Abstract
The differentiation and apoptosis-sensitizing effects of the Bcr-Abl–specific tyrosine kinase inhibitor CGP57148B, also known as STI-571, were determined in human Bcr-Abl–positive HL-60/Bcr-Abl and K562 cells. First, the results demonstrate that the ectopic expression of the p185 Bcr-Abl fusion protein induced hemoglobin in the acute myeloid leukemia (AML) HL-60 cells. Exposure to low-dose cytosine arabinoside (Ara-C; 10 nmol/L) increased hemoglobin levels in HL-60/Bcr-Abl and in the chronic myeloid leukemia (CML) blast crisis K562 cells, which express the p210 Bcr-Abl protein. As compared with HL-60/neo, HL-60/Bcr-Abl and K562 cells were resistant to apoptosis induced by Ara-C, doxorubicin, or tumor necrosis factor-α (TNF-α), which was associated with reduced processing of caspase-8 and Bid protein and decreased cytosolic accumulation of cytochrome c (cyt c). Exposure to CGP57148B alone increased hemoglobin levels and CD11b expression and induced apoptosis of HL-60/Bcr-Abl and K562 cells. CGP57148B treatment down-regulated antiapoptotic XIAP, cIAP1, and Bcl-xL, without affecting Bcl-2, Bax, Apaf-1, Fas (CD95), Fas ligand, Abl, and Bcr-Abl levels. CGP57148B also inhibited constitutively active Akt kinase and NFκB in Bcr-Abl–positive cells. Attenuation of NFκB activity by ectopic expression of transdominant repressor of IκB sensitized HL-60/Bcr-Abl and K562 cells to TNF-α but not to apoptosis induced by Ara-C or doxorubicin. Importantly, cotreatment with CGP57148B significantly increased Ara-C– or doxorubicin-induced apoptosis of HL-60/Bcr-Abl and K562 cells. This was associated with greater cytosolic accumulation of cyt c and PARP cleavage activity of caspase-3. These in vitro data indicate that combinations of CGP57148B and antileukemic drugs such as Ara-C may have improved in vivo efficacy against Bcr-Abl–positive acute leukemia.
Introduction
The dysregulated activity of the tyrosine kinase (TK) encoded by the bcr-abl fusion gene is responsible for the malignant phenotype of the Bcr-Abl–expressing chronic myeloid leukemia (CML) and acute lymphoid leukemia (ALL) blasts.1The fusion gene encodes for either the p210 or p185 TK implicated in the pathogenesis of CML or ALL, respectively.2 Leukemic blasts expressing Bcr-Abl display arrested differentiation, as well as resistance to apoptosis, even when exposed to high doses of antileukemic drugs.3-5 Recent studies from our laboratory demonstrated that Bcr-Abl also exerts its antiapoptotic effect against apoptosis triggered by sphingosine or C2-ceramide or Fas (CD95) receptor-mediated death signaling.6 Ectopic or endogenous expression of Bcr-Abl in HL-60/Bcr-Abl or K562 cells, respectively, blocked the mitochondrial permeability transition (Δψm) and release of cytochrome c (cyt c), thereby inhibiting the activation of caspase-3 and apoptosis.6 Recent reports have indicated that the ectopic expression of Bcr-Abl in interleukin (IL)-3–dependent murine myeloid cells activates p65 NFκB (Rel A), which is known to suppress apoptosis due to a variety of apoptotic stimuli.7-9Conversely, in these cells, antisense oligonucleotides to Rel A were shown to abrogate the antiapoptotic effect of Bcr-Abl against apoptosis induced by IL-3 withdrawal.7 However, whether constitutively active NFκB affected differentiation and apoptosis in human myeloid leukemia cells had not been determined. Bcr-Abl expression has also been shown to up-regulate Bcl-xL levels in HL-60/Bcr-Abl and K562 cells,5,10 but the relative contribution of Bcl-xL up-regulation toward Bcr-Abl—mediated resistance to apoptosis had not been established. Recent studies have shown that the inhibition of Bcr-Abl activity by a relatively specific Bcr-Abl TK inhibitor CGP57148B causes in vitro and in vivo eradication of Bcr-Abl–positive human leukemia cells.11 12 However, whether treatment of Bcr-Abl–positive leukemic cells with CGP57148B would attenuate their resistance to drug-induced apoptosis as well as increase their sensitivity to differentiating agents such as low-dose cytosine arabinoside (Ara-C) had not been investigated.
In the present studies, we investigated the roles of potential downstream effectors of Bcr-Abl TK and its pharmacologic inhibition by CGP57148B in modulating drug-induced differentiation and apoptosis of Bcr-Abl–positive leukemic blasts. Surprisingly, Bcr-Abl expression was noted to induce hemoglobin (Hb) production in HL-60/Bcr-Abl cells. This was further augmented by treatment with low-dose Ara-C (LODAC). Inhibition of NFκB activity by expression of the transdominant repressors of IκBα reduced LODAC-induced Hb production, but had no effect on the resistance of HL-60/Bcr-Abl and K562 cells to apoptosis induced by Ara-C or doxorubicin. In contrast, CGP571418B not only induced Hb and the expression of the myeloid differentiation marker CD11b, but also sensitized HL-60/Bcr-Abl and K562 cells to apoptosis induced by Ara-C or doxorubicin. CGP57148B-induced apoptosis was also associated with inhibition of Akt kinase and NFκB activities as well as down-regulation of the levels of the antiapoptotic Bcl-xL, XIAP, and cIAP1 proteins.
Materials and methods
Reagents
Ara-C was purchased from Sigma Chemical Co (St Louis, MO). Antihuman Abl monoclonal antibodies were purchased from Santa Cruz (Santa Cruz, CA). A monoclonal anti–Bcl-2 antibody was purchased from DAKO (Carpinteria, CA). Polyclonal anti-Bcl-x, anti-Bax, and antipoly (ADP-ribose) polymerase (PARP) antibodies and caspase-8 antibodies were purchased from Pharmingen (San Diego, CA). Fas receptor (CD95) and ligand (FasL) monoclonal antibodies were purchased from Transduction Labs (Lexington, KY). Anti-XIAP antibody was purchased from Boehringer Mannheim (Indianapolis, IN); anti-cIAP antibody was purchased from Pharmingen. Antiphosphotyrosine antibody and Akt kinase assay kit were purchased from New England Biolabs (Beverly, MA). Anti–Apaf-113 and anti-Bid antisera14 were kindly provided by Dr Xiaodong Wang of the University of Texas Southwestern Medical Center (Dallas, TX). CGP57148B was kindly provided by Novartis Pharma AG (Basel, Switzerland).
Cells and transfection of the bcr-ablgene
Human AML HL-60/Bcr-Abl and HL-60/neo cells were created by transfection of the bcr-abl gene encoding the p185 Bcr-Abl and/or neomycin-resistant gene and passaged twice per week, as previously described.6 K562 cells were passaged as previously reported.5 Logarithmically growing cells were used for the studies described below.
Generation of plasmids and IκBα2N or IκBα2NΔ4 expressing K562 and HL-60 cells
The CMVt-rtTA, CMVt-Neo, CMVt-IκBα2N, and CMVt-IκBα2NΔ4 plasmids were created as previously described.15,16 These were kindly provided by Dr John Hiscott of McGill University, Montreal, Quebec, Canada. First, K562 cells were transfected with the CMVt-rtTA plasmid DNA. Then puromycin-resistant clones were transfected with CMVt-Neo, CMVt-2N, and CMVt-2NΔ4 plasmids by a previously described method.5 Transformants were analyzed for inducible IκBα expression, and 3 clones from each transformant pool were selected for Western analyses and further studies.15,16Stable transfectants of HL-60/Bcr-Abl cells containing IκBα2NΔ4 were also created for Western analyses and further studies, as previously described.5
NFκB activity reporter assay
HL-60/neo, HL-60/Bcr-Abl, HL-60/Bcr-Abl with IκBα2NΔ4, as well as K562/rtTA, K562/ IκBα2NΔ4, or K562/ IκBα2NΔ4 were transfected, using Lipofectamine, with the reporter plasmid for pNFκB (Clontech, Palo Alto, CA), which has 3 repeats of the NFκB site upstream of a minimal thymidine kinase promoter and theluciferase gene. After exposure to 2.0 μg/mL doxycycline alone or cotreatment with doxycycline and TNF-α, cells were harvested in phosphate-buffered saline (PBS) and lysed in a luciferase lysis buffer. The lysates were assayed for luciferase using a luminometer.17
Growth inhibitory effects of Ara-C
Logarithmically growing HL-60/neo versus HL-60/Bcr-Abl cells were exposed to low concentrations (10 nmol/L) of Ara-C for 7 days. Following these treatments, aliquots of cells were withdrawn and the cell numbers were determined using a Coulter particle count and size analyzer (Coulter Inc, Hialeah, FL). Suspension culture growth inhibition by Ara-C was determined, as previously described.18
Flow cytometric analysis of apoptosis
The flow cytometric evaluation of apoptosis was performed according to a modification of a previously described method.19 Briefly, untreated or drug-treated cells were centrifuged, washed in Hanks' balanced saline solution, and fixed in 70% ethanol. The tubes containing the cell pellets were stored at −20°C for at least 24 hours. Following this, the cells were centrifuged at 800g for 15 minutes and supernatant was discarded to remove ethanol completely. The pellets were resuspended in 40 μL (for 2-3 × 106 cells) of phosphate-citrate buffer at room temperature for 30 minutes. Following this incubation, cells were washed with 4 to 5 mL PBS and stained with propidium iodide (PI) solution (20 μg/mL PI and 20 μg/mL RNAse A in PBS) for 30 minutes. The samples were read on a Coulter Elite flow cytometer using Elite software program 4.0 for 2-color detection. The percentage of cells in the apoptotic sub-G1 phase was calculated using Multicycle software (Phoenix Flow Systems, San Diego, CA).
Apoptosis assessment by annexin-V staining
After drug treatment, 5 × 105 to 1 × 106 cells were washed in PBS and resuspended in 100 μL staining solution (containing annexin-V fluorescein and PI in a HEPES buffer, Annexin-V-FLUOS Staining Kit, Boehringer-Mannheim). Following incubation at room temperature for 15 minutes, cells were analyzed by flow cytometry. Annexin V binds to those cells that express phosphotidylserine on the outer layer of the cell membrane, and PI stains the cellular DNA of those that have a compromised cell membrane. This allows for the discrimination of live cells (unstained with either fluorochrome) from apoptotic cells (stained only with annexin V) and necrotic cells (stained with both annexin V and PI).20
Western analyses
Western analyses of Bcl-2, Bcl-xL, Bax, Fas receptor (CD95), FasL, Bcr-Abl, IκBα, caspase-8, tyrosine phosphorylated proteins, and β-actin were performed using specific antisera or monoclonal antibodies (see above), as described previously.19 21 Horizontal scanning densitometry was performed on Western blots by using acquisition into Adobe Photo Shop (Apple, Inc, Cupertino, CA) and analysis by the NIH Image Program (National Institutes of Health, Bethesda, MD). The expression of β-actin was used as a control.
Immunophenotyping for differentiation markers and Hb production
The HL-60/neo, HL-60/Bcr-Abl, and K562 cells were treated with Ara-C for 7 days. Cells were then washed with PBS, and resuspended in 100 μL FACS wash buffer (PBS, 0.2% NaN3, 0.1% bovine serum albumin [BSA], 2.0% human AB+ serum, filtered by suction at 0.45 μm). Ten microliters phycoerythrin (PE) antihuman CD11b, CD33, or CD34 antibody (Pharmingen) was added,22,23and the cells were incubated in the dark at 4°C for 30 minutes. The samples were then analyzed by flow cytometry. Alternatively, untreated or drug-treated cells were washed in PBS and intracellular Hb levels were determined by a spectrophotometric assay, as previously described.5
Akt kinase assay
In untreated and CGP57148B-treated cells, Akt kinase activity was determined by using an immunoprecipitation-kinase assay with reagents provided in a commercially available kit (New England Biolabs). Briefly, cell lysates were used to immunoprecipitate Akt utilizing a polycolonal Akt antibody. Immunoprecipitates were then incubated with GSK-3 fusion protein in the presence of adenosine triphosphate (ATP) and kinase buffer, allowing immunoprecipitated Akt to phosphorylate GSK-3, which was analyzed by Western blotting using a phospho-GSK-3α/β (serine 21/9) antibody.24
Morphology of apoptotic cells
After treatment with or without Ara-C, 50 × 103cells were washed with PBS (pH 7.3) and resuspended in the same buffer. Cytospin preparations of the cell suspensions were fixed and stained with Wright stain. Cell morphology was determined by light microscopy. In all, 5 different fields were randomly selected for counting 500 cells. The percentage of apoptotic cells was calculated for each experiment, as described previously.25
Statistical analysis
Significant differences between values obtained in a population of leukemic cells treated with different experimental conditions were determined by paired t test analyses. A one-way ANOVA was also applied to the results of the various treatment groups, andpost hoc analysis was performed using the Bonferroni adjustment method.
Results
Bcr-Abl expression induced Hb but inhibited LODAC-induced myeloid differentiation
Ectopic and stable expression of p185 Bcr-Abl in HL-60 and endogenous expression of p210 Bcr-Abl in K562 cells are associated with high Bcl-xL and barely detectable levels of Bcl-2. These effects of Bcr-Abl expression have been previously reported, although the precise mechanism(s) underlying these effects has not been elucidated.5,6 As previously reported, there was no significant difference in Bax expression in the control HL-60/neo versus HL-60/Bcr-Abl and K562 cells.6 Surprisingly, the ectopic expression of Bcr-Abl induced Hb production (Figure1A), imparting a red color to the pellet of the centrifuged HL-60/Bcr-Abl cells. Hb was barely detectable in HL-60/neo cells and their pellet was colorless. As compared to HL-60/neo, there was no significant alteration in the expression of CD33 or CD34 in HL-60/Bcr-Abl cells (data not shown). Figure 1A also demonstrates that treatment with 10 nmol/L Ara-C (LODAC) for 7 days significantly increased Hb levels in both HL-60/Bcr-Abl and K562 cells (P < .05). This was associated with other morphologic features of erythroid differentiation such as the loss of cytoplasmic granularity and nuclear condensation in approximately 40% of HL-60/Bcr-Abl cells (data not shown). Exposure to LODAC for 7 days markedly increased the expression of the late myeloid differentiation marker CD11b in HL-60/neo (from 33.7% ± 3.8% to 62.7% ± 6.4%), but not in HL-60/Bcr-Abl cells (Figure 1B). This was associated with morphologic features of myeloid differentiation in approximately 60% of HL-60/neo but not in HL-60/Bcr-Abl and K562 cells (data not shown). The effect of the ectopic expression of Bcr-Abl on the differentiation response to phorbol esters was not investigated and remains to be determined. It is noteworthy that K562 cells did not express CD11b, and the ectopic expression of Bcr-Abl was associated with inhibition of CD11b expression in HL-60/Bcr-Abl cells (Figure 1B). Treatment with LODAC for 7 days produced 95% and 97.6% growth inhibition as well as 38% and 5% apoptosis in HL-60/neo and HL-60/Bcr-Abl cells, respectively (means of 2 experiments performed in duplicate).
Low-dose Ara-C (LODAC) increases Hb levels but not CD11b expression in HL-60/Bcr-Abl cells.
Following treatment with 10 nmol/L Ara-C for 7 days, Hb levels (A) or CD11b expression (B) was determined in HL-60/Bcr-Abl and K562 cells.
Low-dose Ara-C (LODAC) increases Hb levels but not CD11b expression in HL-60/Bcr-Abl cells.
Following treatment with 10 nmol/L Ara-C for 7 days, Hb levels (A) or CD11b expression (B) was determined in HL-60/Bcr-Abl and K562 cells.
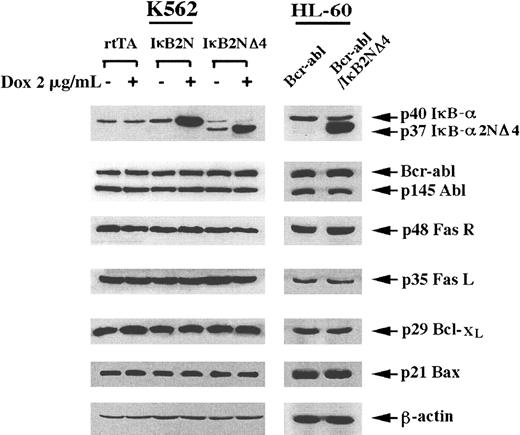
Inhibition of constitutively high NFκB activity reduces erythroid differentiation and increases TNF-α but not apoptosis induced by Ara-C- or doxorubicin
Previous reports had demonstrated that Bcr-Abl expression is associated with increased NFκB activity in the IL-3–dependent murine myeloid cells.7 K562 and HL-60/Bcr-Abl cells also had higher NFκB activity than HL-60/neo cells, which was reduced by the doxycycline-inducible or stable expression of the transdominant repressor of IκBα, that is IκBα2N or IκBα2NΔ4, respectively (data not shown). Clear evidence for the doxycycline-inducible or stable expression of the transdominant repressors of IκBα is provided in the immunoblot analysis presented in Figure 3. In Figures2 and 3, the inducible expression of IκBα2N or IκBα2NΔ4 in K562 cells is representative of 3 separate clones of stable transfectants. The effect of the repression of NFκB activity on LODAC-induced Hb generation was also determined in K562 and HL-60/Bcr-Abl cells. Doxycycline-induced expression of IκBα2N or IκBα2NΔ4 significantly inhibited LODAC-induced Hb levels in K562 cells (P < .05) (Figure 2A). Stable expression of IκBα2NΔ4 had a similar effect in HL-60/Bcr-Abl cells (Figure 2B). We next examined the effects of IκBα2N or IκBα2NΔ4 expression on TNF-α–, Ara-C–, or doxorubicin-induced apoptosis of K562 and HL-60/Bcr-Abl cells. Table1 shows that treatment of the control HL-60/Bcr-Abl and K562 cells (K562/rtTA) with TNF-α (100 ng/mL for 24 hours) only slightly increased the percentage of apoptotic cells. Repression of NFκB activity by inducible (in K562) or stable expression of IκBα2NΔ4 (in HL-60/Bcr-Abl) by itself did not alter the percentage of apoptotic cells. However, it significantly increased TNF-α-induced apoptosis of HL-60/Bcr-Abl and K562 cells (P < .05) (Table 1), although the increment was modest in K562 cells (Table 1). In contrast, the expression of IκBα2NΔ4 did not sensitize K562 or HL-60/Bcr-Abl cells to Ara-C (5-100 μmol/L), etoposide (50 μmol/L), or doxorubicin-induced (0.25-1.0 μmol/L) apoptosis (data not shown). These results suggest that in HL-60/Bcr-Abl and K562 cells NFκB activity contributes to the resistance to apoptosis due to TNF-α but not due to Ara-C, etoposide, or doxorubicin. Immunoblot analyses shown in Figure 3 clearly demonstrate that exposure to 2.0 μg/mL doxycycline induced the levels of either IκBα2N, which has the same molecular weight as IκBα (40 kd), or the levels of the smaller p37 IκBα2NΔ4 in K562/IκBα2N or K562/IκBα2NΔ4 cells, respectively. Inducible (K562) or stable expression of IκBα2NΔ4 (HL-60/Bcr-Abl) reduced the endogenous IκBα levels in K562 and HL-60/Bcr-Abl cells (Figure 3A), since NFκB is known to transactivate its own repressor IκBα.16 Expression of IκBα2N or IκBα2NΔ4 did not alter the levels of Bcr-Abl, Abl, Fas, FasL, Bcl-xL, and Bax in K562 or HL-60/Bcr-Abl cells (Figure 3). Contrary to the reported findings from other cell types,9 intracellular cIAP1 levels were also not affected by inhibition of NFκB activity (data not shown).
Repression of NFκB.
Transdominant (TD) repressors of IκBα inhibit LODAC-induced Hb levels in K562 (A) and HL-60/Bcr-Abl cells (B).
Repression of NFκB.
Transdominant (TD) repressors of IκBα inhibit LODAC-induced Hb levels in K562 (A) and HL-60/Bcr-Abl cells (B).
Immunoblot analyses.
Western analyses of IκBα, IκBα2N, IκBα2NΔ4, Bcr-Abl, Abl, FasR, FasL, Bcl-xL, Bax, and β-actin in K562 and HL-60/Bcr-Abl cells containing doxycycline-inducible and stable expression of transdominant (TD) repressor of IκBα, respectively.
Immunoblot analyses.
Western analyses of IκBα, IκBα2N, IκBα2NΔ4, Bcr-Abl, Abl, FasR, FasL, Bcl-xL, Bax, and β-actin in K562 and HL-60/Bcr-Abl cells containing doxycycline-inducible and stable expression of transdominant (TD) repressor of IκBα, respectively.
Effect of IκBα2NΔ4 on the resistance of K562 and HL-60/Bcr-Abl cells to TNF-α–induced apoptosis
| . | % apoptotic cells* . | |
|---|---|---|
| Control . | TNF-α† (100 ng/mL) . | |
| K562 | ||
| RtTA‡ | 2.8 ± 1.5 | 5.0 ± 1.0 |
| IκBα2NΔ4‡ | 4.1 ± 2.0 | 11.7 ± 2.11-153 |
| HL-60 | ||
| Bcr-Abl | 2.9 ± 1.6 | 6.3 ± 1.4 |
| Bcr-Abl/Iκbα2NΔ4 | 3.3 ± 1.2 | 31.2 ± 3.61-155 |
| . | % apoptotic cells* . | |
|---|---|---|
| Control . | TNF-α† (100 ng/mL) . | |
| K562 | ||
| RtTA‡ | 2.8 ± 1.5 | 5.0 ± 1.0 |
| IκBα2NΔ4‡ | 4.1 ± 2.0 | 11.7 ± 2.11-153 |
| HL-60 | ||
| Bcr-Abl | 2.9 ± 1.6 | 6.3 ± 1.4 |
| Bcr-Abl/Iκbα2NΔ4 | 3.3 ± 1.2 | 31.2 ± 3.61-155 |
Detected by annexin V staining followed by flow cytometry.
These cells were cotreated with 3 μg/mL cycloheximide (CHX).
Pretreated with 1 μg/mL doxycycline for 48 hours.
Value significantly different from those in K562/rtTA cells treated with TNF-α + CHX (P < .05).
Value significantly different from those in HL-60/Bcr-Abl cells treated with TNF-α + CHX (P < .01).
Bcr-Abl TK inhibitor CGP57148B induces Hb and apoptosis of K562 and HL-60/Bcr-Abl cells
Recent reports had indicated that the ATP binding-site antagonist, Bcr-Abl–specific TK inhibitor CGP57148B or STI-571 can suppress growth and induce apoptosis of Bcr-Abl–positive leukemic cells.11,12,26 In the present studies, we determined whether CGP57148B would induce differentiation and apoptosis, as well as sensitize HL-60/Bcr-Abl and K562 cells to high-dose Ara-C (HIDAC)– and doxorubicin-induced apoptosis. First, we examined the effect of 0.25 μmol/L CGP57148B, a dose previously shown to be the IC50 value for the autophosphorylation of vAbl or Bcr-Abl,26 on Bcr-Abl, Abl, Bcl-xL, Bcl-2, Bax, and cellular protein tyrosine phosphorylation levels in HL-60/Bcr-Abl and K562 cells. Twenty-four to 72 hours of exposure to 0.25 μmol/L (or 0.5 μmol/L, not shown) CGP57148B did not alter the intracellular levels of Bcr-Abl, Abl, Bcl-2, and Bax (Figure4A,D,E). CGP57148B treatment also did not affect the intracellular levels of Apaf-1 (not shown). In contrast, exposure to CGP57148B for 48 to 72 hours produced approximately a 5-fold decline in Bcl-xL levels (Figure 4C) and inhibited the tyrosine phosphorylation of cellular proteins (Figure 4B). Leukemic transformation mediated by Bcr-Abl is known to involve an increase in Akt kinase activity that inhibits apoptosis.27 Therefore, we examined Akt kinase activity in untreated and CGP57148B-treated (0.5 μmol/L for 48 hours) HL-60/neo, HL-60/Bcr-Abl, and K562 cells. As shown in Figure 5, the latter 2 cell types demonstrated higher activity of Akt kinase than HL-60/neo cells. CGP57148B inhibited Akt kinase activity in HL-60/Bcr-Abl and K562 but not in HL-60/neo cells (Figure 5). Although not shown, CGP57148B did not affect Akt levels in any of the cell types. CGP57148B treatment also significantly lowered XIAP levels (Figure 5). As shown, exposure to CGP57148B also lowered cIAP1 levels in HL-60/Bcr-Abl cells. Collectively, XIAP and cIAP1 are known to inhibit the activity of caspase 3, 7, 8, and 9.28 29 Although a shorter exposure (24 hours) produced only modest effects, exposure to higher doses of CGP57148B did not augment these effects of CGP57148B (data not shown). Although the mechanism underlying this remains to be firmly established, treatment with CGP57148B (0.25 μmol/L) also modestly inhibited NFκB activity by a mean of 20% (2 experiments) in HL-60/Bcr-Abl and K562 cells (data not shown). There was no effect of CGP57148B on Akt kinase (Figure 5) or NFκB activity (data not shown) in HL-60/neo cells.
CGP57148B (CGP) inhibits tyrosine phosphorylation of proteins and down-regulates Bcl-xL in HL-60/Bcr-Abl and K562 cells.
Cells were treated with 0.25 μmol/L CGP for 24 to 72 hours, cellular proteins were extracted and Western analyses of Bcr-Abl, Abl, Bcl-xL, Bax, β-actin, as well as tyrosine phosphorylated proteins (P-Tyr) were performed (see text). Expression levels are representative of 3 separate experiments.
CGP57148B (CGP) inhibits tyrosine phosphorylation of proteins and down-regulates Bcl-xL in HL-60/Bcr-Abl and K562 cells.
Cells were treated with 0.25 μmol/L CGP for 24 to 72 hours, cellular proteins were extracted and Western analyses of Bcr-Abl, Abl, Bcl-xL, Bax, β-actin, as well as tyrosine phosphorylated proteins (P-Tyr) were performed (see text). Expression levels are representative of 3 separate experiments.
Akt kinase activity in untreated and CGP57148B-treated cells.
Following treatment of HL-60/neo, HL-60/Bcr-Abl, and K562 cells with 0.5 μmol/L CGP57148B (CGP) for 48 hours, cell lysates were used to determine either the Akt kinase activity by measuring phosphorylation of its substrate GSK-3α, or a Western analysis of XIAP, cIAP1, and β-actin was performed (see text). Beta-actin served as the control for protein loading. CGP lowered Akt kinase activity as well as XIAP and cIAP1 levels in HL-60/Bcr-Abl and K562 but not HL-60/neo cells.
Akt kinase activity in untreated and CGP57148B-treated cells.
Following treatment of HL-60/neo, HL-60/Bcr-Abl, and K562 cells with 0.5 μmol/L CGP57148B (CGP) for 48 hours, cell lysates were used to determine either the Akt kinase activity by measuring phosphorylation of its substrate GSK-3α, or a Western analysis of XIAP, cIAP1, and β-actin was performed (see text). Beta-actin served as the control for protein loading. CGP lowered Akt kinase activity as well as XIAP and cIAP1 levels in HL-60/Bcr-Abl and K562 but not HL-60/neo cells.
Figure 6 demonstrates that treatment with 0.25 μmol/L CGP57148B for 7 days also induced Hb levels in HL-60/Bcr-Abl and K562 cells. This increase in Hb was more than that observed following exposure to LODAC (P < .05). A combined treatment with CGP57148B and LODAC for 7 days did not increase Hb levels over those induced by treatment with CGP57148B alone (P > .05) (Figure 6). CGP57148B (0.25 μmol/L for 7 days) also increased the percentage of cells expressing CD11b from 1.0% to 27.4% in HL-60/Bcr-Abl and from 0.4% to 37.5% in K562 cells (mean of 3 experiments; Figure 6). Exposure to LODAC alone for 7 days only modestly increased CD11b expression in K562 cells. In addition, cotreatment with LODAC did not augment the expression of CD11b induced by treatment with CGP57148B alone (Figure 6).
Increases in Hb after treatment with CGP57148B.
CGP57148B (CGP) increases intracellular Hb levels and CD11b expression in HL-60/Bcr-Abl and K562 cells. Following treatment with 0.25 μmol/L CGP, 10 nmol/L Ara-C or CGP plus Ara-C for 7 days, intracellular Hb levels and CD11b expression were determined in HL-60/Bcr-Abl and K562 cells (see text). Values represent the means of 3 separate experiments.
Increases in Hb after treatment with CGP57148B.
CGP57148B (CGP) increases intracellular Hb levels and CD11b expression in HL-60/Bcr-Abl and K562 cells. Following treatment with 0.25 μmol/L CGP, 10 nmol/L Ara-C or CGP plus Ara-C for 7 days, intracellular Hb levels and CD11b expression were determined in HL-60/Bcr-Abl and K562 cells (see text). Values represent the means of 3 separate experiments.
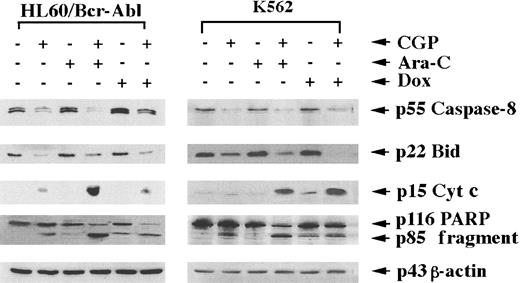
Data in Table 2 describe the apoptotic effects of CGP57148B, Ara-C, and doxorubicin in HL-60/Bcr-Abl and K562 cells. Following an exposure to 0.25 μmol/L CGP57148B for 48 hours approximately 15% of HL-60/Bcr-Abl and 18% of K562 cells were apoptotic as determined by annexin V staining and flow cytometry. CGP57148B had no effect on HL-60/neo cells (data not shown). In Table2, the differences in apoptotic rates detected by the various methods may be attributable to a disparate feature detected by each method to characterize apoptosis. Cotreatment with CGP57148B (0.25 μmol/L) also significantly increased the percentage of apoptotic cells following exposure to Ara-C (5.0 μmol/L) or doxorubicin (0.25 μmol/L) for 48 hours (P < .05; Table 2). This was not observed in HL-60/neo cells (data not shown), which are known to be highly sensitive to apoptosis induced by Ara-C and doxorubicin. Cotreatment with CGP57148B also significantly increased apoptosis of HL-60/Bcr-Abl cells induced by etoposide (5.0 μmol/L) (etoposide: 8.0% ± 0.9% versus CGP57148B plus etoposide: 24.1% ± 1.5%) or HIDAC (100.0 μmol/L) for 4 hours, where Ara-C was added in the final 4 hours of exposure to CGP57148B, versus Ara-C alone (Ara-C: 6.5 ± 1.0% versus CGP7148B plus Ara-C: 28.4% ± 2.2%) (P < .01). Furthermore, we determined the effect of cotreatment with CGP57148B plus Ara-C or doxorubicin on the processing of caspase-8 and cytosolic Bid, as well as the accumulation of cytosolic cyt c and the generation of PARP cleavage activity of caspase-3.10 Figure7 demonstrates that treatment with Ara-C alone did not result in the processing of procaspase-8 and Bid or cause cytosolic accumulation of cyt c and caspase-dependent cleavage of PARP. Doxorubicin treatment produced modest accumulation of cyt c in the cytosol associated with partial cleavage of p116 PARP into its p85 fragment (Figure 7). Treatment with CGP57148B alone clearly induced the processing of procaspase-8 and Bid and was associated with an increase in cytosolic cyt c (Figure 7). Accumulation of cyt c in the cytosol triggers Apaf-1-mediated cleavage and activity of caspase-9, followed by activation of caspase-3.13,30 Caspase-3 results in the cleavage of PARP and expression of phosphatidyl serine on the cell membrane, detected by increased staining by annexin V,13as shown in Figure 7 and Table 2, respectively. Cotreatment with CGP57148B and Ara-C (5.0 μmol/L) or doxorubicin (0.25 μmol/L) for 48 hours produced procaspase-8 and Bid processing, resulting in an increase in the cytosolic cyt c. The results of the immunoblot analyses presented in Figure 7 are representative of 3 separate experiments. Collectively, these data indicate that CGP57148B not only induces phenotypic markers of differentiation and apoptosis but also sensitizes Bcr-Abl–positive cells to antileukemic drugs such as Ara-C, etoposide, and doxorubicin.
Effect of CGP57148B on ara-C– or doxorubicin-induced apoptosis of HL-60/neo, HL-60/Bcr-Abl, and K562 cells
| . | % apoptotic cells . | |||
|---|---|---|---|---|
| HL60/Bcr-Abl . | K562 . | |||
| Annexin V . | Sub-G1 phase . | Annexin V . | Morphology . | |
| Control | 4.0 ± 1.7 | 6.3 ± 2.0 | 2.6 ± 0.7 | 3.6 ± 4.7 |
| CGP | 14.8 ± 2.5 | 14.1 ± 1.6 | 18.3 ± 2.4 | 15.1 ± 1.6 |
| Ara-C | 11.9 ± 3.0 | 11.6 ± 1.3 | 14.5 ± 2.5 | 15.1 ± 1.5 |
| CGP + Ara-C | 25.4 ± 3.3* | 36.8 ± 1.1* | 28.8 ± 1.8* | 35.0 ± 0.3* |
| Dox | ND | 33.6 ± 0.2 | ND | 27.1 ± 2.0 |
| CGP + Dox | ND | 64.4 ± 1.1* | ND | 40.1 ± 1.6* |
| . | % apoptotic cells . | |||
|---|---|---|---|---|
| HL60/Bcr-Abl . | K562 . | |||
| Annexin V . | Sub-G1 phase . | Annexin V . | Morphology . | |
| Control | 4.0 ± 1.7 | 6.3 ± 2.0 | 2.6 ± 0.7 | 3.6 ± 4.7 |
| CGP | 14.8 ± 2.5 | 14.1 ± 1.6 | 18.3 ± 2.4 | 15.1 ± 1.6 |
| Ara-C | 11.9 ± 3.0 | 11.6 ± 1.3 | 14.5 ± 2.5 | 15.1 ± 1.5 |
| CGP + Ara-C | 25.4 ± 3.3* | 36.8 ± 1.1* | 28.8 ± 1.8* | 35.0 ± 0.3* |
| Dox | ND | 33.6 ± 0.2 | ND | 27.1 ± 2.0 |
| CGP + Dox | ND | 64.4 ± 1.1* | ND | 40.1 ± 1.6* |
HL-60/Bcr-Abl and K562 cells were exposed to 0.25 μmol/L CGP57148B (CGP) and/or Ara-C (5 μmol/L) or doxorubicin (Dox) (0.25 μmol/L) for 48 hours. Following this, cells were harvested and percent apoptotic cells was determined by morphology and annexin V or PI staining followed by flow cytometry. Values represent mean ± SEM of 3 separate experiments.
ND indicates not done due to technical difficulties caused by doxorubicin fluorescence.
Values are significantly different from those cells treated with Ara-C or Dox alone (P < .05).
Processing of procaspase-8 and Bid.
Cotreatment with CGP57148 (CGP) sensitizes HL-60/Bcr-Abl and K562 cells to Ara-C–induced or doxorubicin (Dox)-induced caspase-8 and Bid processing, as well as cytosotic accumulation of cyt c and the processing of poly (ADP-ribose) polymerase (PARP). Cells were treated with 0.25 μmol/L CGP, Ara-C, or Dox alone, or with a combination of CGP plus Ara-C or CGP plus Dox for 48 hours. Following this, Western analyses of Bid, caspase-8, 116-kd PARP, and its 85-kd cleavage product and β-actin were performed on cellular proteins, or S100 fractions were used for immunoblot analysis for cyt c (see text). Expression levels are representative of 3 separate experiments.
Processing of procaspase-8 and Bid.
Cotreatment with CGP57148 (CGP) sensitizes HL-60/Bcr-Abl and K562 cells to Ara-C–induced or doxorubicin (Dox)-induced caspase-8 and Bid processing, as well as cytosotic accumulation of cyt c and the processing of poly (ADP-ribose) polymerase (PARP). Cells were treated with 0.25 μmol/L CGP, Ara-C, or Dox alone, or with a combination of CGP plus Ara-C or CGP plus Dox for 48 hours. Following this, Western analyses of Bid, caspase-8, 116-kd PARP, and its 85-kd cleavage product and β-actin were performed on cellular proteins, or S100 fractions were used for immunoblot analysis for cyt c (see text). Expression levels are representative of 3 separate experiments.
Discussion
Previous reports had indicated that Bcr-Abl–mediated transformation and resistance of mouse myeloid cells to apoptosis is associated with constitutively increased activities of Akt kinase and NFκB.7,27,31 In this report we demonstrate that Bcr-Abl expression in the human myeloid leukemia HL-60/Bcr-Abl and K562 cells is also associated with constitutively active Akt kinase and NFκB. Binding of Bcr-Abl to the p85 regulatory subunit of PI-3 kinase (PI-3K) and an intact SH2 domain of Bcr-Abl is known to be necessary for the activation of PI-3K as well as its downstream effector Akt kinase.27 Although not all the potential mechanisms by which Akt kinase activity suppresses apoptosis have been elucidated,32 Akt kinase has been shown to phosphorylate Bad on serine S136.32 This converts the proapoptotic, “BH3 domain-only” containing Bad protein into an inactive moiety sequestered in the cytosol bound to 14-3-3 protein.33,34Because only the active, nonphosphorylated Bad is bound to Bcl-xL or Bcl-2, the phosphorylation of Bad frees up Bcl-xL and Bcl-2 to promote survival.33,34 Akt kinase has also been recently shown to phosphorylate and inactivate caspase-9,35 thereby preventing the cleavage and activities of the executioner caspases and apoptosis.36 In the present studies we have demonstrated that CGP57148B inhibits Akt kinase activity in the HL-60/Bcr-Abl and K562 but not HL-60/neo cells. Whether CGP57148B inhibits the antiapoptotic mechanism of Akt kinase, mediated through Bad and caspase-9, was not determined in the present studies.
Bcr-Abl enhances NFκB activity through a Ras-dependent pathway that may involve Mek kinase-1 activation of the IκBα kinase complex.37-39 NFκB is known to exert its antiapoptotic effect by inducing inhibitor of apoptosis (IAP) proteins that inhibit caspase activity.28,29 Inhibition of NFκB diminishes the chemoresistance of some cell types, but not all cancer cells respond in this manner.8,9,40 In the present studies, we demonstrate that the expressions of the transdominant repressors of IκBα, which inhibit the TNF-α inducibility and activity of NFκB, do not significantly diminish the resistance of HL-60/Bcr-Abl and K562 cells to Ara-C or doxorubicin-induced apoptosis. This suggests that NFκB does not significantly contribute to chemoresistance of human myeloid leukemic cells mediated by Bcr-Abl protein. These findings corroborate previous observations that NFκB activity is not a necessary component of the antiapoptotic effect exerted by Bcr-Abl against DNA damaging agents and IL-3 withdrawal in the mouse myeloid 32D cells.31 However, as has also been previously reported in 32D cells, our data do show that apoptosis induced by TNF-α is significantly enhanced by the inhibition of NFκB activity by the transdominant repressors of IκBα in HL-60/Bcr-Abl and K562 cells.31
Studies have shown that in K562 cells LODAC treatment induces Hb.5 In contrast, in HL-60 cells, LODAC treatment increases the expression of the myeloid differentiation marker CD11b.5,22 Surprisingly, enforced expression of Bcr-Abl induced Hb in HL-60/Bcr-Abl cells, imparting a red color to their cell pellet (Figure 1). A recent report has indicated that Bcr-Abl TK can fully support erythroid development and maturation even in those progenitor cells that lack erythropoietin receptors.41Bcr-Abl expression also sensitized HL-60/Bcr-Abl cells to erythroid but not myeloid differentiation, as evidenced by LODAC-induced Hb but not CD11b or the morphologic features of myeloid differentiation (data not shown). In contrast, the ectopic expression of Bcr-Abl also inhibited LODAC-induced apoptosis of HL-60 cells. Inhibition of NFκB activity by the transdominant repressors of IκBα significantly reduced LODAC-induced Hb in HL-60/Bcr-Abl and K562 cells, suggesting that NFκB may play a role in this process. Parenthetically, LODAC has been shown to have clinical activity against the Bcr-Abl–positive leukemic clone in CML.2,42 Previous reports had also indicated that treatment of K562 cells with CGP57148B could markedly increase the percentage of benzidine- positive cells.26 Our data corroborate these findings, showing that exposure to CGP57148B induced Hb in HL-60/Bcr-Abl and K562 cells. Furthermore, our findings demonstrate that CGP57148B also induced CD11b expression in the Bcr-Abl–positive cells. The differentiating effects of CGP57148B appeared to be more related to the inhibition of Bcr-Abl TK than NFκB activity, because CGP57148B only modestly inhibited NFκB activity in HL-60/Bcr-Abl and K562 cells. Nevertheless, these data argue against a role for NFκB in mediating erythroid differentiation of Bcr-Abl–positive leukemic cells induced by CGP57148B. Cotreatment with LODAC did not significantly augment the differentiating effects of CGP57148B in these cells. This observation may argue against the benefit of adding LODAC to CGP57148B for augmenting its differentiating effect in the treatment of Bcr-Abl–positive leukemia.
Conventional chemotherapy with Ara-C, doxorubicin, and etoposide does not have major clinical efficacy against Bcr-Abl–positive acute leukemia or the blast crisis of CML.2,42 This may be because, as compared with HL-60/neo, HL-60/Bcr-Abl and K562 cells are relatively resistant to antileukemic drug-induced mitochondrial Δψm, cytosolic accumulation of cyt c, and apoptosis.10Recent studies have demonstrated that the activation of the death receptor signaling for apoptosis involves the activation and cleavage of caspase-8 followed by Bid protein.43-45 Cleavage of cytosolic Bid results in its localization to mitochondria, which causes the mitochondrial release and cytosolic accumulation of cyt c.44,45 Caspase-3 activity triggered by the cytosolic cyt c-Apaf-1-caspase-9 “apoptosome” can, in turn, process and activate caspase-8.46 It is noteworthy that treatment of HL-60/Bcr-Abl and K562 cells with concentrations of CGP57148B that approximates the IC50 value for Bcr-Abl TK clearly induces caspase-8 and Bid processing associated with cytosolic accumulation of cyt c, which induced apoptosis of HL-60/Bcr-Abl and K562 cells. Cotreatment with CGP57148B and Ara-C or doxorubicin also results in the processing of caspase-8 and Bid, as well as augmented accumulation of cyt c in the cytosol. However, it should be noted that CGP57148B-mediated processing of caspase-8 and Bid may represent part of an amplification loop in which the primary damaging event is at the level of the mitochondria.47 Consistent with this, cotreatment with CGP57148B significantly increased apoptosis due to Ara-C, etoposide, or doxorubicin. Treatment with CGP57148B also decreased the intracellular levels of Bcl-xL. However, this is unlikely to be the sole or main mechanism by which CGP57148B reverses the resistance to antileukemia drug-induced apoptosis in HL-60/Bcr-Abl and K562 cells. In a previous report, we had shown that the abrogation of the antiapoptotic effect of Bcl-xL may only modestly sensitize Bcr-Abl–positive acute leukemic cells to drug-induced apoptosis.5,10 Present studies also show that CGP57148B treatment did not affect Bcl-2, Bax, Fas, and Fas receptor levels. In a previous report, Apaf-1 levels were demonstrated to modify the threshold for apoptosis induced by anticancer drugs.13However CGP57148B treatment did not alter Apaf-1 levels in HL-60/Bcr-Abl or K562 cells. On the other hand, CGP57148B-mediated lowering of Akt kinase activity as well as the down-regulation of XIAP and cIAP1 levels may be responsible for CGP57148B-induced sensitization of HL-60/Bcr-Abl or K562 cells to antileukemic drug-induced apoptosis.
In summary, data presented here indicate that specific inhibition of Bcr-Abl TK by CGP57148B results in the down-regulation of Bcl-xL, XIAP, and cIAP1 levels as well as inhibition of Akt kinase and NFκB activities. These effects may sensitize Bcr-Abl–positive human leukemic cells to apoptosis induced by antileukemic drugs. Although the precise molecular effectors are not known, inhibition of Bcr-Abl TK activity by CGP57148B also induces differentiation of Bcr-Abl–positive leukemic blasts. These observations have obvious implications for the design of future, anti–Bcr-Abl therapeutic strategies incorporating CGP57148B. Early clinical data show this drug to be safe and to possess promising clinical activity.48
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kapil Bhalla, H. Lee Moffitt Cancer Center, 12902 Magnolia Drive, MRC3E, Room 3056D, Tampa, FL 33612; e-mail:bhallakn@moffitt.usf.edu.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal