold in P/E−/− mice compared with wild-type controls. Higher numbers of circulating progenitors were also observed in splenectomized P/E−/− animals (not shown), suggesting that HPCs did not originate from their enlarged spleens.25Given the multiple abnormalities present in endothelial selectin knockouts, it was possible that deficiency from birth may have indirectly affected the numbers of circulating HPCs.
Deficiency or inhibition of endothelial selectins increase circulating hematopoietic progenitors.
(A), The low-density fraction of blood cells from wild-type and P- and E-selectin-deficient mice was isolated and plated to assay colony-forming units in culture (CFU-Cs). Colonies were scored using an inverted microscope after 7 days in culture. n = 4-5; *P < .05 compared with wild-type. (B), Wild-type (WT) and E-selectin-deficient (E−/−) mice were injected intravenously with either anti-P-selectin antibody or isotype-matched IgG control (1 mg/kg) for 3 consecutive days. Twenty-four hours after the last dose, low-density blood cells were isolated and assayed for CFU-C content. n = 3-4; # P < .05 compared with the other groups.
Deficiency or inhibition of endothelial selectins increase circulating hematopoietic progenitors.
(A), The low-density fraction of blood cells from wild-type and P- and E-selectin-deficient mice was isolated and plated to assay colony-forming units in culture (CFU-Cs). Colonies were scored using an inverted microscope after 7 days in culture. n = 4-5; *P < .05 compared with wild-type. (B), Wild-type (WT) and E-selectin-deficient (E−/−) mice were injected intravenously with either anti-P-selectin antibody or isotype-matched IgG control (1 mg/kg) for 3 consecutive days. Twenty-four hours after the last dose, low-density blood cells were isolated and assayed for CFU-C content. n = 3-4; # P < .05 compared with the other groups.
To address this issue, we used mice lacking only E-selectin that have no significant phenotype.25,26,43 To induce a transient inhibition of one or both endothelial selectins, we injected into the tail vein of wild-type and E−/− mice either an anti-P-selectin antibody or an isotype-matched control IgG. Mice were treated daily for 3 consecutive days and blood and bone marrow were harvested 24 hours after the last dose. This dose schedule using anti-α4 integrin was previously shown to increase circulating HPCs in mice.44Anti-P-selectin antibody administration in wild-type mice or IgG administration in E−/− animals did not significantly alter peripheral neutrophil counts and HPC numbers in comparison with wild-type mice treated with IgG. However, anti-P-selectin antibody treatment of E−/− animals significantly increased circulating progenitors (3.5-fold;P < .006 ANOVA, Figure 1B), as well as the number of mature blood leukocytes (2.8-fold, not shown). There was no significant difference in the number of nucleated cells and HPCs in the bone marrow among the treatment groups (not shown).
Fucoidan rapidly mobilizes hematopoietic progenitors
As mentioned previously, fucoidan can inhibit the function of P-selectin and L-selectin but not E-selectin.18 19 To assess whether this selectin inhibitor would also augment circulating progenitor counts, we treated E−/− mice with fucoidan or with vehicle (PBS) as control for 6 doses over 48 hours. This way, fucoidan administration to E−/− mice transiently inhibits all 3 selectins. Blood and bone marrow were collected 2 hours after the last dose and progenitor content was assessed. As shown in Figure2A, fucoidan treatment increased the number of circulating progenitors in E−/− animals, compared with vehicle alone. Together, the data using antibody or fucoidan inhibition indicate that short-term blockade of more than one selectin increases the numbers of circulating progenitors. Unlike antibody treatment, fucoidan also reduced the numbers of bone marrow nucleated cells (not shown), suggesting that fucoidan might act through mechanism(s) other than blocking P-selectin function.
Fucoidan-induced hematopoietic progenitor mobilization in wild-type and endothelial selectin-deficient mice.
(A), E−/− mice were administered 6 doses (25 mg/kg ip) of fucoidan or vehicle (PBS) over 2 days. Two hours after the last dose, circulating hematopoietic progenitors were assayed. n = 4. (B), Mice were injected with 2 doses (25 mg/kg) of fucoidan (FUC) or PBS over 6 hours. Numbers of circulating progenitors in WT, E−/−, and P/E−/− mice, n = 3-10, *P < .05; #P < .002.
Fucoidan-induced hematopoietic progenitor mobilization in wild-type and endothelial selectin-deficient mice.
(A), E−/− mice were administered 6 doses (25 mg/kg ip) of fucoidan or vehicle (PBS) over 2 days. Two hours after the last dose, circulating hematopoietic progenitors were assayed. n = 4. (B), Mice were injected with 2 doses (25 mg/kg) of fucoidan (FUC) or PBS over 6 hours. Numbers of circulating progenitors in WT, E−/−, and P/E−/− mice, n = 3-10, *P < .05; #P < .002.
Despite its relatively short half-life, fucoidan appeared equally effective in increasing the numbers of circulating HPCs as antiselectin antibody administration (Figures 1B and 2A). To investigate whether fewer doses of fucoidan given at a shorter interval could achieve similar mobilization, E−/− and wild-type mice were treated with 2 doses of the polysaccharide or vehicle over 6 hours. Blood and bone marrow were harvested 2 hours after the second dose. We found that treatment of E−/− mice with fucoidan for only 6 hours greatly increased (37-fold; P < .002) the number of circulating progenitors, compared with vehicle-treated animals (Figure 2B). The effect was more modest (6.6-fold; P < .04) in wild-type mice (Figure 2B), indicating that the absence of E-selectin augments the number of circulating HPCs after fucoidan treatment. A single dose of the sulfated polysaccharide (25 mg/kg) also produced HPC mobilization, albeit less drastically (12.5 ± 5.0 vs 120 ± 35 for E−/− treated with PBS and E−/− mice treated with fucoidan, respectively; n = 2-3). Treatment of E−/− mice with higher doses of fucoidan (2-dose schedule) did not significantly elicit more circulating progenitors (475 ± 128 and 266 ± 51 for 50 and 100 mg, respectively; n = 3), whereas administration of fucoidan for 2 or 3 consecutive days (25 mg 2-dose schedule) was less effective than the single day 2-dose regimen (not shown). Thus, our results suggest that the short (2 doses over 6 hours) protocol is more effective than multiple doses over more than 1 day (Figure 2A,B). Consistent with a critical role for selectins in leukocyte trafficking, fucoidan also drastically increased peripheral leukocyte numbers in E−/− mice and, to a lesser extent, in wild-type animals (Table1). Interestingly, a significant reduction in circulating platelet counts was seen regardless of selectin expression (Table 1).
Effect of fucoidan treatment on blood cell counts
| . | WBC (per μL) . | Neutrophils (per μL) . | Lymphocytes (per μL) . | Monocytes (per μL) . | Eosinophils (per μL) . | Platelets (×103/μL) . |
|---|---|---|---|---|---|---|
| WT PBS | 3 540 ± 256 | 1 022 ± 184 | 2 374 ± 218 | 63 ± 12 | 74 ± 17 | 920 ± 47 |
| WT FUC | 8 020 ± 1094* | 3 078 ± 617* | 4 816 ± 567* | 65 ± 30 | 101 ± 71 | 533 ± 132* |
| E−/− PBS | 3 120 ± 503 | 720 ± 144 | 2 328 ± 398 | 45 ± 15 | 34 ± 17 | 1 019 ± 26 |
| E−/− FUC | 23 333 ± 1 466* | 9 373 ± 901* | 13 695 ± 1 196* | 230 ± 86* | 33 ± 33 | 558 ± 44* |
| P/E−/− PBS | 9 125 ± 1 916 | 5 248 ± 1 239 | 3 423 ± 891 | 132 ± 50 | 71 ± 28 | 966 ± 66 |
| P/E−/− FUC | 26 500 ± 5 804* | 13 140 ± 4 870 | 12 355 ± 1 827* | 704 ± 207* | 177 ± 106 | 561 ± 66* |
| P/E−/− (spl) PBS | 23 016 ± 5 258 | 11 722 ± 2 692 | 12 353 ± 2 830 | 376 ± 55 | 244 ± 211 | 1312 ± 81 |
| P/E−/− (spl) FUC | 40 550 ± 8 584 | 22 119 ± 4 684 | 19 065 ± 3 417 | 485 ± 191 | 216 ± 137 | 954 ± 160 |
| . | WBC (per μL) . | Neutrophils (per μL) . | Lymphocytes (per μL) . | Monocytes (per μL) . | Eosinophils (per μL) . | Platelets (×103/μL) . |
|---|---|---|---|---|---|---|
| WT PBS | 3 540 ± 256 | 1 022 ± 184 | 2 374 ± 218 | 63 ± 12 | 74 ± 17 | 920 ± 47 |
| WT FUC | 8 020 ± 1094* | 3 078 ± 617* | 4 816 ± 567* | 65 ± 30 | 101 ± 71 | 533 ± 132* |
| E−/− PBS | 3 120 ± 503 | 720 ± 144 | 2 328 ± 398 | 45 ± 15 | 34 ± 17 | 1 019 ± 26 |
| E−/− FUC | 23 333 ± 1 466* | 9 373 ± 901* | 13 695 ± 1 196* | 230 ± 86* | 33 ± 33 | 558 ± 44* |
| P/E−/− PBS | 9 125 ± 1 916 | 5 248 ± 1 239 | 3 423 ± 891 | 132 ± 50 | 71 ± 28 | 966 ± 66 |
| P/E−/− FUC | 26 500 ± 5 804* | 13 140 ± 4 870 | 12 355 ± 1 827* | 704 ± 207* | 177 ± 106 | 561 ± 66* |
| P/E−/− (spl) PBS | 23 016 ± 5 258 | 11 722 ± 2 692 | 12 353 ± 2 830 | 376 ± 55 | 244 ± 211 | 1312 ± 81 |
| P/E−/− (spl) FUC | 40 550 ± 8 584 | 22 119 ± 4 684 | 19 065 ± 3 417 | 485 ± 191 | 216 ± 137 | 954 ± 160 |
Mice were treated with 2 doses of fucoidan (FUC; 25 mg/kg) or vehicle (phosphate-buffered saline [PBS]) over 6 hours. Leukocyte and platelet counts were assessed using an automated cell counter and leukocyte subsets were determined using Wright-stained smears.
WT, wild-type; E−/−, E-selectin-deficient; P/E−/−, P- and E-selectin-deficient; spl, splenectomized.
P < .05 compared with PBS, n = 3-10.
To evaluate the role of both endothelial selectins in this activity, mice deficient in P- and E-selectins were treated with 2 doses of fucoidan. As shown in Figure 2B, P/E−/− mice displayed a drastic increase in circulating HPCs, compared with the baseline elevation in vehicle-treated P/E−/− mice. Mobilized HPCs did not originate from splenic release since splenectomized P/E−/− animals showed similar results (329 ± 57 vs 1367 ± 248 for splenectomized P/E−/− mice treated with PBS or fucoidan, respectively; n = 3-4,P < .05). These data suggest that fucoidan rapidly mobilizes HPCs from the bone marrow and that its effect appears greater when endothelial selectins are absent. Because profound mobilization is seen in the absence of both endothelial selectins, our results also suggest that fucoidan acts through other mechanisms.
L-selectin may also participate in fucoidan-induced mobilization
The leukocyte selectin (L-selectin) is expressed on immature progenitor cells.15,16 Because it binds fucoidan, L-selectin is a potential candidate receptor to participate in progenitor mobilization. To test the role of L-selectin in fucoidan-induced HPC mobilization, we treated L-selectin–deficient (L−/−) and wild-type control mice using the 2-dose fucoidan administration protocol. As shown in Table2, fucoidan mobilized progenitors equally well in the absence of L-selectin. Because the spleen of L−/− animals is slightly enlarged,45 we also evaluated progenitor numbers in the spleen of mice treated with vehicle and fucoidan. Although fucoidan administration to L−/− animals tended to increase splenic progenitor numbers (P = .08; Table 2), there was no significant difference in baseline (PBS-treated) splenic progenitor counts between L−/− and wild-type mice. Fucoidan treatment of L−/− mice also produced leukocytosis, reduced platelet counts, and lowered bone marrow nucleated cell numbers to an extent similar to their wild-type counterparts (Table 2). Although the above results suggest no role for L-selectin in fucoidan-induced HPC mobilization, it is possible that other adhesion molecules have compensated in L−/− animals and masked a potential difference.
Cell and progenitor counts after mobilization in L-selectin-deficient mice
| . | Blood CFU-Cs (per mL) . | BM CFU-Cs (×103/femur) . | Spleen CFU-Cs (×103/spleen) . | WBC (per μL) . | BMNC (×106/femur) . | Platelets (×103/μL) . |
|---|---|---|---|---|---|---|
| WT PBS | 19 ± 6 | 72.5 ± 3.8 | 9.8 ± 0.8 | 2 700 ± 441 | 17.4 ± 0.9 | 853 ± 49 |
| WT fucoidin | 271 ± 61* | 65.4 ± 2.5 | 10.7 ± 1.8 | 22 510 ± 3 141* | 14.8 ± 0.7* | 435 ± 45* |
| L−/− PBS | 19 ± 12 | 62.1 ± 5.1 | 8.4 ± 0.9 | 3 460 ± 533 | 16.2 ± 0.6 | 837 ± 44 |
| L−/− fucoidin | 269 ± 63* | 70.8 ± 4.9 | 11.4 ± 1.3 | 19 033 ± 1 777* | 13.7 ± 0.5* | 448 ± 46* |
| . | Blood CFU-Cs (per mL) . | BM CFU-Cs (×103/femur) . | Spleen CFU-Cs (×103/spleen) . | WBC (per μL) . | BMNC (×106/femur) . | Platelets (×103/μL) . |
|---|---|---|---|---|---|---|
| WT PBS | 19 ± 6 | 72.5 ± 3.8 | 9.8 ± 0.8 | 2 700 ± 441 | 17.4 ± 0.9 | 853 ± 49 |
| WT fucoidin | 271 ± 61* | 65.4 ± 2.5 | 10.7 ± 1.8 | 22 510 ± 3 141* | 14.8 ± 0.7* | 435 ± 45* |
| L−/− PBS | 19 ± 12 | 62.1 ± 5.1 | 8.4 ± 0.9 | 3 460 ± 533 | 16.2 ± 0.6 | 837 ± 44 |
| L−/− fucoidin | 269 ± 63* | 70.8 ± 4.9 | 11.4 ± 1.3 | 19 033 ± 1 777* | 13.7 ± 0.5* | 448 ± 46* |
Wild-type (WT) and L-selectin-deficient (L−/−) mice were treated with 2 sequential doses of fucoidan (25 mg/kg). Six hours after the initial dose, blood, bone marrow (BM), and spleen were harvested. Blood nucleated cell (white blood cell [WBC]), platelets, and bone marrow nucleated cell (BMNC) counts were determined using an automated cell counter. An aliquot of cells was plated in methylcellulose media and read 8 days after cultivation to assess progenitor counts.
P < .05; n = 9-10 mice, except spleen where n = 7.
CFU-Cs, colony-forming units in culture.
To further evaluate the function of L-selectin in HPC mobilization, we developed a competitive assay to compare within the same animal the ability of L−/− and wild-type (L+/+) progenitors to be mobilized after fucoidan treatment. In this assay, chimeric animals harboring green fluorescent progenitors (from transgenic mice expressing the EGFP [EGFP-Tg]) and nonfluorescent wild-type control (L+/+) or L−/− progenitors were generated by bone marrow transplantation. To generate chimeric animals, bone marrow nucleated cells from L−/−, L+/+, and EGFP-Tg mice were harvested, and a mixture containing equal numbers of nucleated cells (1 × 106 cells each) from L+/+ and EGFP-Tg or L−/− and EGFP-Tg were injected into lethally irradiated P/E−/− mice. The proportion of “green” progenitors in the injected mixtures was determined by assessment of colonies plated from the injected cells using an inverted microscope equipped with both light and fluorescent sources, and was similar between both chimeric samples (45.7% ± 2.6% “green” CFU-Cs in L+/+/ EGFP and 45.7% ± 6.3% in L−/−/EGFP). Once steady-state hematopoiesis is established, L+/+ nonfluorescent and green fluorescent progenitors should have an equal chance of exiting the bone marrow and the percentage of green colonies from the blood should be approximately the same as that from the bone marrow (therefore, the ratio % green CFU-Cs blood / % green CFU-Cs BM ≈ 1).
Progenitor mobilization in these chimeric mice was induced by administering 2 doses of fucoidan (25 mg/kg) or vehicle alone. Blood and bone marrow nucleated cells were plated to assay their progenitor content. After 8 days of culture, the numbers of nonfluorescent and green fluorescent colonies were determined (Figure3A,B). To verify L-selectin expression in the chimeras, CFU-Cs were sampled, stained for L-selectin, and analyzed by FACS. As predicted, both “green” and nonfluorescent colonies derived from L+/+/EGFP chimeras expressed L-selectin at similar levels, whereas in L−/−/EGFP chimeras, the “green” CFU-Cs stained positively for L-selectin, whereas the vast majority of nonfluorescent colonies did not express L-selectin (not shown). In mice transplanted with bone marrow cells expressing L-selectin (L+/+/EGFP), approximately half of the CFU-Cs were green fluorescent and the ratios of percentage green colonies in the blood over percentage green colonies in the bone marrow were approximately equal to 1 even after fucoidan treatment (Figure 3C). This result indicates that EGFP expression does not influence the egress of HPCs from the bone marrow. However, in chimeric mice harboring L−/− and EGFP progenitors, fucoidan administration significantly decreased the proportion of green progenitors that occupied the bone marrow (52% ± 3% in PBS-treated group vs 38% ± 3% in fucoidan-treated group, P = .01), whereas it tended to increase the proportion of green progenitors in the blood (36 ± 8 in PBS-treated group vs 53 ± 4 in fucoidan-treated group,P = .09). The blood over bone marrow progenitor ratios in chimeric L−/−/EGFP mice were significantly altered after fucoidan administration (Figure 3C). Thus, these results suggest that in a competitive environment, progenitors expressing L-selectin are preferentially mobilized from the bone marrow into the blood after administration of fucoidan and that L-selectin may participate in this activity.
Progenitor mobilization in chimeric mice.
Animals chimeric for L-selectin (L+/+ or L−/−), and green fluorescence protein (EGFP) expression were generated by adoptive bone marrow transfer. Lethally irradiated P- and E-selectin doubly deficient recipient mice were injected with a mixture of bone marrow nucleated cells obtained from transgenic mice expressing EGFP and wild-type (L+/+) or L-selectin-deficient (L−/−) mice. Two weeks after transplantation, the mice were splenectomized and allowed to recover for 1 month. EGFP expressing progenitors also express L-selectin and can be distinguished from L+/+ and L−/− progenitors by their green fluorescence. L+/+ / EGFP and L−/−/EGFP splenectomized chimeric mice were then treated with 2 doses of fucoidan (FUC) or PBS over 6 hours. Blood mononuclear and bone marrow cells were plated to assess their progenitor content. The numbers of nonfluorescent and green fluorescent progenitors were determined using an inverted microscope equipped with a fluorescence source. A representative area containing colonies grown in methylcellulose media is shown using (A) light and (B) both light and fluorescence microscopy. The numbers of green fluorescent (arrow) and nonfluorescent (L+/+ in this photograph) colonies (arrowhead) were determined after 8 days of culture. (C), The ratios of green colonies in the blood and bone marrow were calculated by dividing the percentage green colonies in the blood by the percentage green colonies in the bone marrow. n = 3-5; *P < .05 compared with PBS-treated.
Progenitor mobilization in chimeric mice.
Animals chimeric for L-selectin (L+/+ or L−/−), and green fluorescence protein (EGFP) expression were generated by adoptive bone marrow transfer. Lethally irradiated P- and E-selectin doubly deficient recipient mice were injected with a mixture of bone marrow nucleated cells obtained from transgenic mice expressing EGFP and wild-type (L+/+) or L-selectin-deficient (L−/−) mice. Two weeks after transplantation, the mice were splenectomized and allowed to recover for 1 month. EGFP expressing progenitors also express L-selectin and can be distinguished from L+/+ and L−/− progenitors by their green fluorescence. L+/+ / EGFP and L−/−/EGFP splenectomized chimeric mice were then treated with 2 doses of fucoidan (FUC) or PBS over 6 hours. Blood mononuclear and bone marrow cells were plated to assess their progenitor content. The numbers of nonfluorescent and green fluorescent progenitors were determined using an inverted microscope equipped with a fluorescence source. A representative area containing colonies grown in methylcellulose media is shown using (A) light and (B) both light and fluorescence microscopy. The numbers of green fluorescent (arrow) and nonfluorescent (L+/+ in this photograph) colonies (arrowhead) were determined after 8 days of culture. (C), The ratios of green colonies in the blood and bone marrow were calculated by dividing the percentage green colonies in the blood by the percentage green colonies in the bone marrow. n = 3-5; *P < .05 compared with PBS-treated.
Long-term bone marrow repopulating cells are mobilized after fucoidan treatment
To evaluate the ability of fucoidan-mobilized progenitors to reconstitute the bone marrow of a lethally irradiated recipient, we used congenic mice that harbor a leukocyte antigen (CD45.1) distinguishable from that of selectin knockout mice backcrossed in the C57BL/6 background (CD45.2) by FACS analysis. Mice lacking E-selectin were treated with either fucoidan or vehicle for 2 doses over 6 hours (Figure 4A). After the treatment period, blood was harvested from the retroorbital venous plexus, pooled (5 mice per group), and erythrocytes were lysed. Concomitantly, fresh bone marrow cells were obtained from the femora of CD45.1 congenic mice. These fresh bone marrow cells were used as competitor cells and were mixed with mobilized blood nucleated cells (1 × 105 bone marrow competitor cells [CD45.1] mixed with nucleated cells from 1 mL of blood [CD45.2]). The 1 × 105 bone marrow cells contain sufficient numbers of HPCs to allow recipient mice to survive if no long-term reconstituting stem cells were present in the tested sample. The cell mixture was injected into the tail vein of lethally irradiated CD45.1 mice. Transplanted mice were allowed to recover for 1 month, at which point CD45 expression on leukocytes was assessed by flow cytometry. In 2 independent experiments pooled in Figure 4B, fucoidan-mobilized progenitors contributed greatly to the blood leukocyte composition at all timepoints tested. At 6 months, the competitive repopulating ability of fucoidan-mobilized blood cells was approximately 700-fold greater than that of the PBS group. In addition, CD45.2-positive cells were represented among the various subsets of leukocytes defined by light scatter characteristics (Figure 4C). These data indicate that administration of fucoidan can indeed mobilize long-term repopulating stem cells.
Competitive reconstitution.
(A), E−/− mice, which harbor the leukocyte antigen CD45.2, were treated with 2 doses of fucoidan or PBS. Two hours after the last dose, blood was harvested and the volume measured. Erythrocytes were lysed and nucleated cells were pooled within each group. Concomitantly, bone marrow nucleated cells from congenic mice expressing the leukocyte antigen CD45.1 were obtained. Blood (CD45.2) and bone marrow (CD45.1) nucleated cells were mixed and injected into lethally irradiated CD45.1 congenic mice. Each recipient received nucleated cells from 1 mL of blood obtained from PBS or fucoidan-treated E−/− mice with 1 × 105 bone marrow cells (competitor) from normal CD45.1 donor. A sample of blood from transplanted mice was obtained monthly from the retroorbital venous plexus. Leukocytes were stained for both CD45.1 and CD45.2, and leukocyte expression was assessed by flow cytometric analysis. (B), Competitive repopulating units (CRUs) of donor (CD45.2) fucoidan-mobilized and PBS blood. (C) Percentage of CD45.2 expression in total leukocytes and among the various leukocyte subsets, as judged by their scatter characteristics, 6 months after transplantation in mice receiving blood nucleated cells from E−/− mice treated with PBS or fucoidan. Donor mice, n = 10; recipients, n = 6-7.
Competitive reconstitution.
(A), E−/− mice, which harbor the leukocyte antigen CD45.2, were treated with 2 doses of fucoidan or PBS. Two hours after the last dose, blood was harvested and the volume measured. Erythrocytes were lysed and nucleated cells were pooled within each group. Concomitantly, bone marrow nucleated cells from congenic mice expressing the leukocyte antigen CD45.1 were obtained. Blood (CD45.2) and bone marrow (CD45.1) nucleated cells were mixed and injected into lethally irradiated CD45.1 congenic mice. Each recipient received nucleated cells from 1 mL of blood obtained from PBS or fucoidan-treated E−/− mice with 1 × 105 bone marrow cells (competitor) from normal CD45.1 donor. A sample of blood from transplanted mice was obtained monthly from the retroorbital venous plexus. Leukocytes were stained for both CD45.1 and CD45.2, and leukocyte expression was assessed by flow cytometric analysis. (B), Competitive repopulating units (CRUs) of donor (CD45.2) fucoidan-mobilized and PBS blood. (C) Percentage of CD45.2 expression in total leukocytes and among the various leukocyte subsets, as judged by their scatter characteristics, 6 months after transplantation in mice receiving blood nucleated cells from E−/− mice treated with PBS or fucoidan. Donor mice, n = 10; recipients, n = 6-7.
Critical role of sulfation
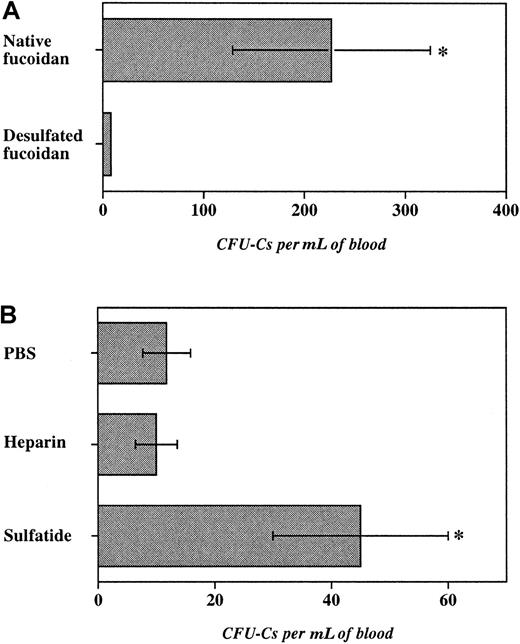
To investigate the role of sulfate groups in fucoidan-induced HPC mobilization, fucoidan was desulfated by solvolysis. This treatment led to approximately 99% desulfation as measured by barium sulfate precipitation. E−/− mice were treated with 2 doses (25 mg/kg) of desulfated or native fucoidan. As shown in Figure5A, desulfated fucoidan was ineffective in inducing progenitor mobilization, compared with native fucoidan. Importantly, desulfated fucoidan did not produce leukocytosis and thrombocytopenia suggesting that sulfate groups are critical for the various hematologic effects of fucoidan in mice.
Role of fucoidan sulfation and of other sulfated glycans.
Progenitor mobilization was induced in E−/− mice with 2 doses of glycans over 6 hours. The number of progenitors per milliliter of blood recovered after administration of (A) native or desulfated fucoidan (25 mg/kg), (B) heparin (100 units per mouse), sulfatide (25 mg/kg), or PBS. n = 3-6, *P < .05.
Role of fucoidan sulfation and of other sulfated glycans.
Progenitor mobilization was induced in E−/− mice with 2 doses of glycans over 6 hours. The number of progenitors per milliliter of blood recovered after administration of (A) native or desulfated fucoidan (25 mg/kg), (B) heparin (100 units per mouse), sulfatide (25 mg/kg), or PBS. n = 3-6, *P < .05.
Mammalian cells also harbor sulfated glycans that have been shown to bind selectins. For example, cell membrane-associated sulfated glycosphingolipid (3′-sulphogalactosylceramide, or sulfatide) is found on myeloid cells46 and heparin is characteristically stored by mast cells.47 Although not fucosylated, both sulfatide and heparin, like fucoidan, have been shown to interact with P- and L-selectins.18 19 To evaluate the function of these sulfated molecules in HPC mobilization, we treated E−/− mice with either sulfatide (25 mg/kg per dose), heparin (100 units per mouse per dose), or vehicle (PBS) for 2 doses over 6 hours. Although heparin administration did not significantly increase circulating progenitors, treatment with sulfatide produced a 4-fold augmentation in blood progenitors compared with vehicle-treated mice. Sulfatide administration also produced a profound leukocytosis compared with vehicle treatment, but no reduction in platelet counts (not shown). These results suggest that sulfation but not fucosylation is indeed critical for fucoidan-induced progenitor mobilization as desulfated fucoidan is inert and a nonfucosylated sulfated glycosphingolipid can also mobilize HPCs.
Discussion
In this study, we evaluated the role of selectins and sulfated inhibitors of selectin function in the mobilization of hematopoietic progenitor cells from the bone marrow to the blood compartment. We found that antibody blockade and/or absence of endothelial selectins led to peripheralization of HPCs. The combination of anti-P-selectin antibody administration in E−/− mice produced changes similar to those seen in mice lacking both selectins from birth, suggesting that increased circulating HPCs likely arose from selectin function blockade and not from the secondary abnormalities described in double-deficient mice.25 The rapid and profound mobilization response induced by sulfated polysaccharides in mice lacking E-selectin or both P- and E-selectins suggests a role for endothelial selectins in this process. Importantly, fucoidan treatment of E−/− mice led to a drastic increase in long-term (more than 6 months) bone marrow repopulating cells, indicating that this treatment also mobilized hematopoietic stem cells.
Endothelial selectins may influence progenitor mobilization in different ways. It is conceivable that P- and E-selectins expressed on the bone marrow endothelium might retain progenitors in the bone marrow. This is suggested by the varying degrees of mobilization achieved, depending on the type and the extent of selectin blockade. However, their restricted expression in the bone marrow stroma (eg, endothelial only) argues against this possibility. As we previously described, endothelial selectins play a role in progenitor homing to bone marrow.32 It is likely that under steady state conditions or during induction of mobilization, traffic between the bone marrow and the blood compartments is bidirectional. Therefore, blocking or absence of endothelial selectins may prevent progenitors from reentering the bone marrow and tilt this equilibrium toward the circulating HPCs. Our studies indeed suggest that blocking adhesion receptors acting on progenitor homing increases circulating progenitor numbers and that it may represent a very useful addition to other agents currently used to mobilize HPCs for clinical bone marrow transplantation.
Although the lack of one or both endothelial selectins enhanced the effect of fucoidan, expression of the leukocyte selectin appeared to be an advantage in a competitive setting as progenitors expressing L-selectin were mobilized in greater numbers than those lacking it (Figure 3C). L-selectin expression was not required because fucoidan-induced HPC mobilization was similar between wild-type and L−/− mice (Table 2). How does L-selectin expression influence mobilization? Two major possibilities are apparent. L-selectin adhesion might be important for progenitors to migrate from the areas of hematopoiesis to the blood. The presence of L-selectin ligand activity on progenitors supports this possibility.48 Second, fucoidan ligation to L-selectin might trigger signaling events, which could then result in the egress of progenitors into the circulation. Notably, L-selectin ligation by antibodies, fucoidan, or sulfatides have been shown to induce tyrosine phosphorylation and activate the MAP kinase pathway49,50 and sulfatide binding of L-selectin on neutrophils induce messenger RNA (mRNA) expression of IL-8,51 a potent HPC mobilizer.9 Whether similar signaling events occur in progenitor cells after L-selectin ligation is unknown. Thus, our results suggest distinct and opposite roles for selectins converging to enhance progenitor mobilization after fucoidan administration: Endothelial selectin inhibition or deficiency and leukocyte selectinexpression appear to promote circulating hematopoietic progenitors.
Several lines of evidence suggest that fucoidan acts through mechanism(s) other than interacting with selectins alone. First, the effect of fucoidan appears more powerful than the influence of antiselectin antibody administration or selectin gene knockouts (compare Figure 2B and Figure 1). Second, fucoidan treatment produces a reduction in the total nucleated cell numbers in the bone marrow, whereas these changes were not observed in nontreated P/E−/− mice25 or in E−/− animals treated with anti-P-selectin antibody (current study). Last, as suggested by mobilization in mice chimeric for EGFP and L-selectin expression, fucoidan-mediated HPC mobilization requires neither endothelial nor the leukocyte selectins because many L−/− progenitors were mobilized in P/E−/− mice.
Arguably, the most striking observation of our studies is the profound effect of fucoidan on progenitor mobilization. Fucoidan was first shown by Stoolman and Rosen52 to inhibit lymphocyte binding to high endothelial venules in a Stamper-Wooddruff assay. Subsequent work confirmed this finding,53 and it was later demonstrated that this sulfated polysaccharide could influence leukocyte-endothelial interactions and inflammatory responses through inhibition of P-selectin and L-selectin function.20-22 Although our study clearly suggests a target other than selectins for fucoidan-induced mobilization, the targeted bone marrow cell or compartment and the nature of this “ligand” are unclear at present. It is noteworthy that fucoidan can bind thrombospondin and laminin,54 extracellular matrix (ECM) proteins expressed in the bone marrow, and that low concentrations of fucoidan may produce endothelial cell retraction in vitro.55 Thus, fucoidan might compete with HPCs for binding to ECM proteins or proteoglycans that could contribute to HPC release. It is also conceivable that the sulfated polysaccharide might directly interact with progenitor or mature hematopoietic cells via nonselectin ligands. Evidence exists for such ligands on lymphocytes.56-58 Surprisingly, administration of multiple doses of fucoidan to E−/− mice elicited fewer circulating progenitors than the 2-dose regimen. The reasons for this observation are unclear at present. Chief possibilities include a phenomenon of tachyphylaxis on the target cell(s) or other alterations in the actual mobilized pool of bone marrow HPCs.
Of note is the 2-fold reduction in platelet counts after fucoidan treatment. Although, to our knowledge, a reduction in platelet numbers in fucoidan-treated animals has not been previously described, this effect was highly reproducible in our studies and was independent of selectin expression (Tables 1 and 2). Interestingly, Durig and colleagues59 showed that a low concentration (10 μg/mL) of fucoidan could induce platelet activation in vitro, and this effect was more prominent in high molecular weight fractions. In addition, fucoidan exhibits an anticoagulant activity similar to that of heparin.60-62 During our studies, however, mice appeared to tolerate fucoidan therapy very well and no spontaneous bleeding was observed. This may result from the fact that the reduction in platelet counts is relatively small and the anticoagulant potency of fucoidan is much lower than that of heparin.62
Our data also indicate that sulfation, but not fucosylation, of the glycan appears to be critical for its effect because the desulfated fucose polymer is inactive and sulfated glycosphingolipids display activity in HPC mobilization (Figure 5). Anionic charges provided by sulfated groups are not likely to be solely responsible for the effect of fucoidan because the charge density per saccharide residue is greater in heparin than in fucoidan,47 63 and heparin was not capable of significant mobilization (Figure 5). The fact that sulfated galactosylceramide (sulfatide) also induced HPC mobilization, albeit to a lesser extent than fucoidan, suggests that the proper spacial arrangement of the anionic groups rather than the type of saccharide is important.
Despite their structural disparities, fucoidan and sulfatides share many characteristics such as binding P- and L-selectins,18,19 inducing tyrosine phosphorylation,49,50 and interacting with extracellular matrix proteins.54 In contrast to fucoidan, which is not found in mammals, sulfatides are produced by human myeloid cells.46 It has been estimated that 108granulocytes may excrete as much as 5 μg of sulfatides in 12 hours.64 Interestingly, recent data indicate that mature leukocytes have a critical role in chemokine-induced HPC mobilization. For example, IL-8–induced mobilization is inhibited by anti-αLβ2 integrin,65 in mice lacking the G-CSF receptor,66 and by an antibody against gelatinase B, a metalloproteinase produced by myeloid cells.67 It remains to be evaluated whether endogenous sulfatides released by myeloid elements of the bone marrow participate in the cascade of events culminating in progenitor cell mobilization. Our studies suggest, however, that exogenous administration of sulfated glycans can profoundly alter the behavior of hematopoietic progenitor cells in vivo.
Acknowledgments
We thank Drs Richard Hynes and Masaru Okabe for their gifts of breeding pairs of L-selectin knockout and EGFP transgenic mice, respectively. We are also grateful to Sylviu Tamasdan for his help in carbohydrate determination and desulfation, Drs Schickwann Tsai and Rona Weinberg for providing cell lines and reagents for CFU-C assays, Dr Juan Badimon for sharing his automated cell counter, and Drs George Atweh and Denisa Wagner for their helpful comments.
Supported in part by a Junior Faculty Scholarship from the American Society of Hematology and a scholarship from the Comprehensive Manhattan Sickle Cell Center (P60 HL28381).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Paul S. Frenette, Mount Sinai School of Medicine, Department of Medicine, 1 Gustave L. Levy Pl, Box 1079, New York, NY 10029; e-mail: paul.frenette@mssm.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal