Abstract
The role of glutathione peroxidase in red cell anti-oxidant defense was examined using erythrocytes from mice with a genetically engineered disruption of the glutathione peroxidase-1 (GSHPx-1) gene. Because GSHPx-1 is the sole glutathione peroxidase in the erythrocyte, all red cell GSH peroxidase activity was eliminated. Oxidation of hemoglobin and membrane lipids, using the cis-parinaric acid assay, was determined during oxidant challenge from cumene hydroperoxide and H2O2. No difference was detected between wild-type red cells and GSHPx-1–deficient cells, even at high H2O2 exposures. Thus, GSHPx-1 appears to play little or no role in the defense of the erythrocyte against exposure to peroxide. Simultaneous exposure to an H2O2 flux and the catalase inhibitor 3-amino-1,2,4-triazole supported this conclusion. Hemoglobin oxidation occurred only when catalase was depleted. Circulating erythrocytes from the GSHPx-1–deficient mice exhibited a slight reduction in membrane thiols, indicating that high exposure to peroxides might occur naturally in the circulation.
Introduction
The role of glutathione peroxidase (GSHPx) in cellular defense against oxidant attack has been discussed for many years.1-13 The red cell has been a central focus of this research,1,2,4-6,8,9,11,12,14 because it is thought to undergo a high endogenous rate of H2O2production from hemoglobin autoxidation,15-21 which can be markedly increased in cells with unstable hemoglobins.22-24 In addition, the red cell is probably exposed to H2O2 from stimulated macrophages and, under certain circumstances, from pathogenic bacteria or malarial parasites.6,25 It is believed that the 2 enzymes, catalase and glutathione peroxidase, protect the erythrocyte against peroxides that are generated intracellularly or exogenously. The relative importance of catalase and GSHPx in defending the cell has been debated since the discovery of glutathione peroxidase.1 Arguments have been presented for a predominant role for GSHPx1,2,18or for catalase4,8,9,11,12 or for both playing a role in detoxifying peroxide.3,5,6 The existence of naturally occurring acatalasemia in humans and mice has aided in resolving this question, allowing the observation that acatalasemic red cells have enhanced sensitivity to exogenous peroxides.25-27 However, a definitive solution to the question has been hindered by the absence of an effective inhibitor of GSHPx, so that the effect of eliminating GSHPx activity cannot be determined. Hochstein et al2,18attempted to circumvent this problem by eliminating GSH, the obligatory substrate of GSHPx, from the red cell. However, this approach suffers from relative nonspecificity because GSH may have additional roles within the red cell.28
The production of a mouse in which the GSHPx-1 gene has been disrupted29 allows a definitive answer to the question of GSHP function within the red cell. There are 4 distinct GSHPx enzymes,13 but GSHPx-1 appears to be the only one to occur in the erythrocyte. Thus, deletion of the gene for GSHPx-1 eliminates all GSHPx activity in the red cell. We here describe the response of GSHPx-1–negative erythrocytes to exogenous peroxides. Our results suggest that GSHPx has a role in anti-oxidant defense, but only in situations of high peroxide flux.
Materials and methods
Erythrocyte assays
For these experiments, blood was obtained from the hearts of GSHPx-1–deficient mice and matched controls after Nembutol (Abbott Laboratories) was administered for anesthesia. White cells were removed by filtration through cellulose,30 and the erythrocytes were washed twice in phosphate-buffered saline (PBS) (145 mmol/L NaCl, 5 mmol/L NaPi, 1 mmol/L EDTA, pH 7.4). GSH and membrane thiols were determined as described earlier.31 For glutathione peroxidase activity, cellulose-filtered cells were washed once in PBS. Packed cells were diluted 1:20 in 0.01% β-mercaptoethanol and 1 mmol/L EDTA, pH 7.4, and were flash frozen in dry ice–methanol to lyse the cells. These hemolysates were assayed for glutathione peroxidase as described by Beutler,32 with one modification. To eliminate interference from methemoglobin,33 10 mg NaCN and 30 mg K3Fe(CN)6 was added to 10 mL of the 1 mol/L Tris, 5 mmol/L EDTA, pH 8.0 buffer used in the assay.
Catalase was assayed as described by Aebi.34 To estimate the catalase concentration, it was assumed that the specific activity of mouse catalase is similar to that of human, and a rate constant of 3.4 × 107 (mol/L)−1 s−1 was used. H2O2 was assayed by the method of Green and Hill.35 The concentration of H2O2 in the stock solution was quantitated36 using an A240 value of 43.6 (mol/L)−1 cm−1. Methemoglobin (metHb) was determined by a standard method37 or spectroscopically.15 Unless otherwise noted, reagents were obtained from Sigma.
Knockout mice
The construction of the knockout mice has been described29 and will be briefly outlined. The mouse genomic clone for GSHPx-1 was isolated from a genomic library of the 129/SVJ mouse. A 5.3-kb genomic fragment was selected and subcloned into pBluescript SK for mapping and sequencing. The GSHPx-1 gene has 2 exons, and the second was disrupted by insertion of a Neo cassette. The HSV thymidine kinase gene was inserted downstream of the GSHPx-1 gene to allow positive-negative selection. The vector was transfected into R1 embryonic stem cells. Cells resistant to both ganciclovir and G418 were screened by Southern analysis with a sequence 3′ to the targeted sequence. Chimeric mice were obtained by injecting homologous recombinants of R1 cells into C57BL/6 blastocysts. Homozygous GSHPx-1–deficient mice were obtained by breeding heterozygous GSHPx(±) mice.
Parinaric acid assay for lipid oxidation
The procedures of van den Berg et al38 were followed exactly. The washed erythrocytes were adjusted to exactly 0.10% hematocrit in PBS containing 10 mmol/L glucose. A cuvette with 2 mL of this suspension and a small stir bar were placed in a spectrofluorometer (model 8000; SLM/Aminco Instruments). The excitation wavelength was 320 nm (slit width, 5 nm), and the emission wavelength was 415 nm (slit width, 20 nm). Temperature was 25°C. Readings of fluorescence were begun, and at 30 seconds, 2 μL 1 mmol/Lcis-parinaric acid (Molecular Probes, Eugene, OR) was added to make the final concentration 1 μmol/L. At 150 seconds, appropriate volumes of 1 mmol/L cumene hydroperoxide (Aldrich Chemicals, St Louis, MO) dissolved in ethanol were added. Fluorescence was recorded for 10 minutes. Controls were treated in the same way, except that neat ethanol replaced the cumene hydroperoxide solution. As noted earlier,38 there was a gradual decline in fluorescence even in the control because of the high light emission of the SLM instrument.
Hemoglobin oxidation in response to exogenous H2O2
A continuous flux of H2O2 was generated with glucose oxidase.5,8,9,12,39 40 Washed, cellulose-filtered red cells were resuspended to 20% hematocrit in Krebs-Ringer buffer (143 mmol/L NaCl, 5.7 mmol/L KCl, 1.4 mmol/L MgCl2, 18 mmol/L NaPi, pH 7.4) with 10 mmol/L glucose and 50 μg/mL gentamicin. To begin the exposure to H2O2, glucose oxidase was added. The rate of H2O2 production under these conditions was linear with added glucose oxidase and was equal to 0.123 μmol/L of H2O2 produced per minute for each mU glucose oxidase/mL. Both glucose concentration and pH were monitored, and did not change during the incubation.
Results
Erythrocyte oxidation in response to organic peroxides
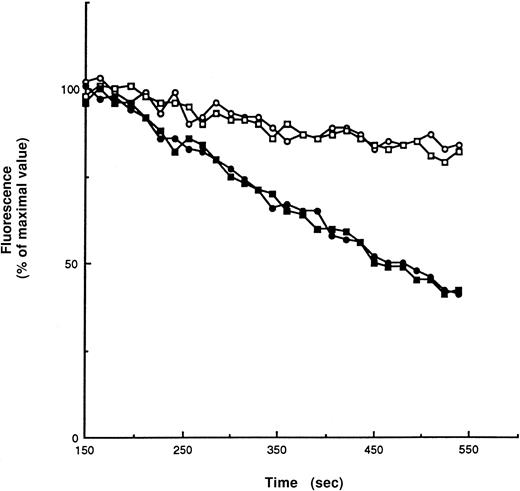
Addition of cumene hydroperoxide to lipid bilayers leads to the oxidation of membrane-lipid components. This can be conveniently assayed by monitoring the disappearance of the fluorescent signal ofcis-parinaric acid intercalcated into the bilayer.41 Van den Berg et al38 have carefully optimized the cis-paranaric acid assay for use with intact red cells. Using their procedures, we detected no difference between GSHPx-1–deficient and wild-type red cells in their responses to cumene hydroperoxide challenge (Figure 1), indicating that GSHPx-1 plays no role in protecting red cell membrane lipids from oxidant attack. In addition, analysis of the hemoglobin in these cells demonstrated that metHb formation in response to cumene hydroperoxide was negligible in both types of erythrocytes.
Fluorescence intensity of 50 μmol/L cis-parinaric acid in a 2 mL suspension of red cells, 10% hematocrit, in PBS with 10 mmol/L glucose and 50 μg/mL gentamycin.
Readings were taken every 15 seconds. After 150 seconds of equilibration, either 50 μL cumene hydroperoxide in ethanol was added (100 μmol/L final concentration) (filled symbols), or 50 μL ethanol (open symbols) was added to controls. There was no difference between the rate of fluorescence loss in wild-type red cells (circles) and GSHPx-1–deficient red cells (squares).Two runs are shown.
Fluorescence intensity of 50 μmol/L cis-parinaric acid in a 2 mL suspension of red cells, 10% hematocrit, in PBS with 10 mmol/L glucose and 50 μg/mL gentamycin.
Readings were taken every 15 seconds. After 150 seconds of equilibration, either 50 μL cumene hydroperoxide in ethanol was added (100 μmol/L final concentration) (filled symbols), or 50 μL ethanol (open symbols) was added to controls. There was no difference between the rate of fluorescence loss in wild-type red cells (circles) and GSHPx-1–deficient red cells (squares).Two runs are shown.
Hemoglobin oxidation in response to H2O2
Single additions of H2O2 to red cell suspensions were rapidly destroyed. This was true for both wild-type and GSHPx-1–deficient erythrocytes. Therefore, a glucose oxidase–glucose system was used to produce a steady flux of H2O2 in the test solution.
H2O2 fluxes were varied over a 50-fold range from 0.12 μmol/L/min to 6.15 μmol/L/min (Table1). Despite the high rates of H2O2 generation, steady state H2O2 levels in the supernatant were undetectably low when red cells were present. In most experiments, the rate of hemoglobin oxidation was negligible, though some variability in oxidation rates was noted. Significantly, however, no difference was detected in the rate of hemoglobin oxidation between wild-type and GSHPx-1–deficient cells in these experiments, indicating that GSHPx plays no role in protecting erythrocyte hemoglobin against oxidation by peroxides.
MetHb formation in erythrocytes exposed to a constant flux of H2O2
| Experiment . | Rate . | % metHb (wild-type cells) . | % metHb (GSHPx-1-deficient cells) . | ||||
|---|---|---|---|---|---|---|---|
| 0 h . | 5 h . | Increase . | 0 h . | 5 h . | Increase . | ||
| 1 | 0.12 | 0.67 | 4.20 | 3.53 | 1.0 | 4.1 | 3.10 |
| 2 | 0.12 | 1.3 | 1.6 | 0.30 | 1.8 | 1.8 | 0.00 |
| 3 | 0.12 | 1.4 | 1.7 | 0.30 | 1.8 | 1.8 | 0.00 |
| 4 | 0.62 | 1.6 | 1.7 | 0.10 | 1.6 | 1.7 | 0.10 |
| 5 | 0.62 | 1.6 | 1.7 | 0.10 | 1.6 | 1.9 | 0.30 |
| 6 | 1.23 | 1.7 | 1.8 | 0.10 | 1.4 | 3.9 | 2.50 |
| 7 | 1.23 | 1.2 | 1.4 | 0.20 | 1.6 | 1.6 | 0.00 |
| 8 | 6.15 | 1.3 | 1.9 | 0.60 | 1.5 | 2.5 | 1.00 |
| Experiment . | Rate . | % metHb (wild-type cells) . | % metHb (GSHPx-1-deficient cells) . | ||||
|---|---|---|---|---|---|---|---|
| 0 h . | 5 h . | Increase . | 0 h . | 5 h . | Increase . | ||
| 1 | 0.12 | 0.67 | 4.20 | 3.53 | 1.0 | 4.1 | 3.10 |
| 2 | 0.12 | 1.3 | 1.6 | 0.30 | 1.8 | 1.8 | 0.00 |
| 3 | 0.12 | 1.4 | 1.7 | 0.30 | 1.8 | 1.8 | 0.00 |
| 4 | 0.62 | 1.6 | 1.7 | 0.10 | 1.6 | 1.7 | 0.10 |
| 5 | 0.62 | 1.6 | 1.7 | 0.10 | 1.6 | 1.9 | 0.30 |
| 6 | 1.23 | 1.7 | 1.8 | 0.10 | 1.4 | 3.9 | 2.50 |
| 7 | 1.23 | 1.2 | 1.4 | 0.20 | 1.6 | 1.6 | 0.00 |
| 8 | 6.15 | 1.3 | 1.9 | 0.60 | 1.5 | 2.5 | 1.00 |
Red cells were exposed to a continuous flux of H2O2 generated by a GO/glucose system in the presence of gentamicin. The metHb values at the beginning of the exposure period (0 hours) and after 5 hours are shown.
Rate, μmol/L H2O2 produced per minute.
Relative importance of catalase and GSHPx
This result implied that GSHPx was unnecessary to protect red cells against exogenous H2O2 fluxes, a conclusion that was supported by experiments with the catalase inhibitor, 3-amino-1,2,4-triazole (3-AT). The compound irreversibly inhibits catalase, but only when catalase is actively reducing H2O2. Catalysis by catalase proceeds in 2 steps:
Catalase (ground state) + H2O2 → Compound I + H2O
Compound I + H2O2 → Catalase + H2O + O2
Margoliash42 found that 3-AT irreversibly combines with Compound I, which is the product of the reaction between catalase and the first molecule of H2O2. Thus, loss of catalase activity in the presence of 3-AT is an indication that catalase is actively catabolizing H2O2.
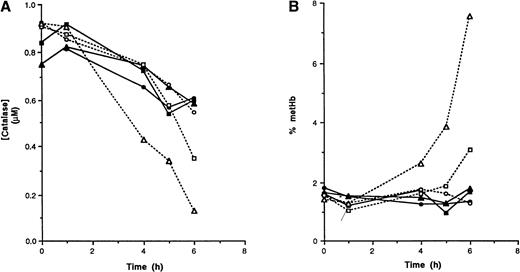
As has been found with erythrocytes from other species, exposure of wild-type mouse red cells to a continuous flux of H2O2 in the presence of 3-AT led to a slow inactivation of catalase (Figure 2A). When H2O2 fluxes were low to moderate, the rate of catalase inactivation was similar in both wild-type and GSHPx-deficient erythrocytes. At high H2O2 flux rates, however, catalase was more rapidly inactivated in GSHPx-deficient red cells than in controls. Thus, catalase is sufficient to deal with low to moderate H2O2fluxes because there is no effect of GSHPx-1 deletion. At high fluxes, however, the more rapid rate of catalase inactivation in GSHPx-deficient cells than in the wild type strongly implies that GSHPx-1 assists in eliminating H2O2 under these circumstances. The data are consistent with a model in which wild-type cells exposed to high H2O2 levels use both catalase and GSHPx for H2O2 removal. In GSHPx-deficient cells, the entire exogenous H2O2 flux will combine with catalase, thus raising the level of Compound I, leading to a more rapid inactivation by 3-AT.
Catalase inactivation (A) and hemoglobin oxidation (B) in red cells exposed to 50 mmol/L 3-AT and a continuous flux of H2O2.
Hematocrit 10%, Krebs-Ringer buffer. The rate of H2O2 production was 0.123μmol/L per minute (circles), 0.615 μmol/L per minute (squares), and 1.23 μmol/L per minute (triangles). Solid lines, wild-type red cells; dashed lines, GSHPx-1–deficient red cells.
Catalase inactivation (A) and hemoglobin oxidation (B) in red cells exposed to 50 mmol/L 3-AT and a continuous flux of H2O2.
Hematocrit 10%, Krebs-Ringer buffer. The rate of H2O2 production was 0.123μmol/L per minute (circles), 0.615 μmol/L per minute (squares), and 1.23 μmol/L per minute (triangles). Solid lines, wild-type red cells; dashed lines, GSHPx-1–deficient red cells.
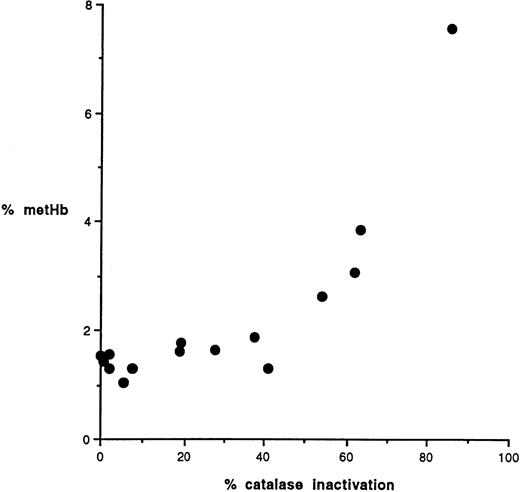
The importance of catalase is also indicated by the observation that metHb rose only after catalase was inactivated (Figure 2B). Hemoglobin oxidation was seen when approximately 50% of the catalase was inactivated (Figure 3).
Hemoglobin oxidation as a function of catalase inactivation.
The percentage methemoglobin in GSHPx-1–deficient cells is plotted versus the residual catalase activity (percentage of starting activity).
Hemoglobin oxidation as a function of catalase inactivation.
The percentage methemoglobin in GSHPx-1–deficient cells is plotted versus the residual catalase activity (percentage of starting activity).
Membrane thiols
Table 2 shows that the levels of endogenous GSH and metHb were normal in GSHPx-1–deficient red cells. However, there was a statistically significant decrease in membrane protein thiols in red cells obtained from GSHPx-1–deficient mice (P < .001). Each table entry shows values for the pooled blood from 2 to 3 mice.
Membrane thiols in GSHPx-1-deficient red cells
| . | GSH μmol/gHb . | % metHb . | Membrane thiols nmol/mg protein . |
|---|---|---|---|
| Wild-type cells | 8.9 | 1.47 | 62.5 ± 0.80 |
| 8.6 | 0.98 | 61.3 ± 1.0 | |
| GSHPx-1-deficient cells | 7.2 | 1.09 | 53.9 ± 0.5 |
| 8.0 | 2.58 | 54.1 ± 2.42 | |
| 8.0 | 1.89 | 53.4 ± 0.6 |
| . | GSH μmol/gHb . | % metHb . | Membrane thiols nmol/mg protein . |
|---|---|---|---|
| Wild-type cells | 8.9 | 1.47 | 62.5 ± 0.80 |
| 8.6 | 0.98 | 61.3 ± 1.0 | |
| GSHPx-1-deficient cells | 7.2 | 1.09 | 53.9 ± 0.5 |
| 8.0 | 2.58 | 54.1 ± 2.42 | |
| 8.0 | 1.89 | 53.4 ± 0.6 |
Hb, hemoglobin.
Discussion
The defenses of the red cell against oxidant attack include catalase and glutathione peroxidase. There has been a long debate about the relative importance of these 2 enzymes.1-6,8,9,11,12,18,43 Catalase, the first discovered, was thought to play a major role in protecting the cell, but the discovery of glutathione peroxidase and experiments demonstrating an essential role for glutathione in protecting intracellular hemoglobin from oxidation led to the proposal that GSHPx was the major defensive enzyme.1,2,18,44 However, considerations of kinetic constants4,9,11,12 and reconstitution studies with resealed ghosts14 43 are more consistent with a predominant role for catalase.
The observations presented here with red cells lacking GSHPx-1 indicate that under most conditions, GSHPx-1 is a dispensable enzyme. No oxidation of hemoglobin or membrane lipid was observed when GSHPx-deficient red cells were exposed to exogenous peroxides. A difference between wild-type and GSHPx-deficient erythrocytes was detected only in the presence of 3-AT and high exogenous H2O2 exposures. Thus, GSHPx-1 in the red cell has a functional role only under conditions of severe oxidant stress. This finding confirms the earlier inferences of Scott et al,8 who used resealed ghosts containing mixtures of catalase and GSHPx, and it confirms the conclusions of Gaetani et al11 and Mueller et al,12 based on the kinetics of mixtures of purified catalase and GSHPx. All these groups modeled low to moderate levels of oxidant stress and concluded that GSHPx has little role in the erythrocyte's oxidant defense. Our results with the GSHPx-1–deficient mouse verify their models, providing direct evidence that GSHPx plays little or no role in red cell anti-oxidant defense, except possibly when oxidant exposures are high.
It is difficult to ascertain whether the in vivo levels of oxidant exposure are ever high enough to bring GSHPx into play. The results depicted in Table 2, showing that the circulating red cells of GSHPx-deficient mice exhibit some degree of membrane protein oxidation, suggests that oxidant exposures in the circulation may sometimes be high enough to damage GSHPx-deficient red cells. It has been speculated6,25 that peroxides and nitric oxide produced by macrophages and endothelial cells or by pathogenic bacteria or parasites can generate localized high concentrations of H2O2, which might explain the observed endogenous red cell membrane oxidation. However, this inference does not affect the main conclusion of this work, which is that red cell GSHPx-1 plays little or no role in erythrocyte anti-oxidant defenses, at least in the mouse. Although absolute concentrations differ,7 the array of antioxidant enzymes is similar in mouse and other mammals, including humans, suggesting that this result will be of general applicability. In support of this idea, it can be noted that sporadic cases of GSHPx deficiency have been noted in humans without any accompanying clinical symptoms.45 Thus, genetic, enzymologic, and clinical evidence all suggest that GSHPx is of minor significance for red cell function.
Supported by National Institutes of Health grant HL56421 (Y-S.H.) and the Ginopolis Fund of Children's Hospital of Michigan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Robert M. Johnson, Department of Biochemistry and Molecular Biology, Wayne State Medical School, 540 E. Canfield, Detroit, MI 48201; e-mail: rmjohns@med.wayne.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal