Abstract
In current clinical trials, chimeric antibody-like receptors fused to signaling domains derived from TCR-ζ or Fc(ε)RIγ-chain are tested for their ability to lyse tumor cells in vivo. In this study, the function of primary T cells expressing such receptors has been investigated in transgenic mice. These receptors cannot induce proliferation of resting T cells or trigger the production of optimal amounts of cytokines. It is further demonstrated that an initial low presence of cytokine message and protein is disappearing rather fast, whereas the triggering of endogenous TCR/CD3 in the same cells leads to normal prolonged cytokine production. The direct clinical relevance of these findings is further underlined by the increased in vivo tumor rejection by T cells expressing chimeric receptors in presence of exogenous interleukin-2. Therefore, adoptive T-cell therapy using primary T cells transfected with single chain receptors might benefit substantially from the accompanying administration of cytokines.
Introduction
Chimeric receptors with extracellular antibody-binding domains (Fv) connected to signaling portions of TCR-ζ1,2 or Fc(ε)RIγ-chain2 enable T cells to recognize native antigen (Ag). These artificial receptors induced lysis of Ag-expressing target cells in vitro and in vivo.2-10 The ultimate goal is to transfect primary T cells from cancer patients with tumor antigen-specific Fv-receptors for use in adoptive transfers.10,11 Despite TCR/CD3-like signaling capacities of Fv-ζ receptors in T-cell hybridomas, clones, and activated T cells,1,2 these receptors were not sufficient to activate resting primary T cells.9 These findings suggested a limited signaling potential of Fv-receptors and led us to study Fv-receptor effector functions more in detail. This report describes the inability of Fv-ζ receptors to induce cytokine production in primary T lymphocytes. Coexpression of an additional signaling domain derived from TCR/CD3ε could not overcome this defect. Because of the absence of sufficient interleukin-2 (IL-2) production by Fv-receptor triggered T cells, a supportive effect of exogenous IL-2 on the antitumor effect of these T cells can be demonstrated in a tumor animal model in vivo.
Study design
DNA construct
To construct Fv-ε, we performed linking of polymerase chain reaction (PCR) fragments as described previously for Fv-ζ.9 The transgenic Fv-ε construct is identical to the previously described Fv-ζ plasmid,9 where position 114 to 168 from CD3e12 replaces the cytoplasmic tail of ζ. The sequences of the oligonucleotide primers were as follows: oligo1 TCCTGTCGACAGTGGAATTCACTACTACCAAGCCAGTGCTGC oligo2 GCCTTGGCCTTCCTATTCTTCAGGTACAGGGCTGTGATGA oligo3 TCATCACAGCCCTGTACCTGAAGAATAGGAAGGCCAAGGC oligo4 AGTCAGGGCCCAGATCTCCAGCCAATACACTTTATTG(underlinedsequences = CD3ε).
DNA injection into fertilized C57BL/6 oocytes was performed as previously described.9
Flow cytometry
Preparation and staining of cells was performed as described before1 with mAbs purchased from PharMingen (San Diego, CA). Cells were analyzed using a FACScan (Becton Dickinson, Mountain View, CA).
Stimulation of T cells
T-cell stimulation was quantified by [3H]-thymidine incorporation as previously described9 on a Betaplate system (1205, Wallacoy, Turku, Finland). For prestimulation, T cells were incubated with 5 μg/mL mAb 2C11 plus anti-CD28 coated to plastic.
Metabolic labeling of lymphokines
The 2 × 106 prestimulated T cells/mL were triggered for 4 hours in presence of 3.7 MBq/mL (100 μCi/mL) [35S]-methionine (Amersham International, Little Chalfont, UK). Immunoprecipitations with IL-specific mAbs (PharMingen, San Diego, CA) were performed as described before.1
Messenger RNA extraction, complementary DNA synthesis, and specific polymerase chain reaction amplification
Reverse transcriptase-polymerase chain reaction (RT-PCR) was performed using a RT-PCR kit (GibcoBRL, Karlsruhe, Germany) according to manufacturer's instructions. For PCR amplification, we used the following oligonucleotides: HPRT: 5′GCTGGTGAAAAGGACCTCT3′, 5′CACAGGACTAGAACACCTGC3′; IL-2: 5′-GCAGGATGGAGAATTACAGG-3′, 5′-AGCGCTTACTTTGTGCTGTC-3′.
Tumor animal model
The tumor used in this study was EL4, a C57BL/6 derived lymphoma, transfected with human CD3ε-cDNA as described previously (huCD3-EL49). Human CD3ε is the native antigen, recognized by the chimeric Fv-receptor.9 The 104 huCD3-EL4 intraperitoneally (ip) corresponded to the 100-fold lethal dosis. Effector T cells were preactivated as described above and expanded for 4 days in the presence of low doses of IL-2 (100 IU/mL) before injection.
Results and discussion
Mice transgenic for Fv-ζ9 and Fv-ε receptor constructs showed Fv-expression on all CD4+ and CD8+ T cells (Figure 1A). Western blot analysis indicated that T cells from Fv-εζ double transgenic animals expressed both receptors (data not shown). To test the proliferative capacities of Fv-εζ double transgenic primary T cells, they were activated with plastic-coated mAb. T cells did proliferate, when stimulated by anti-CD3 mAb, but not by anti-Fv mAb (Figure 1B). A similar unresponsiveness has been observed for T cells expressing only Fv-ε (data not shown) or Fv-ζ.9Equally, costimuli via anti-CD28 mAb, anti-CD4 mAb, or major histocompatibility complex (MHC) classII+antigen-presenting cells did not help to induce activation of resting T cells via the chimeric Fv-receptors (data not shown).
Fv-receptor expression and triggering.
(A) Expression of Fv-receptor transgenes leads to surface expression of chimeric Fv-ζ and Fv-ε receptors. Lymph node cell suspensions of nontransgenic littermates (wt), Fv-ζ– (Fv-ζ), and Fv-ε– (Fv-ε) transgenic mice were analyzed by 3-color flow cytometry using CD4-PE (not shown), CD8-FITC (not shown), and anti-Fv-biotin plus Streptavidin-Red 613. Histograms show CD4+ cells (thin line) and CD8+ cells (bold line). (B, upper panel) Triggering of Fv-receptors is not sufficient to induce proliferation in resting T lymphocytes. T cells of lymph nodes from nontransgenic littermates (■, ▪), or Fv-εζ (○, ●) double transgenic mice were incubated for 20 hours at 2 × 104 cells per well. [3H]-thymidine was then added and the radioactivity incorporation measured after another 12 hours. Cultures were performed in 96-well plates previously coated with mAb2C11 (■, ○), specific for TCR/CD3 or anti-Fv–specific mAb (▪, ●) at the indicated concentrations. (B, lower panel) Preactivated T cells from Fv-ζ–transgenic mice were stimulated with TCR- (■, ▪) or Fv-specific mAb (○, ●). Before addition of [3H]-thymidine for measurements of the proliferative response (■, ○), culture supernatant was removed from each well and tested for its content of IL-2. Results for proliferation (left y-axis, ■, ○) and cytokine measurements (right y-axis, ▪, ●) were overlayed within the same graph. (C) Analysis of cytokine mRNA in differentially stimulated transgenic T cells. Preactivated Fv-ζ+ T cells were restimulated in presence of plastic-coated mAb specific for TCR (TCR), chimeric receptor (Fv), or medium alone (no Ab) for 4 or 24 hours. Then cDNA was analyzed by RT-PCR, equal amounts of 10-, 100-, or 1000-fold dilutions (10 ×, 100 ×, and 1000 ×) of cDNA were amplified with oligonucleotides specific for IL-2 or HPRT (H). (D) Differential production and secretion of IL-2 in Fv-ζ–expressing T cells. 2 × 106preactivated T cells were cultured with optimal concentrations of anti-Fv (Fv; 8 μg/mL, lanes 2, 5), anti-TCR/CD3 (TCR; 5 μg/mL, lanes 3, 6) mAb, or no mAb (lanes 1, 4) in the presence of [35S]-methionine for 4 hours. Culture supernatants (lanes 4, 5, 6) and activated cells (lanes 1, 2, 3) were processed separately. Cell lysates and culture supernatants were submitted to immunoprecipitations with IL-2–specific mAb and analyzed on 11% SDS-PAGE under reducing conditions. The gels were dried and autoradiography on x-ray film revealed the radioactively labeled cytokines.
Fv-receptor expression and triggering.
(A) Expression of Fv-receptor transgenes leads to surface expression of chimeric Fv-ζ and Fv-ε receptors. Lymph node cell suspensions of nontransgenic littermates (wt), Fv-ζ– (Fv-ζ), and Fv-ε– (Fv-ε) transgenic mice were analyzed by 3-color flow cytometry using CD4-PE (not shown), CD8-FITC (not shown), and anti-Fv-biotin plus Streptavidin-Red 613. Histograms show CD4+ cells (thin line) and CD8+ cells (bold line). (B, upper panel) Triggering of Fv-receptors is not sufficient to induce proliferation in resting T lymphocytes. T cells of lymph nodes from nontransgenic littermates (■, ▪), or Fv-εζ (○, ●) double transgenic mice were incubated for 20 hours at 2 × 104 cells per well. [3H]-thymidine was then added and the radioactivity incorporation measured after another 12 hours. Cultures were performed in 96-well plates previously coated with mAb2C11 (■, ○), specific for TCR/CD3 or anti-Fv–specific mAb (▪, ●) at the indicated concentrations. (B, lower panel) Preactivated T cells from Fv-ζ–transgenic mice were stimulated with TCR- (■, ▪) or Fv-specific mAb (○, ●). Before addition of [3H]-thymidine for measurements of the proliferative response (■, ○), culture supernatant was removed from each well and tested for its content of IL-2. Results for proliferation (left y-axis, ■, ○) and cytokine measurements (right y-axis, ▪, ●) were overlayed within the same graph. (C) Analysis of cytokine mRNA in differentially stimulated transgenic T cells. Preactivated Fv-ζ+ T cells were restimulated in presence of plastic-coated mAb specific for TCR (TCR), chimeric receptor (Fv), or medium alone (no Ab) for 4 or 24 hours. Then cDNA was analyzed by RT-PCR, equal amounts of 10-, 100-, or 1000-fold dilutions (10 ×, 100 ×, and 1000 ×) of cDNA were amplified with oligonucleotides specific for IL-2 or HPRT (H). (D) Differential production and secretion of IL-2 in Fv-ζ–expressing T cells. 2 × 106preactivated T cells were cultured with optimal concentrations of anti-Fv (Fv; 8 μg/mL, lanes 2, 5), anti-TCR/CD3 (TCR; 5 μg/mL, lanes 3, 6) mAb, or no mAb (lanes 1, 4) in the presence of [35S]-methionine for 4 hours. Culture supernatants (lanes 4, 5, 6) and activated cells (lanes 1, 2, 3) were processed separately. Cell lysates and culture supernatants were submitted to immunoprecipitations with IL-2–specific mAb and analyzed on 11% SDS-PAGE under reducing conditions. The gels were dried and autoradiography on x-ray film revealed the radioactively labeled cytokines.
In contrast, when TCR/CD3-preactivated primary T cells were restimulated with Fv-specific mAb, T cells showed a proliferative response similar to a TCR-stimulus (Figure 1B, data not shown and Brocker and Karjalainen9). In contrast, only the triggering of TCR lead to production of IL-2 (Figure 1B). Further analysis also showed the absence of IL-3, IL-4, and IL-6 (data not shown).
To investigate IL-2 production on messenger RNA (mRNA)-level, RT-PCR was performed on Fv-ζ+ T cells, which produced approximately 10- to 20-fold more IL-2 (Figure 1C, 4 hours), IL-3, and IL-4 message (data not shown) when triggered via TCR/CD3, compared with Fv-ζ. After a 24-hour stimulus (Figure 1C, 24 hours), the signal for IL-2 mRNA in the TCR/CD3-stimulated cells decreased approximately 10-fold. In contrast, the Fv-ζ–triggered T cells had completely lost their IL-2 (Figure 1C, 24 hours) and IL-3 signals (data not shown).
This lack of efficient cytokine production was further confirmed by biochemical lymphokine analysis (Figure 1D). Immunoprecipitation with IL-2–specific mAb and subsequent SDS-PAGE resulted in a prominent band of approximately 25 kd, corresponding to IL-2 (Figure 1D). The same protein is precipitated from the lysate of the cells, indicating that IL-2 is produced by TCR/CD3-triggered Fv-ζ+ T cells (Figure 1D). In contrast, when the same cells were triggered via their Fv-ζ receptor, only a small amount of IL-2 was present in the cell lysate or secreted into the supernatant (Figure 1D). Similarly, the T-cell lymphokines IL-3 (data not shown) and IL-4 (data not shown) remained completely undetectable in the same analysis after Fv-ζ triggering (data not shown). In contrast, IFN-γ was detectable in 2- to 3-fold lower amounts, compared with a stimulus via TCR/CD3 (data not shown). Taken together, the biochemical analysis confirmed the RT-PCR and bioassay data, showing that a stimulus via Fv-receptors does result in minimal cytokine response.
We then tested whether the lack of autocrine cytokine supply might have an effect on the use of these modified T cells in a syngeneic mouse tumor model.
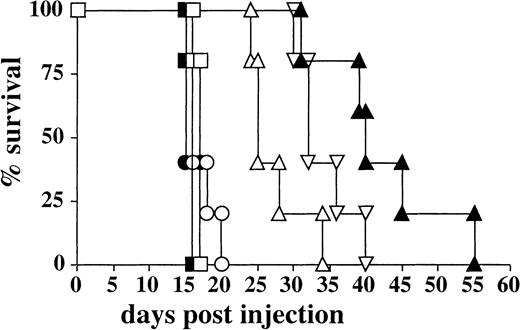
Activated T cells from Fv-ζ–transgenic mice were injected ip into recipient mice. These also received a tumor, previously transfected with cDNA encoding the surface-Ag (human CD3ε), which is recognized in its native form by the Fv-receptor.1,9 13 The administration of Fv-ζ+ T cells delayed the onset of tumor growth significantly (Figure 2). This effect was not seen when nontransfected EL4 cells (data not shown) or Fv-ζ− T cells were used (Figure 2). The administration of recombinant IL-2, together with the Fv-ζ+ T cells, delayed tumor growth further (Figure 2). This effect was specific, because IL-2 had no significant effect on mouse survival, when given either with tumor alone or together with nontransgenic Fv-ζ− T cells (Figure 2). When Fv-ζ+ T cells were administered twice (day 0 and day 7), this treatment also augmented the survival time in the absence of IL-2 treatment, albeit less strong as a single T-cell treatment accompanied by IL-2 (Figure 2).
IL-2 enhances the antitumor activity of Fv-ζ+ T cells in vivo.
Five C57BL/6 mice per group were injected ip with 104huCD3-EL4 lymphoma cells. The recipient mice received either tumor cells alone (■) or tumor and IL-2 (▪), 107Fv-ζ–negative T cells plus IL-2 (○), 107Fv-ζ–negative T cells repeatedly (●),107Fv-ζ+ T cells (▵), 107 Fv-ζ+T cells plus IL-2 (▴), or 107 Fv-ζ+ T cells repeatedly (▿). When exogenous IL-2 was given, each animal was injected with 80 000 U/d during the first week of the experiment. When repeated injections were performed, 107 T cells were injected a second time at day 6.
IL-2 enhances the antitumor activity of Fv-ζ+ T cells in vivo.
Five C57BL/6 mice per group were injected ip with 104huCD3-EL4 lymphoma cells. The recipient mice received either tumor cells alone (■) or tumor and IL-2 (▪), 107Fv-ζ–negative T cells plus IL-2 (○), 107Fv-ζ–negative T cells repeatedly (●),107Fv-ζ+ T cells (▵), 107 Fv-ζ+T cells plus IL-2 (▴), or 107 Fv-ζ+ T cells repeatedly (▿). When exogenous IL-2 was given, each animal was injected with 80 000 U/d during the first week of the experiment. When repeated injections were performed, 107 T cells were injected a second time at day 6.
The above findings suggest that Fv-receptors with signal transducing domains of TCR-ζ or CD3ε are not able to replace the native endogenous TCR/CD3 complex completely. The ζ-chain might be wired differentially to the signal-transducing machinery or has only a partial task within the TCR/CD3-complex. Other components such as CD3γ, δ, and ε might be necessary for efficient interleukin production. Eventually, only the whole TCR/CD3 complex with its defined sterical arrangement, but not isolated single chains taken from the whole, are able to transduce the full signal. As suggested recently,14 modifications in the design of chimeric receptors might be necessary to augment their chances for a successful use in clinical therapy. These findings have implications for the use of T cells expressing Fv-receptors in current clinical trials.10 11 A life- and function-supporting effect by sufficient autocrine IL-2 production can, according to the above results, most likely not be expected. Thus, an adoptive therapy with Fv-receptor+ T cells might need to be supported by exogenous IL-2 as shown in this report.
Acknowledgments
I thank M. Riedinger for expert technical assistance and Drs T. Blankenstein and A. Hombach for carefully reading the manuscript.
The Basel Institute for Immunology was founded and is supported by Hoffmann-LaRoche, Ltd., Basel. T.B. was supported by the Deutsche Forschungsgemeinschaft (Leibniz-Program to M. Reth and a Heisenberg-Stipend to T.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Institute for Immunology, LMU Munich, Goethestr. 31, D-80336 Munich, Germany; e-mail: tbrocker@gmx.de.

![Fig. 1. Fv-receptor expression and triggering. / (A) Expression of Fv-receptor transgenes leads to surface expression of chimeric Fv-ζ and Fv-ε receptors. Lymph node cell suspensions of nontransgenic littermates (wt), Fv-ζ– (Fv-ζ), and Fv-ε– (Fv-ε) transgenic mice were analyzed by 3-color flow cytometry using CD4-PE (not shown), CD8-FITC (not shown), and anti-Fv-biotin plus Streptavidin-Red 613. Histograms show CD4+ cells (thin line) and CD8+ cells (bold line). (B, upper panel) Triggering of Fv-receptors is not sufficient to induce proliferation in resting T lymphocytes. T cells of lymph nodes from nontransgenic littermates (■, ▪), or Fv-εζ (○, ●) double transgenic mice were incubated for 20 hours at 2 × 104 cells per well. [3H]-thymidine was then added and the radioactivity incorporation measured after another 12 hours. Cultures were performed in 96-well plates previously coated with mAb2C11 (■, ○), specific for TCR/CD3 or anti-Fv–specific mAb (▪, ●) at the indicated concentrations. (B, lower panel) Preactivated T cells from Fv-ζ–transgenic mice were stimulated with TCR- (■, ▪) or Fv-specific mAb (○, ●). Before addition of [3H]-thymidine for measurements of the proliferative response (■, ○), culture supernatant was removed from each well and tested for its content of IL-2. Results for proliferation (left y-axis, ■, ○) and cytokine measurements (right y-axis, ▪, ●) were overlayed within the same graph. (C) Analysis of cytokine mRNA in differentially stimulated transgenic T cells. Preactivated Fv-ζ+ T cells were restimulated in presence of plastic-coated mAb specific for TCR (TCR), chimeric receptor (Fv), or medium alone (no Ab) for 4 or 24 hours. Then cDNA was analyzed by RT-PCR, equal amounts of 10-, 100-, or 1000-fold dilutions (10 ×, 100 ×, and 1000 ×) of cDNA were amplified with oligonucleotides specific for IL-2 or HPRT (H). (D) Differential production and secretion of IL-2 in Fv-ζ–expressing T cells. 2 × 106preactivated T cells were cultured with optimal concentrations of anti-Fv (Fv; 8 μg/mL, lanes 2, 5), anti-TCR/CD3 (TCR; 5 μg/mL, lanes 3, 6) mAb, or no mAb (lanes 1, 4) in the presence of [35S]-methionine for 4 hours. Culture supernatants (lanes 4, 5, 6) and activated cells (lanes 1, 2, 3) were processed separately. Cell lysates and culture supernatants were submitted to immunoprecipitations with IL-2–specific mAb and analyzed on 11% SDS-PAGE under reducing conditions. The gels were dried and autoradiography on x-ray film revealed the radioactively labeled cytokines.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/5/10.1182_blood.v96.5.1999/5/m_h81700075001.jpeg?Expires=1767739280&Signature=HJ7UVbv4KJdygxuABKn600Cj8qBEZgqh6Xv3AAxsYA5nkpzNEfS5PAadAugmASV8PnPa9W8pX6VHL~gAvOo8QN-jklLzRr7L~2UCrhJ73~ds8VWMky0HMTLxmxaX5a6XvylVuNPQ7vk0sajDwlq49RkUWncSzsEFExxxsARm6wFAALJL4b8OPsB6w7NB1uHTjCCDrGaWArQgEsOEdsW7zlZ7wB-FGpV8NrIfvrq1FPPsLmGQdrjx3lZx3NS1ErjDyVy~BxEiJ1N8Siy5wNokP6WyAYqUCCfMqYoiENbUGxaQJ2~aw67plXlIbmhPsPX5XQyTmUEnxV00PjcHQPjIiw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal