Abstract
Several tyrosine kinase oncogenes have been associated with myeloproliferative diseases, including Bcr/Abl, Tel/Abl, Tel/Jak2, and Tel/PDGFR. One target molecule shared by these oncogenes is known to be STAT5. We generated sublines of Ba/F3 cells in which either wild-type STAT5 or a constitutively active mutant of STAT5 (STAT5-1*6) were expressed under the control of a tetracycline-inducible promoter. These cell lines were compared with a Ba/F3 cell line in which the expression of p210Bcr/Abl was made inducible by a similar promoter. Before induction, all cells were dependent on interleukin 3 (IL-3) for growth and survival. Both STAT5-1*6 and Bcr/Abl enhanced viability and induced proliferation in the absence of IL-3. We found that the proviability protein Bcl-XL, but not Bcl-2, was induced by both p210Bcr/Abl and STAT5-1*6. Using a Bcl-X gene promoter construct fused to a luciferase complementary DNA (cDNA), both p210Bcr/Abl and STAT5-1*6 were shown to induce transcription of Bcl-X. The increase in transcription of the Bcl-X promoter and the increase in Bcl-X protein, due to p210Bcr/Abl, were blocked by expression of a dominant negative STAT5 mutant. Interestingly, however, STAT5-1*6 required the continued presence of IL-3 to cause a significant increase in Bcl-XL protein, whereas p210Bcr/Abl did not need IL-3. Studies with enzyme inhibitors suggest that the extra signal supplied by IL-3 may be supplied by the PI3K pathway. Overall, these data suggest that constitutively activated STAT5 can increase viability and proliferation of Ba/F3 cells. This may contribute to, but is not likely sufficient for, the enhanced viability associated with Bcr/Abl transformation.
Introduction
Chronic myelogenous leukemia (CML) is a myeloproliferative disorder associated with the t(9:22) translocation, which fuses the BCR and ABL genes.1The oncogene product, p210Bcr/Abl, displays enhanced tyrosine kinase activity, transforms primary murine hematopoietic cells,2 and converts interleukin 3 (IL-3)–dependent cell lines to growth factor independence.3 A number of signaling pathways are known to be activated by Bcr/Abl, including p21Ras,4 PI3-kinase,5 c-jun and c-myc.6,7 The STAT5 transcription factor is also known to be constitutively phosphorylated and activated in Bcr/Abl transformed cells,8 9 and is also known to be activated by other tyrosine kinase oncogenes associated with myeloproliferative disorders, such as Tel/PDGFR. We have recently shown that overexpression of a c-terminal truncated dominant negative form of STAT5 reduces viability and growth of Bcr/Abl-transformed Ba/F3 murine hematopoietic cells. These results suggested that STAT5 might play a role in transformation by Bcr/Abl, but its significance and target genes remain unclear.
STAT5 is a transcription factor that is activated in response to multiple hematopoietic cytokines. STAT5A and STAT5B are the products of 2 different genes that may play different roles in signal transduction.10 Tyrosine phosphorylation of STAT transcription factors is believed to induce dimerization, translocation to the nucleus, and binding to specific DNA motifs.11 Only a small number of STAT5-regulated genes are known, including β-casein,12CIS-1,13OSM,14 and Bcl-X.15 The role of STAT5 in hematopoiesis of adult mice remains unclear, since mice with a targeted disruption of the STAT5A/B genes display largely normal blood counts.16 However, these mice had reduced myeloid progenitor counts suggesting STAT5 might be important for maintenance of the stem cell compartment. Also, recent studies support a role for STAT5 and Bcl-XL in fetal erythropoiesis and survival of myeloid progenitors.15 17
In this study, we have attempted to dissect out the function of the STAT5 pathway from the functions of the many other signaling pathways activated by Bcr/Abl. A constitutively active mutant of STAT5, termed STAT5-1*6, was recently described by Onishi et al.18 This mutant has 2 point mutations, at positions H299R and S711F, and was discovered using a random mutagenesis strategy, followed by selection for mutants conferring a growth advantage. Recent studies suggest that the mutant is defective in binding to a nuclear corepressor, explaining in part its mechanism of activation.19
Using the murine IL-3–dependent Ba/F3 cell line as a parent, we generated stable sublines in which either wild-type STAT5, STAT5-1*6, or p210Bcr/Abl were placed under the control of a tetracycline-inducible promoter. The cell lines were then compared with each other for biologic properties and gene induction. STAT5-1*6 enhanced viability and induced slow proliferation of Ba/F3 cells, whereas overexpression of wild-type STAT5 had no effect. The effects of STAT51*6 and Bcr/Abl on a proviability gene, Bcl-X, are described in detail.
Materials and methods
Plasmid constructs
The complementary DNA (cDNA) encoding the constitutively active mutant form of STAT5 (STAT5-1*6) was obtained from Onishi.18 The STAT5-1*6 cDNA was digested byEcoR1 and ligated into pTRE, containing a tet-responsive promoter, forming pTRE-STAT5-1*6. pTRE-STAT5-1*6 was checked for sense orientation by restriction enzyme digestion. A cDNA encoding the wild-type form of STAT5 (STAT5-WT) was obtained from A. Mui (DNAX Research Institute, Palo Alto, CA), and the same method as described previously was used to construct a pTRE-STAT5-WT plasmid. pTRE-Δ-STAT5 plasmid was constructed as previously described.20 PGL2 vectors containing fragments of various lengths of the Bcl-X gene promoter fused to a luciferase reporter gene was obtained from G. Nunez (University of Michigan Medical School21). The plasmid pCMV-βGAL was purchased from Invitrogen (Carlsbad, CA).
Cell lines and culture conditions
The IL-3–dependent Ba/F3 cells line was maintained in RPMI-1640 (Mediatech Cellgro, Herndon, VA), supplemented with 10% fetal calf serum (FCS), 1 mg/mL L-glutamine and penicillin-streptomycin, and 10% WEHI-3B conditioned medium (WEHI-3B-CM) as a source of IL-3. The p210Bcr/Abl transformed Ba/F3 cells (Ba/F3-p210) are IL-3–independent and maintained in culture without IL-3. All cells were grown at 37°C, in a 5% CO2 humidified incubator. Ba/F3 cells expressing the reverse tet-transactivator pUHD172-1 (Ton.B.1) and Ton.B.210 cells in which p210Bcr/Abl is induced by the addition of doxycycline were obtained from G. Daley, Whitehead institute, Cambridge, MA.22 The p210Bcr/Abl transformed Ton.B.1 cells inducibly expressing the ΔSTAT5 transgene were described in a previous paper.20 To generate Ton.B.1 cells inducibly expressing the STAT5-1*6, or the STAT5-WT proteins, 15 μg pTRE-STAT5-1*6, or pTRE-STAT5-WT, respectively, and 5 μg pTK-Hygro (Clontech, Palo Alto, CA) were electroporated into these cells (0.35 kV, 960 μF) using a gene pulser (Biorad, Hercules, CA). Cells were grown for 48 hours in 10% FCS-RPMI 1640, supplemented with 10% WEHI-3B-CM before addition of hygromycin-B (Boehringer Mannheim, Indianapolis, IN), 400 μg/mL. After 2 to 3 weeks of selection, a polyclonal population regrew, and individual clones were obtained by limiting dilution.
Antibodies and protein analysis
Anti-Abl monoclonal antibody was a gift from R. Salgia (Dana Farber Cancer Institute). Anti–P-Tyr monoclonal antibody, clone 4G10, was a kind gift of B. J. Druker (Oregon Health Sciences University). Monoclonal antibodies against Bcl-XL (B22620) and Bcl-2 (B46620) and N-terminal STAT5 (S21520) were purchased from Transduction Laboratories (Pharmingen/Transduction Laboratories, San Diego, CA). Anti-p85 antiserum (06-195) was obtained from Upstate Biotechnology Inc (Lake Placid, NY). For protein analysis, cells were washed in phosphate-buffered saline (PBS) and lysed at 5 × 107 cells/mL in cold lysis buffer (50 mmol/L Tris, pH 7.5, 150 mmol/L NaCl, 0.5% Triton X100,10 mmol/L NaF, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L PMSF, 1 mmol/L NaVO3, 1 mg/mL leupeptin and aprotinin) for 30 minutes. Lysates were clarified by centrifugation at 15 000 × g for 20 minutes at 4°C. Protein amount was evaluated by Bradford assay before analysis. Equal amounts of lysates were loaded onto SDS-polyacrylamide gels, and transferred to a PVDF membrane (Millipore, Bedford, MA). Filters were blocked for 2 hours at room temperature with either 5% nonfat dry milk or 3% bovine serum albumin in Tris-buffered saline (TBS), 0.5% Tween (TBS-T). Filters were washed 3 times in TBS-T and incubated for 1 hour with optimal concentrations of primary antibodies, diluted in TBS-0.1% Tween. After 4 additional washes in TBS-T, filters were further incubated 45 minutes with horseradish peroxidase-conjugated secondary antibodies (Amersham Pharmacia Biotech Inc, Piscataway, NJ). Visualization was performed using NEN Renaissance system and Kodak X-Omat blue film (Eastman Kodak, Rochester, NY).
Reporter gene assays
Transcriptional activities were measured by using different luciferase-based reporter gene constructs. A pCMV-βGal plasmid was used as a control for transfection efficiency. For each experiment, 25 μg of the indicated luciferase plasmid and the pCMV-βGal plasmid were electrotransfected into 10 × 106 cells. When using the tetracycline-inducible cell lines, the cells were split into 2 equal cultures 8 hours after transfection and maintained in either the presence or absence of 1 μg/mL doxycycline for 24 hours. After being washed twice with cold PBS, cells were resuspended in 100 μL buffer-A (25 mmol/L Tris PO4, pH 7.8; 2 mmol/L EDTA; 105 glycerol; 15 Triton X100; 2 mmol/L DTT), and freeze-thawed 3 times. Lysates were clarified by centrifugation at 15 000 × g for 10 minutes. Immediately before measurement, 20 μL of lysates were incubated with 300 μL buffer-B (25 mmol/L glycylglycine, pH 7.8; 15 mmol/L potassium phosphate, pH 7.8; 15 mmol/L MgSO4; 4 mmol/L EGTA; 2 mmol/L ATP; 1 mmol/L DTT) and 100 μL d-luciferin. Luciferase activity was assessed with an automated luminometer (Lumat LB 9507, EG&G Berthold, Gaithersburg, MD). The βGal activity was measured from these lysates with a βGal assay kit (Invitrogen). In each case, reporter gene activity was reported as a ratio of luciferase activity to βGal activity.
Combined annexin V–propidium iodide staining
Viability was assessed using annexin V staining (annexinV-FLUOS, Boehringer Mannheim) following the manufacturer's specifications. Binding of fluorescein-conjugated annexin V and propidium iodide (PI) was assessed by fluorescence-activated cell separation (Coulter EPICS XL, Miami, FL).
In vivo studies
Ton.B.STAT5 or Ton.B.1*6 cells were resuspended at a cell density of 50 × 106 cells/mL in PBS and 100 mL per mouse were injected in the tail vein of nude mice (Taconic, Germantown, NY). One week before injection, the mice were separated into 2 groups, one group receiving water plus 1% sucrose and one group receiving water plus 1% sucrose plus 500 μg/mL doxycyline. Water containing doxycycline was changed every 2 days. For each cell line used, 5 mice were injected in each group. At the indicated time point (6 weeks after injection), one mouse of each group was killed. Mice were killed after 6 weeks, blood smears were prepared and stained with Wright-Giemsa stain, and spleen and lymph nodes were sectioned and smears stained with hematoxylin and eosin.
Polymerase chain reaction analysis
Bcl-XL RNA levels were assessed by semiquantitative polymerase chain reaction (PCR). G3PDH RNA levels were used as an internal control. Cells were treated as indicated and total RNA was recovered by Trizol (GIBCO, Gaithersburg, MD) extraction. Reverse transcription was performed on 100 ng total RNA, with 50 pmol oligo-dT, 2.5 mmol/L dNTP and 2 units of AMV-RT (Promega, Madison, WI), in a total volume of 20 μL for 90 minutes at 42°C. PCR (30 cycles) was performed on 5 μL of the reverse transcription products, in presence of 30 pmol of the specific primers, 2 units of Taq polymerase (Perkin Elmer, Norwalk, CT), and 125 μmol/L dNTP, in a final volume of 100 μL. The following primers were used: Bcl-XL -1: 5′-CCG GAG AGC GTT CAG TGA TC-3′; Bcl-XL: 5′-TCA GGA ACC AGC GGT TGA AG-3′; GAPDH-1: 5′-TGA AGG TCG GTG TGA ACG GAT TTG GC-3′ and GAPDH-2 : 5′-CTC CTT GGA GGC CAT GTA GGC CAT GAG G-3′. Linearity of the PCR was assessed by titration of the sample.
Results
Inducible expression of STAT5, STAT5-1*6, and p210Bcr/Abl in Ba/F3 cells
Onishi et al18 recently described a constitutively active mutant of STAT5, named STAT5-1*6, obtained by random mutagenesis. The STAT5-1*6 cDNA and a wild-type STAT5 cDNA were cloned into the pTRE plasmid, under the control of a tetracycline-inducible promoter. These constructs were then individually cotransfected, as described in “Materials and methods,” with a pTK-hygro plasmid in the Ton.B.1 cell line, already expressing the reverse tet-transactivator. The transfected cells were selected for 2 to 3 weeks in the presence of 400 μg/mL hygromycin, in medium containing IL-3 to avoid selecting for factor-independence. Individual clones were obtained by limiting dilution and screened for increased STAT5 expression in response to doxycycline. Several clones were obtained for each construct. The Ton.B.210 Ba/F3 cell line has been previously described.22 Each of the cell lines were normally cultured in the absence of doxycycline and in the presence of IL-3 to avoid selection of sublines with growth advantages. Cell lysates prepared 24 hours after the addition of doxycycline demonstrated more than a 10-fold increase in Bcr/Abl, STAT5, or STAT5-1*6, respectively (Figure 1A). Gene expression was reversible after withdrawal of doxycycline (not shown).
Generation of Bcr-Abl and STAT5 inducible cell lines.
(A) Ton.B.210, Ton.B.STAT5, and Ton.B.1*6 cells were cultured in RPMI 1640 medium supplemented with 10% FCS and incubated 24 hours, with (+) or without (−) doxycyline. Cells were harvested and lysed, and the lysates were separated by SDS-PAGE. After transfer to a PVDF filter, the inducible expression was evaluated by immunoblotting with the specified antibodies. (B) 1 × 107 Ton.B.210, Ton.B.STAT5, or Ton.B.STAT51*6 cells were cotransfected with GAS-luc and pCMV-βGal constructs (25 μg each). Transfected cells were then split and incubated in the same medium with or without doxycycline for 24 hours. Cells were harvested and lysed for reporter gene assays as described in “Material and methods.” Results are reported as the activity in induced cells compared with the activity in noninduced cells for each cell line (% of control).
Generation of Bcr-Abl and STAT5 inducible cell lines.
(A) Ton.B.210, Ton.B.STAT5, and Ton.B.1*6 cells were cultured in RPMI 1640 medium supplemented with 10% FCS and incubated 24 hours, with (+) or without (−) doxycyline. Cells were harvested and lysed, and the lysates were separated by SDS-PAGE. After transfer to a PVDF filter, the inducible expression was evaluated by immunoblotting with the specified antibodies. (B) 1 × 107 Ton.B.210, Ton.B.STAT5, or Ton.B.STAT51*6 cells were cotransfected with GAS-luc and pCMV-βGal constructs (25 μg each). Transfected cells were then split and incubated in the same medium with or without doxycycline for 24 hours. Cells were harvested and lysed for reporter gene assays as described in “Material and methods.” Results are reported as the activity in induced cells compared with the activity in noninduced cells for each cell line (% of control).
Bcr/Abl has previously been shown to induce tyrosine phosphorylation and DNA binding of STAT5.8 In the current studies, STAT5 activity was measured by a reporter gene assay using a construct containing 4 tandem β-casein–like GAS elements from the β-globin locus control region fused to a luciferase reporter gene. Inducible cell lines were transfected with this construct and then further cultured in the presence or absence of 1 μg/mL doxycycline for 24 hours. Expression of either Bcr/Abl or STAT5-1*6 induced transcription of this STAT5-dependent reporter gene, GAS-luc (Figure 1B). These data suggested this cell line model might be useful to further investigate the function of STAT5 in Bcr-Abl–mediated transformation.
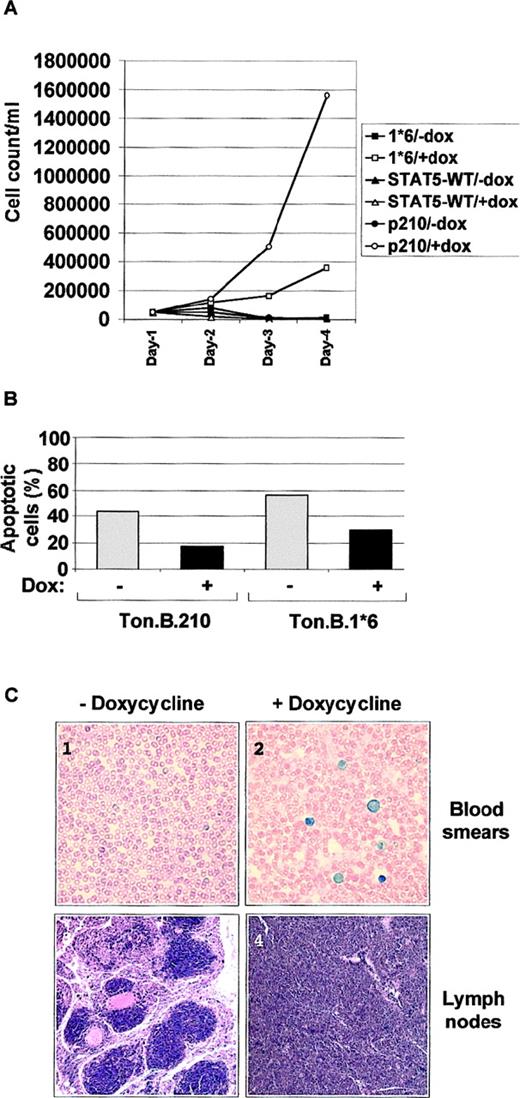
The effects of STAT5-1*6, STAT5, and Bcr/Abl on growth and viability of the IL-3–dependent Ba/F3 cells were then examined (Figure2). In the absence of doxycycline, all 3 lines were indistinguishable from untransfected Ba/F3 cells, and rapidly underwent apoptosis when deprived of IL-3. After adding doxycycline for 24 hours, Ba/F3 cells inducibly expressing STAT5-1*6 (Ton.B.1*6) proliferated slowly in the absence of added IL-3, whereas Ba/F3 cells expressing the wild-type STAT5 (Ton.B.WT) could not proliferate and died rapidly. Thus, the observed effect of STAT5-1*6 was related to enhanced STAT5 activity, and not to overexpression of STAT5 protein. However, the Ton.B.1*6 cells grew much more slowly than doxycyline-induced Ton.B.210 cells (Figure 2A). These observations suggest that constitutive activation of STAT5 alone is sufficient to induce growth in the absence of IL-3 in Ba/F3 cells, but also that Bcr/Abl is more significant as a mitogen than STAT5-1*6.
Constitutive activation of STAT5 mediates growth factor independence and increased in vitro and in vivo.
(A) 5 × 105 Ba/F3 tet-on cells, inducibly expressing either p210Bcr/Abl or wild-type STAT5 (STAT5-WT) or the constitutive active mutant STAT5-1*6 were cultured in RPMI 1640 + 10% FCS with or without doxycycline, as indicated. The number of cells was counted by trypan blue exclusion at the indicated time points. (B) Ton.B.210 and Ton.B.1*6 cells were cultured for 30 hours in RPMI 1640 + 10% FCS with or without doxycycline. 1 × 106 cells were harvested and used for an annexin-V–PI staining procedure, and analyzed as described in “Material and methods.” Results are represented as the percentage of apoptotic cells. (C) 5 × 106 Ton.B.STAT5 or Ton.B.1*6 cells were intraveneously injected into nude mice. For each cell line, the mice were split into 2 groups (5 mice in each group), receiving regular water or water supplemented with 500 μg/mL doxycycline. Mice were killed after 6 weeks, blood smears were prepared and stained with Wright-Giemsa stain, and spleen and lymph nodes were sectioned and smears stained with hematoxylin and eosin. Here are shown blood smears and a lymph node section from Ton.B.1*6 injected mice, treated (pictures 2 and 4) or not (pictures 1 and 3) with doxycycline. Magnification: 60 ×.
Constitutive activation of STAT5 mediates growth factor independence and increased in vitro and in vivo.
(A) 5 × 105 Ba/F3 tet-on cells, inducibly expressing either p210Bcr/Abl or wild-type STAT5 (STAT5-WT) or the constitutive active mutant STAT5-1*6 were cultured in RPMI 1640 + 10% FCS with or without doxycycline, as indicated. The number of cells was counted by trypan blue exclusion at the indicated time points. (B) Ton.B.210 and Ton.B.1*6 cells were cultured for 30 hours in RPMI 1640 + 10% FCS with or without doxycycline. 1 × 106 cells were harvested and used for an annexin-V–PI staining procedure, and analyzed as described in “Material and methods.” Results are represented as the percentage of apoptotic cells. (C) 5 × 106 Ton.B.STAT5 or Ton.B.1*6 cells were intraveneously injected into nude mice. For each cell line, the mice were split into 2 groups (5 mice in each group), receiving regular water or water supplemented with 500 μg/mL doxycycline. Mice were killed after 6 weeks, blood smears were prepared and stained with Wright-Giemsa stain, and spleen and lymph nodes were sectioned and smears stained with hematoxylin and eosin. Here are shown blood smears and a lymph node section from Ton.B.1*6 injected mice, treated (pictures 2 and 4) or not (pictures 1 and 3) with doxycycline. Magnification: 60 ×.
In addition to proliferation, induction of STAT5-1*6 and Bcr/Abl, but not STAT5, prevented cell death after IL-3 withdrawal. Viability was directly examined in these cell lines using a combined annexin V–PI staining method (Figure 2B). Thirty hours after IL-3 withdrawal, approximately 60% and 40% of noninduced Ton.B.1*6 and Ton.B.210 cells, respectively, stained with annexin-V-FITC, whereas less than 30% of cells induced with doxycycline to express STAT5-1*6 and less than 20% of cells induced to express Bcr/Abl were annexin-V positive. These results indicate that STAT5 activity alone is sufficient to rescue most cells from apoptosis after abrupt withdrawal of IL-3, and is approximately as effective as Bcr/Abl in this biologic effect.
The effects of STAT5-1*6 observed in tissue culture could also be replicated in an in vivo murine model. Injection of Ton.B.210 cells has been shown to induce leukemia within 4 weeks in nude mice that were provided with drinking water containing doxycycline.22Following this protocol, the Ton.B.1*6 and Ton.B.WT cells (5 × 106 cells) were injected intravenously into nude mice. Mice not given doxycycline remained healthy for the 6-week observation period. Also, mice given Ton.B.WT cells with doxycycline remained healthy for the observation period. However, mice injected with Ton.B.1*6 cells and provided with doxycycline, showed a dramatic weight loss (about 50% total weight) and developed visibly enlarged lymph nodes and at 6 weeks were found to have high numbers of circulating immature hematopoietic cells in the blood, as well as replacement of marrow and spleen with immature hematopoietic cells with the morphology of Ba/F3 cells (Figure 2C). Thus, the ability of STAT5-1*5 to induce factor-dependent growth and viability in culture is reflected in the generation of doxycyline-dependent leukemia in this nude mouse model. We did not attempt to directly compare the effects of Bcr/Abl and STAT5-1*6 in vivo, but limited experience would suggest that Bcr/Abl is a more virulent oncogene in this system than is STAT51*6. Together, these data show that constitutive activation of STAT5 alone, at least in the form of STAT5-1*6, is sufficient to induce IL-3–independent growth and survival of Ba/F3 cells, in vitro and in vivo, although to a lesser extent than p210Bcr/Abl.
Effects of Bcr/Abl and STAT5-1*6 on Bcl-XL protein levels
Although the increase in viability associated with Bcr/Abl transformation is widely appreciated, the signaling pathways responsible for viability have not been clearly defined. One source of variability is that cell lines containing Bcr/Abl have a tendency to acquire additional mutations, and thus reflect both the effects of Bcr/Abl and any accumulated mutations in other genes. We took advantage of the unique properties of these inducible cell lines to study which of the Bcl-2 family members are specifically induced by Bcr/Abl. Through the use of STAT5 A and B double-knockout mice, it has recently been demonstrated that STAT5 deficiency leads to a fetal anemia, possibly because of a lack of Bcl-XL protein expression in EPO signaling.15 Furthermore, it has been shown that the Bcl-X gene promoter contains several STAT binding sites.23
Ton.B.210 cells were deprived of IL-3 and induced with doxycycline for 0 to 72 hours. In the Ton.B.210 cells, Bcr/Abl protein was expressed as early as 6 hours after induction and maximum levels obtained at 24 hours (Figure 3A). Bcl-2 expression was not affected by increased Bcr/Abl, whereas the levels of Bcl-XL increased steadily starting at 24 hours of induction. Levels of the p85 subunit of PI3K were used to demonstrate approximately equal loading of the gel.
Bcr/Abl and STAT5-1*6 increase Bcl-XLprotein expression.
(A) Ton.B.210 cells were cultured in RPMI 1640 + 10% FCS. At Time 0, 1 μg/mL doxycycline was added to the cell culture and part of the cells were harvested at the indicated time points. Cells were lysed, and an equal amount of protein was separated by SDS-PAGE, and transferred to PVDF membrane. The membrane was cut in strips and probed with the indicated antibodies. The p85-PI3K blot is used as a control for equal loading. (B) Ton.B.1*6 cells were induced with 1 μg/mL doxycycline in RPMI 1640 10% FCS alone or supplemented with IL-3. Part of the cells were harvested at the indicated time points. Cells were lysed, and an equal amount of protein was separated by SDS-PAGE and transferred to PVDF membrane. The membrane was cut in strips and probed wit the indicated antibodies.
Bcr/Abl and STAT5-1*6 increase Bcl-XLprotein expression.
(A) Ton.B.210 cells were cultured in RPMI 1640 + 10% FCS. At Time 0, 1 μg/mL doxycycline was added to the cell culture and part of the cells were harvested at the indicated time points. Cells were lysed, and an equal amount of protein was separated by SDS-PAGE, and transferred to PVDF membrane. The membrane was cut in strips and probed with the indicated antibodies. The p85-PI3K blot is used as a control for equal loading. (B) Ton.B.1*6 cells were induced with 1 μg/mL doxycycline in RPMI 1640 10% FCS alone or supplemented with IL-3. Part of the cells were harvested at the indicated time points. Cells were lysed, and an equal amount of protein was separated by SDS-PAGE and transferred to PVDF membrane. The membrane was cut in strips and probed wit the indicated antibodies.
Similarly, Ton.B.STAT5-1*6 cells were treated with doxycycline. STAT5-1*6 levels were detectable at 6 hours, and were again maximum at about 24 hours (Figure 3B). Interestingly, in the absence of IL-3, no significant accumulation of Bcl-XL was detected. In contrast, when IL-3 was left in the medium, the induction of STAT51*6 was associated with a steady accumulation of Bcl-XLprotein. This required induction of STAT5-1*6 because no increase was seen without doxycycline (Time 0 with IL-3, Figure 3B, and not shown). Also, no change in Bcl-2 levels was detected (not shown). These results suggest that constitutive activation of STAT5 can contribute to Bcl-X induction, but STAT5 by itself is not sufficient. A second pathway, activated through the IL-3 receptor or Bcr/Abl, is required to increase Bcl-XL protein expression.
Bcr/Abl increases Bcl-Xgene transcription and protein levels through STAT5
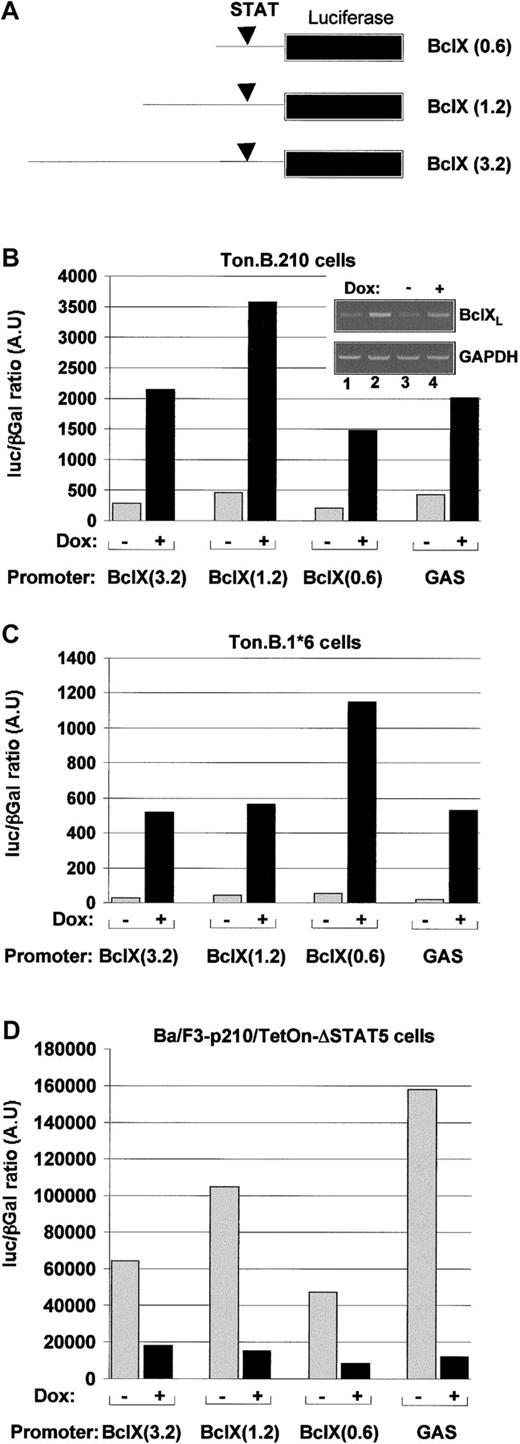
To better understand the role of STAT5 in Bcl-X gene expression, we compared the effects of Bcr/Abl and STAT5-1*6 on the activity of a Bcl-X promoter (in the absence of IL-3). Induction of p210Bcr/Abl by doxycyline resulted in a greater than 5-fold induction of Bcl-X promoter activity in all promoter constructs tested (Figure 4B). This increase in transcriptional activity was confirmed by semiquantitative PCR on Bcl-XL transcripts compared with GAPDH transcripts (Figure4B insert), demonstrating a clear increase of Bcl-XL RNA in Bcr/Abl expressing cells (ie, Ba/F3-Bcr/Abl, lane 2, or doxycycline-induced Ton.B.210 cells, lane 4) compared with nontransformed cells (ie, Ba/F3 cells, lane 1, or noninduced Ton.B.210 cells, lane 4). The GAS-luc construct was used in these experiments as a positive control for STAT5 activity in Ton.B.p210 cells.
Bcr/Abl increases
Bcl-X gene transcription through STAT5. (A) Scheme representing the various constructs containing fragments of different length of the Bcl-X gene promoter fused to a luciferase gene reporter construct. Ton.B.210 (B) Ton.B.1*6 (C) or Ton.p210.Δ.STAT5 (D) cells were cotransfected with the indicated constructs and a pCMV βGal plasmid. The cells were resuspended in RPMI 1640 + 10% FCS and left untreated or treated with doxycycline for 24 hours. The reporter gene activity was measured as described in “Materials and methods.” Insert. In parallel to the luciferase assay, the specific transcripts for Bcl-XL were estimated by semi quantitative PCR as described in “Materials and methods.”
Bcr/Abl increases
Bcl-X gene transcription through STAT5. (A) Scheme representing the various constructs containing fragments of different length of the Bcl-X gene promoter fused to a luciferase gene reporter construct. Ton.B.210 (B) Ton.B.1*6 (C) or Ton.p210.Δ.STAT5 (D) cells were cotransfected with the indicated constructs and a pCMV βGal plasmid. The cells were resuspended in RPMI 1640 + 10% FCS and left untreated or treated with doxycycline for 24 hours. The reporter gene activity was measured as described in “Materials and methods.” Insert. In parallel to the luciferase assay, the specific transcripts for Bcl-XL were estimated by semi quantitative PCR as described in “Materials and methods.”
Various Bcl-XL promoter constructs were used in this study. All these constructs contain the STAT5 binding site, as described by Dumon et al24 (Figure 4A). Induction of STAT5-1*6 induced a greater than 5-fold increase in Bcl-X promoter activity (Figure4C). Similar results were obtained in presence of IL-3; however, there was a higher level of promoter activity in the noninduced cells (not shown). Bcr/Abl-transformed cells were not studied in the presence and absence of IL-3.
Because both Bcr/Abl and STAT51*6 induced Bcl-X promoter activity, we asked if STAT5 was required for the activity of Bcr/Abl. A dominant negative STAT5 mutant, ΔSTAT5, was expressed in Ba/F3 cells, along with p210Bcr/Abl, as previously described.20In these cells, the expression of ΔSTAT5 was under the control of a tetracycline-inducible promoter, whereas Bcr/Abl was constitutively expressed. We have previously shown in these cells that ΔSTAT5 is expressed in the presence, but not the absence, of doxycycline, and that ΔSTAT5 can partially inhibit the activity of endogenous STAT5 on a GAS-luciferase promoter assay.20 Induction of ΔSTAT5 reduced Bcl-X promoter activity in these Bcr/Abl transformed Ba/F3 cells by 3- to 10-fold (Figure 4D). The expected inhibition of a GAS-luciferase promoter construct is shown for comparison. Furthermore, the inhibitory effect of Δ-STAT5 on Bcl-XL expression was also observed at the protein level (Figure5). Using the same inducible Δ-STAT5 expressing cell line as described in Figure 4D, Bcl-XLexpression decreased after 24 hours of induction with doxycycline. Expression of Δ-STAT5 at levels higher than that of the endogenous STAT5 appeared to be necessary to cause reduced expression of Bcl-X.
Expression of a dominant negative STAT5 mutant inhibits Bcl-XL expression.
Ton.p210.ΔSTAT5 cells were kept in culture in RPMI 1640 + 10% FCS and left untreated or treated with 1 μg/mL for 48 hours. Cells were then harvested, washed, and lysed. Lysates were separated by SDS-PAGE and the level of Bcl-XL was assessed by Western blot. The equal loading is assessed either by the endogenous level of STAT5 (upper panel, STAT5) or by the level of expression of p85 (middle panel).
Expression of a dominant negative STAT5 mutant inhibits Bcl-XL expression.
Ton.p210.ΔSTAT5 cells were kept in culture in RPMI 1640 + 10% FCS and left untreated or treated with 1 μg/mL for 48 hours. Cells were then harvested, washed, and lysed. Lysates were separated by SDS-PAGE and the level of Bcl-XL was assessed by Western blot. The equal loading is assessed either by the endogenous level of STAT5 (upper panel, STAT5) or by the level of expression of p85 (middle panel).
Together, these data indicate that STAT5 activity contributes to the increased expression of Bcl-X induced by Bcr/Abl. However, other pathways appear to be important because STAT5-1*6 requires a small amount of IL-3 to obtain optimal increases in Bcl-X protein accumulation.
Cooperation between STAT5 and the PI3K pathway in the induction of Bcl-XL
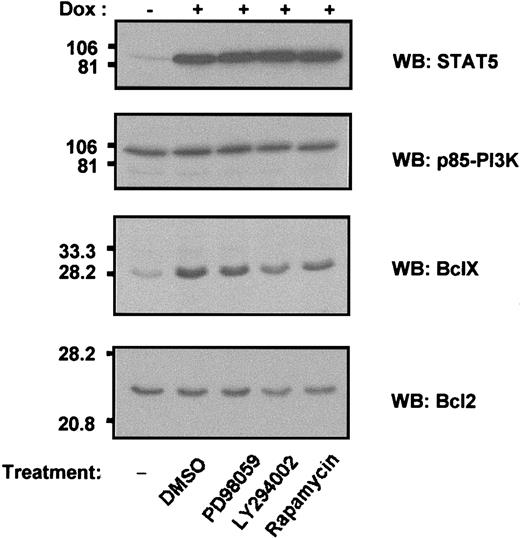
The results shown in Figures 3B and 4B indicate that, although STAT5 can activate Bcl-X promoter activity, it is insufficient by itself to cause maximum accumulation of Bcl-XL protein. An additional signal, supplied by IL-3, is required. In an initial effort to identify the second pathway(s), Ton.B.1*6 cells cultured in the presence of IL-3 were exposed to several drugs believed to specifically inhibit various signaling pathways (Figure6). Bcl-X expression induced by STAT5-1*6 expression was not affected by the addition of the diluent, DMSO, or PD98059, an inhibitor of MAPK, indicating that the ERK/MAPK pathway is not involved in this mechanism. However, 2 drugs known to act at different levels in the PI3K/Akt pathway did have a reproducible effect. The PI3K inhibitor, LY294002, significantly decreased Bcl-XL expression, suggesting the requirement of PI3K in Bcl-XL regulation. Second, and to a lessor extent, Bcl-XL expression was reduced in response to the p70 S6 kinase inhibitor, rapamycin. These results suggest that signals provided potentially by the PI3K pathway can synergize with STAT5 to enhance Bcl-X expression, possibly by decreasing protein degradation, enhancing translation, or through another mechanism.
Bcl-XL expression is mediated by cooperation between STAT5 and PI3K.
Ba/F3 cells inducibly expressing STAT5-1*6 were incubated in RPMI 1640 10% FCS, suplemented with 10% WEHI supernatant as a source of IL-3. The cells were either left untreated or induced with doxycycline for 8 hours before additional drug treatment. Doxycycline-induced cells were treated with DMSO (0.05 % v/v), PD98059 (20 μmol/L), LY 294002 (25 μmol/L), or rapamycin (5 nmol/L) for 18 hours. The cells were then processed as described above for immunoblot analysis with the indicated antibodies. Equal loading is verified by p85-PI3K blot.
Bcl-XL expression is mediated by cooperation between STAT5 and PI3K.
Ba/F3 cells inducibly expressing STAT5-1*6 were incubated in RPMI 1640 10% FCS, suplemented with 10% WEHI supernatant as a source of IL-3. The cells were either left untreated or induced with doxycycline for 8 hours before additional drug treatment. Doxycycline-induced cells were treated with DMSO (0.05 % v/v), PD98059 (20 μmol/L), LY 294002 (25 μmol/L), or rapamycin (5 nmol/L) for 18 hours. The cells were then processed as described above for immunoblot analysis with the indicated antibodies. Equal loading is verified by p85-PI3K blot.
Discussion
CML is a clonal disorder of hematopoietic stem cells caused by the Bcr/Abl oncogene and characterized by expansion of myeloid cells in the blood, spleen, and bone marrow.25 In contrast to acute myeloid leukemias, differentiation of CML myeloid cells appears to be normal, resulting in the overproduction of mature, functional, neutrophils and other hematopoietic elements. Although the exact biologic effects of Bcr/Abl on hematopoietic cells have been controversial, most investigators agree that the oncogene enhances proliferation,26 prolongs viability,27 and alters adhesion and mobility.28 These effects require the constitutively active tyrosine kinase activity of Bcr/Abl, which presumably functions by activating cellular signaling pathways. Although a number of target molecules downstream of Bcr/Abl have been identified, few have so far been linked to specific aspects of the transformed phenotype.
STAT5 is phosphorylated and activated in Bcr/Abl-transformed cells, and also in leukemia cells containing other activated tyrosine kinase oncogenes, including v-abl, Tel/Jak2, Tel/Abl, and Tel/PDGFR.29,30 In these leukemias, STAT5 may be a direct phosphorylation target of the oncogenes, but this has not been proven. Interestingly, STAT5 is phosphorylated in cells from many patients with acute myeloid leukemias, suggesting that secondary activation of endogenous tyrosine kinases may also be sufficient to activate STAT5.31 In both AML and CML, the contribution of STAT5 to transformation is currently unknown. In a previous study, we showed that expression of a truncation mutant of STAT5 lacking the transcriptional activation domain (ΔSTAT5) reduced endogenous STAT5 transcriptional activity in Bcr/Abl transformed cells. Expression of ΔSTAT5 slowed the growth rate of Bcr/Abl transformed cells, primarily due to a modest, but reproducible, reduction in viability. Similar results were reported by Nieborowska-Skorska et al32 in a different Bcr/Abl transformed cell line. These results suggested that one effect of STAT5 might be a contribution to viability, but such studies are limited by the effectiveness and relative expression levels of the dominant negative mutants.
In an effort to better define the potential contribution of STAT5 to transformation, we generated several comparable cell lines in which either wild-type STAT5, STAT5-1*6, or Bcr/Abl were placed under the control of a tetracycline inducible promoter. The parent cell line, Ba/F3, undergoes cell cycle arrest and apoptosis in the absence of IL-3. Treatment of the inducible cell lines with doxycycline resulted in rapid, high-level, expression of the respective genes, and allowed for very precise determination of biologic effects and signal pathway activation. These inducible cell lines have the advantage that any observed effects on biology or signaling are necessarily due to the induced gene and cannot occur because of a mutation in an unknown gene. Induction of wild-type STAT5 had no discernable effect on growth or viability, and was not further studied in any detail. In contrast, both Bcr/Abl and STAT5-1*6 resulted in factor-independent proliferation and enhanced viability. STAT51*6 was not as potent as Bcr/Abl, and would only support slow proliferation by itself, but maintained high cell viability for a prolonged period. Interestingly, these tissue culture effects were also observed in vivo. The Ba/F3 cell line does not form tumors in nude mice, whereas Bcr/Abl-transformed Ba/F3 cells proliferate rapidly in nude mice, accumulating particularly in marrow, spleen, nodes, and blood (our results and Klucher et al22). Ba/F3 cells expressing the doxycycline-inducible STAT51*6 were injected into nude mice, which were then given either regular drinking water or water containing doxycycline. Mice given doxycycline, but not mice given regular water, developed enlarged lymph nodes, leukocytosis, and a marrow infiltrated with cells identical in morphology to Ba/F3 cells. Thus, the signaling pathways activated by STAT5-1*6 appear to have some overlap with those of Bcr/Abl, at least at a functional level.
Studies looking for downstream targets of STAT5 and Bcr/Abl that might contribute to transformation are therefore of interest. In this study, we focused on genes involved in regulating viability, because both Bcr/Abl and STAT5-1*6 had a prominent effect on the viability of Ba/F3 cells. The increase in viability associated with Bcr/Abl is likely to be an important aspect of transformation. Bcr/Abl can rapidly convert IL-3–dependent cell lines to complete factor independence.3 This effect is a direct effect of Bcr/Abl as shown by studies with inducible Bcr/Abl and with the Abl kinase inhibitor, STI571.22,33,34 In addition, Bcr/Abl has been reported to improve viability after exposure to chemotherapy drugs and to ionizing radiation, suggesting that viability is enhanced in the setting of several different types of apoptotic stimuli.27 35
Here, we report that both Bcr/Abl and STAT5-1*6 induce expression of Bcl-XL, a known proviability protein. Further, we demonstrate that this occurs in part at the level of transcription of the Bcl-X gene, which enhanced Bcl-X gene transcription because Bcr/Abl can be significantly inhibited by the dominant negative inhibitor of STAT5, ΔSTAT5. In contrast, neither Bcr/Abl nor STAT5 induced expression of Bcl-2, although this proviability gene has been previously linked to Bcr/Abl in another cell line.2 Bcl-XL is known to enhance viability in hematopoietic cells when overexpressed, and it is reasonable to expect that constitutively elevated levels of Bcl-XL could contribute to the pathogenesis of a myeloproliferative disorder. Overall, our data suggest that activation of STAT5 by Bcr/Abl may contribute to the known viability effects of this oncogene through regulation of Bcl-XL expression. While the present manuscript was under review, Horita et al36 reported similar results, showing that BclXL expression is under the control of STAT5 activity in the human Ph-positive cell line K562.
However, we found that the regulation of Bcl-XL protein expression also requires signaling pathways other than STAT5. Although both Bcr/Abl and STAT5-1*6 could induce transcription of theBcl-X gene by themselves in Ba/F3 cells, only Bcr/Abl could induce an increase in the level of Bcl-XL protein. STAT5-1*6 required the continued presence of IL-3 to induce accumulation of Bcl-XL. Thus, in these cells, Bcl-XL protein accumulation is likely to be regulated by 2 or more pathways, one involving STAT5 and Bcl-X transcription, and a second unknown signal, possibly affecting Bcl-X translation or protein metabolism. In contrast to STAT1*6, Bcr/Abl supplies both signals. One possibility for the second pathway involves the PI3K pathway, because the addition of an inhibitor of PI3K reduced STAT5-1*6/IL-3–induced expression of Bcl-XL protein. Bcr/Abl is known to activate PI3K, and therefore could supply this second signal independently of IL-3. However, there may be other redundant pathways, because the addition of the PI3K inhibitor LY294002 did not effectively block Bcr/Abl-induced accumulation of Bcl-XL. The PI3K pathway has been repeatedly linked to viability signaling, and in one popular model, Bcr/Abl activates PI3K, leading to activation of Akt, which then phosphorylates and inactivates Bad, a pro-death molecule.5 37
Overall, our studies suggest that STAT5 activation can have significant effects on the biological behavior of Ba/F3 hematopoietic cells, and can mimic some, but not all, of the activities of Bcr/Abl. Identification of other genes regulated by STAT5, and of other pathways cooperating with STAT5, will be important to understand the survival and proliferative function of Bcr/Abl and related tyrosine kinase oncogenes in hematopoietic cells.
Supported by a National Institutes of Health grant CA66996 (J.D.G.) and a fellowship from the Association pour la Recherche sur le Cancer (F.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
James D. Griffin, Dana Farber Cancer Institute, Department of Adult Oncology, 44 Binney St, Boston MA 02115; e-mail: james_griffin@dfci.harvard.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal