Abstract
A novel Hoechst 33342 dye efflux assay was recently developed that identifies a population of hematopoietic cells termed side population (SP) cells. In the bone marrow of multiple species, including mice and primates, the SP is composed primarily of CD34−cells, yet has many of the functional properties of hematopoietic stem cells (HSCs). This report characterizes SP cells from human umbilical cord blood (UCB). The SP in unfractionated UCB was enriched for CD34+ cells but also contained a large population of CD34− cells, many of which were mature lymphocytes. SP cells isolated from UCB that had been depleted of lineage-committed cells (Lin− UCB) contained CD34+ and CD34− cells in approximately equivalent proportions. Similar to previous descriptions of human HSCs, the CD34+Lin− SP cells were CD38dimHLA-DRdimThy-1dimCD45RA−CD71−and were enriched for myelo-erythroid precursors. In contrast, the CD34−Lin− SP cells were CD38−HLA-DR−Thy-1−CD71−and failed to generate myelo-erythroid progeny in vitro. The majority of these cells were CD7+CD11b+CD45RA+, as might be expected of early lymphoid cells, but did not express other lymphoid markers. The CD7+CD34−Lin− UCB SP cells did not proliferate in simple suspension cultures but did differentiate into natural killer cells when cultured on stroma with various cytokines. In conclusion, the human Lin− UCB SP contains both CD34+ multipotential stem cells and a novel CD7+CD34−Lin− lymphoid progenitor. This observation adds to the growing body of evidence that CD34− progenitors exist in humans.
Introduction
The characterization and manipulation of hematopoietic stem cells (HSCs) for transplantation and gene therapy purposes has been intensely studied in recent years.1,2The most primitive HSCs have an extensive potential for self-renewal and can give rise to all blood-cell lineages.3A number of in vitro and xenochimeric transplantation studies have demonstrated that primitive human HSCs express CD34, a cell-surface protein of unknown function.4-6 A small number of primate studies have confirmed that CD34+ bone marrow cells can durably reconstitute multilineage hematopoiesis following transplantation.7 On the basis of this evidence, a number of autologous and allogeneic clinical bone marrow transplantation trials have used CD34+–enriched cell preparations.8 In these trials, multilineage hematopoietic engraftment occurred in the majority of patients, further supporting the contention that HSCs express CD34.
Recently, several experimental observations have suggested that HSCs that do not express CD34 may also exist. Murine CD34lo/−HSCs with long-term repopulating ability have been isolated from Lin−Sca-1+ fractions of bone marrow.9,10 Similarly, murine CD34lo/− HSCs have been fractionated from bone marrow by virtue of their aldehyde dehydrogenase activity or by virtue of their capacity to efflux various dyes.11,12 One recent study has indicated that the majority of HSCs in resting murine bone marrow are CD34lo/− but that CD34 expression on HSCs is increased following cell activation.13 This observation raises the possibility that expression of CD34 on HSCs is dynamic and may vary depending on the physiologic status of the donor.14
In humans, the expression pattern of CD34 on HSCs is less clear because of the absence of simple and authentic human transplant models. Human CD34− cells have reconstituted multiple hematopoietic cell lineages in xenotransplant animal models including fetal sheep and nonobese diabetic/severe combined immune-deficient (NOD/SCID) mice.15,16 In addition, a subset of Lin−CD34− cells isolated from both bone marrow and mobilized peripheral blood gave rise to long-term culture (LTC)–initiating cells following incubation with high doses of certain cytokines.17 Finally, a Lin−CD34− population has been shown to acquire CD34 expression, clonogenic activity, and NOD/SCID repopulating activity following a brief period in culture on stroma supplemented with several cytokines.18 Although these studies provide provocative evidence that CD34− HSCs may exist in humans, these cells have been difficult to study because they are obscured by a much larger number of mature CD34− cells. In addition, the Lin− CD34− populations studied to date appear to be heterogeneous so that multiple-lineage–committed progenitors rather than individual multilineage HSCs may have generated the observed stem cell activity.
A recently developed technique for isolating HSCs based on Hoechst 33342 dye efflux may prove useful for addressing these issues.12,19 This technique identifies a small subpopulation of cells, termed the side population (SP), on the basis of its unique fluorescence emission properties. In mice, bone marrow SP cells had the phenotypic and functional properties of HSCs. These cells were highly enriched for long-term hematopoietic reconstitution activity despite expressing low to no levels of CD34.12,19SP cells have also been identified in primate and human bone marrow and in human umbilical cord blood (UCB).19 CD34−SP cells isolated from rhesus bone marrow lacked the expression of obvious lineage commitment markers, acquired CD34 expression in vitro, were enriched for cells capable of generating clonogenic progeny in LTCs, and could generate T-cells on rhesus thymic stroma.19
These observations suggest that using Hoechst dye efflux to isolate SP cells may be a useful method for enriching rare populations of CD34− HSCs. Since UCB is increasingly being used for transplantation purposes,20 21 we sought to characterize UCB SP cells and to determine whether the UCB SP contains primitive CD34− hematopoietic cells.
Materials and methods
UCB processing
Staff members of the Carolina Cord Blood Bank collected human UCB after obtaining institutional-review-board–approved informed consent. UCB was diluted with Dulbecco's phosphate-buffered saline (PBS), and red blood cells were agglutinated at room temperature with the use of Hespan (DuPont Pharma, Wilmington, DE) at a final concentration of 1%. Nonagglutinated white blood cells were harvested, and residual red cells were hemolyzed at 37°C in 0.17 mol/L NH4Cl, 20 mmol/L Tris-HCl, pH 7.2, and 200 μmol/L EDTA. Lineage-committed cells were then removed from the white-cell fraction with the use of the CD34+ StemSep enrichment cocktail (StemCell Technologies Inc, Vancouver, British Columbia, Canada) according to kit instructions. The recovered cells, termed Lin− cells, were washed in Iscove's Modified Dulbecco's Medium (IMDM)/10% fetal calf serum (FCS) and then held on ice until they were analyzed or further fractionated with fluorescence-activated cell sorting (FACS).
Hoechst 33342 and antibody staining
To identify and isolate SP cells, undepleted or Lin− UCB was resuspended at 106 cells per milliliter in IMDM/2% FCS (staining media) and preincubated at 37°C for 30 minutes. The cells were then labeled with 2.5 μg/mL Hoechst 33342 (Molecular Probes; Eugene, OR) in staining media at 37°C for 90 minutes. When verapamil (American Regent Laboratories; Shirley, NY) was used, it was included at 50 μM. After staining, the cells were washed, resuspended in ice-cold staining media, and then maintained on ice until their analysis or sorting. For staining of cell-surface antigens, cells were resuspended in 100 μL of staining media, and antibodies were added directly to the cell suspensions. The cells were incubated on ice for 15 minutes and then washed with ice-cold staining media. Immediately prior to FACS analysis or sorting, 1 to 2 μg/mL propidium iodide (PI) (Sigma Chemical, St Louis, MO) was added to the cell suspensions.
Directly conjugated fluorescent antibodies used in these studies were antibodies directed against CD2 (clone S5.2), CD3 (clone SK7), CD5 (clone L17F12), CD7 (clone 4H9), CD16 (clone NKP15), CD34 (clone 8G12, ie, HPCA-2), CD38 (clone HB7), and CD56 (clone MY31) from Becton Dickinson Immunocytometry Systems (BDIS) (San Jose, CA). In addition, an anti-CD7 (CD7-6B7) antibody was obtained from CalTag Laboratories (Burlingame, CA); anti-CD45 (clones KC-56 and J33) as well as pooled anti-CD34 antibodies (clones QBEnd10, Immu-133, and Immu-134) were obtained from Coulter/Immunotech (Miami, FL); an anti-CD94 antibody (clone HP-3D9) was obtained from PharMingen (San Diego, CA); an anti-CD38 antibody (clone B-A6) was obtained from BioSource International (Camarillo, CA); and an anti-CD45RA antibody (clone F8-11-13) was obtained from Southern Biotechnology Associates, Inc (Birmingham, AL).
Fluorescence-activated cell sorting
Cells were analyzed and sorted on a FACStar Plus (BDIS) cell sorter equipped with dual Coherent I-90 lasers. Hoechst 33342 was excited at 351 nm, and fluorescence emission was detected with the use of 450DF20 (blue) and LP675 (far-red) filters (Omega Optical Inc, Brattleboro, VT). A 610-nm short pass dichroic mirror was used to separate these emission wavelengths (Omega Optical Inc). Fluorescence from the Hoechst dye was acquired in linear scales. Dead and dying cells were excluded on the basis of PI uptake. Fluorochrome-conjugated antibodies were excited at 488 nm, and their fluorescence emission was detected by means of standard filters.
In some experiments, CD34+ and CD34− SP cells were isolated directly from unfractionated UCB that had been stained simultaneously with Hoechst and phycoerythrin (PE)–conjugated anti-CD34. For these assays, the SP was defined as 0.02% to 0.03% of the total white-cell content of the unfractionated UCB. To isolate CD34+ and CD34− fractions from the Lin− SP, multiple sequential sorts were employed to optimize purity. After lineage depletion, the cells were stained with Hoechst, and the SP region was defined on the cytometer on the basis of its low fluorescence emission in both blue and far-red wavelengths (representative profiles appear in Figure 3). SP cells were sorted as the dimmest 1% of the Hoechst-stained Lin− UCB. For assays for myeloid growth, the Lin− SP cells were then washed and restained with anti-CD34 PE and anti-CD38 fluorescein isothiocyanate (FITC) and re-sorted to isolate CD34+CD38dimLin− SP and CD34−CD38dimLin− SP fractions. The CD38− gate was defined on the basis of a signal intensity equivalent to, or less than, an FITC isotype control. On reanalysis, purified CD34−Lin− SP cells did not express CD34 as defined by monoclonal antibodies raised against multiple different epitopes of the protein. For in vitro lymphoid assays, the Lin− SP cells were stained with anti-CD7 FITC, with anti-CD34 allophycocyanin, and simultaneously with anti-CD2 PE, CD3 PE, CD5 PE, and CD56 PE. The cells were then re-sorted to isolate CD7+CD34−Lin− SP and CD34+CD7±Lin− SP subfractions.
Short-term and long-term colony-forming unit assays
Hematopoietic progenitor colony assays (HPCAs) were performed by plating 100 to 200 cells in MethoCult H4431 containing agar-leukocyte–conditioned media and recombinant human erythropoietin (StemCell Technologies). The cells were incubated in a humidified chamber at 37°C with 5% CO2, and hematopoietic colonies (greater than 100 cells) were scored at 14 to 18 days after the cultures were initiated. LTC assays were maintained on either irradiated allogeneic bone marrow stroma or MS5 cells (graciously provided by Dr Tadashi Sudo of the Kirin Pharmaceutical Research Laboratory, Gunma, Japan). The MS5 stromal layers were established by seeding 24-well plates (Corning Costar Corp, Cambridge, MA) with 6 to 7 × 104 MS5 cells per well in DMEM/10% FCS. Allogeneic bone marrow stromal cells were seeded at similar densities in Myelocult H5100 (StemCell Technologies) containing 1 μmol/L hydrocortisone (succinate salt; Sigma Chemical). Stromal cells were cultured at 37°C in a humidified incubator until the cultures approached approximately 80% confluence. The monolayers were then γ-irradiated from a cesium source (30 to 40 Gy for MS5 stromal layers; 17.5 Gy for allogeneic bone marrow stroma). LTCs established with SP cells derived from unfractionated UCB were initiated with 150 to 350 cells per well. LTCs established from SP fractions derived from Lin− UCB were initiated with 400 to 2000 hematopoietic progenitor cells per well on the irradiated MS5 cells. LTCs on MS5 cells were maintained in Myelocult H5100 medium (StemCell Technologies) at 33°C in a humidified chamber with 5% CO2. LTCs on allogeneic stroma were maintained in Myelocult H5100 medium supplemented with 1 μmol/L hydrocortisone, 25 ng/mL Kit ligand (KL), 10 ng/mL interleukin 3 (IL-3), and 10 ng/mL IL-6 (R&D Systems, Minneapolis, MN) at 37°C in a humidified chamber with 5% CO2. For all LTCs, half the media from each well were removed at weekly intervals and replenished with fresh media. Adherent and nonadherent cells were harvested after 5 weeks and plated into HPCAs as described above.
Lymphoid cell cultures
For lymphocyte suspension cultures, 100 to 500 sorted cells were plated in duplicate either in serum-free media (BIT 9500, StemCell Technologies) or in RPMI 1640/10% FCS with IL-2 (100 U/mL), IL-7 (10 ng/mL, R&D Systems), or IL-12 (10 ng/mL; R&D Systems). In some cultures, 10% conditioned medium from phytohemagglutinin [PHA]–stimulated leukocytes (T-Stim without PHA; Collaborative Biomedical Products, Bedford, MA) was added. For lymphoid development on stroma, Lin− SP cells were seeded onto γ-irradiated (20 Gy) MS5 stroma at 100 to 2000 cells per well. Cells were cultured either in MEMα medium supplemented with 10% FCS or in HAMS F12 medium supplemented with 1% bovine serum albumin, 2% FCS, 1 μmol/L ZnSO4, 1 μmol/L CuSO4, 5 μmol/L β-mercaptoethanol, and a mixture of insulin, transferrin, and selenium (ITS-G; Gibco BRL, Gaithersburg, MD). These cultures were supplemented with KL, Flt3 ligand (F3L), IL-2, IL-7, and/or IL-15 (all from R&D Systems), as described in Table1.
Culture conditions for NK-cell development
| Condition . | Media and cytokines . |
|---|---|
| A | MEMα |
| 5 weeks: KL, F3L, IL-2, IL-7, IL-15 | |
| 2 weeks: IL-2 | |
| B | HAMS F12 |
| 1 week: KL, F3L, IL-2, IL-3, IL-7 | |
| 5 weeks: KL, F3L, IL-2, IL-7 | |
| 2 weeks: IL-2 | |
| C | HAMS F12 |
| 6 weeks: KL, IL-15 | |
| 2 weeks: IL-2 | |
| D | HAMS F12 |
| 2 weeks: KL, F3L, IL-2, IL-7, IL-15 | |
| 4 weeks: KL, IL-15 | |
| 2 weeks: KL, IL-2, IL-15 |
| Condition . | Media and cytokines . |
|---|---|
| A | MEMα |
| 5 weeks: KL, F3L, IL-2, IL-7, IL-15 | |
| 2 weeks: IL-2 | |
| B | HAMS F12 |
| 1 week: KL, F3L, IL-2, IL-3, IL-7 | |
| 5 weeks: KL, F3L, IL-2, IL-7 | |
| 2 weeks: IL-2 | |
| C | HAMS F12 |
| 6 weeks: KL, IL-15 | |
| 2 weeks: IL-2 | |
| D | HAMS F12 |
| 2 weeks: KL, F3L, IL-2, IL-7, IL-15 | |
| 4 weeks: KL, IL-15 | |
| 2 weeks: KL, IL-2, IL-15 |
Kit ligand (KL), Flt3 ligand (F3L), interleukin (IL)-2, IL-7, and/or IL-15 were used to propagate CD+ and CD34+Lin− SP cells on irradiated (20 Gy) MS5 stroma. IL-2 was used at 1000 U/mL, and IL-3 was used at 5 ng/mL. All other cytokines were at 10 ng/mL except under condition B, where KL and IL-7 were both used at 20 ng/mL.
Flow cytometric assay for natural killer cell function
A protocol for natural killer (NK) cell function, which measures target cell death through the uptake of membrane impermeable DNA dyes, was modified to evaluate NK-cell function in small numbers of cells.22 The target cells used in these assays included NK-sensitive K562 cells as well as NK-resistant Raji cells. K562 cells were labeled with 3 μmol/L carboxyfluorescein succinimidylester (CFSE) (Molecular Probes) in PBS at 106 cells per milliliter for 10 minutes at room temperature, and Raji cells were labeled with 0.5 μmol/L CFSE in a similar fashion. These concentrations of CFSE achieved similar fluorescence intensities for the 2 target cell lines. After CFSE labeling, the target cells were incubated overnight at 37°C with 5% CO2 in RPMI 1640 medium supplemented with 10% FCS. For lysis assays, the target cells were plated in 50 μL RPMI 1640/10% FCS to deliver 1000 cells per well in 96-well V-bottom culture plates. Effector cells were isolated from the lymphoid development cultures (see above) by FACS sorting human CD45+ cells from the murine MS5 stroma. The sorted cells were collected into RPMI 1640/10% FCS supplemented with 1000 U/mL IL-2 and plated in triplicate at the various effector-to-target ratios described in the Figure legends. To facilitate cell-to-cell interactions, the microtiter plates were centrifuged briefly at 700g. The cocultures were then incubated at 37°C with 5% CO2. After 4 hours in coculture, 0.5 μg 7-AAD (Molecular Probes) was delivered to each well in 50 μL RPMI 1640/10% FCS. After an additional 45 to 60 minutes, the cells were pelleted, washed once with PBS/2% FCS, and fixed in 1% formaldehyde prepared in PBS/2% FCS.
Results
A Hoechst 33342 SP is present in human umbilical cord blood
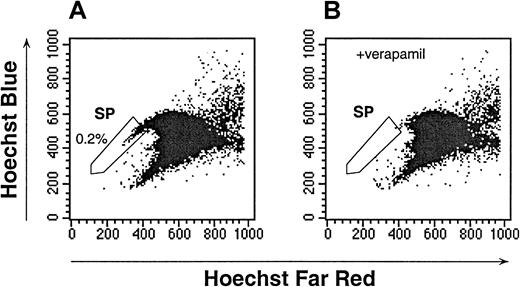
To identify SP cells in human UCB, initial studies were conducted to establish an optimal Hoechst dye concentration and staining duration (data not shown). Several conditions produced a similar pattern; however, incubation of UCB with 2.5 μg/mL of Hoechst for 90 minutes consistently identified a population of cells with a staining and fluorescence-emission pattern similar to that of murine bone marrow SP (Figure 1A, and data not shown). Goodell et al12 had previously shown that the Hoechst SP profile in murine bone marrow was blocked by staining in the presence of verapamil, indicating that the dim staining of SP cells was at least partially due to the efflux of Hoechst by a multidrug resistance (MDR)–like protein. The Hoechst staining of human UCB was also sensitive to verapamil (Figure 1B). The verapamil-sensitive UCB SP subpopulation represented 0.40% ± 0.29% (n = 28) of the total white-cell content of UCB. Because in murine studies, the dimmest SP cells had the highest capacity for long-term hematopoietic reconstitution, in subsequent UCB studies the dimmest 0.05% to 0.1% of the total mononuclear-cell content was defined as the human UCB SP (Figure 1A-B). In all cases, the fluorescent staining of the SP cells was easily distinguishable from the majority of the UCB cells.
Hoechst dye efflux by human UCB.
The SP cells, as defined by verapamil-sensitive cells, are indicated in the enclosed box. The data are representative of 28 experiments. (A) The Hoechst 33342 staining and emission patterns of human UCB in the absence of verapamil. (B) The Hoechst 33342 staining and emission patterns of human UCB in the presence of verapamil.
Hoechst dye efflux by human UCB.
The SP cells, as defined by verapamil-sensitive cells, are indicated in the enclosed box. The data are representative of 28 experiments. (A) The Hoechst 33342 staining and emission patterns of human UCB in the absence of verapamil. (B) The Hoechst 33342 staining and emission patterns of human UCB in the presence of verapamil.
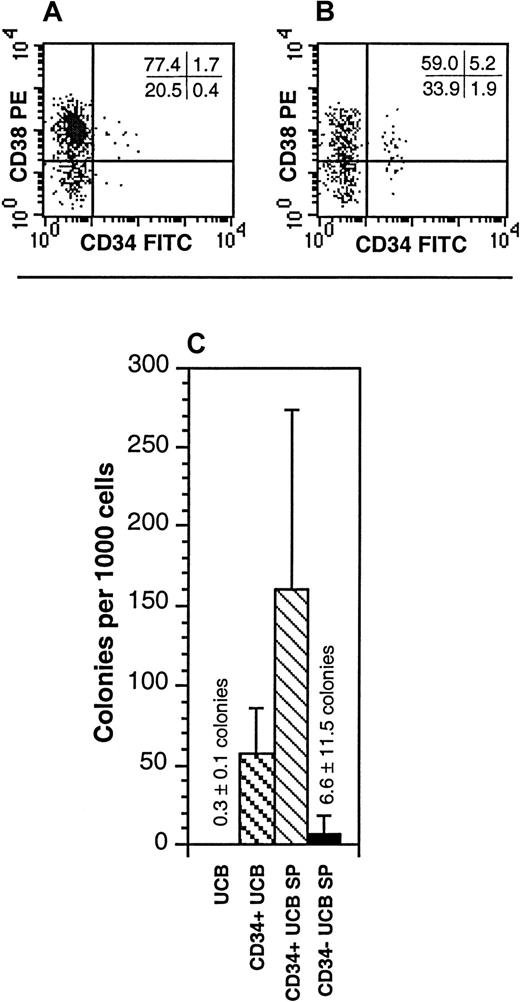
To characterize the cells within the SP, unfractionated UCB was stained with Hoechst in conjunction with antibodies directed against a variety of cell-surface markers. Cell surface antigens for mature myeloid cells, B cells, and erythroid cells were absent on UCB SP cells (Table2). In contrast, relatively high proportions of the CD34− UCB SP cells expressed markers commonly found on mature lymphoid cells, including CD2, CD3, CD5, CD16, and CD56 (Table 2). These antigens were detected exclusively on the CD34− SP cells. The UCB SP contained 11.7% ± 8.7% CD34+ cells and was approximately 6-fold enriched for these cells relative to unfractionated UCB (Figure 2A-B; Table3). Similarly, the UCB SP contained 3.05% ± 2.9% CD34+CD38lo/− cells and was approximately 15-fold enriched for these cells over the unfractionated UCB (Figure 2A-B; Table 3). Despite this, 88.3% ± 8.7% of the UCB SP cells were CD34−. To determine whether the CD34+ or the CD34− fractions of the UCB SP contained any progenitor-cell activity, CD34+ SP and CD34− SP cells were isolated and placed into LTC colony-forming unit assays. Whereas the CD34+ SP fraction was highly enriched in LTC progenitors relative to unfractionated UCB, the CD34− SP contained only a very low proportion of LTC progenitors (Figure 2C).
Analyses of surface markers on cells present within the UCB CD34− SP
| Immunophenotype . | UCB CD34− SP . |
|---|---|
| CD(16/56)+ | 15%-32% |
| CD2+ | 18%-38% |
| CD3+ | 10%-29% |
| CD4+ | 1.7%-8.1% |
| CD5+ | 12%-51% |
| CD14+ | none detected |
| CD33+ | none detected |
| CD19+ | none detected |
| CD71+ | none detected |
| Immunophenotype . | UCB CD34− SP . |
|---|---|
| CD(16/56)+ | 15%-32% |
| CD2+ | 18%-38% |
| CD3+ | 10%-29% |
| CD4+ | 1.7%-8.1% |
| CD5+ | 12%-51% |
| CD14+ | none detected |
| CD33+ | none detected |
| CD19+ | none detected |
| CD71+ | none detected |
The UCB SP was analyzed for the expression of various lineage-specific antigens with the use of a highly stringent gate to define the SP region (n = 2 to 3 for each marker examined).
UCB indicates umbilical cord blood; SP, side population.
Characterization of the UCB SP for properties of HSCs.
(A,B) The expression of CD34 and CD38 on unfractionated UCB (A) and the UCB SP (B). The SP represented the dimmest 0.05% to 0.1% of the Hoechst-stained UCB. Quadrant statistics are provided for the individual experiment depicted. These data are representative of 5 experiments. (C) Hematopoietic progenitors from unfractionated UCB, CD34+ UCB, CD34+ UCB SP cells, and CD34− UCB SP cells were enumerated in LTCs on allogeneic bone marrow stroma. The data represent the mean ± SD of 3 experiments. Cultures of CD34+ UCB SP cells represent only 2 UCB samples; LTCs detected from CD34− SP cells were derived from 1 well of 8 wells plated.
Characterization of the UCB SP for properties of HSCs.
(A,B) The expression of CD34 and CD38 on unfractionated UCB (A) and the UCB SP (B). The SP represented the dimmest 0.05% to 0.1% of the Hoechst-stained UCB. Quadrant statistics are provided for the individual experiment depicted. These data are representative of 5 experiments. (C) Hematopoietic progenitors from unfractionated UCB, CD34+ UCB, CD34+ UCB SP cells, and CD34− UCB SP cells were enumerated in LTCs on allogeneic bone marrow stroma. The data represent the mean ± SD of 3 experiments. Cultures of CD34+ UCB SP cells represent only 2 UCB samples; LTCs detected from CD34− SP cells were derived from 1 well of 8 wells plated.
Hematopoietic progenitor subsets present in the UCB SP
| Immunophenotype . | UCB . | UCB SP . |
|---|---|---|
| CD34+ | 1.83% ± 0.88% | 11.7% ± 8.7% |
| CD34+CD38− | 0.23% ± 0.15% | 3.05% ± 2.9% |
| CD34−CD38− | 15.7% ± 6.3% | 37.6% ± 8.9% |
| Immunophenotype . | UCB . | UCB SP . |
|---|---|---|
| CD34+ | 1.83% ± 0.88% | 11.7% ± 8.7% |
| CD34+CD38− | 0.23% ± 0.15% | 3.05% ± 2.9% |
| CD34−CD38− | 15.7% ± 6.3% | 37.6% ± 8.9% |
The UCB SP was analyzed by flow cytometry for the expression of CD34 and CD38 (n = 10). For these studies, the SP region was defined as 0.047% ± 0.011% of the UCB.
UCB indicates umbilical cord blood; SP, side population.
The Lin− SP contains CD34+ and CD34−cells
One potential explanation for the low frequency of detectable progenitors within the CD34− SP is that they may have been diluted by the mature cells within the SP. To enrich for possible primitive CD34− cells, the lineage-committed cells were depleted from the UCB by means of an immunoabsorbance technique. The resulting Lin− UCB was enriched 21.7-fold ± 38.7-fold for SP cells relative to the unfractionated UCB (Figure 3A-B; n = 39; median, 7.7-fold; range, 0.1-fold to 124-fold). The Lin− SP cells remained sensitive to verapamil (Figure 3C), and the verapamil-sensitive gate represented 1.02% ± 0.47% (n = 6) of the Lin− UCB. For these studies, the analyses of the Lin− SP were restricted to those cells with the dimmest Hoechst staining, which typically represented 1% of the Lin− UCB or less. The Lin− SP was depleted of cells expressing CD2, CD3, CD4, CD5, CD16, or CD56, and it did not contain cells expressing CD19, CD33, or CD71 (data not shown).
Hoechst dye efflux by Lin− UCB.
The SP cells, as defined by verapamil-sensitive cells, are indicated in the enclosed box. The data are representative of 39 experiments. (A) The Hoechst 33342 staining and emission patterns of unfractionated human UCB. (B) The Hoechst 33342 staining and emission patterns of Lin− UCB in the absence of verapamil. (C) The Hoechst 33342 staining and emission patterns of Lin− UCB in the presence of verapamil.
Hoechst dye efflux by Lin− UCB.
The SP cells, as defined by verapamil-sensitive cells, are indicated in the enclosed box. The data are representative of 39 experiments. (A) The Hoechst 33342 staining and emission patterns of unfractionated human UCB. (B) The Hoechst 33342 staining and emission patterns of Lin− UCB in the absence of verapamil. (C) The Hoechst 33342 staining and emission patterns of Lin− UCB in the presence of verapamil.
The Lin− SP contained nearly equivalent proportions of CD34+ (60.5% ± 23.3%) and CD34−(39.7% ± 23.3%) cells (n = 29; Figure4A-B). In addition, the Lin−SP was enriched for the brightest CD34+ cells and for CD34−CD38− cells (Figure 4B). When reanalyzed, the purified CD34−Lin− SP cells did not express CD34 even when stained with a pool of monoclonal antibodies raised against 3 different epitopes of CD34 (QBEnd10, Immu-133, and Immu-409), indicating that these cells did not merely express an alternative isoform of CD34 (data not shown). The CD34+Lin− SP cells had additional phenotypic features typical of primitive progenitors, including low levels of Thy-1 and HLA-DR expression as well as absent CD45RA and CD71 expression (Figure 4C-F).23,24 In contrast, the CD34−Lin− SP cells were predominantly CD38−, HLA-DR−, Thy-1−, and CD71− but did express CD45RA (Figure 4C-F). The lack of expression of CD38, HLA-DR, and Thy-1 on the CD34−Lin− SP cells is similar to the phenotype of the Lin−CD34− populations previously reported by Bhatia et al15 and Nakamura et al.18 By both FACS and microscopic analysis, the CD34+ and CD34−Lin− SP cells were small blast cells with minimal internal complexity and cytoplasm; however, the CD34−Lin− SP cells were distinctly smaller than the CD34+Lin− SP cells (data not shown).
Characterization of the Lin− UCB SP for HSC properties.
(A,B) The expression of CD34 and CD38 on Lin− (A) and on Lin− SP UCB cells (B). The SP represented the dimmest 0.5% to 1.0% of the Hoechst-stained Lin− UCB cells. Quadrant statistics are provided for the individual experiment depicted. (C-F) The expression of Thy-1 (CDw90; C), HLA-DR (D), CD45RA (E), or CD71 (F) versus that of CD34 within the Lin− SP. In all panels, CD34 is depicted on the x-axis regardless of the fluorochrome used. Quadrant statistics are provided for the individual experiments depicted. Each panel is representative of at least 3 experiments. (G,H) Myelo-erythroid hematopoietic progenitors were quantified from unfractionated UCB, Lin− UCB, the Lin− SP, the CD34+Lin− SP, and the CD34−Lin− SP fractions. These were enumerated in HPCAs (G) and in 5-week LTC assays on MS5 cells (H). These data are the mean ± SD of 5 experiments. All but 2 of the HPCAs detected from CD34−Lin− SP cells were derived from cells isolated from a single sort.
Characterization of the Lin− UCB SP for HSC properties.
(A,B) The expression of CD34 and CD38 on Lin− (A) and on Lin− SP UCB cells (B). The SP represented the dimmest 0.5% to 1.0% of the Hoechst-stained Lin− UCB cells. Quadrant statistics are provided for the individual experiment depicted. (C-F) The expression of Thy-1 (CDw90; C), HLA-DR (D), CD45RA (E), or CD71 (F) versus that of CD34 within the Lin− SP. In all panels, CD34 is depicted on the x-axis regardless of the fluorochrome used. Quadrant statistics are provided for the individual experiments depicted. Each panel is representative of at least 3 experiments. (G,H) Myelo-erythroid hematopoietic progenitors were quantified from unfractionated UCB, Lin− UCB, the Lin− SP, the CD34+Lin− SP, and the CD34−Lin− SP fractions. These were enumerated in HPCAs (G) and in 5-week LTC assays on MS5 cells (H). These data are the mean ± SD of 5 experiments. All but 2 of the HPCAs detected from CD34−Lin− SP cells were derived from cells isolated from a single sort.
To determine whether myelo-erythroid progenitors were present in either the CD34+ or the CD34− fractions of the Lin− SP, these populations were isolated and placed into both short-term HPCAs and LTC assays. To ensure the purity of the cell preparations, Lin− SP cells were first isolated on the basis of dim Hoechst staining and then restained and re-sorted on the basis of their expression of CD34. For HPCAs, the Lin− UCB was enriched approximately 80-fold over the unfractionated UCB (Figure4G). The CD34+Lin− SP cells had a clonogenic frequency similar to that of the Lin− UCB cells, whereas the CD34−Lin− SP cells were nearly devoid of progenitors detectable in the HPCA. Similar to the HPCA results, the Lin− UCB and the CD34+Lin− SP cells were both highly enriched for LTC activity, whereas no colonies were generated from the CD34−Lin− SP (Figure4H).
The CD34−Lin−SP contains NK-cell progenitors
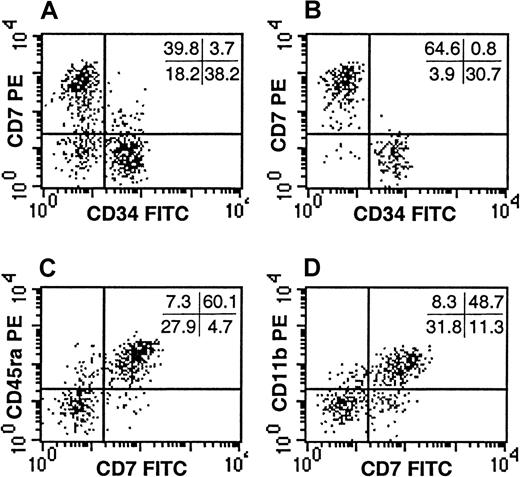
Despite the observation that the CD34−Lin− SP failed to generate progeny in standard assays of myelo-erythroid progenitors, it remained possible that this population contained additional progenitors not detectable by these assays. Because the majority of the CD34−Lin− SP cells expressed CD45RA (Figure4E), an isoform of CD45 common to lymphocytes, we evaluated whether the CD34−Lin− SP contained lymphoid progenitors. In addition to CD45RA, the CD34−Lin− SP expressed high levels of CD7 and CD11b (Figure5), cell-surface markers expressed on various lymphoid progenitors.25-27 The CD34−Lin− SP cells did not express CD10 or CD19 (data not shown), indicating these cells were not obvious early B-lymphocytes. In addition, the CD34−Lin− SP cells did not express the T-lymphocyte–associated antigens CD1a, CD3, CD4, or CD8 or the mature NK-cell markers CD56 or CD16 (data not shown). They also did not express CD2 or CD5 (data not shown), antigens found on very early progenitors of both the T- and the NK-cell lineages.
CD7 expression by the CD34−Lin− SP cells.
The expression of CD34 and CD7 are depicted for Lin− UCB (A) and compared with that of the Lin− SP (B). Co-expression of CD7 with CD45RA (C) and CD11b (D) is demonstrated within the Lin− SP. The SP represented the dimmest 0.5% to 1.0% of the Hoechst-stained Lin− UCB cells. Quadrant statistics are provided for the individual experiments depicted. Each panel is representative of at least 3 experiments.
CD7 expression by the CD34−Lin− SP cells.
The expression of CD34 and CD7 are depicted for Lin− UCB (A) and compared with that of the Lin− SP (B). Co-expression of CD7 with CD45RA (C) and CD11b (D) is demonstrated within the Lin− SP. The SP represented the dimmest 0.5% to 1.0% of the Hoechst-stained Lin− UCB cells. Quadrant statistics are provided for the individual experiments depicted. Each panel is representative of at least 3 experiments.
Because the CD7+CD34−Lin− SP cells expressed markers found on lymphoid cells but did not obviously fit into current lymphoid developmental schemas, we tested whether these cells could generate lymphoid progeny in vitro. In preliminary studies, CD34−Lin− SP cells were placed in suspension cultures that support the growth of relatively mature lymphoid progenitors.28 Whereas Lin− UCB and CD34+Lin− SP cells grew under some of these conditions, none of the conditions supported the growth of CD34−Lin− SP cells (data not shown). This observation, coupled with the absence of markers found on mature lymphoid cells, suggested that the CD7+CD34−Lin− SP cells were not mature lymphoid cells.
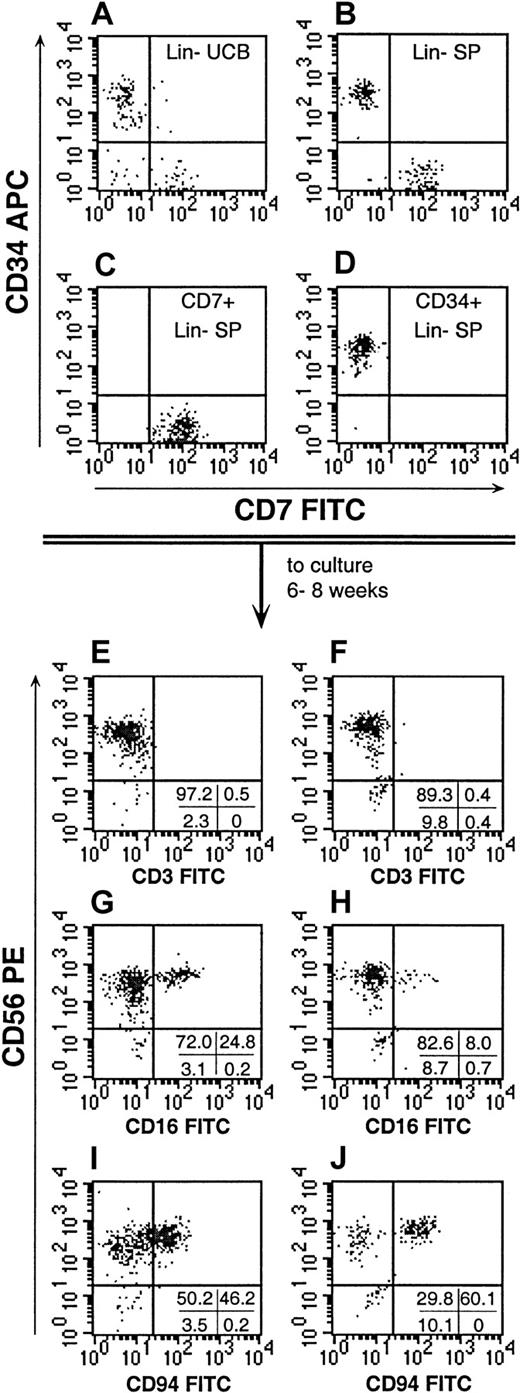
To determine whether the CD7+CD34−Lin− SP contained more primitive lymphoid progenitors, these cells were cultured on stroma using modifications of previously described in vitro culture systems that support the growth of primitive NK-cell progenitors (Table 1, conditions A-D).29-31 All of these conditions except condition B contained IL-15, a cytokine known to enhance the maturation of NK cells. As previously described, the stromal cells or condition B may provide factors that replace the effects of IL-15 on NK-cell development.29-31 In our studies, Lin− SP cells were isolated and then re-sorted into CD7+ and CD34+ subfractions. CD34+ cells, as well as cells expressing CD2, CD3, CD5, and CD56, were stringently excluded from the CD7+CD34−Lin− SP subfraction to ensure that CD34+ HSCs and committed lymphoid progenitors would not contribute to the culture results (Figure 6). After 6 to 8 weeks of growth, the stroma-based cultures were assessed for total cell expansion and for the proportion of cells expressing CD56+ and other NK-cell markers (Figure 6; Table 4). In all conditions tested, both the CD34+Lin− SP and the CD7+CD34−Lin− SP fractions proliferated. However, both purified cell fractions exhibited wide ranges in their expansion under each of the culture conditions tested (Table 4). Under culture condition C, the CD7+CD34−Lin− SP cells expanded approximately 3-fold more than the CD34+Lin−SP fraction. Under all of the conditions tested, the CD7+Lin− SP consistently gave rise to higher proportions of CD56+ progeny than were derived from the CD34+Lin− SP cells, and under condition C, the increased proportion of CD56+ cells derived from the CD7+Lin− SP was statistically significant (P < .05, Table 4). This finding argues that the generation of CD56+ cells from the CD7+Lin− SP was not due to contamination with CD34+Lin− SP cells.
Characterization of NK cells derived from Lin− SP cells.
Lin− SP cells were isolated and re-sorted on the basis of their expression CD7 or CD34 (B). The Lin− SP resolved 2 distinct subpopulations when compared with Lin− UCB (A). The reanalysis of sorted CD7+ and CD34+ SP cells (C,D, respectively) consistently demonstrated the purity of the fractions. The CD7+CD34−Lin− SP cells and CD34+Lin− SP cells were then grown in the different stroma-based cultures described in Table 1. The immunophenotype of the progeny of CD7+CD34−Lin− SP cells (E, G, I) and CD34+Lin− SP cells (F, H, J) grown under culture condition D (Table 1) are shown. For each culture, one quarter of the human CD45+ progeny were used to confirm the presence and maturity of CD56+ cells by their immunophenotype. Quadrant statistics are provided for the individual experiments depicted. These data are representative of 4 cultures derived from CD7+Lin− SP cells and 2 cultures of CD34+Lin− SP cells. An additional 2 cultures of CD34+Lin− SP expanded but did not yield CD56+ cells after 8 weeks in culture.
Characterization of NK cells derived from Lin− SP cells.
Lin− SP cells were isolated and re-sorted on the basis of their expression CD7 or CD34 (B). The Lin− SP resolved 2 distinct subpopulations when compared with Lin− UCB (A). The reanalysis of sorted CD7+ and CD34+ SP cells (C,D, respectively) consistently demonstrated the purity of the fractions. The CD7+CD34−Lin− SP cells and CD34+Lin− SP cells were then grown in the different stroma-based cultures described in Table 1. The immunophenotype of the progeny of CD7+CD34−Lin− SP cells (E, G, I) and CD34+Lin− SP cells (F, H, J) grown under culture condition D (Table 1) are shown. For each culture, one quarter of the human CD45+ progeny were used to confirm the presence and maturity of CD56+ cells by their immunophenotype. Quadrant statistics are provided for the individual experiments depicted. These data are representative of 4 cultures derived from CD7+Lin− SP cells and 2 cultures of CD34+Lin− SP cells. An additional 2 cultures of CD34+Lin− SP expanded but did not yield CD56+ cells after 8 weeks in culture.
NK-cell development from Lin−SP cells
| Condition . | CD7+Lin−SP . | CD34+Lin− SP . | ||||
|---|---|---|---|---|---|---|
| Fold-expansion (range) . | % CD56+ (range) . | % CD56+ CD94+ (range) . | Fold-expansion (range) . | % CD56+ (range) . | % CD56+ CD94+ (range) . | |
| A | 4.3 ± 5.8 | 74.9 ± 19 | 25.1 ± 6.5 | 14.8 ± 17 | 68.4 ± 12 | 45.6 ± 8.3 |
| (0.1-13) | (53-87)4-150 | (18-29)4-150 | (0.1-35) | (55-78)4-150 | (40-55)4-150 | |
| B | 7.6 ± 11 | 55.9 ± 34 | 4-151 | 47.3 ± 67 | 5.1 ± 7.9 | 4-151 |
| (1.6-25) | (8.2-86) | (3.5-145) | (0.3-17) | |||
| C | 9.3 ± 7.0 | 91.0 ± 10‡ | 5.2 ± 6.7 | 2.8 ± 0.9 | 9.5 ± 13‡ | 4.2 ± 7.7 |
| (1.6-17) | (76-99) | (0-15) | (1.5-3.4) | (1.4-29) | (0-16) | |
| D | 20 ± 28 | 85.8 ± 16‡ | 28.1 ± 23 | 35.9 ± 74 | 34 ± 41‡ | 14 ± 25 |
| (4.4-68)4-153 | (62-96.8) | (4-41) | (1.4-168)4-153 | (0.9-86)4-155 | (0.3-52) | |
| Condition . | CD7+Lin−SP . | CD34+Lin− SP . | ||||
|---|---|---|---|---|---|---|
| Fold-expansion (range) . | % CD56+ (range) . | % CD56+ CD94+ (range) . | Fold-expansion (range) . | % CD56+ (range) . | % CD56+ CD94+ (range) . | |
| A | 4.3 ± 5.8 | 74.9 ± 19 | 25.1 ± 6.5 | 14.8 ± 17 | 68.4 ± 12 | 45.6 ± 8.3 |
| (0.1-13) | (53-87)4-150 | (18-29)4-150 | (0.1-35) | (55-78)4-150 | (40-55)4-150 | |
| B | 7.6 ± 11 | 55.9 ± 34 | 4-151 | 47.3 ± 67 | 5.1 ± 7.9 | 4-151 |
| (1.6-25) | (8.2-86) | (3.5-145) | (0.3-17) | |||
| C | 9.3 ± 7.0 | 91.0 ± 10‡ | 5.2 ± 6.7 | 2.8 ± 0.9 | 9.5 ± 13‡ | 4.2 ± 7.7 |
| (1.6-17) | (76-99) | (0-15) | (1.5-3.4) | (1.4-29) | (0-16) | |
| D | 20 ± 28 | 85.8 ± 16‡ | 28.1 ± 23 | 35.9 ± 74 | 34 ± 41‡ | 14 ± 25 |
| (4.4-68)4-153 | (62-96.8) | (4-41) | (1.4-168)4-153 | (0.9-86)4-155 | (0.3-52) | |
One hundred to 2000 CD7+Lin− SP cells or CD34+Lin− SP cells were cultured on MS5 stroma under the culture conditions defined in Table 1. The expansions and percentages of CD56+ or CD56+ CD94+progeny of CD7+Lin− SP or CD34+Lin− SP cells were derived from the total CD45+ cells present in the immunophenotyping studies. The data are the mean and SD of 4 (conditions A, B, and C) or 6 (condition D) separate experiments.
SP indicates side population.
One of 4 cultures failed to generate CD56+ progeny.
Too few CD56+ cells were elicited for an accurate determination.
P ≤ .05 for differences between the values for CD7+Lin− SP and CD34+Lin− SP.
Data are derived from 4 of 6 cultures with expansion.
Two of 4 cultures with expansion failed to generate CD56+ progeny.
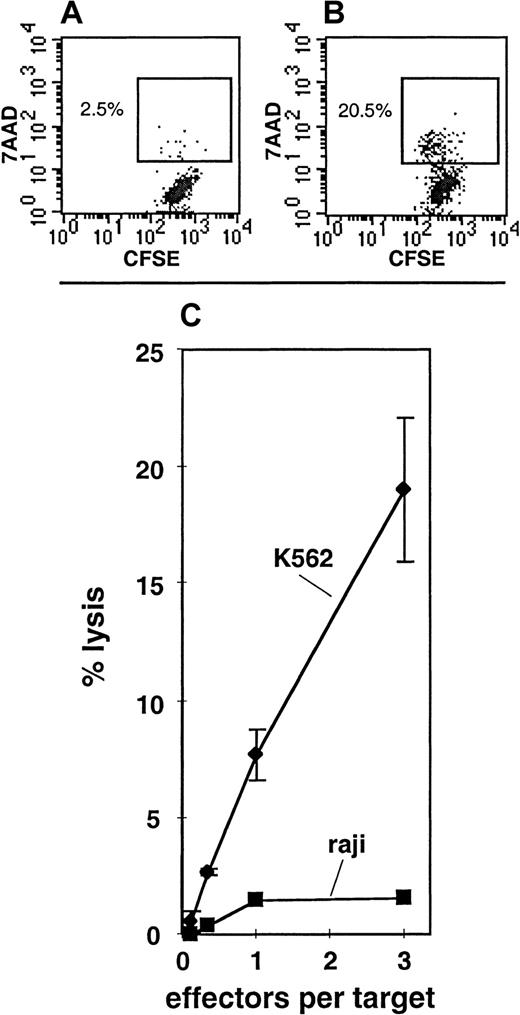
Under culture conditions A, B, or C, either the total cell expansion or the degree of maturation of the progeny was insufficient to perform NK-cell functional assays. In order to more effectively generate mature NK cells, a fourth culture condition, condition D, was designed. Condition D included a 2-week “initiation” phase in the presence of KL, F3L, IL-2, IL-7, and IL-15. This was followed by a 4-week “expansion” phase in which the media were supplemented with only KL and IL-15, and finally by a 2-week “maturation” phase in which the media contained KL, IL-2, and IL-15. In 4 of 6 experiments, the CD34+Lin− SP cells expanded under these conditions (Table 4), and in 2 of these 4 experiments, CD56+ progeny were detected (Table 4; Figure 6). The CD7+CD34−Lin− SP cells expanded in 4 of 6 experiments under condition D, and CD56+ progeny were detected in all of these cultures (Table 4; Figure 6). In condition D, 28.1% ± 6.5% of the total cells generated from the CD7+CD34−Lin− SP fraction had the CD56+CD3−CD94+ phenotype, indicating that these were mature NK cells.29-31 The remainder of the cells had phenotypes more consistent with those of immature NK cells or non-NK cells (Figure 6). To confirm that the cells generated from the CD7+CD34−Lin−SP under condition D were NK cells, the cultured cells were tested for their ability to lyse NK-sensitive target cells by means of a newly developed FACS assay for NK-cell activity (Figure7). In 2 of 3 cultures assayed, lysis of the NK-sensitive cell line K562 was readily observed, whereas the NK-insensitive cell line Raji was not lysed (Figure 7C). K562 lysis was observed even at relatively low effector-to-target ratios, suggesting that a large proportion of the cultured cells possessed NK functional activity.
NK cytotoxic activity in the progeny of CD7+CD34−Lin− SP cells.
Panels A and B depict 7AAD uptake by CFSE-labeled K562 cells when cultured 4 hours without effectors (A) as compared with those cultured 4 hours with putative NK cells generated from CD7+CD34−Lin− SP under condition D (B). Panel C depicts the percentage of cell lysis for K562 or Raji target cells at various effector-to-target ratios. The effector cells were putative NK cells derived from CD7+CD34−Lin− SP cells grown under condition D (Table 1). These data are representative of 2 cultures (of 3 tested) where lysis of K562 target cells was clear.
NK cytotoxic activity in the progeny of CD7+CD34−Lin− SP cells.
Panels A and B depict 7AAD uptake by CFSE-labeled K562 cells when cultured 4 hours without effectors (A) as compared with those cultured 4 hours with putative NK cells generated from CD7+CD34−Lin− SP under condition D (B). Panel C depicts the percentage of cell lysis for K562 or Raji target cells at various effector-to-target ratios. The effector cells were putative NK cells derived from CD7+CD34−Lin− SP cells grown under condition D (Table 1). These data are representative of 2 cultures (of 3 tested) where lysis of K562 target cells was clear.
Discussion
In the current study, we found that human UCB SP cells were largely CD34−, similar to SP cells derived from murine, rhesus, and human bone marrow. In contrast to the rhesus bone marrow SP, up to 50% of the CD34− UCB SP expressed markers found on mature NK cells and T cells. This finding was not surprising since some human T and NK cells can express high levels of MDR activity that could result in the efflux of the Hoechst dye.32 The differences in the content of the human UCB SP and the rhesus bone marrow SP could be due to technical differences in the Hoechst staining and analysis procedures, differences between bone marrow and UCB, or differences between species. The depletion of cells expressing lineage-commitment markers eliminated the contaminating mature lymphocytes from the UCB SP. The resulting Lin− UCB SP was composed of distinct CD34+ and CD34−populations. The phenotype and in vitro behavior of the CD34+Lin− SP cells was consistent with that of primitive multipotent human hematopoietic progenitors previously described by a number of groups.4-6,23 33 The CD34+Lin− SP cells were small blast cells with high levels of CD34 expression, dim to undetectable levels of CD38 and Thy-1 expression, intermediate levels of HLA-DR expression, and undetectable levels of CD33, CD45RA, and CD71 expression. These cells were enriched for myelo-erythroid progenitors in both short-term and 5-week long-term colony-forming unit assays and gave rise to NK cells on stroma supplemented with various cytokines. While the CD34+Lin− SP cells were not more enriched in short-term or 5-week long-term colony-forming units than Lin− UCB (Figure 4), the culture conditions used in these studies were not optimized for primitive progenitors. Future studies using more extended LTCs or other conditions designed to support the growth of very primitive hematopoietic progenitors will be useful for determining whether the SP fractionation procedure segregates primitive CD34+ progenitors from more mature progenitors within the Lin− fraction.
In contrast to the CD34+Lin− SP cells, the CD34−Lin− SP cells failed to grow in standard short-term or long-term myelo-erythroid colony-forming unit assays. The majority of the CD34−Lin− SP cells coexpressed CD7, CD45RA, and CD11b, yet failed to express CD38 or a variety of lineage-commitment markers, including CD1a, CD2, CD3, CD4, CD5, CD8, CD10, CD16, CD19, CD33, CD56, CD71, HLA-DR, and Thy-1. This suggested that the CD34−Lin− SP cells may have been an early lymphoid progenitor population. In support of this, the CD7+CD34−Lin− SP fraction readily expanded into NK cells on stroma supplemented with cytokines, indicating that the CD7+CD34−Lin−SP contained primitive NK progenitors. A similar CD7+CD34−Lin− population has not been previously described, and this cell population cannot be easily placed into current models of lymphocyte ontogeny. NK progenitors isolated from fetal liver are CD7brightCD34−but are also typically more than 95% CD38+CD56+.25 Of the T-cell and NK-cell progenitors described to date, the CD7+CD34−Lin− SP cell is phenotypically most similar to a putative shared T-cell and NK-cell progenitor that was cloned from fetal liver.28 This cell is CD7brightCD11b+CD1−CD3−CD4−CD8−CD56−; however, unlike the CD7+CD34 Lin− SP cells, the fetal liver cells express CD2 and HLA-DR and respond to IL-2. Similarly, the CD7+Lin− SP cells are not obvious T-cell progenitors. Most primitive prethymic T-cell progenitors are derived from CD34+CD7dim cells detectable in either bone marrow or fetal liver,27,30 and fetal thymic T-cell progenitors generally coexpress CD38, CD2, CD5, CD1a, or CD3 during their development.25 On the basis of these prior observations, it remains unclear whether the CD7+CD34−Lin− SP is a previously unreported population of committed NK-cell progenitors or whether this population represents a novel multipotent cell whose full activity was not detected in the assays used in this report. Detecting myelo-erythroid, B-cell, T-cell, and dendritic-cell growth from the CD7+CD34−Lin− SP may require different assays, different culture conditions, or longer culture durations than those used in this report. Additional studies using both in vitro and in vivo models for T-cell, B-cell and myelo-erythroid growth are ongoing to further delineate the full developmental potential of the CD7+CD34−Lin− SP cells.
In addition to the CD34+Lin− SP and CD7+CD34−Lin− SP fractions, a small population of CD34−CD7−Lin− SP cells were routinely detected (Figure 5B, lower left quadrant). This fraction comprised approximately 5% of the Lin− SP (4.9% ± 3.3%), or approximately 0.00025% of the total UCB. This subfraction of the SP was excluded from our studies directed at lymphoid development and may have been present at too low a frequency to be consistently detectable in the assays for myeloid development. Consequently, it remains possible that this rare cell type has progenitor or HSC activity that was not effectively analyzed in the studies described in this report. Studies are also ongoing to determine whether this subset of Lin−CD34− SP cells may have progenitor or stem cell activity as well.
In summary, our observations provide evidence that the SP region of UCB contains a mixture of early hematopoietic progenitors. The CD34+ compartment of the Lin− SP contains both myeloid and lymphoid progenitors detectable in HPCAs and in LTC and NK-cell–progenitor cell assays. The CD7+CD34−Lin− SP fraction contains a previously undescribed primitive NK-cell progenitor. This observation provides further evidence that primitive CD34−hematopoietic progenitors exist in humans. Since NK cells may generate important antitumor effects following transplantation, the elimination of CD34− cells by means of CD34+ selection procedures may need to be reconsidered.34
Acknowledgments
We thank Drs Michael Cook, Joanne Kurtzberg, Barton Haynes, O. Michael Colvin, and Lola Reid for their helpful discussions and comments, and the staff of the Labor and Delivery ward of Duke University Hospital for their support and commitment.
Supported by grants 5KO8-AI-01121-03 from the National Institutes of Health and 30006-17-GT from the Pediatric AIDS Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Clay Smith, H. Lee Moffitt Cancer Center, University of South Florida, 12902 Magnolia Dr, MRC-3E, Tampa, FL; e-mail: smithca@moffitt.usf.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal