Abstract
Interleukin-9 (IL-9) has been implicated in the pathogenesis of allergic disorders. To examine the interaction between IL-9 and eosinophils, we evaluated mature peripheral blood eosinophils for their expression of the specific α-subunit of the IL-9 receptor (IL-9R–α). The expression of IL-9R–α by human eosinophils was detected at the messenger RNA (mRNA) and protein levels by reverse transcriptase–polymerase chain reaction (RT-PCR), flow cytometry, and immunocytochemical analysis, respectively. Functional analyses demonstrated that recombinant human (rh)IL-9 inhibited in vitro peripheral blood human eosinophil apoptosis in a concentration-dependent manner. We then examined the role of IL-9 in eosinophil differentiation using the human cord blood CD34+cells and human promyelocytic leukemia cells (HL-60). The addition of IL-9 to CD34+ cells cultured in IL-3 and IL-5 enhanced eosinophil development, and IL-9 alone induced the expression of IL-5R–α. IL-9 also up-regulated the IL-5R–α chain cell surface expression during terminal eosinophil differentiation of the HL-60 cell line. Our findings suggest that IL-9 may potentiate in vivo eosinophil function by increasing their survival and IL-5–mediated differentiation and maturation. Taken together, these results suggest a mechanism by which IL-9 potentiates airway and tissue eosinophilia.
Introduction
Airway eosinophilia have been recognized as a predominant feature of allergic asthma, and elevated numbers within the inflammatory infiltrate are often associated with disease severity.1-3 These cells are believed to contribute to the pathophysiology of asthma through release of cationic granule proteins, reactive oxygen metabolites, and proinflammatory and profibrotic cytokines.4 In addition to eliciting tissue damage, eosinophil-derived proinflammatory mediators can perpetuate the inflammatory reaction and lead to chronic changes in airway function.5 Asthmatic airways also play host to an increased number of CD4+ T cells, which appear to orchestrate the specific immune response occurring within the lungs. These cells produce a range of Th2-type cytokines, particularly interleukin-5 (IL-5), with regulatory effects on eosinophil growth, differentiation, and activation.6-8
IL-9 is a Th2-type cytokine first described in the mouse as a T-cell and mast-cell growth factor.9-11 This regulatory cytokine inhibits lymphokine production by interferon-γ (IFN-γ)–producing CD4+ T cells and enhances the growth of CD8+ T cells.12 In addition, IL-9 promotes the production of immunoglobulin E (IgE) by B cells13 and the proliferation of mast cells.10 Recently, a number of observations have suggested that this cytokine may play a role in asthma. Linkage analysis has demonstrated an association between theIL-9 gene and both elevated serum IgE levels and airway hyper-responsiveness.14-17 The IL-9 gene has also been shown to reside within the Th2 cytokine cluster on the long arm of chromosome 5 (5q), within close proximity ofIL-4 and IL-13.18-21 More recently, the development of transgenic mice overexpressing IL-9 has suggested potential for this cytokine in the development of airway eosinophilia.22-24 Like all cytokines, IL-9 functions through cognate interactions with its own distinct receptor (IL-9R), which consists of a ligand-specific α-subunit and a common γ-chain that is shared with IL-2, IL-7, and IL-15.25,26 Most recently, Holroyd et al27 have found genetic linkage of asthma and airway hyper-responsiveness to the IL-9R locus in humans.
There is extensive literature on the complex interactions between the eosinophil and the CD4+ T lymphocyte, which are fundamental to the development of allergic inflammation. While it has been shown that IL-9 promotes eosinophilia in vivo,22-24 the mechanism remains obscure. To further investigate the role of IL-9 on eosinophils, we examined the expression of functional IL-9R on the surface of human eosinophils and whether IL-9 could influence eosinophil function, survival, and/or development. In this report we show that eosinophils from human peripheral blood express IL-9R on their surface. Furthermore, stimulation of eosinophils with IL-9 up-regulates the cell surface expression of the IL-5R–α and increases their survival. In addition IL-9 potentiates eosinophil development from CD34+progenitors by mechanisms that include up-regulation of the IL-5R–α.
Materials and methods
Isolation of eosinophils from peripheral human blood
Blood was collected into sterile heparinized syringes from the peripheral veins of 10 asthmatic donors28 and 5 nonasthmatic nonatopic donors following informed consent. After the erythrocytes were sedimented with dextran for 45 minutes at room temperature, the granulocyte fraction was obtained by centrifugation through a cushion of 1.077 g/mL Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) of the buffy coat at 350g for 30 minutes. After the hypotonic lysis of residual erythrocytes, the eosinophils were separated from neutrophils by negative immunomagnetic selection with a magnetic-activated cell sorter (MACS) column (Miltenyi Biotech, Montreal, Quebec, Canada).29 In brief, the erythrocyte-depleted granulocyte pellet was incubated for 45 minutes at 4°C with an anti-CD16 monoclonal antibody (mAb) bound to immunomagnetic beads. When the mixture was applied to a steel wire column in a strong magnetic field, the CD16+ neutrophils were retained, whereas the CD16− eosinophils were highly purified in the fraction that flowed through the column. Contaminating T lymphocytes were further removed by incubating the CD16 fraction for another 15 minutes with saturating concentrations of anti-CD3 magnetic beads. Cytocentrifugation slides of the eosinophils stained with Wright-Giemsa showed that the purity of the isolated eosinophils was greater than 97%.
Reverse transcriptase–polymerase chain reaction and Southern blot analysis
Total cellular RNA was extracted from highly purified eosinophils using the Trizol method (Gibco BRL Life Technologies, Gaithersburg, MD). Reverse transcriptase (RT) was performed by using 2 μg total RNA from each patient in a first-strand complementary DNA (cDNA) synthesis reaction with Super Script RT (Gibco) as recommended by the supplier. Polymerase chain reaction (PCR) was performed by adding 1 μL of the RT product into 50 μL total volume reaction containing 1 times the buffer; 200 μmol of each dNTP (nucleoside 5′-triphosphate) (Gibco); 20 pmol of each oligonucleotide primer; and 0.2 U Ampli-Taq polymerase (Gibco). Oligonucleotides specific for the IL-9R–α sequences on either side of a splice junction were used in the PCR reaction to preclude amplification of a possible contaminating genomic DNA.30
The oligonucleotide primers were synthesized on the basis of the entire coding region of the IL-9R–α (GenBank accession No.M8474726) as follows: 5′-primer, 5′-GCAACATCAGTTCTGGCCAC-3′; internal primer, 5′-CCTTGAGCTGGACCCTG-3′; and 3′-primer, 5′-CCCAGAGACAAGGCCCTCT-3′. PCR (35 cycles) was carried out in a thermal cycler (PTC100; MJ Research Inc, Watertown, MA). Each cycle consists of the following incubations: denaturation for 1 minute at 94°C, annealing for 2 minutes at 60°C, and primer extension for 1 minute 30 seconds at 72°C. The initial denaturation period was 5 minutes, and the final extension was 10 minutes. Amplified products were analyzed by DNA gel electrophoresis in 2% agarose gel, visualized by ethidium bromide staining, and blotted on Hybond N membrane (Amersham International, Little Chalfont, England) using standard methods.31 Oligonucleotide probes were labeled with 3-deoxy digoxigenin–labeled adenosine 5′-triphosphate (ATP) using terminal transferase.31 Hybridization was carried out as recommended by the supplier (Boehringer Mannheim, Mannheim, Germany).
Cytofluorographic analysis of surface expression of IL-9R on human eosinophils
Samples of 2 × 105 cells were incubated with saturating concentrations of the primary antibodies (5 μg/mL purified mouse mAb anti–IL-9R–α chain or isotype control IgG1) in the presence of 2 mg/mL affinity-purified human IgG in phosphate-buffered saline (PBS) and 5% fetal calf serum (FCS) for 30 minutes on ice. The cells were washed twice with PBS/2%FCS and incubated in the dark for 30 minutes with fluorescein isothiocyanate (FITC) goat antimouse IgG at a final dilution of 1:200. The cells were then washed and resuspended in 300 μL PBS and analyzed on a fluorescence-activated cell sorter (FACS) (FACScan; Becton Dickinson, Oxnard, CA). The results were analyzed using Cell Quest software (Becton Dickinson).
Cytospin preparations
Cytospin slides were prepared from peripheral blood eosinophils or bronchoalveolar lavage (BAL),32 fixed in 4% paraformaldehyde for 20 minutes at room temperature, and washed with 0.05 mol/L Tris-HCl (tris[hydroxymethyl] aminomethane–hydrochloride)–buffered isotonic saline (TBS) (pH.7.6). After drying, the slides were stored at −20°C before immunocytochemistry.
Immunocytochemistry
The cytopreparations of purified eosinophils or BAL slides32 were washed with TBS. After saturation for 20 minutes with TBS containing 10% normal human serum and 5% normal goat serum, the cells were incubated with 1 μg/mL rabbit polyclonal antibody (pAb) anti–IL-9R–α, which is specific to the C-terminus intracellular domain of the IL-9R–α, in antibody dilution buffer (Dako SA, Glostrup, DK) overnight at 4°C. As a negative control, a dilution of 1:500 normal rabbit serum was used as a primary antibody. After washing, a concentration of 1:200 biotinylated swine antirabbit IgG was added for 30 minutes at 37°C followed by 1:200 streptavidin-conjugated alkaline phosphatase for 1 hour at room temperature. The slides were developed using Fast Red and counterstained with Mayer's hematoxylin.
Determination of eosinophil apoptosis
Morphological assessment of nuclei alteration.
Freshly isolated human eosinophils were incubated in complete Roswell Park Memorial Institute medium (RPMI 1640) with 10 ng/mL recombinant human (rh)IL-9, rhIL-5, or medium alone for 18 hours. Apoptosis was determined by morphological assessment.33 In this system, cytospin preparations were made and stained by Diff Quick (American Scientific Products, McGray, IL), and cells exhibiting apoptotic nuclei were enumerated in different fields in a blinded fashion using a random coded order. A minimum of 500 total cells was counted. This was achieved using a Nikon light microscope (Nikon, Japan) at the original magnification × 400. The number of apoptotic cells was calculated as a percentage of the total cell count. The results are reported as the mean percentage of apoptotic cells plus or minus SD.
Determination of eosinophil viability.
The percentage of necrotic eosinophils was determined by counting trypan blue positive cells.
DNA fragmentation assay
Apoptosis was also assessed by analysis of the staining characteristics of fixed permeabilized cells exposed to the DNA-binding dye, propidium iodide (PI), as described previously.34Briefly, after 18 or 36 hours of culture, 0.3 × 106eosinophils were washed with PBS and fixed with 70% ethanol for 30 minutes at 4°C. The fixed cells were then washed twice with 2 mL cold PBS and resuspended in 200 μL PBS containing 200 U/mL ribonuclease A (RNase A) for 30 minutes at 37°C. PI was then added to the suspension and analyzed using cell cycle parameters on the FACScan machine.
Competitive RT-PCR
Competitive PCR was optimized to allow rapid estimation of the ratios of soluble and membrane-associated IL-5R–α isoforms. A forward primer was designed for the extracellular portion of the receptor (position 1033-1056) and 2 reverse primers for a soluble isoform (position 1279-1298) and a membrane-anchored isoform (position 1539-1561), as previously described.35
Cell line and culture conditions
The human cell line HL-60 (clone 15; American Type Culture Collection, Rockville, MD) was used in the study. The cells were cultured at 37°C in humidified 5% carbon dioxide (CO2) in RPMI 1640 supplemented with 10% heat-inactivated FCS, 100 U/mL penicillin, and 100 μg/mL streptomycin. Eosinophil differentiation was induced as described previously.36 Briefly, the cells were grown in RPMI 1640 and 10% FCS in the presence of 0.3 mmol/L butyric acid for 7 days to generate eosinophils.
Effect of IL-9 on IL-5R–α expression in peripheral blood eosinophils
Freshly prepared peripheral blood eosinophils and eosinophil-differentiated HL-60 cells (day 7) were incubated with 10 ng/mL IL-9 protein in complete RPMI 1640 or with the appropriate vehicle control (medium alone). This concentration of IL-9 was chosen from preliminary concentration-response experiments with the HL-60 cell line. The cells were then harvested at 18 hours after stimulation, and surface expression of the IL-5R–α chain was then performed by flow cytometry analysis using 5 μg/mL mouse mAb anti–IL-5–α chain as described above.
Eosinophil differentiation of human umbilical cord blood progenitors and HL-60 cells
Human umbilical cord blood was collected from the maternity unit of Chelsea and Westminster Hospital (London, UK) with approval of the local ethics committee. Mononuclear cells were isolated after centrifugation over Histopaque (Sigmal Chemical Co, Oakville, Ontario, Canada) followed by red cell lysis in sterile water and 1-hour adherence to plastic. The CD34+ cells were isolated from nonadherent cells by MACS immunomagnetic cell separation using anti-CD34 beads. The cells were then cultured in Iscove's modified Dulbecco's medium (IMDM) with 15% FCS, penicillin, streptomycin, amphotericin mix, and 50 μmol/L β-mercaptoethanol in 24-well plates (Nunc, Roskilde, Denmark) at 1 × 105 cells per well. The cytokines were added at 1 ng/mL for IL-3 and IL-5 and at 1, 5, or 10 ng/mL for IL-9. The cells were counted, and the medium and cytokines were replenished at 7 and 14 days of culture, when flow cytometry was performed. To detect the presence of soluble and membrane-bound forms of IL-5R–α messenger RNA (mRNA) in these cells over time, RNA was extracted at days 0, 3, 5, 7, and 14 for RT-PCR.
To assess the ability of IL-9 to influence the eosinophilic differentiation of the HL-60 cell line, the cells were taken on day 6, washed twice with RPMI 1640, and incubated with 10 ng/mL IL-9 protein in complete RPMI 1640 or with the vehicle control (medium alone). The cells were then harvested at 4, 6, 8, 18, or 24 hours after stimulation. For umbilical cord progenitors and HL-60 cells, the eosinophilic phenotype of the cells was confirmed by May-Grünwald-Giemsa and chromotrope 2R staining and by immunocytochemistry to detect a major basic protein with mAb BMK-13. Surface expression of IL-5R–α was determined using 5 μg/mL mAb anti–IL-5–α chain or IgG1 control as described above.
Quantification and statistics
The differences between groups were analyzed using the Studentt test, and P < .05 was considered statistically significant.
Reagents and antibodies
The following was used in the study: rabbit pAb affinity-purified anti–IL-9R–α directed to C-terminal intracellular domain specific and FITC-conjugated goat antimouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA); mAb antihuman IL-5R–α chain (α-16, prepared by J.T.); rhIL-9 (Calbiochem, La Jolla, CA); mouse mAb affinity-purified antihuman IL-9R–α, which was directed to N-terminal extracellular domain specific and was used for cord blood culture IL-3 (Genzyme Corporation, West Malling, England); IL-5 (PharMingen, San Diego, CA); mAb antimajor basic protein (BMK-13) (Sanbio b.v., Uden, The Netherlands); normal rabbit serum and PI (Cedarlane, Toronto, Ontario, Canada); affinity-purified human IgG and IgG1 isotype control (clone MOPC1), chromotrope 2R, and Histopaque (Sigma); alkaline phosphatase antialkaline phosphatase (APAAP), biotinylated and unconjugated Fab′-2 swine antirabbit IgG, Fast Red, and streptavidin-conjugated alkaline phosphatase (Dako, Glostrup, Denmark); anti-CD16, anti-CD34, and anti-CD3 immunomagnetic beads (Miltenyi Biotech); FCS (Hyclone Laboratories, Logan, UT); and β-mercaptoethanol (Life Technologies, Inc, Grand Island, NY)
Results
Detection of mRNA encoding IL-9R in human peripheral blood eosinophils
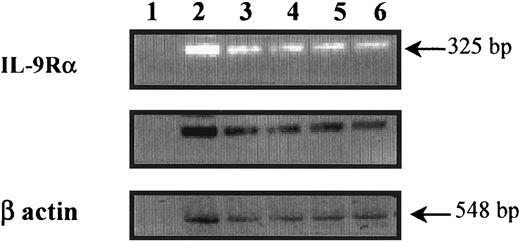
To determine whether freshly isolated peripheral blood eosinophils express the IL-9R–α chain transcript, mRNA preparation from highly purified eosinophils was analyzed by RT-PCR. As shown in Figure 1, a specific band at the expected size (325 base pair [bp]) corresponding to the IL-9R–α chain mRNA was detected in eosinophils from both nonasthmatic controls (lanes 3 and 4) and asthmatic patients (lanes 5 and 6), and the eosinophil-differentiated HL-60 cell line, used as a positive control (lane 2). There was no specific band seen in the absence of cDNA (lane 1). Amplification products specific for β-actin were of similar intensity between all samples, which suggests the equality of the RNA preparations.
Detection of IL-9R–α transcripts in human peripheral blood eosinophils.
Preparation of human peripheral blood eosinophils from nonasthmatics (lanes 3 and 4), asthmatics (lanes 5 and 6), and eosinophil-differentiated HL-60 cell lines (lane 2) showed IL-9R–α–specific amplified fragments. The specificity of the amplified fragments was confirmed by Southern blot analysis using an internal primer. The control was β-actin, and cDNA was not used in lane 1. Eosinophil RNA was isolated, and first-strand cDNA synthesis was performed. Human IL-9R was amplified using PCR and IL-9R–α–specific primers on either side of a splice junction to preclude amplification of possible contaminating genomic DNA.
Detection of IL-9R–α transcripts in human peripheral blood eosinophils.
Preparation of human peripheral blood eosinophils from nonasthmatics (lanes 3 and 4), asthmatics (lanes 5 and 6), and eosinophil-differentiated HL-60 cell lines (lane 2) showed IL-9R–α–specific amplified fragments. The specificity of the amplified fragments was confirmed by Southern blot analysis using an internal primer. The control was β-actin, and cDNA was not used in lane 1. Eosinophil RNA was isolated, and first-strand cDNA synthesis was performed. Human IL-9R was amplified using PCR and IL-9R–α–specific primers on either side of a splice junction to preclude amplification of possible contaminating genomic DNA.
Cell surface expression of IL-9R–α chain in human eosinophils
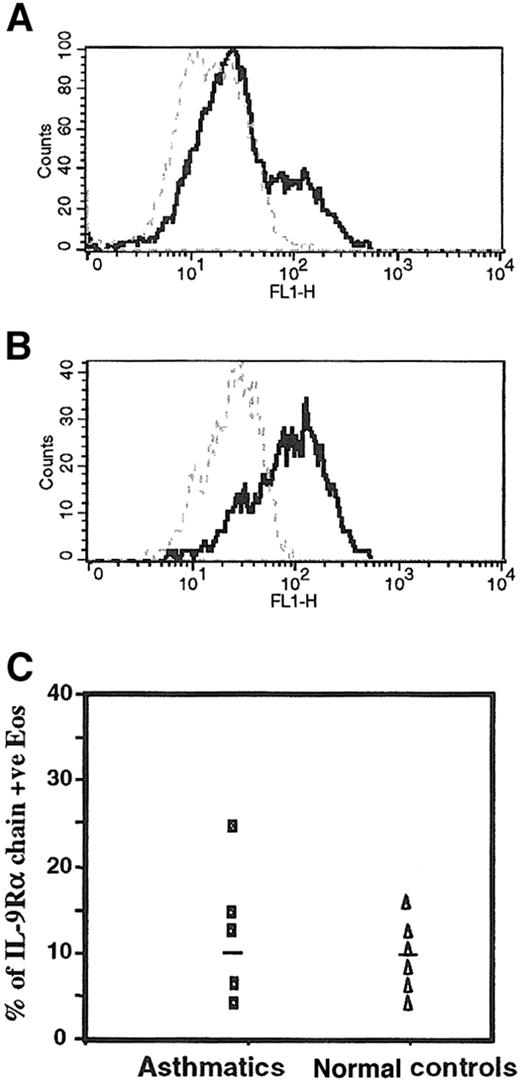
To examine whether the IL-9R–α chain is expressed on the cell surface of freshly isolated peripheral blood eosinophils, we performed flow cytometry analyses of human eosinophils from different subjects stained with mouse mAb to the human IL-9R–α chain. Purified human eosinophils from an asthmatic patient and the eosinophil-differentiated HL-60 cell line showed positive reactivity using the mouse mAb anti–IL-9R–α, with a mean percentage positivity of 23% and 65%, respectively (Figure 2A,B). We also confirmed the expression of the IL-9R–α chain in eosinophils from healthy controls, but there was no significant difference in the level of the IL-9R–α expression eosinophils from 5 asthmatics (10.0% ± 12.7%) and 5 nonasthmatic healthy control subjects (8.9% ± 8.2%) (P > .05), as depicted in Figure2C.
Surface expression of IL-9R on human peripheral blood eosinophils.
(A) Highly purified peripheral blood eosinophils and (B) eosinophil-differentiated HL-60 cell lines were analyzed with a mouse affinity purified mAb antihuman IL-9R–α chain (5 μg/mL isotype IgG1) (solid line) followed by a FITC-conjugated goat antimouse IgG (dilution 1:200). Mouse IgG1 (5 μg/mL clone MOPC-1) was used as an isotype-matched control antibody (broken line). (C) The surface expression of IL-9R–α showed no significant difference between asthmatics and normal (nonasthmatic) controls using flow cytometry analysis (P > .05). Flow cytometry analysis was performed as described in “Materials and methods.” +ve, positive; FL1, fluorescence 1.
Surface expression of IL-9R on human peripheral blood eosinophils.
(A) Highly purified peripheral blood eosinophils and (B) eosinophil-differentiated HL-60 cell lines were analyzed with a mouse affinity purified mAb antihuman IL-9R–α chain (5 μg/mL isotype IgG1) (solid line) followed by a FITC-conjugated goat antimouse IgG (dilution 1:200). Mouse IgG1 (5 μg/mL clone MOPC-1) was used as an isotype-matched control antibody (broken line). (C) The surface expression of IL-9R–α showed no significant difference between asthmatics and normal (nonasthmatic) controls using flow cytometry analysis (P > .05). Flow cytometry analysis was performed as described in “Materials and methods.” +ve, positive; FL1, fluorescence 1.
Detection of IL-9R protein in human peripheral blood and BAL eosinophils by immunocytochemistry
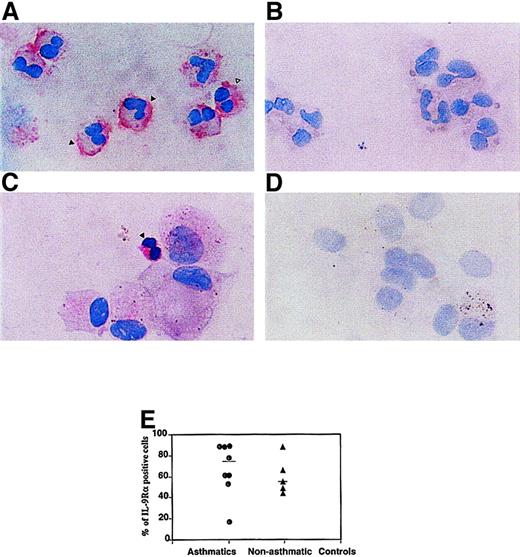
To further investigate the protein expression of the IL-9R–α by human eosinophils, immunocytochemistry was performed with rabbit pAb anti–IL-9R–α chain on human peripheral blood eosinophils from 12 donors. Specific staining with anti–IL-9R–α antibodies was observed in peripheral blood (Figure 3A) and BAL eosinophils from an asthmatic subject (Figure 3C). Substitution of the primary antibody with normal rabbit serum eliminated the positive immunoreactivity, demonstrating the specificity of the analysis (Figure 3B,D). Our immunohistochemical studies demonstrated the presence of IL-9R–α on cells that showed the eosinophilic phenotype. Furthermore, there was no significant difference observed between the percentage of IL-9R–α+ eosinophils from 8 asthmatics (68.0% ± 24.7%) and 5 nonasthmatic controls (55.0% ± 18.9%) (P > .05), as seen in Figure 3E. However, in both groups of subjects it was noted that higher numbers of IL-9R–α+ eosinophils were detected by immunocytochemistry compared to flow cytometry analysis. This suggests that the surface expression of IL-9R–α is under regulatory control, and it can be speculated that factors, such as cytokines, can increase the surface expression of IL-9R, which may potentiate the function of recruited eosinophils during an inflammatory reaction.2
Detection of IL-9R immunoreactivity on peripheral blood and BAL eosinophils.
(A) Specific staining was detected on the cell membrane (solid arrow) as well as the cytoplasm (open arrow) of purified peripheral blood human eosinophils. (C) Positive staining of BAL eosinophil (solid arrow) from asthmatic individual. Normal rabbit serum was used as the negative controls for the (B) peripheral blood eosinophils and (D) BAL cells. (E) Quantification of IL-9R–α+ cells in asthmatics and nonasthmatic controls. The percentage of IL-9R–α+ peripheral blood eosinophils from asthmatics and nonasthmatic controls was determined by counting 500 cells per slide. There was no significant difference between the 2 groups using immunocytochemical methods to detect the IL-9R–α (P > .05). The staining was performed using the affinity-purified rabbit pAb anti–IL-9R–α (directed to C-terminus specific domain; final dilution, 1 μg/mL) followed by biotin-labeled swine antirabbit Ig and streptavidin phosphatase alkaline. Counterstaining was performed using hematoxylin.
Detection of IL-9R immunoreactivity on peripheral blood and BAL eosinophils.
(A) Specific staining was detected on the cell membrane (solid arrow) as well as the cytoplasm (open arrow) of purified peripheral blood human eosinophils. (C) Positive staining of BAL eosinophil (solid arrow) from asthmatic individual. Normal rabbit serum was used as the negative controls for the (B) peripheral blood eosinophils and (D) BAL cells. (E) Quantification of IL-9R–α+ cells in asthmatics and nonasthmatic controls. The percentage of IL-9R–α+ peripheral blood eosinophils from asthmatics and nonasthmatic controls was determined by counting 500 cells per slide. There was no significant difference between the 2 groups using immunocytochemical methods to detect the IL-9R–α (P > .05). The staining was performed using the affinity-purified rabbit pAb anti–IL-9R–α (directed to C-terminus specific domain; final dilution, 1 μg/mL) followed by biotin-labeled swine antirabbit Ig and streptavidin phosphatase alkaline. Counterstaining was performed using hematoxylin.
Effect of IL-9 on eosinophil survival
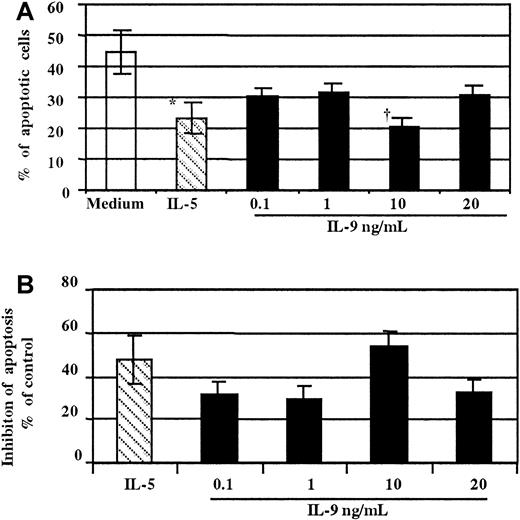
As demonstrated in thymic lymphoma cell lines37and T cell lines,38 the major activity of IL-9 on T cells has been proposed to be protection from apoptosis. To date, there have been no studies that have examined the effect of this cytokine on human eosinophil survival. To test whether stimulation of the IL-9R influenced eosinophil apoptosis, human purified blood eosinophils were treated with increased concentration of either rhIL-9, rhIL-5, or medium alone for 18 hours. The extent of cellular apoptosis was then evaluated by morphological assessment (Figure4).33 As observed in Figure4A, treatment with IL-9 significantly decreased, in a concentration-dependent manner, the percentage of eosinophils undergoing apoptosis compared to untreated cells. The minimal effective concentration of IL-9 was 0.1 ng/mL, and the maximum effect was observed at 10 ng/mL. The mean percentage of apoptotic cells was 20.8% ± 2.5% vs 44.5% ± 6.2% with medium (P < .01). Similarly, as expected, the percentage of eosinophils undergoing apoptosis decreased significantly in the presence of IL-5 (P < .01) (Figure 4A,B).
Concentration-dependent effect of IL-9 on eosinophil survival.
(A) Cells stimulated with IL-5 (▧,10 ng/mL) were used as a positive control (*P < .05). Freshly purified peripheral blood eosinophils stimulated for 18 hours with a graded amount of IL-9 (▪) showed fewer apoptotic cells compared to medium alone (■, †P < .01 at 10 ng/mL of IL-9). (B) Data from panel A are presented as the percentage of inhibition compared to the control (medium alone). The assays were performed in duplicate on eosinophils from the same donor. These results are representative of 3 independent experiments performed under the same conditions.
Concentration-dependent effect of IL-9 on eosinophil survival.
(A) Cells stimulated with IL-5 (▧,10 ng/mL) were used as a positive control (*P < .05). Freshly purified peripheral blood eosinophils stimulated for 18 hours with a graded amount of IL-9 (▪) showed fewer apoptotic cells compared to medium alone (■, †P < .01 at 10 ng/mL of IL-9). (B) Data from panel A are presented as the percentage of inhibition compared to the control (medium alone). The assays were performed in duplicate on eosinophils from the same donor. These results are representative of 3 independent experiments performed under the same conditions.
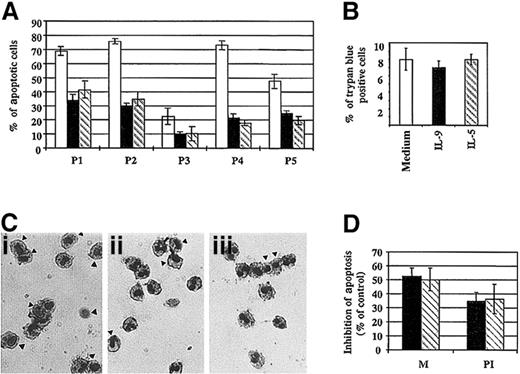
The antiapoptotic effect of IL-9 was then confirmed in additional subjects, and significant inhibition of eosinophil apoptosis was obtained when the cells were stimulated with IL-5 or IL-9 for 18 hours (Figure 5A,C). No difference in the percentage of necrotic cells could be detected between different samples at 18 hours (Figure 5B), which suggests that the untreated cells are at the first stage of the apoptosis process.33Using a DNA fragmentation assay, we demonstrated that IL-9 inhibited apoptotic cell death. We found that treatment of eosinophils with IL-9, as well as IL-5, significantly inhibited DNA fragmentation compared to untreated cells at 18 hours. The mean percentage of inhibition was 34.7% ± 6.3% and 36.4% ± 10.4%, respectively (Figure 5D). Furthermore, comparable results were observed when apoptotic cells were assessed by morphology analysis (Figure 5D).
Effect of IL-9 on eosinophil survival.
(A) Percentage of apoptotic eosinophils from different patients treated for 18 hours with IL-9 (solid bar), IL-5 (broken bar), or medium alone (open bar). Morphological assessment was performed as described in “Materials and methods.” (B) There was no significant difference in cell viability observed between the 3 culture conditions at 18 hours, as assessed by trypan blue exclusion. (C) Morphological alteration of nuclei was evaluated by Diff Quick staining. Freshly purified peripheral blood eosinophils stimulated with 10 ng/mL IL-9 (panel ii) or IL-5 (panel iii) showed less apoptotic cells (indicated by arrows) compared to medium alone (panel i). (D) Similar results were obtained using either morphology assessment (M) or DNA fragmentation assay using PI and flow cytometry analysis. DNA fragmentation was assessed by analysis of the staining characteristics of fixed permeabilized cells exposed to the DNA-binding dye, PI. Analysis was performed by flow cytometry on a FACScan machine. The data are the mean ± SD of 3 independent experiments.
Effect of IL-9 on eosinophil survival.
(A) Percentage of apoptotic eosinophils from different patients treated for 18 hours with IL-9 (solid bar), IL-5 (broken bar), or medium alone (open bar). Morphological assessment was performed as described in “Materials and methods.” (B) There was no significant difference in cell viability observed between the 3 culture conditions at 18 hours, as assessed by trypan blue exclusion. (C) Morphological alteration of nuclei was evaluated by Diff Quick staining. Freshly purified peripheral blood eosinophils stimulated with 10 ng/mL IL-9 (panel ii) or IL-5 (panel iii) showed less apoptotic cells (indicated by arrows) compared to medium alone (panel i). (D) Similar results were obtained using either morphology assessment (M) or DNA fragmentation assay using PI and flow cytometry analysis. DNA fragmentation was assessed by analysis of the staining characteristics of fixed permeabilized cells exposed to the DNA-binding dye, PI. Analysis was performed by flow cytometry on a FACScan machine. The data are the mean ± SD of 3 independent experiments.
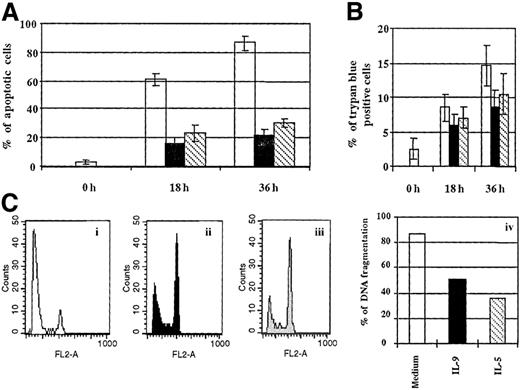
We next investigated whether IL-9 could increase human eosinophil survival at 36 hours. In this system fewer apoptotic eosinophils were detected in preparations cultured with IL-9 or IL-5 compared to untreated cells, as showed by morphology assessment and DNA fragmentation (P < .05) (Figure6A,C). A significant difference in the percentage of necrotic cells could be observed between IL-9 or IL-5 compared to untreated cells at 36 hours (Figure 6B). Altogether these results demonstrate that IL-9 increases human eosinophil survival.
IL-9 increases eosinophil survival.
(A) Eosinophils were cultured as described in Figure 5 for 18 and 36 hours. Morphological alterations of (A) nuclei and (B) trypan blue exclusion were determined as described in Figure 5. A significant difference in the percentage of apoptotic cells was observed between cells cultured with IL-9, IL-5, and medium at 18 hours as well as 36 hours (P < .05). The percentage of trypan blue cells is significantly decreased in IL-5– or IL-9–treated cells compared to medium only at 36 hours of culture (P < .05). (C) DNA fragmentation was determined by DNA staining as described in Figure 5D. Eosinophils were cultured with 10 ng/mL each (i) medium alone, (ii) IL-9, and (iii) IL-5 for 36 hours. (iv) The values are representative of 3 independent experiments.
IL-9 increases eosinophil survival.
(A) Eosinophils were cultured as described in Figure 5 for 18 and 36 hours. Morphological alterations of (A) nuclei and (B) trypan blue exclusion were determined as described in Figure 5. A significant difference in the percentage of apoptotic cells was observed between cells cultured with IL-9, IL-5, and medium at 18 hours as well as 36 hours (P < .05). The percentage of trypan blue cells is significantly decreased in IL-5– or IL-9–treated cells compared to medium only at 36 hours of culture (P < .05). (C) DNA fragmentation was determined by DNA staining as described in Figure 5D. Eosinophils were cultured with 10 ng/mL each (i) medium alone, (ii) IL-9, and (iii) IL-5 for 36 hours. (iv) The values are representative of 3 independent experiments.
Effect of IL-9 stimulation on human eosinophil IL-5R–α surface expression
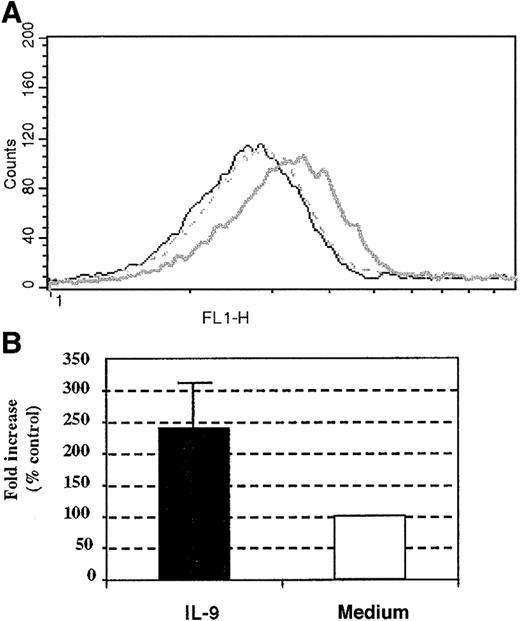
To further investigate the mechanisms underlying the ability of IL-9 to increase eosinophil survival, we evaluated the surface expression of IL-5R–α following IL-9 incubation. Compared to unstimulated cells, stimulation for 18 hours with IL-9 of the peripheral blood human eosinophils increased the expression of the IL-5R–α chain by 2.4 ± 0.6-fold compared with baseline cells (Figure 7A,B). Similarly, the expression of IL-5R–α increased the baseline level by 4-fold when differentiated HL-60 cells (harvested on day 7) were stimulated with IL-9 for 18 hours (data not shown). Therefore, IL-9 augmented the expression of IL-5R–α on human eosinophils. Taken together, these results suggest that the antiapoptotic effect exerted by IL-9 on human eosinophils may be accounted, at least partially, by the increased surface expression of the IL-5R–α chain.
Effect of IL-9 stimulation on human eosinophil IL-5R–α chain surface expression.
(A) Increased surface expression of IL-5R–α on human peripheral blood eosinophils. (B) The results from 3 other individual experiments given as the means of fold increase ± SD. The IL-5R–α chain surface expression was performed with anti–IL-5R–α chain mAb at 18 hours following stimulation with 10 ng/mL IL-9 (bold line) or control stimulation (broken line). Mouse IgG1 (5μg/mL clone MOPC-1) was used as an isotype-matched control antibody (thin line).
Effect of IL-9 stimulation on human eosinophil IL-5R–α chain surface expression.
(A) Increased surface expression of IL-5R–α on human peripheral blood eosinophils. (B) The results from 3 other individual experiments given as the means of fold increase ± SD. The IL-5R–α chain surface expression was performed with anti–IL-5R–α chain mAb at 18 hours following stimulation with 10 ng/mL IL-9 (bold line) or control stimulation (broken line). Mouse IgG1 (5μg/mL clone MOPC-1) was used as an isotype-matched control antibody (thin line).
Effect of IL-9 on IL-5R–α expression and development of eosinophils from cord blood progenitors
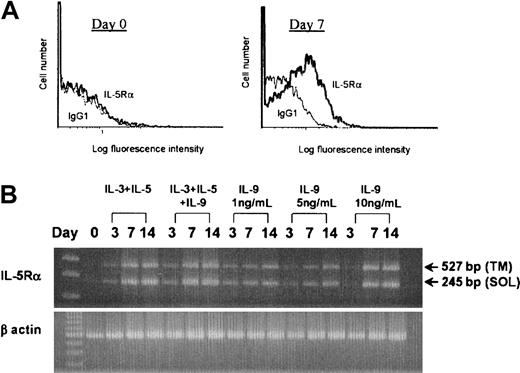
Previous studies have suggested that IL-5 is the most important cytokine for the terminal differentiation of the committed eosinophil precursors.6-8 This function is mediated through the membrane-bound IL-5R, which is composed of the ligand-specific α-subunit and common β-subunit.6 We next tested whether IL-9 has an effect on IL-5R–α expression. As previously reported, human umbilical cord blood CD34+ cells cultured in IL-3 and IL-5 developed into eosinophils by day 14, as determined by the presence of eosinophilic granules using the May-Grünwald-Giemsa stain (mean, 84.5%; range, 75%-88%; n = 10 independent cultures). This showed a switch to the mRNA expression for the transmembrane isoform of the IL-5R–α accompanied by surface expression of the IL-5R–α.39Culture of CD34+ cells in IL-9 alone lead to surface IL-5R–α expression at day 7 and day 14 (Figure8A). The specific mean fluorescence intensity (SMFI) for IL-5R–α staining at day 7 for cells cultured in IL-9 alone was 0.122 (SE, 0.06), and the SMFI for cells cultured in IL-3 and IL-5 was 0.135 (SE, 0.09) (n = 5). This was confirmed by RT-PCR, which showed mRNA for both soluble and membrane-associated IL-5R–α isoforms at days 7 and 14 of culture in IL-9 alone (Figure 8B). IL-9 supported modest cell survival, with a 2.18-fold mean expansion in cell number by day 14 (n = 3).
Effect of IL-9 on IL-5R–α expression by human CD34+
progenitor cells. Human umbilical cord blood CD34+ progenitor cells were cultured with 1 ng/mL IL-9 alone or 1 ng/mL each of IL-3 and IL-5. Initial plating density was 1 × 105 cells per mL. (A) Flow cytometric analysis of IL-5R–α expression by CD34+ cells at day 0 and after culture in 1 ng/mL IL-9 for 7 days. One representative experiment of 5 is shown. (B) RT-PCR products showing amplification of mRNA for both soluble (SOL) and transmembrane (TM) IL-5R–α in cord blood cells grown in IL-3 and IL-5 or IL-9 alone for 3, 7, or 14 days. There was no IL-5R–α mRNA detected in freshly isolated CD34+ cells at day zero. Data is shown for 1 of 3 independent experiments.
Effect of IL-9 on IL-5R–α expression by human CD34+
progenitor cells. Human umbilical cord blood CD34+ progenitor cells were cultured with 1 ng/mL IL-9 alone or 1 ng/mL each of IL-3 and IL-5. Initial plating density was 1 × 105 cells per mL. (A) Flow cytometric analysis of IL-5R–α expression by CD34+ cells at day 0 and after culture in 1 ng/mL IL-9 for 7 days. One representative experiment of 5 is shown. (B) RT-PCR products showing amplification of mRNA for both soluble (SOL) and transmembrane (TM) IL-5R–α in cord blood cells grown in IL-3 and IL-5 or IL-9 alone for 3, 7, or 14 days. There was no IL-5R–α mRNA detected in freshly isolated CD34+ cells at day zero. Data is shown for 1 of 3 independent experiments.
IL-9 increases IL-5R–α during terminal differentiation of an eosinophilic cell line
We then differentiated the HL-60 cell line into eosinophils and determined the effect of IL-9 at a late stage of culture (day 6) by following the surface expression of IL-5R–α. We could not detect surface expression of the IL-5R–α chain on eosinophil-differentiated HL-60 cells before day 6. A small percentage of cells expressed the membrane-bound IL-5R–α after 6 days of culture with the vehicle control for IL-9 (SMFI, 3.4 ± 1.8). Kinetic experiments revealed a significant and progressive increase in the surface expression of the IL-5R–α chain at 4, 6, 8, and 18 hours, which reached a maximum expression after 18 hours of culture (SMFI, 58 ± 6.5). As suggested for eosinophil-derived CD34+ cells,60this showed that surface expression of the IL-5R–α is a feature of the differentiation and maturation of HL-60 cells. The introduction of 10 ng/mL IL-9 in the culture on day 6 induced a 6.2-fold increase in the IL-5R–α chain surface expression on the eosinophil-differentiated HL-60 cell line by 6 hours (SMFI, 119 ± 13.5), which then decreased with time to reach the baseline expression by 18 hours (Figure 9A,B). These results suggest that IL-9 may potentiate the terminal differentiation and maturation of eosinophils by increasing the surface expression of the IL-5R–α.
Effect of IL-9 on terminal differentiation of the HL-60 cell line.
(A) The time course of the IL-5R–α chain expression on the HL-60 cell line in the presence of IL-9. The HL-60 cell line was differentiated toward eosinophils as described in “Materials and methods” and examined for the presence of IL-5R–α by flow cytometry. We added 10 ng/mL IL-9 or the vehicle control (medium alone) to medium at day 6 (t0 = time 0). The surface expression of the IL-5R–α chain was investigated at 4, 6, 8, 18, and 24 hours following IL-9 stimulation (bold line) or control stimulation (broken line) using mAb anti–IL-5R–α. The IgG1 control isotype is also shown (thin line). (B) The increase in the mean ± SD of SMFI with 10 ng/mL IL-9 over control stimulation. The maximal effect of IL-9 was observed after 6 hours of stimulation. The results represent the mean ± SD of 2 independent experiments.
Effect of IL-9 on terminal differentiation of the HL-60 cell line.
(A) The time course of the IL-5R–α chain expression on the HL-60 cell line in the presence of IL-9. The HL-60 cell line was differentiated toward eosinophils as described in “Materials and methods” and examined for the presence of IL-5R–α by flow cytometry. We added 10 ng/mL IL-9 or the vehicle control (medium alone) to medium at day 6 (t0 = time 0). The surface expression of the IL-5R–α chain was investigated at 4, 6, 8, 18, and 24 hours following IL-9 stimulation (bold line) or control stimulation (broken line) using mAb anti–IL-5R–α. The IgG1 control isotype is also shown (thin line). (B) The increase in the mean ± SD of SMFI with 10 ng/mL IL-9 over control stimulation. The maximal effect of IL-9 was observed after 6 hours of stimulation. The results represent the mean ± SD of 2 independent experiments.
Discussion
This study set out to examine the expression of the IL-9R on eosinophils and the functional implications of its stimulation with respect to eosinophil viability. Using flow cytometry and immunohistochemistry, our results demonstrate that human peripheral blood eosinophils express the specific α-chain of the IL-9R on their cell surface. Furthermore, these cells were also positive for IL-9R–α mRNA when examined using RT-PCR. Incubation with IL-9 enhanced the viability of peripheral blood eosinophils, and this was associated with an increase in the expression of the specific α-chain of the IL-5R. In addition, IL-9 enhanced eosinophil development from cord blood progenitors, increased their expression of the IL-5R–α and up-regulated IL-5R–α expression during terminal eosinophil differentiation of the HL-60 cell line. The results of this study not only have functional implications for eosinophil and T-cell directed inflammation, but they also provide a novel mechanism that may contribute to the development of tissue eosinophilia.
Although originally described as a T-cell growth factor with very narrow specificity for certain T cells, IL-9–mediated activities have been described on erythroid progenitors; B cells; mast-cell fetal thymocytes9-13; and most recently, airway epithelia.24 Recently, IL-9 has received considerable attention by virtue of the genetic studies linking the IL-9and IL-9R gene loci to indices of airway hyper-responsiveness and asthma.15-17,27 Moreover, IL-9 has been shown to be produced by activated CD4+ T lymphocytes of the Th2-like phenotype11 and is implicated in the Ig isotype switching to favor IgE production.13 The finding that human peripheral blood eosinophils express a functional receptor for IL-9 suggests that IL-9 may influence the differentiation and activation of effector cells linked to the pathogenesis of allergic disease.
In our initial experiments, the expression of the IL-9R–α was established by RT-PCR, flow cytometry, and immunohistochemistry using freshly isolated human peripheral blood eosinophils and differentiated HL-60 cells. The expression of the IL-9R on human eosinophils was widely heterogeneous according to individual donors, and there was no significant difference between the percentage of IL-9R+eosinophils in asthmatics and normal controls analyzed either by flow cytometry or immunohistochemistry. Interestingly, the numbers of cells expressing IL-9R–α by immunocytochemical techniques exceeded those enumerated by flow cytometry, which suggests that at least some of the IL-9R–α is found within the intracellular environment. This intracellular IL-9R–α may be under regulatory control, and it can be speculated that factors, such as cytokines, can increase the surface expression of the IL-9R. This in turn may potentiate the function of recruited eosinophils during an inflammatory reaction.2
In this study, we provided evidence of increased survival of human peripheral blood eosinophils stimulated with IL-9. Using different criteria to determine apoptotic cell death, we found that IL-9 has a consistent and significant effect on human eosinophil survival compared to medium alone, both at 18 and 36 hours. Furthermore, the percentage of necrotic eosinophils differs significantly between IL-5 or IL-9 and medium-treated cells at 36 hours but not at 18 hours. At 18 hours the cells are at the early stage of the apoptotic process, in which membrane integrity is still conserved.33 However, at 36 hours cells undergoing apoptosis reach secondary necrosis, in which the integrity of the cell membrane is compromised. These results not only confirm the presence and the functional integrity of the IL-9R on human eosinophils, but they also provide a novel mechanism to explain the continued presence of eosinophils within inflamed tissues such as asthmatic airways. In agreement with our findings, airway eosinophilia were observed in transgenic mice that overexpress IL-9 selectively within the lungs22,23 and in naive mice administered with recombinant IL-9 intratracheally.21 These previous studies suggested a mechanism where IL-9 could influence IL-5 activity on eosinophils. To further address this issue, we determined whether IL-9 was able to influence the expression of the specific α-chain of the IL-5R.
Previous studies have suggested that IL-5 is the most important cytokine for the terminal differentiation of the committed eosinophil precursors and is a potent inducer of eosinophil survival.39-42 These actions are mediated via a membrane-bound IL-5R that is composed of a ligand-specific α-subunit chain and a common β-chain.6 Our results demonstrated that IL-9 up-regulates the cell surface expression of the IL-5R–α chain in peripheral blood human eosinophils, CD34+progenitors developing toward the eosinophil lineage, and butyric acid–differentiated HL-60 cells. These data further suggest that IL-9 may have a direct effect on eosinophil survival and/or an indirect effect through the up-regulation of the IL-5R expression. This action of IL-9 is not limited to eosinophils; previously it was suggested that IL-9 exerts an antiapoptotic influence on T cell lines.37,38 While the intracellular mechanism underlying this effect on human eosinophils has not been investigated, an in vitro study in a T cell line has suggested that IL-9–mediated prevention of apoptosis depends upon dimerization of the α-subunit and γ-c–subunit of the IL-9R,43 which involve STAT1,44 STAT3,45 and STAT5.46These molecules have also been implicated in antiapoptotic effects in other signaling systems.47 48 Whether these transcription factors are activated following stimulation of the IL-9R in eosinophils remains to be determined.
From in vitro studies little is known about the factors regulating the expression of the IL-5R–α. Indeed, investigating the nature of the signals responsible for modulating the expression of the IL-5R–α on human eosinophil progenitors may provide insight into the control of eosinophil differentiation. Early studies suggested that preincubation with granulocyte-macrophage–colony-stimulating factor (GM-CSF) could enhance IL-5 binding to specific high-affinity sites on mature eosinophils.49 In a study by Zanders,50 the IL-5R–α was down-regulated on an erythroleukemic cell line only by tumor growth factor (TGF)–β1 and not subject to modulation by a variety of cytokines including IL-9. More recently, IL-3, IL-5, and GM-CSF have all been shown to selectively inhibit the transcription of IL-5R–α mRNA in human blood eosinophils.51 These studies, however, do not address the expression of the IL-5R–α on immature eosinophils, which is possibly regulated via alternative mechanisms. Indeed, as yet we have no direct indication as to the factors responsible for the increased expression of IL-5R–α, which was detected on bone marrow progenitor (CD34+) cells in response to an allergen challenge in atopic asthmatics.40 Our data show that IL-9 is a potential candidate because it enhanced the IL-5R–α in cord blood cell cultures and increased eosinophil yield. Although IL-9 alone did support survival of CD34+ cord blood cells, the effect of IL-9 on cell yield was modest compared to IL-3 and IL-5.
However IL-9 alone did up-regulate the IL-5R–α expression, and this effect on early stages of development may greatly amplify subsequent lineage expansion by IL-5. Whether these effects are direct or are due to the induction of autocrine IL-5 synthesis by progenitors remains to established. We also showed that stimulation of the HL-60 cell line on day 6, a late stage of differentiation toward eosinophils, with rhIL-9 significantly increases the surface expression IL-5R compared to unstimulated cells. In addition, IL-9 has been shown to stimulate myeloid progenitor cells; this therefore suggests a role for IL-9 in myeloid cell maturation,52 which is in support of our results. Because the airway IL-5R–α chain expression is closely correlated to measurements of lung function of asthmatic individuals,53 and CD34+/IL-5R–α cells could be detected in the airway of asthmatic subjects,54finding regulatory mediators, such as IL-9, provides a novel therapeutic target for future research.
Taken together, our results show for the first time the presence of a functional receptor for IL-9 on human eosinophils and suggest that IL-9 may induce airway eosinophilia both by promoting eosinophil differentiation and inhibiting cellular apoptosis. These actions may be attributed to its ability to up-regulate the IL-5R–α which in turn may potentiate IL-5–mediated differentiation and maturation. These data also provide a novel therapeutic target for controlling the development of the eosinophilic component of the allergic inflammatory response.
Acknowledgment
The authors would like to acknowledge Ms Elsa Schotman for technical assistance.
Supported by grant MT13273 from the Medical Research Council of Canada, Canada; the Wellcome Trust, Glaxo-Wellcome plc; and Magainin Pharmaceuticals Inc., Philadelphia, PA.
Submitted November 8, 1999; accepted May 12, 2000.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Qutayba Hamid, Meakins-Christie Laboratories, 3626 St Urbain Street, Montreal, Quebec, Canada H2X 2P2; e-mail: hamid@meakins.lan.mcgill.ca.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal