Abstract
Previous studies have demonstrated that combinations of all-trans retinoic acid (ATRA) with either granulocyte-colony stimulating factor (G-CSF) or lithium chloride (LiCl) produced synergistic terminal differentiation of WEHI-3B myelomonocytic leukemia (D+) cells. It was found that steady-state retinoic acid receptor alpha (RARα) protein levels were markedly reduced in these cells after exposure to ATRA. Because the presence of receptors for a hormone ligand is required for its action, differentiation therapy with ATRA may be self-limiting. The combination of G-CSF with ATRA significantly attenuated the loss of RARα protein, and synergistic terminal differentiation occurred. LiCl was more effective than G-CSF in preserving RARα pools and synergized with ATRA more strongly than G-CSF. These findings suggested that the prevention of RARα protein loss by G-CSF or LiCl in ATRA-treated cells functioned to extend the differentiation response to the retinoid and was responsible, at least in part, for the observed synergism. D+ cells transfected with an expression plasmid containing RARα cDNA had a 6- to 8-fold increase in steady-state RARα mRNA compared with vector-transfected cells and showed a 2- to 3-fold increase in RARα protein. ATRA caused a reduction, but not a complete loss, of RARα protein in these transfectants, which were considerably more responsive than parental D+ cells to ATRA as a single agent, supporting the concept that the protection of RARα pools results in a heightened differentiation response to ATRA.
Introduction
Successful chemotherapy of the acute leukemias requires the use of cytotoxic drugs to kill the neoplastic cells. Because these agents lack selectivity for leukemia cells, their use is often accompanied by serious adverse side effects for the patient. Clearly, alternatives to the use of cytotoxic regimens are desirable. One such approach involves the concept that a leukemia cell is one that fails to complete its normal maturation program, thereby retaining infinite proliferative capacity. If the block or defect in the maturation process were overcome, the cell could possibly mature to a functional end-stage cell with a finite life span.1Perhaps the most compelling evidence supporting the induction of differentiation as a viable mode of cancer therapy arises from clinical trials with all-trans retinoic acid (ATRA), in which complete remissions were attained in patients with acute promyelocytic leukemia (APL)2 in a process that clearly involved terminal differentiation.3
Tempering the success of ATRA-based differentiation therapy are several problems that attend its use.4 Serious side effects occur in some patients treated with ATRA, but these are usually successfully managed with steroid therapy.5 Of greater concern is the fact that the remissions produced in patients treated with ATRA alone are of short duration because of the rapid development of resistance6 and the inability of ATRA to convert the entire leukemic cell population to mature end-stage cells. Another limitation of the differentiation therapy of the leukemias using ATRA is that its effectiveness is limited to APL cells carrying the t(15;17) rearrangement,7 which fuses the PML (promyelocytic leukemia–associated) gene to the retinoic acid receptor alpha (RARα) gene.8 Strategies aimed at avoiding retinoid resistance, increasing the percentage of leukemia cells that undergo terminal differentiation, and broadening the spectrum of clinical activity to other subtypes of acute myelocytic leukemia (AML) are important objectives.
Clinically, the development of resistance to ATRA is associated with decreases in the plasma concentration of drug while patients are actively treated.9 This may result from increased catabolism or increased sequestration of ATRA by retinoid binding proteins (see Warrell6 for references). In studies in cell culture, relatively low concentrations of ATRA are capable of inducing the terminal differentiation of leukemia cells when combined with granulocyte-colony stimulating factor (G-CSF),10-15 a cytokine that, through the binding of its receptor (G-CSFR), regulates the production of neutrophils and enhances their maturation, or LiCl,16 which also improves neutrophil production (see Boggs and Joyce17 for review). Thus, it is conceivable that the inclusion of G-CSF or LiCl in treatment regimens may allow the effective use of relatively low levels of ATRA, possibly reducing toxicity, delaying the development of retinoid resistance, and extending the duration of remissions produced by ATRA through the terminal differentiation of a larger proportion of the neoplastic cell population. Given that, as a single agent, ATRA has produced clinical usefulness only in patients with t(15;17) APL, it is of potential importance that major differentiation responses to the retinoid have been obtained in vitro with several subtypes of myeloid leukemia—including, but not limited to, t(15;17) APL—when ATRA is used in combination with G-CSF or LiCl.10-16 Thus, the use of differentiation-inducing agents such as G-CSF and LiCl in combination with ATRA might well extend the spectrum of activity of the retinoid to other forms of myeloid leukemia, enlarging the therapeutic range of the vitamin.
An understanding of the molecular basis for the synergistic induction of terminal differentiation of malignant cells exhibited by mixtures of agents such as G-CSF or LiCl and ATRA is essential for the optimum use of differentiation therapy regimens. Retinoids such as ATRA are known to exert most of their effects through the binding of 2 classes of nuclear receptors, RARs and RXRs.18,19 The natural ligands for the RARs and RXRs are ATRA and 9-cis retinoic acid (9-cis-RA), respectively.18,20 Association of these receptors, usually as RAR/RXR heterodimers, with specific DNA sequences (retinoic acid response elements, RAREs) in the promoter regions of target genes provides for the ligand-dependent modulation of gene expression. Cells that do not express RARs and RXRs, or that express mutant forms of the receptors, are unable to respond appropriately to retinoids. The current mechanistic concept of retinoid receptor regulation of gene expression involves the recruitment to the RARE of chromatin remodeling multiprotein complexes whose constituents and activities differ depending on ligand binding by the retinoid receptors (for reviews, see Johnson and Turner,21 Minucci and Pelicci22). Because G-CSF also exerts its effects through the binding of its receptor, it is conceivable that the complementary modulation of receptor concentration or activation by these agents could contribute to the synergistic effects observed with these combinations. We now report that the modulation of RARα expression occurs in cells treated with G-CSF and ATRA or LiCl and ATRA. ATRA induced a loss of RARα protein in D+ cells, G-CSF and LiCl attenuated this loss, and synergistic terminal differentiation was produced. Because exogenously enforced expression of RARα in these cells also resulted in a heightened differentiation response to ATRA alone, we conclude that the modulation of RARα protein pools by G-CSF or LiCl in ATRA-treated cells is relevant to the synergistic induction of the terminal differentiation of myelomonocytic leukemia cells produced by these combinations.
Materials and methods
Cell culture and differentiation
D+ cells were maintained in suspension culture in McCoy 5A modified medium supplemented with 15% fetal bovine serum at 37°C in a humidified atmosphere containing 5% CO2. Cells used for RNA and protein analyses were subcultured at 0.2 × 106 cells/mL before the addition of drugs and were maintained between 0.2 × 106 and 1 × 106cells/mL by the daily addition of fresh medium with or without drugs.
The capacity of cells to undergo functional maturation was assessed by nitro blue tetrazolium (NBT) dye reduction. Approximately 1 × 106 cells were collected by centrifugation at 300g for 5 minutes and resuspended in 1.0 mL complete medium containing 0.1% NBT and 1.0 μmol/L of 12-O-tetradecanoylphorbol 13-acetate (TPA). The cell suspension was incubated at 37°C for 30 minutes, and the percentage of cells containing blue–black formazan deposits, indicative of a TPA-stimulated respiratory burst, was determined by microscopic visualization of at least 200 cells using a hemacytometer. To determine whether the interaction between G-CSF or LiCl and ATRA is truly synergistic, experiments were conducted in which the concentrations of G-CSF or LiCl and ATRA were each varied differently in combination, and the extent of differentiation of D+ cells was measured by determining the percentage of NBT-positive cells after 72 hours of incubation. The data were analyzed by isobologram and combination index (CI) methodologies using computer-based programs.23 24
Northern blotting
Total cellular RNA was isolated from 3 × 106cells using TRIzol reagent (Gibco BRL, Gaithersburg, MD), or poly A+ RNA was isolated from 5 × 106 cells using the Micro-FastTrack system (Invitrogen, Carlsbad, CA) according to the manufacturer's protocols, separated by 1.2% agarose formaldehyde gel electrophoresis and transferred onto nitrocellulose membranes by capillary elution. The membranes were hybridized with [α32P]dCTP random primer labeled probes. For G-CSFR mRNA analyses, the entire mouse cDNA, kindly provided by Dr Shigekagu Nagata (Osaka Bioscience Institute, Osaka, Japan) was used in the random primer labeling reaction. The probe used in the RARα Northern blot analyses was derived from the variable F region of the RARα cDNA. The murine RARα cDNA25 was provided by A. Krust and P. Chambon (Institute de Chimie Biologique, Strasbourg, France).
Western blotting
For Western blot analysis, 3 × 106 cells were collected by centrifugation and resuspended in Tris-buffered saline (10 mmol/L Tris-HCl, pH 7.6, 0.1 mol/L NaCl, 1 mmol/L EDTA) containing a mixture of protease inhibitors (2 mmol/L phenylmethylsulfonyl fluoride, 1 μg/mL leupeptin, and 1 μg/mL aprotinin), lysed by the addition of an equal volume of 2 × sodium dodecyl sulfate (SDS) gel-loading buffer (100 mmol/L Tris-HCl, pH 6.8, 200 mmol/L dithiothreitol, 4% SDS, 0.2% bromphenol blue, 20% glycerol), placed in a boiling water bath for 5 minutes, and vortexed vigorously. Extracts were separated by electrophoresis on a 10% SDS-polyacrylamide gel and transferred onto nitrocellulose membranes. The membranes were blocked with 5% dry milk in TBST (20 mmol/L Tris-HCl, pH 7.6, 137 mmol/L NaCl, 0.01% Tween 20) for 30 minutes, incubated overnight with rabbit polyclonal anti-RARα or anti-RXRα antibody, diluted to 1 μg/mL in TBST containing 5% milk, washed with 3 changes of TBST for a total of 30 minutes, and then incubated with horseradish peroxidase-conjugated donkey antirabbit IgG for 1 hour and washed for 30 minutes with TBST (3 changes). Immunoreactive proteins were visualized by enhanced chemiluminescence (ECL; Amersham, Arlington Heights, IL).
Chemicals and antibodies
ATRA and 9-cis-RA were purchased from Biomol (Plymouth Meeting, PA). NBT and Ultrapure LiCl were purchased from Sigma (St. Louis, MO). The RAR-specific ligand (TTNPB; 4-[(E)-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1-propenyl]benzoic acid) and the RXR-specific ligand (LG100346; 4-[(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthyl) carbonyl]benzoic acid methoxy-oxime) were synthesized by Stacie Canan-Koch and kindly provided by Dr Elizabeth Allegretto (Ligand Pharmaceuticals, San Diego, CA). Retinoid receptor subfamily-specific antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and Biomol.
Results
Differentiation responses of D+ cells to G-CSF or LiCl and ATRA
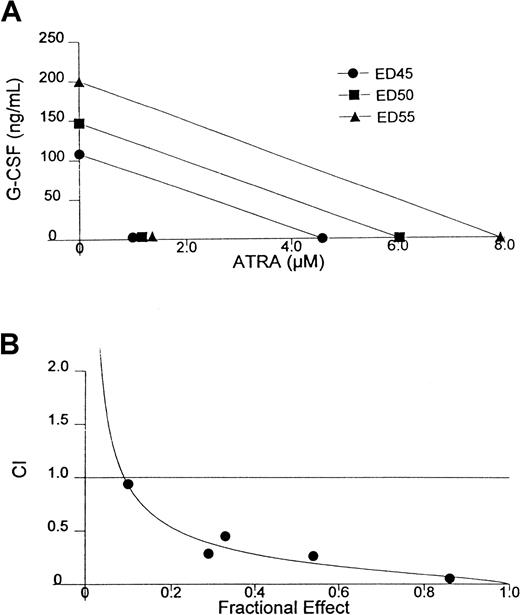
In previous studies we observed an enhanced induction of the differentiation of D+ cells by the combination of ATRA and G-CSF that permits the use of exceedingly low doses of ATRA to achieve a terminally differentiated state.13 A similar phenomenon is exhibited in HL-60 and AML-193 cells and in cells from patients with APL and AML.10,12 26 To determine whether the interaction between G-CSF and ATRA is truly synergistic, we have conducted experiments in which the concentrations of G-CSF and ATRA were each varied differently in combination and the extent of differentiation of D+ cells was measured by determining the percentage of NBT-positive cells. Isobologram and CI analyses of data from one of these experiments, demonstrating the particularly strong synergism exhibited by this combination, is shown in Figure1. Note that the synergistic interaction of G-CSF and ATRA is so strong that all the points shown in the isobologram obtained with the combination are clustered in the lower left corner of the plot (Figure 1A). Analysis of data generated from an alternative effect-oriented study by CI methodology confirmed the strong synergism exhibited between these agents to produce terminal differentiation (Figure 1B). These studies also show that the success of retinoid-based differentiation therapy may not be limited to t(15;17) rearranged APL. Thus, the differentiation responses in this case were demonstrated in WEHI-3B cells, which is a non-APL myeloid leukemia cell line, when ATRA was combined with the differentiation-inducing cytokine, G-CSF.
Isobologram analysis and combination index analysis of the induction of differentiation in D+ cells treated with the combination of G-CSF and ATRA for 72 hours.
(A) Isobologram analysis. (B) Synergism is indicated when combination index (CI) < 1, additivity is indicated when CI = 1, and antagonism is indicated when CI > 1.
Isobologram analysis and combination index analysis of the induction of differentiation in D+ cells treated with the combination of G-CSF and ATRA for 72 hours.
(A) Isobologram analysis. (B) Synergism is indicated when combination index (CI) < 1, additivity is indicated when CI = 1, and antagonism is indicated when CI > 1.
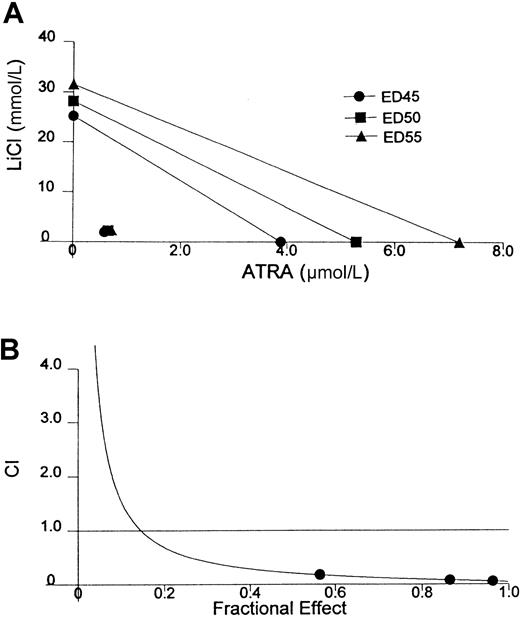
Millimolar concentrations of LiCl have previously been shown to induce terminal differentiation of both HL-60 and D+cells.16 Furthermore, in these studies, the differentiation-inducing effects of LiCl were markedly enhanced by the addition of low levels of ATRA. We evaluated the interaction between LiCl and ATRA in producing differentiation of D+ cells using the NBT reduction assay. Isobologram and CI evaluation of the results, shown in Figure 2, indicated that synergistic differentiation induction was clearly produced. Notably, when used in an admixture, significant differentiation responses were achieved at concentrations of these agents that are attainable in vivo.27
Isobologram analysis and combination index analysis of the induction of differentiation in D+ cells treated with the combination of LiCl and ATRA for 72 hours.
(A) Isobologram analysis. (B) Synergism is indicated when combination index (CI) < 1, additivity is indicated when CI = 1, and antagonism is indicated when CI > 1.
Isobologram analysis and combination index analysis of the induction of differentiation in D+ cells treated with the combination of LiCl and ATRA for 72 hours.
(A) Isobologram analysis. (B) Synergism is indicated when combination index (CI) < 1, additivity is indicated when CI = 1, and antagonism is indicated when CI > 1.
Effects of G-CSF and ATRA on G-CSFR expression in D+ cells
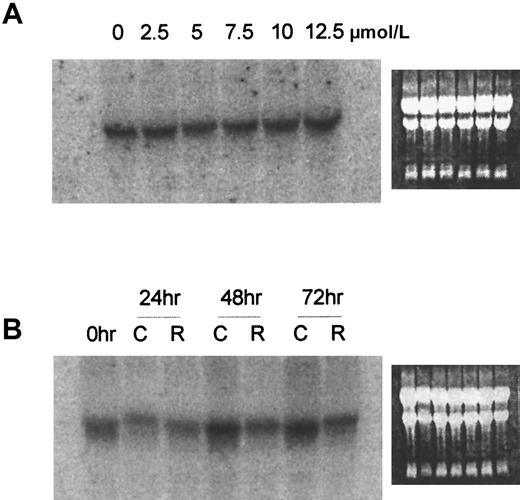
Because both G-CSF and ATRA exert their effects through the binding of their cognate receptors, receptor presence and abundance in/on target cells are important determinants of cellular response and may correlate with the success of treatment with these agents. We were, therefore, interested in whether complementary modulation of receptor concentration was produced by these agents. It has been reported that ATRA increased G-CSFR mRNA in NB4 leukemia cells and in HL-60 leukemia cells.14 15 We were unable to duplicate this result in the HL-60 cell line routinely used in our laboratory. Moreover, increased G-CSFR mRNA expression was not induced by ATRA in our studies with WEHI-3B leukemia cells. Thus, Northern blot analyses showed that ATRA, over a range of concentrations that effectively produced terminal differentiation, did not significantly alter the expression of G-CSFR mRNA in D+ cells after 48 hours of treatment (Figure3A). Even after 24 to 72 hours of exposure to the optimum concentration of ATRA, producing differentiation of D+ cells when used as a single agent (7 μmol/L), G-CSFR mRNA was not increased but appeared to be slightly decreased (Figure 3B). G-CSFR expression was also not significantly affected in D+ cells treated with the combination of G-CSF and ATRA (data not shown).
Northern blot analysis of G-CSFR in D+ cells treated with various concentrations of ATRA for 48 hours or after 24, 48, or 72 hours' exposure to 7 μmol/L ATRA.
(A) 48 hours, various concentrations. (B) Exposure to 7μmol/L ATRA. Ethidium bromide fluorescence of the gel before transfer shows the presence of approximately equal amounts of RNA. C indicates untreated control; R, ATRA treated.
Northern blot analysis of G-CSFR in D+ cells treated with various concentrations of ATRA for 48 hours or after 24, 48, or 72 hours' exposure to 7 μmol/L ATRA.
(A) 48 hours, various concentrations. (B) Exposure to 7μmol/L ATRA. Ethidium bromide fluorescence of the gel before transfer shows the presence of approximately equal amounts of RNA. C indicates untreated control; R, ATRA treated.
Effects of G-CSF and ATRA on steady-state RARα mRNA and protein levels in D+ cells
Because the expression of the G-CSFR appeared not to change significantly during G-CSF– and ATRA-induced differentiation, the impact of treatment with these agents on the retinoic acid receptors was examined. D+ cells were treated with G-CSF, ATRA, or the combination of the 2 inducers. The differentiation response typically produced in D+ cells under these treatment conditions is shown in Table 1. RARβ protein was not detected in these cells either before or after treatment. RARγ, expressed almost exclusively in epithelial tissues,25 was not examined in these studies. RARα was detected, however, and its levels were modulated by treatment. Figure4A shows a representative RARα Western blot of G-CSF– and ATRA-treated D+ cells. As a single agent, G-CSF did not appreciably alter levels of RARα protein in D+ cells, and only a weak differentiation response was induced (Table 1). The effects of ATRA alone were more notable. The retinoid alone produced a modest differentiation response in the D+ cells and caused a reduction in the steady-state levels of RARα protein such that the protein was barely detectable after 24 hours of treatment. Of greater interest, when G-CSF was used in combination with ATRA, the ATRA-induced loss of RARα protein was decreased and a synergistic differentiation response was elicited. The basis for the attenuation of the ATRA-induced loss of RARα by coexposure to G-CSF was investigated by Northern blot analyses of RARα in drug-treated D+ cells (Figure 4B). Exposure to ATRA alone induced an increase in the steady-state levels of RARα mRNA even though RARα protein levels were markedly reduced. Surprisingly, exposure to the combination of ATRA and G-CSF produced an increase in the steady-state levels of RARα mRNA to a degree similar to that found in cells exposed to ATRA alone. Recent studies have definitively shown that ATRA induces proteasome-dependent degradation of RARα in a variety of cell types.28 The transcriptional up-regulation of RARα may be the cells' response to counter this loss of receptor protein. Because the addition of G-CSF with ATRA did not produce a further increase in RARα mRNA yet protected the RARα pool, G-CSF signaling may interfere with some aspect of the proteasomal degradation cascade.
Capacity of D+ cells to reduce NBT after treatment with relatively low levels of G-CSF and ATRA
| Treatment duration (hr) . | % NBT positivity . | |||
|---|---|---|---|---|
| C . | G . | R . | GR . | |
| 48 | 1 ± 1 | 1 ± 1 | 3 ± 3 | 14 ± 5 |
| 72 | 1 ± 1 | 9 ± 3 | 18 ± 4 | 59 ± 11 |
| 96 | 1 ± 0 | 5 ± 2 | 14 ± 5 | 56 ± 13 |
| Treatment duration (hr) . | % NBT positivity . | |||
|---|---|---|---|---|
| C . | G . | R . | GR . | |
| 48 | 1 ± 1 | 1 ± 1 | 3 ± 3 | 14 ± 5 |
| 72 | 1 ± 1 | 9 ± 3 | 18 ± 4 | 59 ± 11 |
| 96 | 1 ± 0 | 5 ± 2 | 14 ± 5 | 56 ± 13 |
C indicates untreated control; G, G-CSF (10 ng/mL); R, ATRA (3 μmol/L); GR, G-CSF + ATRA. Average values ± SD from 3 experiments are shown.
Analyses of RARα expression in D+ cells treated for 24 hours with ATRA, G-CSF, or their combination.
(A) Western blot. Loading was standardized by equal cell numbers. Coomassie staining of a duplicate gel shows approximately equal amounts of protein in each lane. (B) Northern blot. Ethidium bromide staining of the gel shows approximately equal loading. C indicates untreated control; G, G-CSF (10 ng/mL); R, ATRA (3 μmol/L); GR, combination of G-CSF and ATRA.
Analyses of RARα expression in D+ cells treated for 24 hours with ATRA, G-CSF, or their combination.
(A) Western blot. Loading was standardized by equal cell numbers. Coomassie staining of a duplicate gel shows approximately equal amounts of protein in each lane. (B) Northern blot. Ethidium bromide staining of the gel shows approximately equal loading. C indicates untreated control; G, G-CSF (10 ng/mL); R, ATRA (3 μmol/L); GR, combination of G-CSF and ATRA.
Effects of G-CSF and ATRA on steady-state levels of RXRα
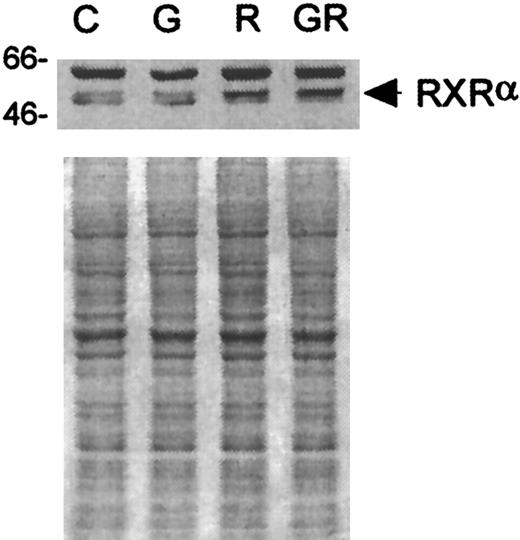
It was possible that retinoid receptors other than RARs (ie, RXRs) were involved in the differentiation process. Therefore, analyses of the expression of RXRs in response to G-CSF and ATRA treatment were performed using receptor subfamily-specific antibodies. RXRβ was detected in D+ cells, but its steady-state levels were not changed after treatment with G-CSF, ATRA, or the combination thereof (data not shown). Modest changes in the levels and mobility of RXRα protein, however, were detected in ATRA-treated cells (see Figure5 for a representative blot). Laser densitometric scanning of films exposed to Western blots (ECL detection) indicated that the steady-state levels of RXRα protein in D+ cells treated for 24 hours with ATRA or G-CSF + ATRA were increased by approximately 50% over those in untreated control cells or in cells treated with G-CSF alone. The migration of most RXRα protein from cells incubated with ATRA was also slightly slower in the SDS-polyacrylamide gel than that from either untreated control cells or from cells treated with G-CSF alone. Adding G-CSF to ATRA did not appear to enhance or diminish this effect.
Western blot analyses of RXRα in D+ cells after incubation with G-CSF, ATRA, or G-CSF + ATRA.
Loading was standardized by equal cell numbers. Coomassie staining of a duplicate gel shows approximately equal amounts of protein in each lane. C indicates untreated control; G, G-CSF (10 ng/mL); R, ATRA (3 μmol/L); GR, G-CSF + ATRA treatment.
Western blot analyses of RXRα in D+ cells after incubation with G-CSF, ATRA, or G-CSF + ATRA.
Loading was standardized by equal cell numbers. Coomassie staining of a duplicate gel shows approximately equal amounts of protein in each lane. C indicates untreated control; G, G-CSF (10 ng/mL); R, ATRA (3 μmol/L); GR, G-CSF + ATRA treatment.
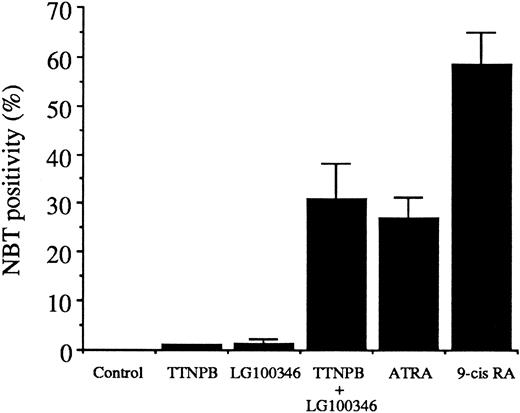
Evaluation of the induction of terminal differentiation by retinoid receptor–specific ligands
RARs bind either ATRA or 9-cis-RA, whereas RXRs bind only 9-cis-RA. However, because these naturally occurring retinoids may be interconverted in target tissues,20 it was not possible to ascertain whether RARs, RXRs, or both contributed to the synergistic induction of differentiation produced by G-CSF and ATRA. Retinoid receptor subfamily-specific agonists and antagonists (see Fitzgerald et al29 for references) permitted a dissection of the roles of RARs and RXRs in the synergism exhibited by G-CSF and ATRA. Thus, RAR (TTNPB; 4-[(E)-2-(5,6,7,8-tetrahydro-5,5,8,8-tetramethyl-2-naphthalenyl)-1-propenyl]benzoic acid) and RXR (LG100346; 4-[(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthyl)carbonyl]benzoic acid methoxyoxime) -specific agonists provided evidence that both RAR and RXR participation were essential for induction of the differentiation of D+ cells (Figure6). Given that neither RAR nor RXR activation alone was sufficient to produce differentiation of these cells, a heterodimer of RAR/RXR is likely to be the species involved. Moreover, these results suggest that both monomer partners must be bound by their respective ligands. This assumption is supported by the superior effectiveness of 9-cis-RA, which can bind and activate RARs and RXRs.
Differentiation of D+ cells induced by various retinoids (all at 5 μmol/L), as determined by NBT reduction after 72 hours of treatment.
TTNPB indicates RAR-specific agonist; LG100346, RXR-specific agonist; 9-cis RA, 9-cisretinoic acid. Data are the average values of 2 experiment ± the difference between values.
Differentiation of D+ cells induced by various retinoids (all at 5 μmol/L), as determined by NBT reduction after 72 hours of treatment.
TTNPB indicates RAR-specific agonist; LG100346, RXR-specific agonist; 9-cis RA, 9-cisretinoic acid. Data are the average values of 2 experiment ± the difference between values.
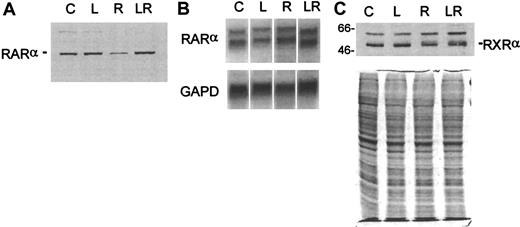
Effects of LiCl on retinoid receptor expression in ATRA-treated D+cells
When combined with ATRA, LiCl, like G-CSF, produced synergistic differentiation of D+ cells. We ascertained whether the effects of LiCl on RARα expression were comparable to those produced by G-CSF. Western blot analyses showed that LiCl was at least as effective as G-CSF in preventing ATRA-induced loss of RARα protein in these cells (Figure 7A). Like G-CSF, LiCl did not noticeably alter the levels of RARα mRNA (Figure 7B), and the expression of RXRα protein was also not affected by LiCl (Figure 7C). Interestingly, both G-CSF and LiCl were capable of protecting RARα pools in ATRA-treated cells by what appeared to be a nontranscriptional mechanism while producing synergistic terminal differentiation of the leukemia cells.
Analysis of retinoid receptor expression in D+ cells treated for 24 hours with LiCl, ATRA, or their combination.
(A) Western blot analysis of RARα. (B) Northern blot analysis of RARα. The blot was probed for GAPD to show approximately equal loading of RNA. (C) Western blot analysis of RXRα. The blot in A was stripped and reprobed with an antibody to RXRα. Loading was standardized by equal cell numbers. Coomassie staining of a duplicate gel shows approximately equal amounts of protein in each lane. C indicates untreated control; L, LiCl (2.5 mmol/L); R, ATRA (3 μmol/L); LR, combination of LiCl and ATRA.
Analysis of retinoid receptor expression in D+ cells treated for 24 hours with LiCl, ATRA, or their combination.
(A) Western blot analysis of RARα. (B) Northern blot analysis of RARα. The blot was probed for GAPD to show approximately equal loading of RNA. (C) Western blot analysis of RXRα. The blot in A was stripped and reprobed with an antibody to RXRα. Loading was standardized by equal cell numbers. Coomassie staining of a duplicate gel shows approximately equal amounts of protein in each lane. C indicates untreated control; L, LiCl (2.5 mmol/L); R, ATRA (3 μmol/L); LR, combination of LiCl and ATRA.
Effects of the prevention of the loss of RARα protein on the differentiation response of D+ cells to ATRA
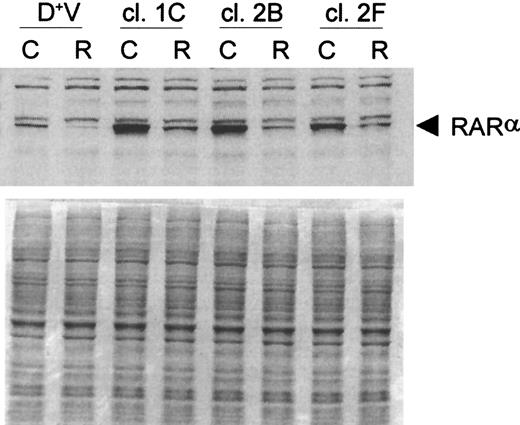
Based on the results obtained with the retinoid receptor–selective ligands, it is clear that both RARs and RXRs are essential for the differentiation response in D+ cells. We reasoned that the loss of one of these receptors (ie, RARα) could markedly limit the capacity of ATRA to produce terminal differentiation of these cells. Thus, we postulated that the attenuation of the ATRA-induced loss of RARα protein by coexposure to G-CSF or LiCl could extend the response to the retinoid and thereby lead to the synergistic induction of differentiation. Support for this concept would be provided if prevention of the loss of RARα protein by other means resulted in a heightened response to ATRA. To accomplish this, D+ cells were transfected with an expression plasmid containing RARα cDNA. Northern and Western blot analyses of 3 separate transfections are shown in Figure8. Populations of these cells had a 6- to 8-fold increase in steady-state levels of RARα mRNA compared to vector-transfected control cells (Figure 8A) and showed a small but significant increase (2- to 3-fold) in RARα protein (Figure 8B). Single-cell clones were derived from these transfected populations by flow cytometry, and clones with high RARα protein expression were chosen for further evaluation. ATRA treatment was found to cause a reduction in, but not a complete loss of, RARα protein in these clones (Figure 9), which were found to be considerably more responsive than parental WEHI-3B cells to ATRA as a single agent (Table 2). These findings support the concept that the basis for the synergistic interaction between G-CSF or LiCl and ATRA in producing terminal differentiation of D+ cells is at least in part caused by the prevention by G-CSF and LiCl of the complete loss of RARα protein in ATRA-treated cells.
Analyses of RARα expression in 3 populations of RARα-transfected D+ cells.
(A) Northern blot. The blot was probed for β-actin to show approximately equal loading of RNA. (B) Western blot. Loading was standardized by equal cell numbers. p indicates parental D+ cells; v, vector-transfected D+ cells.
Analyses of RARα expression in 3 populations of RARα-transfected D+ cells.
(A) Northern blot. The blot was probed for β-actin to show approximately equal loading of RNA. (B) Western blot. Loading was standardized by equal cell numbers. p indicates parental D+ cells; v, vector-transfected D+ cells.
Western blot analysis of RARα in selected RARα-transfected D+ cell clones after 24-hours incubation with 3 μmol/L ATRA.
Loading was standardized by equal cell numbers. Coomassie staining of a duplicate gel shows approximately equal amounts of protein in each lane. D+V indicates vector-transfected D+ cells; C, untreated control; R, ATRA (3 μmol/L).
Western blot analysis of RARα in selected RARα-transfected D+ cell clones after 24-hours incubation with 3 μmol/L ATRA.
Loading was standardized by equal cell numbers. Coomassie staining of a duplicate gel shows approximately equal amounts of protein in each lane. D+V indicates vector-transfected D+ cells; C, untreated control; R, ATRA (3 μmol/L).
Differentiation responsiveness of RARα-transfected D+ cell lines to ATRA as determined by NBT reduction
| Cell line . | % NBT positivity . | |
|---|---|---|
| Untreated . | ATRA treated . | |
| D+V | 0.2, 0.5 | 21.2, 18.5 |
| D+RAR pop 1 | 0.5, 0.3 | 50.0, 49.4 |
| D+RAR pop 2 | 0.3, 0.7 | 39.5, 46.6 |
| D+RAR pop 3 | 0.2, 0.2 | 43.9, 47.3 |
| D+RAR clone 1C | 0.3, 0.5 | 72.7, 45.1 |
| D+RAR clone 2B | 0.5, 0.0 | 82.9, 72.5 |
| D+RAR clone 2F | 0.3, 0.3 | 81.1, 65.3 |
| Cell line . | % NBT positivity . | |
|---|---|---|
| Untreated . | ATRA treated . | |
| D+V | 0.2, 0.5 | 21.2, 18.5 |
| D+RAR pop 1 | 0.5, 0.3 | 50.0, 49.4 |
| D+RAR pop 2 | 0.3, 0.7 | 39.5, 46.6 |
| D+RAR pop 3 | 0.2, 0.2 | 43.9, 47.3 |
| D+RAR clone 1C | 0.3, 0.5 | 72.7, 45.1 |
| D+RAR clone 2B | 0.5, 0.0 | 82.9, 72.5 |
| D+RAR clone 2F | 0.3, 0.3 | 81.1, 65.3 |
Cells were seeded at a density of 1 × 104/mL and treated with 3 μmol/L ATRA for 72 hr in McCoy's modified 5A medium supplemented with 15% fetal bovine serum. Values from 2 separate experiments are shown.
Discussion
Numerous investigations have established correlations between the loss of retinoic acid receptors and malignant progression.29-38 Similar to previous studies using other cell lines,28 we have shown that ATRA induces a marked decrease in the levels of RARα protein in WEHI-3B leukemia cells. We hypothesize that this loss of receptor may limit the ability of the cell population to differentiate fully in response to ATRA. Thus, if the loss of RARα protein limits the effectiveness of ATRA as a single agent, it is reasonable to assume that the ability of G-CSF or LiCl to attenuate this loss would increase the effectiveness of ATRA as an inducer of differentiation and thereby can account, at least in part, for the synergistic interaction of these combinations. The correlation between prevention of the ATRA-induced loss of RARα in RARα transfectants and the enhancement of the differentiation response to ATRA in these clones supports this notion.
The RXRs also play important roles in retinoid action, and their activation appears to be required for the maturation of D+cells exposed to the retinoid. Western blot analyses of RXRα showed that ATRA treatment caused a modest increase in the levels of RXRα and a slight shift to a slower mobility form of the protein. The importance of these changes are unclear at this time, however, because these effects were brought about by the retinoid alone, and, because these changes were not enhanced or diminished by the inclusion of either G-CSF or LiCl with ATRA, they are not considered relevant to the synergism exhibited by these combinations.
G-CSF, initially identified by Nicola et al39 as a factor that induced the terminal differentiation of D+cells, is widely used to stimulate neutrophil production after chemotherapy and in other syndromes accompanying neutropenia.40,41 Cases have been reported in which G-CSF, when used in combination with ATRA and chemotherapy, contributed to remission induction in patients with refractory APL,42-44 presumably because of the induction of terminal differentiation.42,45 The combination of these agents has also been used recently with some success in the treatment of myelodysplastic syndromes.46 47 These clinical responses, however modest, justify the search for more effective regimens of differentiation therapy.
Lithium salts have long been known to have the capacity to improve neutrophil production (see Boggs and Joyce17 for a review). Thus, the use of this monovalent cation has been proposed as an approach to minimize the myelosuppressive effects of anticancer and antiviral therapies in humans. Previous work from our laboratory examined the possibility that lithium increased the formation of granulocytes from immature leukemic precursors.16 LiCl, at millimolar concentrations, was found to induce the maturation of both HL-60 human promyelocytic leukemia and D+ murine myelomonocytic leukemia cells in culture. In preliminary studies of the mechanism(s) involved, we found that KCl, but not NaCl or MgCl2, could antagonize the differentiation-inducing effects of LiCl alone or when combined with ATRA (data not shown). The significance of this observation is unclear. LiCl has been demonstrated to act on second messengers, blocking phosphatidyl inositol signaling pathways (see Berridge48 for a review). More recent studies with lithium in the developmental field have shown that some of the effects of lithium are attributable to its inhibition of glycogen synthase kinase-3β (GSK-3β), which regulates cell fate determination in various organisms.49 GSK-3β activity is normally inhibited through activation of the Wnt signaling pathway (see Peifer and Polakis50 for a review). The possible involvement of these pathways in the LiCl-induced maturation of leukemia cells is under investigation in our laboratory.
One criticism of in vitro differentiation studies is that the concentrations of inducers required to elicit meaningful responses are not attainable in vivo. The use of agents such as G-CSF and LiCl in combination with retinoids may favorably impact on this limitation of differentiation therapy by allowing relatively low concentrations to be used. Drug combinations that include G-CSF are limited to blood cells that express G-CSFR. Combinations having LiCl as a constituent presumably will not be subject to the same limitation. This possibility entices speculation that retinoid-based differentiation therapy regimens for other forms of leukemia or for tumors originating from other tissue types (eg, lung, squamous cells of the head and neck) may be markedly improved by including LiCl to protect receptor pools.
Supported in part by United States Public Health Service grant CA-02817 from the National Cancer Institute. R.A.F. is a Special Fellow of the Leukemia and Lymphoma Society.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alan C. Sartorelli, Department of Pharmacology, Yale University School of Medicine, 333 Cedar Street, New Haven, CT 06520; e-mail: alan.sartorelli@yale.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal