Abstract
Angiogenesis has been associated with the growth, dissemination, and metastasis of solid tumors. The aims of this study were to evaluate the vascularity and the levels of angiogenic factors in patients with acute and chronic leukemias and myelodysplastic syndromes (MDS). The numbers of blood vessels were measured in 145 bone marrow biopsies and the levels of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), tumor necrosis growth factor-α (TNF-α), tumor growth factor-α (TGF-α), and hepatocyte growth factor (HGF) were determined in 417 plasma samples. Except for chronic lymphocytic leukemia (CLL), vascularity was significantly higher in all leukemias and MDS compared with control bone marrows. The highest number of blood vessels and largest vascular area were found in chronic myeloid leukemia (CML). VEGF, bFGF, and HGF plasma levels were significantly increased in acute myeloid leukemia (AML), CML, CLL, chronic myelomonocytic leukemia (CMML), and MDS. HGF, TNF-α, and bFGF but not VEGF were significantly increased in acute lymphoblastic leukemia (ALL). TNF-α levels were significantly increased in all diseases except for AML and MDS. No significant increase was found in TGF-α in any leukemia or MDS. The highest plasma levels of VEGF were in CML, and the highest plasma levels of bFGF were in CLL. The levels of HGF were highest in CMML. These data suggest that vascularity and angiogenic factors are increased in leukemias and MDS and may play a role in the leukemogenic process.
Introduction
Angiogenesis is the formation of new blood vessels from an existing vasculature.1 It involves degradation of extracellular matrix proteins and activation, proliferation, and migration of endothelial cells and pericytes in a multistep process.2-4 In addition to its physiologic role in vascularization during ovulation, placentation, and embryogenesis,1 angiogenesis has been associated with the growth, dissemination, and metastasis of solid tumors.5-7Several positive and negative regulatory molecules have been reported to be involved in the angiogenic process.8 The 2 most potent and specific positive regulators are vascular endothelial growth factor (VEGF)8,9 and basic fibroblast growth factor (bFGF).8,10-13 Other cytokines such as tumor necrosis factor-α (TNF-α),14 tumor growth factor-β (TGF-β), tumor growth factor-α (TGF-α),15 and hepatocyte growth factor (HGF)16 have also been reported to have angiogenic activity. Increased levels of these molecules have been correlated with poor prognosis in several solid tumors.17-20 Measurement of intratumoral microvessel density (IMD) by immunohistochemistry appears to be the most reliable method of measuring angiogenic activity.21-24 In recent studies, increased microvessel density in areas where neovascularization was most intense was a significant and independent prognostic indicator in early stage breast cancer.22,24 Studies in prostate cancer,25melanoma,26 gastrointestinal cancer,23 and ovarian carcinoma27 also suggested the angiogenesis index to be a useful prognostic factor.
Little is known about angiogenesis and angiogenesis-related molecules in leukemia. The normal vascular bed in bone marrow forms a sinusoidal network supporting the hematopoietic cells, similar to cellular support in other organs such as kidney and spleen.28 Perez-Atayde and colleagues29 studied 61 bone marrow biopsies (BMBs) of 40 children with untreated acute lymphoid leukemia (ALL) and 10 control biopsies.29 They found a significantly higher bone marrow microvessel density in ALL as well as a higher level of urinary bFGF. Increased vascularity was also reported in 20 bone marrow samples from patients with acute myeloid leukemia (AML).30 Expression of VEGF in leukemic cells of patients with AML was found by Fiedler and colleagues31 and Hussong and coworkers.30 We have reported that intracellular levels of VEGF in AML32and chronic lymphocytic leukemia (CLL)33 are of prognostic significance. These data suggest that angiogenesis may have a role in the pathophysiology of leukemias and that antiangiogenesis therapy could have an anticancer effect.34 In this study, we expanded on these observations by evaluating vascularity in BMBs of patients with acute leukemias, chronic leukemias, and myelodysplastic syndromes (MDS). We measured the number of blood vessels in BMBs and the plasma levels of VEGF, bFGF, HGF, TNF-α, and TGF-α.
Patients, materials, and methods
Blood vessels in the bone marrow
We evaluated 208 BMBs from 192 consecutive patients referred to the Leukemia Department at the University of Texas M. D. Anderson Cancer Center, and from 16 patients (the control group) being evaluated at the institution for metastatic solid tumor without evidence of bone marrow involvement. Of the samples taken from the 192 leukemia patients, 63 were not evaluable, 27 because of superficial or otherwise inadequate biopsy and 36 because the patients did not have active disease or had a diagnosis other than leukemia or MDS.
Immunohistochemical preparation.
All blood vessels were highlighted by staining endothelial cells with anti–factor VIII (FVIII)–related antigen antibody using a standard immunoperoxidase technique described previously.35-37 Factor VIII–related antigen antibodies were purchased from Dako Corporation (Santa Barbara, CA) and used at a dilution of 1:400.
Measurement of bone marrow microvessel density.
Microvessel density was assessed blindly. All BMBs were evaluated for cellularity by light microscopy with a 10 × power ocular lens. Five cellular representative areas were chosen randomly and examined by 20 × power objective lens. Pictures of the 5 fields were digitized. Individual microvessels (stained in brown) were counted in each field, and the vascular area was measured using National Institutes of Health shared image analysis software. The relationship between the total area of blood vessels selected (expressed in squared pixels) and the total picture taken by 20 × power objective lens (313 956 squared pixels) was calculated and expressed as a percentage. The average number of blood vessels and average area of the blood vessels were obtained for the 5 fields. Neither vessel lumens nor red blood cells in vessel lumens were used to define a blood vessel in the absence of FVIII staining. Megakaryocytes were stained with FVIII but were easily distinguishable and not counted.
Measurement of angiogenic factors
Plasma and serum collection.
Plasma and serum samples from 417 patients with newly diagnosed or relapsed AML, ALL, MDS, CLL, chronic myeloid leukemia (CML), and chronic myelomonocytic leukemia (CMML) were collected and stored according to approved protocols. Consent forms were obtained according to institutional guidelines. These samples were used to measure various angiogenic factors and compared with 11 healthy individuals used as controls.
Enzyme-linked immunosorbent assay.
The enzyme-linked immunosorbent assays (ELISAs) for VEGF, bFGF, HGF, and TNF-α were performed using commercially available kits from R&D Systems (Minneapolis, MN). Measurement of TGF-α was performed using a kit from Oncogene Research Products (Boston, MA). We followed the protocols recommended by the manufacturer. Briefly, plasma was collected using EDTA as anticoagulant and stored at −82°C. Patient samples were added to separate microplates each containing a specific monoclonal antibody. The mixtures were incubated at room temperature for 2 hours. The plates were washed 3 times to remove any unbound substances. Enzyme-linked polyclonal antibodies specific for each protein were added to the wells, and the mixtures were incubated at room temperature for 2 hours followed by another washing to remove any unbound antibody or enzyme reagent. A substrate solution was added to the wells, and a blue color developed. The intensity of the blue was proportionate to the amount of cytokine bound in the initial step. The color development was stopped, and the intensity of the color was measured and compared with a standard curve. Reading was done at 450-nm wavelength for the VEGF, bFGF, HGF, and TNF-α and at 490 nm for the TGF-α as per the manufacturer's recommendations.
Statistical analysis
The relative vascular area was obtained by dividing the vascular area by the average total marrow area in 5 (20 ×) fields. The median, minimum, and maximum values were obtained for each group of patients and compared with those for controls. Box plots were used to show the median, minimum, and maximum values and 25th to 75th percentiles for each group. To correlate the vascular area and number of blood vessels with other bone marrow characteristics, we used the SpearmanR correlation. The Kruskal-Wallis test was used to test the null hypothesis between groups. For plasma levels, we compared the values between groups using Kruskal-Wallis analysis. The Spearman correlation was used to correlate between the variables.
Results
Increased vascularity in ALL, AML, CML, and MDS
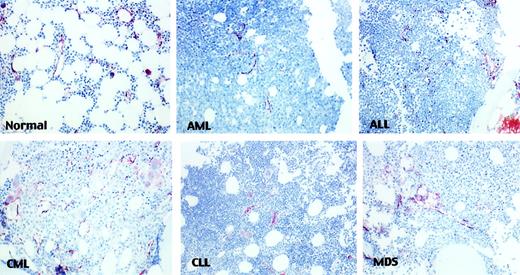
The 129 BMBs from patients with leukemia or MDS were evaluated and compared with 16 control marrows. Twenty-three patients had CLL, 20 had ALL, 24 had CML, 30 had AML, and 32 had MDS. Of the patients with MDS, 1 had refractory anemia (RA), 3 had RA with ringed sideroblasts (RARS), 9 had RA with excess blasts (RAEB), 5 had RA with excess blasts in transformation (RAEBt), and 14 had CMML. Blood vessels were well visualized using FVIII staining (Figure1) and easily distinguishable from megakaryocytes.
Microvessels in leukemias.
Representative fields of various leukemias show microvessels stained with anti-FVIII and highlighted in brown color (× 20).
Microvessels in leukemias.
Representative fields of various leukemias show microvessels stained with anti-FVIII and highlighted in brown color (× 20).
Table 1 shows the peripheral blood and bone marrow characteristics of the leukemia patients and the control group. A good correlation between vascular area size and number of blood vessels was found in control marrows and in marrows of leukemia patients (R = 0.84 and 0.77, respectively;P < .001 for both).
Characteristics of the study group (median and range)
| . | Controls (Control) . | CLL . | CML . | ALL . | AML . | MDS . |
|---|---|---|---|---|---|---|
| No. blood vessels | 11.2 (1.2-24) | 9 (1.3-36.7) | 21.4 (4.7-41.7) | 18 (5.2-45.2) | 16.1 (1.2-32.8) | 20.4 (2.7-41.6) |
| Area (%) | 2.8 (.17-8.7) | 2.4 (.12-11.2) | 6.2 (.9-13.1) | 4.4 (.5-15.4) | 4.3 (.34-23.1) | 5.2 (.4-13.1) |
| PB Hb (g/dL) | 12.9 (9.2-16.8) | 12.8 (6.8-16) | 12.1 (10.1-15.1) | 10.9 (6.1-12.2) | 10.2 (7.1-14.7) | 9.7 (7.2-14.2) |
| PB WBC (×109/L) | 5.4 (1.8-13.1) | 17.3 (3.4-249.9) | 22.8 (2.4-307.4) | 13 (.4-79.8) | 7.9 (.8-124) | 6.6 (.7-64.9) |
| PB Plts | 171 (20-527) | 118 (21-232) | 252 (39-1430) | 36 (5-230) | 41.4 (11-203) | 44.5 (1-413) |
| PB Nt (% of WBC) | 62 (24-76) | 18 (0-73) | 58 (20-85) | 8 (0-89) | 6 (0-76) | 34.5 (3.74) |
| PB Ly (% of WBC) | 21 (14-72) | 71 (10-97) | 10.5 (1.36) | 12 (0-66) | 28 (2-93) | 30.5 (2-90) |
| BM cellularity (%) | 20 (10-50) | 50 (15-95) | 90 (20-100) | 85 (10-95) | 80 (15-100) | 63 (15-100) |
| BM blasts (%) | 1 (0-4) | 0 (0-0) | 2 (0-47) | 92 (79-99) | 70 (1-99) | 8.5 (2-29) |
| BM progranulocytes (%) | 2 (1-4) | 0 (0-2) | 2 (0-12) | 0 (0-4) | 1 (0-90) | 2 (0-10) |
| BM granulocytes (%) | 19 (12-31) | 5 (0-22) | 23 (10-63) | 0 (0-7) | 1.5 (0-29) | 14.5 (0-41) |
| BM Ly (%) | 13 (4-26) | 76 (7-93) | 4 (0-14) | 1 (0-7) | 5.5 (0-51) | 8 (2.57) |
| BM basophils (%) | 0 (0-2) | 0 (0-1) | 1 (0-11) | 0 (0-0) | 0 (0-1) | 0 (0-7) |
| BM prolymph (%) | 0 (0-0) | 1 (0-48) | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0 (0-0) |
| . | Controls (Control) . | CLL . | CML . | ALL . | AML . | MDS . |
|---|---|---|---|---|---|---|
| No. blood vessels | 11.2 (1.2-24) | 9 (1.3-36.7) | 21.4 (4.7-41.7) | 18 (5.2-45.2) | 16.1 (1.2-32.8) | 20.4 (2.7-41.6) |
| Area (%) | 2.8 (.17-8.7) | 2.4 (.12-11.2) | 6.2 (.9-13.1) | 4.4 (.5-15.4) | 4.3 (.34-23.1) | 5.2 (.4-13.1) |
| PB Hb (g/dL) | 12.9 (9.2-16.8) | 12.8 (6.8-16) | 12.1 (10.1-15.1) | 10.9 (6.1-12.2) | 10.2 (7.1-14.7) | 9.7 (7.2-14.2) |
| PB WBC (×109/L) | 5.4 (1.8-13.1) | 17.3 (3.4-249.9) | 22.8 (2.4-307.4) | 13 (.4-79.8) | 7.9 (.8-124) | 6.6 (.7-64.9) |
| PB Plts | 171 (20-527) | 118 (21-232) | 252 (39-1430) | 36 (5-230) | 41.4 (11-203) | 44.5 (1-413) |
| PB Nt (% of WBC) | 62 (24-76) | 18 (0-73) | 58 (20-85) | 8 (0-89) | 6 (0-76) | 34.5 (3.74) |
| PB Ly (% of WBC) | 21 (14-72) | 71 (10-97) | 10.5 (1.36) | 12 (0-66) | 28 (2-93) | 30.5 (2-90) |
| BM cellularity (%) | 20 (10-50) | 50 (15-95) | 90 (20-100) | 85 (10-95) | 80 (15-100) | 63 (15-100) |
| BM blasts (%) | 1 (0-4) | 0 (0-0) | 2 (0-47) | 92 (79-99) | 70 (1-99) | 8.5 (2-29) |
| BM progranulocytes (%) | 2 (1-4) | 0 (0-2) | 2 (0-12) | 0 (0-4) | 1 (0-90) | 2 (0-10) |
| BM granulocytes (%) | 19 (12-31) | 5 (0-22) | 23 (10-63) | 0 (0-7) | 1.5 (0-29) | 14.5 (0-41) |
| BM Ly (%) | 13 (4-26) | 76 (7-93) | 4 (0-14) | 1 (0-7) | 5.5 (0-51) | 8 (2.57) |
| BM basophils (%) | 0 (0-2) | 0 (0-1) | 1 (0-11) | 0 (0-0) | 0 (0-1) | 0 (0-7) |
| BM prolymph (%) | 0 (0-0) | 1 (0-48) | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0 (0-0) |
BM indicates bone marrow; Hb, hemoglobin; Ly, lymphocytes; Nt, neutrophils; PB, peripheral blood; Plts, platelets; Prolymph, prolymphocytes; WBC, white blood cells.
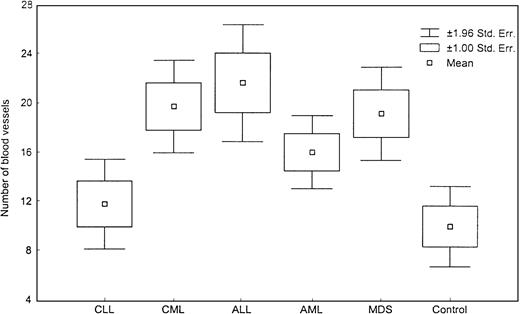
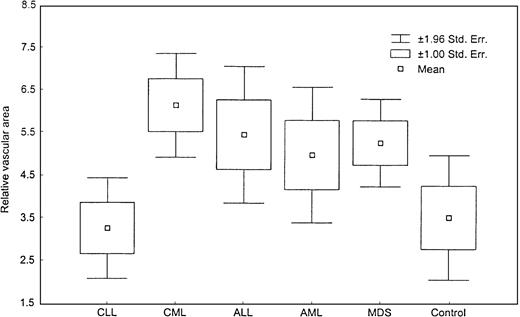
We found a significant increase in vascularity in CML, AML, ALL, and MDS patients but not in CLL patients as compared with the controls (P < .05). The relative vascular area was marginally increased in MDS (Table 2; Figure 2). We did not find a significant difference between ALL and AML. When CMML patients were considered separately from MDS patients, their vascularity was significantly higher than that of the control group (0.04) but was not different from that of the other MDS (RA, RARS, RAEB, and RAEBt) patients.
Comparison of vascularity (P value when compared with the control group)
| Patient group . | Patients (no.) . | Blood vessels (median no.) . | P value . | Relative area (median %) . | P value . |
|---|---|---|---|---|---|
| Control | 16 | 11.2 | 2.8 | ||
| CLL | 23 | 9 | .56 | 2.4 | .78 |
| CML | 24 | 21.4 | .003 | 6.2 | .02 |
| ALL | 20 | 18 | .005 | 4.4 | .07 |
| AML | 30 | 16.1 | .02 | 4.3 | .23 |
| MDS | 32 | 20.4 | .004 | 5.2 | .06 |
| Patient group . | Patients (no.) . | Blood vessels (median no.) . | P value . | Relative area (median %) . | P value . |
|---|---|---|---|---|---|
| Control | 16 | 11.2 | 2.8 | ||
| CLL | 23 | 9 | .56 | 2.4 | .78 |
| CML | 24 | 21.4 | .003 | 6.2 | .02 |
| ALL | 20 | 18 | .005 | 4.4 | .07 |
| AML | 30 | 16.1 | .02 | 4.3 | .23 |
| MDS | 32 | 20.4 | .004 | 5.2 | .06 |
Number of blood vessels.
This box plot shows significant difference in number of blood vessels among various diseases (P = .0005, Kruskal-Wallis test).
Number of blood vessels.
This box plot shows significant difference in number of blood vessels among various diseases (P = .0005, Kruskal-Wallis test).
Differences between plasma and serum levels of VEGF and FGF
Initially we tested the differences in VEGF and bFGF levels between plasma and serum in 67 patients. The median platelet count in this group of patients was 173 × 109/L (range, 9-890). VEGF levels were significantly higher in the serum as compared with plasma (P < .001, Kruskal-Wallis test). The median VEGF in plasma was 49.9 pg/mL (range, 24.1-2767.2 pg/mL); it was 163.5 pg/mL (range, 27.6-2461.9 pg/mL) in serum. There was no significant difference (P = .13) in the levels of bFGF between plasma and serum, 8.5 pg/mL (range, 4.4-465 pg/mL) and 7.36 pg/mL (range, 3.7-487 pg/mL), respectively. Overall VEGF levels in plasma and serum correlated with platelet counts (Spearman R = 0.68,P < .001, and 0.5, P < .001, respectively). We found a similar correlation with total white blood cells (WBC). The bFGF levels in plasma and serum also correlated with platelets (R = 0.5, P < .001, andR = 0.32, P = 0.01, respectively), but there was no correlation between bFGF plasma or serum and WBC (P = .14 and P = .49, respectively). High levels of VEGF have been reported in platelets, and it is possible that during the clotting process and the separation of the serum, VEGF is released from the platelets and WBC leading to the detection of high levels.38,39 High levels of bFGF have been reported in platelets, as demonstrated above, without significant effects on the levels of bFGF during clotting. This may suggest that the mechanisms responsible for releasing the VEGF from platelets during serum separation are different from those for bFGF. The other possibility is that the VEGF levels in serum are affected by its release from the WBC, whereas bFGF is not released from the cells. This discrepancy between serum and plasma levels of VEGF has been reported by other investigators,38 39 who recommended the use of plasma rather than serum for analyzing these angiogenic factors. This is an important issue in leukemias and MDS because of the significant variation in the number of platelets among leukemia patients. Measurements of all angiogenic factors in this study were performed using plasma rather than serum.
Elevated levels of angiogenic factors in leukemia and MDS
Levels of VEGF, bFGF, HGF, TGF-α, and TNF-α in plasma samples collected from patients with various leukemias and MDS were evaluated before therapy or during relapsed disease. These levels in each disease are shown in Table 3. Except for TGF-α, levels of these factors were significantly higher in patients with leukemia and MDS. TGF-α was not detectable in any of the normal samples despite the high sensitivity of the assay (25 pg/mL). Rare samples of leukemias showed expression of TGF-α, but overall there was no significant increase in TNF-α in leukemia or MDS (Table 3).
Levels (median and range) of angiogenic factors in various leukemias and MDS
| Patients . | VEGF . | bFGF . | HGF . | TNF-α . | TGF-α . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | pg/mL . | (range) . | No. . | pg/mL . | (range) . | No. . | pg/mL . | (range) . | No. . | pg/mL . | (range) . | No. . | pg/mL . | (range) . | |
| Control | 11 | 26.74 | (23.17-53.88) | 11 | 5.37 | (4.56-13.65) | 11 | 336.1 | (164-522) | 11 | 8.6 | (7.1-10.3) | 11 | 0 | (0-0) |
| CLL | 157 | 47.17 | (22.23-720.89) | 155 | 47.77 | (5.02-751.27) | 48 | 799.2 | (101.1-1657.3) | 48 | 14.4 | (7.3-41) | 31 | 0 | (0-846.8) |
| CML | 59 | 76.30 | (23.90-1634.8) | 53 | 8.54 | (4.22-478.1) | 58 | 684 | (286.9-5027.7) | 57 | 10.4 | (8.9-22.1) | 58 | 0 | (0-36) |
| ALL | 28 | 28.16 | (20.64-178.31) | 28 | 7.28 | (5.13-25.04) | 22 | 747.7 | (265.4-5148.8) | 22 | 10.6 | (7.7-97.3) | 9 | 0 | (0-0) |
| AML | 115 | 30.43 | (21.47-439.25) | 113 | 6.48 | (4.66-203.74) | 63 | 899.6 | (101.9-12819) | 63 | 8.9 | (7.3-19) | 49 | 0 | (0-199.4) |
| MDS | 40 | 31.05 | (22.45-408.7) | 40 | 6.61 | (4.7-49.74) | 31 | 737.6 | (192.3-3533.6) | 31 | 8.4 | (7.1-11.7) | 12 | 0 | (0-0) |
| CMML | 18 | 65.1 | (24.33-1092) | 19 | 6.54 | (5.47-60.95) | 11 | 1444.3 | (448.2-8657.4) | 11 | 9.9 | (7.3-48.2) | 10 | 0 | (0-87.7) |
| Patients . | VEGF . | bFGF . | HGF . | TNF-α . | TGF-α . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | pg/mL . | (range) . | No. . | pg/mL . | (range) . | No. . | pg/mL . | (range) . | No. . | pg/mL . | (range) . | No. . | pg/mL . | (range) . | |
| Control | 11 | 26.74 | (23.17-53.88) | 11 | 5.37 | (4.56-13.65) | 11 | 336.1 | (164-522) | 11 | 8.6 | (7.1-10.3) | 11 | 0 | (0-0) |
| CLL | 157 | 47.17 | (22.23-720.89) | 155 | 47.77 | (5.02-751.27) | 48 | 799.2 | (101.1-1657.3) | 48 | 14.4 | (7.3-41) | 31 | 0 | (0-846.8) |
| CML | 59 | 76.30 | (23.90-1634.8) | 53 | 8.54 | (4.22-478.1) | 58 | 684 | (286.9-5027.7) | 57 | 10.4 | (8.9-22.1) | 58 | 0 | (0-36) |
| ALL | 28 | 28.16 | (20.64-178.31) | 28 | 7.28 | (5.13-25.04) | 22 | 747.7 | (265.4-5148.8) | 22 | 10.6 | (7.7-97.3) | 9 | 0 | (0-0) |
| AML | 115 | 30.43 | (21.47-439.25) | 113 | 6.48 | (4.66-203.74) | 63 | 899.6 | (101.9-12819) | 63 | 8.9 | (7.3-19) | 49 | 0 | (0-199.4) |
| MDS | 40 | 31.05 | (22.45-408.7) | 40 | 6.61 | (4.7-49.74) | 31 | 737.6 | (192.3-3533.6) | 31 | 8.4 | (7.1-11.7) | 12 | 0 | (0-0) |
| CMML | 18 | 65.1 | (24.33-1092) | 19 | 6.54 | (5.47-60.95) | 11 | 1444.3 | (448.2-8657.4) | 11 | 9.9 | (7.3-48.2) | 10 | 0 | (0-87.7) |
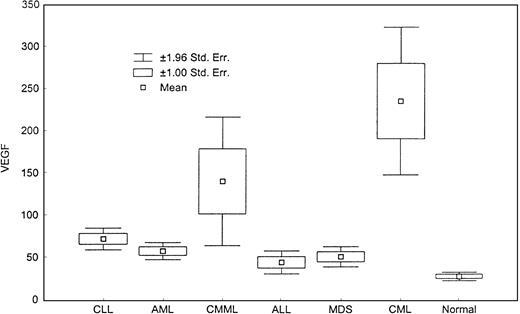
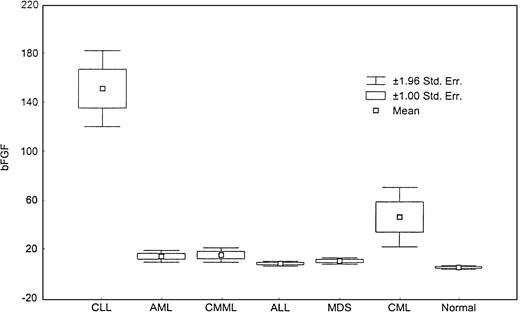
There was a significant difference in the levels of VEGF (Figure 4) and bFGF (Figure5) among groups (P < .001). CML patients had the highest levels of VEGF, and CLL patients had the highest levels of bFGF. Except for ALL patients, all groups displayed a significantly higher level of VEGF compared with healthy controls, with CML and CMML patients showing the highest levels of VEGF (Table 3).
Relative areas of vascular beds.
This box plot compares the relative area of vascular bed in the bone marrows of various diseases (P = .008, Kruskal-Wallis test).
Relative areas of vascular beds.
This box plot compares the relative area of vascular bed in the bone marrows of various diseases (P = .008, Kruskal-Wallis test).
Levels of VEGF.
This box plot compares median levels of VEGF in various types of leukemia and MDS (P < .0001, Kruskal-Wallis test).
Levels of VEGF.
This box plot compares median levels of VEGF in various types of leukemia and MDS (P < .0001, Kruskal-Wallis test).
Levels of bFGF.
This box plot compares levels of bFGF in various types of leukemia and MDS (P < .0001, Kruskal-Wallis test).
Levels of bFGF.
This box plot compares levels of bFGF in various types of leukemia and MDS (P < .0001, Kruskal-Wallis test).
There was no significant difference in VEGF levels between AML and MDS patients, but there was a significant difference between AML and CMML patients (P = .1). The bFGF levels were also significantly higher in all diseases compared with healthy controls (Table 3 and Figure 5), but the highest levels were detected in CLL (Table 3). There was a direct correlation between VEGF and bFGF plasma levels in CLL, CML (P < .001 for both), and AML (P = .007) patients. No correlation was found between the 2 angiogenic factors in ALL (P = .44), MDS (P = .17), or CMML (P = .32).
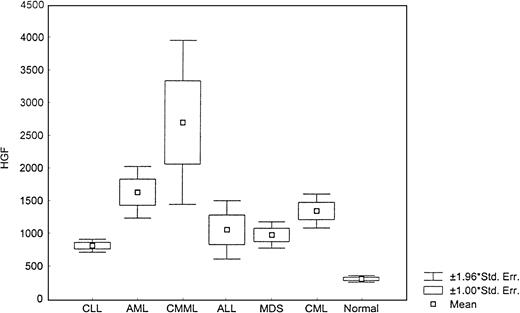
The level of HGF was also increased in leukemia and MDS (Figure6) as compared with normal samples (P < .0001, Kruskal-Wallis test). However, the highest levels were detected in CMML (1444 pg/mL; range, 448-8657.4) (Table 3).
Levels of HGF.
This box plot compares median levels of HGF between various types of leukemia and MDS (P < .0001, Kruskal-Wallis test).
Levels of HGF.
This box plot compares median levels of HGF between various types of leukemia and MDS (P < .0001, Kruskal-Wallis test).
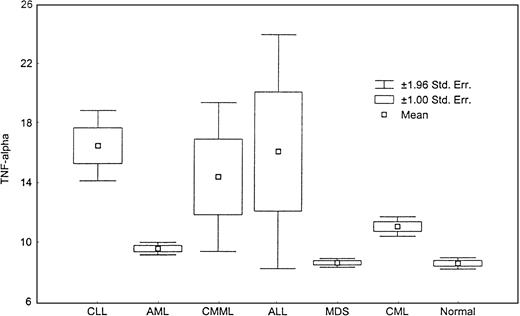
The level of TNF-α was significantly increased in CLL, CML, CMML, and ALL compared with healthy controls (Figure7) (P < .000, Kruskal-Wallis test). Although TNF-α levels were mildly increased in AML and MDS, they were not statistically significant from those detected in healthy controls (P = .18, andP = .67, respectively).
Levels of TNF-α.
This box plot compares median levels of TNF-α between various types of leukemia and MDS (P < .0001, Kruskal-Wallis test).
Levels of TNF-α.
This box plot compares median levels of TNF-α between various types of leukemia and MDS (P < .0001, Kruskal-Wallis test).
To test the reproducibility of ELISA, 44 random samples were assayed in duplicate and a Spearman R correlation of 0.98 was demonstrated. To test the stability of the angiogenic factors in plasma, we used 63 random samples after thawing and refreezing and tested for the levels of VEGF. The Spearman R correlation was 0.97. All values obtained in this study were in the linear ranges of the ELISA, reported by the manufacturer to be 31.2 to 2000 pg/mL for VEGF, 5 to 640 pg/mL for bFGF, 15.6 to 1000 pg/mL for TNF-α, 0 to 1000 pg/mL for TGF-α, and 125 to 8000 pg/mL for HGF.
Discussion
Angiogenesis has a major role in tumor growth, dissemination, and metastasis in solid tumors.5-7 Clearly, angiogenic factors and angiogenesis play a significant role in the course and disease process of some leukemias. The reported increased vascularity in pediatric ALL,29 AML,30 and MDS40; the prognostic importance of VEGF in AML32; and the detection of angiogenic factor receptors in leukemia cell lines41 suggest that angiogenic factors may have a direct effect on marrow vascularity as well as on leukemic cells.
In our study we observed a significant increase in the number of blood vessels in CML, AML, ALL, and MDS, and a borderline increase in the relative vascular area in ALL, MDS, and AML. When we compared bone marrow cellularity with vascularity, there was no correlation, and vascularity appeared to be independent of cellularity. Increased vascularity cannot be explained by the relative increase in cellularity in marrow alone (median cellularity in controls = 20% versus 85% in ALL, 80% in AML, and 90% in CML) (Figure 1). Furthermore, there was no increase in vascularity in CLL bone marrow, despite cellularity. This suggests that vascularity in hematologic malignancies is an active and controlled process.
Increased vascularity was associated with a significant increase in angiogenic factors, including VEGF, bFGF, TNF-α, and HGF. However, levels showed heterogeneity in different diseases. Despite increased vascularity in CML, only VEGF was increased, but not bFGF. Angiogenic factors were increased in CLL, but vascularity was not increased. Vascularity is increased in AML and MDS, but TNF-α was not increased in these 2 diseases. This suggests that angiogenic factors may play different roles in various leukemias. This reflects the complex nature of the interactions between these angiogenic factors and bone marrow stroma interacting with and “feeding” the leukemic process. In an ongoing prospective study of AML we could not demonstrate a direct correlation between the levels of any individual angiogenic factor studied here and the number of blood vessels (unpublished data). This may suggest that other factors in the bone marrow stroma or leukemic process may be important in determining the level of vascularity in these diseases, or that combinations of 2 or more factors may be responsible for overall vascularity.
Patients with CMML showed VEGF levels similar to CML, which may reflect the proliferative nature of the CMML despite its frequent classification as a subgroup of MDS.
The clinical significance of marrow vascularity and plasma levels of angiogenic factors individually or combined in leukemia and MDS needs further investigation and may suggest novel therapeutic approaches in these diseases. Several new antiangiogenic agents are now available, which may have role in treating leukemia and MDS.
In summary, our data suggest that angiogenic factors play a significant role in the leukemic process. Understanding their roles may help in designing new therapeutic strategies for leukemias and MDS.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Maher Albitar, Department of Hematopathology, University of Texas, M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Box 72, Houston, TX 77030-4095; e-mail:malbitar@mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal