Abstract
Solid tumors are dependent on preexisting vasculature and neovascularization for their growth. Successful cancer therapies targeting the tumor vasculature would be expected to block the existing tumor blood supply and to prevent tumor neovascularization. We tested the antitumor activity of experimental therapy with 2 distinct antiangiogenic drugs. Vasostatin inhibits endothelial cell growth and neovascularization, and interleukin-12 (IL-12) targets the tumor vasculature acting through interferon-γ (IFN-γ) and the downstream chemokines interferon-inducible protein-10 (IP-10) and monokine induced by IFN-γ. Individually, vasostatin and IL-12 produced distinct efficacy profiles in trials aimed at reducing tumor growth in athymic mice. In combination, these inhibitors halted the growth of human Burkitt lymphoma, colon carcinoma, and ovarian carcinoma. Thus, cancer therapy that combines distinct inhibitors of angiogenesis is a novel, effective strategy for the experimental treatment of cancer.
Introduction
The survival, growth, and spread of tumors are dependent on an adequate blood supply achieved through cooption of existing blood vessels, neovascularization, and formation of blood channels.1-6 Drugs that selectively inhibit angiogenesis may offer a treatment modality that is complementary to drugs that target tumor cells directly.
Endothelial cell disruption of intercellular junctions, changes in vessel permeability, proteolysis of the extracellular matrix proteins, endothelial cell proliferation and migration into the interstitial matrix, and assembly into patent tubes represent angiogenesis steps potentially amenable to selective regulation.7-10 Mature vascular endothelium is heterogeneous with respect to expression of surface determinants, patterns of cell growth, and expression of metalloproteinases that degrade the extracellular matrix.11-13 The tumor vasculature can display peculiar morphologies,14 in part because of decreased densities of periendothelial support cells,15 increased permeability,14 and enhanced endothelial cell proliferation.16 Tumor tissues may contain abnormally high levels of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) that promote angiogenesis, as well as decreased levels of thrombospondin and IFN-γ that can act as inhibitors of angiogenesis.17-20
Current efforts at targeting the tumor vasculature exploit the existence of distinct stages of angiogenesis, the heterogeneity of the vascular endothelium, and potential alterations of the tumor vasculature. Neutralizing antibodies directed at VEGF, VEGF receptor 2, or soluble VEGF receptors can block VEGF-induced endothelial cell proliferation,21-23 and antisense targeting of bFGF and FGF receptor 1 can suppress bFGF-induced intratumoral angiogenesis.24 Antibodies to the integrin αvβ3, which is expressed at high levels on proliferating endothelium, can promote apoptosis in proliferating endothelial cells.25,26 Vasostatin, the N-terminal domain of human calreticulin, selectively inhibits endothelial cell proliferation and angiogenesis in response to growth factors, and suppresses tumor growth.27,28 Specific inhibitors of matrix metalloproteinases (MMP), enzymes that selectively degrade components of the extracellular matrix, can prevent endothelial cells from sprouting and organizing into networks.29Angiostatin, a fragment of plasminogen,19,30 endostatin, a fragment of collagen XVIII,31 antithrombin and a cleavage fragment of antithrombin,32 appear to inhibit angiogenesis at multiple levels. IL-12, a multifunctional cytokine with profound effects on T and natural killer (NK) cells, can inhibit angiogenesis through the downstream chemokines interferon-inducible protein-10 (IP-10) and monokine induced by interferon-γ (Mig).33-35
We tested the anticancer efficacy of a novel therapeutic approach that combines distinct antiangiogenic agents. Individually, vasostatin, which specifically inhibits proliferating endothelial cells, and IL-12, which inhibits angiogenesis indirectly, produced distinct efficacy profiles. In combination, these drugs halted tumor growth in mice.
Materials and methods
Cell cultures
The human Burkitt lymphoma cell line CA46 was grown as previously described.28 The human colon carcinoma cell line SW620 and the human ovarian carcinoma cell line OVCAR were grown in RPMI 1640 medium supplemented with 10% fetal calf serum (BioWhittaker, Walkersville, MD) and 5 μg/mL gentamycin (Gibco BRL, Gaithersburg, MD). All cell lines were used in exponential growth phase when more than 90% of cells were viable.
Matrigel angiogenesis assay
The Matrigel assay was performed as described previously.33 Matrigel (0.5 mL, Becton Dickinson Labware, Bedford, MA) was injected subcutaneously alone or with additives into the midabdominal region of female athymic nude mice 6 weeks of age. After 7 days, the mice were killed and the Matrigel plugs processed for histology. Quantitative analysis of angiogenesis in Matrigel plugs used a computerized semiautomated digital analyzer (Optomax, Hollis, NH).
Mouse tumor models
BALB/c nu/nu or athymic nude mice 6 weeks of age (National Cancer Institute, Frederick, MD) maintained in pathogen-limited conditions received 400 rad (1 rad = 0.01 Gy) total body irradiation and 24 hours later were injected subcutaneously in the right abdominal quadrant with human Burkitt lymphoma (8 × 106 CA46 cells), colon carcinoma (107 SW620 cells), or human ovarian carcinoma (8 × 106 OVCAR cells). Beginning 1 to 5 days after cell inoculation and continuing daily thereafter, 6 days a week the mice received subcutaneously inoculations (0.1 mL total injection volume) of test drugs or formulation buffer control proximal to the site of original cell injection. Formulation buffer consisted of sterile saline solution containing 50 mg/mL human albumin and 5 mg/mL mannitol (endotoxin less than 5 EU/mL). Recombinant vasostatin (MBP-vasostatin) was purified from Escherichia coli as previously described28 and was found to contain less than 5 EU/10 μg protein. Recombinant murine IL-12 was a gift of Genetics Institute Inc (Cambridge, MA) and contained less than 8 EU/mg protein. Tumor size was estimated in centimeters squared (cm2) as the product of 2-dimensional caliper measurements (longest perpendicular length and width). Tumor weight was measured after the tumors were removed in toto from the animals.
Immunohistochemistry
Paraffin-embedded tissue sections were deparaffinized twice in xylene and rehydrated through graded ethanol washes as previously described.33 Staining for the human Ki-67 antigen with MIB-1 monoclonal antibody (Immunotech, Westbrook, ME) followed previously described methods.36 Staining for murine CD31 was performed on trypsin-treated sections using a rat monoclonal primary antibody (PharMingen, San Diego, CA) and a biotinylated goat antirat secondary antibody (Vector Labs, Burlingame, CA), followed by a Vectastain ABC peroxidase complex (Vector Labs). Apoptosis was detected on tissue sections by Tumor TACS in situ kit (R&D Systems, Inc, Minneapolis, MN). Staining for murine perforin was performed on paraffin-embedded tumor tissue sections using a rat antimouse perforin monoclonal antibody (Kamiya Biomedical Co, Seattle, WA) and a biotynilated goat antirat secondary antibody (Pharmingen), as previously described.37
Semiquantitative reverse transcriptase-polymerase chain reaction
Total cellular RNA was extracted from tumor tissues by Trizol (Gibco-BRL, Gaithersburg, MD); 4 μg RNA was reverse transcribed using Rnase H-RT (Supercript, Gibco/BRL) as described.33 The primers for murine IP-10 and perforin (not cross-reactive with human) were previously described.33 The murine mitocondrial cytochrome oxidase subunit II gene (MOXII, GenBank accession noJ01420), was selected as the housekeeping gene for murine tissues38; the primer pair sequence was 5′: TGGCCTACCCATTCCAACTT and 3′: GGTTAACGCTCTTAGCTTCA. The semiquantitative polymerase chain reaction (PCR) assays for murine IP-10 and perforin were previously described.33 The semiquantitative PCR assay for MOXII (25 cycles at 60 °C), amplified selectively murine complementary DNA (cDNA).
Statistical analysis
The means, least square means, and standard deviations were derived using conventional formulas. The slopes of tumor growth were estimated by fitting linear regression lines to tumor size data. Slopes of tumor growth, tumor measurements at completion of the experiments, and tumor weights were analyzed by 2-way analysis of variance with interaction. Dunnett's method was used for pairwise comparisons with controls. Additional pairwise comparisons of the combined effect of drugs were made by t test, using estimates of variance from the 2-way analysis of variance.39
Results
Vasostatin can directly inhibit the proliferation of primary cultures of human umbilical vein endothelial cells (HUVEC) and fetal bovine heart endothelial (FBHE) cells induced by either bFGF or VEGF,27,28 whereas human and murine IL-12 do not.34,35 As expected, neither vasostatin nor murine IL-12 inhibited the spontaneous proliferation in vitro of the human Burkitt lymphoma (CA46), colon carcinoma (SW620), and ovarian carcinoma (OVCAR) cell lines (not shown). A Matrigel-based assay was used to evaluate the effects of vasostatin and murine (mu) IL-12, alone and together, on bFGF-induced angiogenesis in vivo (Table1). In this assay, bFGF promotes the invasion of von Willebrand factor-positive endothelial cells and vascular structures into the Matrigel plug.33 Vasostatin at concentrations of 50 or 10 μg/mL inhibited bFGF-induced neovascularization by 57% and 25%, respectively, whereas muIL-12 at concentrations of 50 and 10 ng/mL inhibited bFGF-induced neovascularization by 44% and 29%, respectively. When added together at the higher and lower concentrations, vasostatin and muIL-12 inhibited neovascularization by 79% and 58%, respectively.
Effects of vasostatin and murine interleukin-12 on angiogenesis in vivo
| Additions to Matrigel . | Mean surface area (SEM) occupied by cells (μm2/1.26 × 105 μm2) . | Inhibition (%) . |
|---|---|---|
| None | 373 (67.5) | |
| bFGF | 7337 (1170.4) | |
| bFGF + vasostatin (50 μg/mL) | 3163 (777.7) | 56.9 |
| bFGF + muIL-12 (50 ng/mL) | 4119 (442.4) | 43.9 |
| bFGF + vasostatin (50 μg/mL) + muIL-12 (50 ng/mL) | 1522 (213.4) | 79.3 |
| bFGF + vasostatin (10 μg/mL) | 5528 (338.5) | 24.7 |
| bFGF + muIL-12 (10 ng/mL) | 5205 (363.6) | 29.1 |
| bFGF + vasostatin (10 μg/mL) + muIL-12 (10 ng/mL) | 3059 (442.0) | 58.3 |
| Additions to Matrigel . | Mean surface area (SEM) occupied by cells (μm2/1.26 × 105 μm2) . | Inhibition (%) . |
|---|---|---|
| None | 373 (67.5) | |
| bFGF | 7337 (1170.4) | |
| bFGF + vasostatin (50 μg/mL) | 3163 (777.7) | 56.9 |
| bFGF + muIL-12 (50 ng/mL) | 4119 (442.4) | 43.9 |
| bFGF + vasostatin (50 μg/mL) + muIL-12 (50 ng/mL) | 1522 (213.4) | 79.3 |
| bFGF + vasostatin (10 μg/mL) | 5528 (338.5) | 24.7 |
| bFGF + muIL-12 (10 ng/mL) | 5205 (363.6) | 29.1 |
| bFGF + vasostatin (10 μg/mL) + muIL-12 (10 ng/mL) | 3059 (442.0) | 58.3 |
Mice were injected subcutaneously with Matrigel alone, Matrigel with bFGF (150 ng/mL), Matrigel plus bFGF (150 ng/mL) plus vasostatin (10 or 50 μg/mL), Matrigel plus bFGF (150 ng/mL) plus murine IL-12 (10 or 50 ng/mL), or Matrigel plus bFGF (150 ng/mL) plus vasostatin (10 or 50 μg/mL) plus muIL-12 (10 or 50 ng/mL). Plugs were removed after 7 days, and histologic sections were stained by Masson trichrome. The results reflect the mean surface area (expressed in μm2) occupied by cells within a surface area of 1.26 × 105μm2; 12 to 15 nonoverlapping fields were scanned in each plug; there were 8 plugs per group. Determinations of surface areas were performed by a semiautomated digital analyzer.
bFGF, basic fibroblast growth factor; mu, murine.
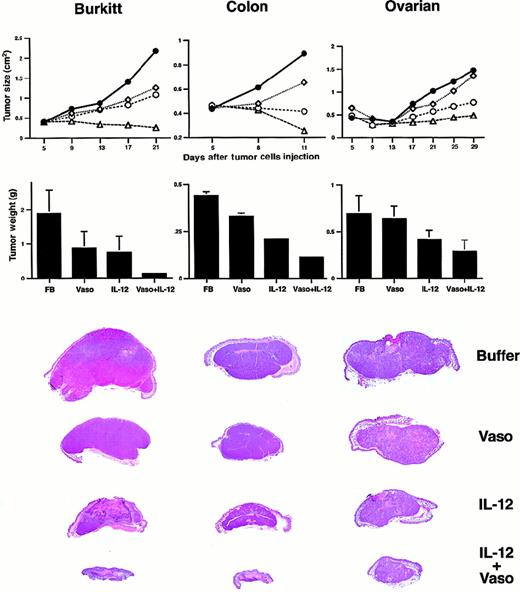
We tested the effects of vasostatin and muIL-12, alone and together, on human Burkitt lymphoma, colon carcinoma, and ovarian carcinoma established in nude mice (Figure 1). These tumors were selected on the basis of previous experiments in which vasostatin was either very effective (Burkitt lymphoma, CA46 cell line), moderately effective (colon carcinoma, SW620 cell line), or marginally effective (ovarian carcinoma, OVCAR cell line) at reducing tumor growth under similar experimental conditions. The Burkitt cell line CA46 was inoculated subcutaneously (sc) into athymic mice, and 5 days later the established tumors were treated with daily sc inoculations (6 days per week) of either formulation buffer (buffer) alone, vasostatin alone (100 μg/d), muIL-12 alone (200 ng/d for 8 days, followed by 100 ng/d), or the combination of vasostatin and muIL-12 (same dose used as single agents). All mice were killed after 16 days of treatment (Figure 1). The rate of tumor growth and tumor size were significantly reduced by treatment with vasostatin, muIL-12, or the combination of vasostatin plus muIL-12, compared with the control group treated with buffer alone (P < .05 for all comparisons). In addition, the combination treatment of vasostatin plus muIL-12 reduced tumor growth more effectively than vasostatin or muIL-12 alone (P < .05, both comparisons).
Treatment of malignant tumors with vasostatin, IL-12, and vasostatin plus IL-12.
All treatments were given by means of daily (6 days per week) sc injections of either buffer alone, vasostatin (100 μg per mouse), murine IL-12 (100 ng per mouse), or vasostatin (100 μg per mouse) plus murine IL-12 (100 ng per mouse) at a site proximal to the tumor. Tumor size was estimated as the product of 2-dimensional caliper measurements. Mean tumor size is shown for each time point. Mice were killed and autopsied; tumors were removed in toto, weighed, and formalin fixed. Mean tumor weight and standard deviations are shown for each group. Gross morphology of representative tumors (hematoxylin and eosin stain) from each group is shown. CA46 Burkitt lymphoma cell line was inoculated subcutaneously into athymic mice (8 × 106cells per mouse), and 5 days later, tumor treatment was initiated; there were 18 mice in the group treated with vasostatin plus IL-12; 11 mice in each of the other groups. SW620 colon carcinoma cell line was inoculated subcutaneously into athymic mice (107 cells per mouse); treatment with buffer alone and vasostatin alone was started 1 day after cell injection, and IL-12 was started 1 day later. There were 11 mice in each group. OVCAR ovarian carcinoma cell line was inoculated subcutaneously into athymic mice (8 × 106 cells per mouse); all treatment was started 8 hours after cell injection. There were 9 mice in the buffer-treated group, and 10 mice in each of the other groups. Buffer (●—●); vasostatin (⋄—⋄); murine IL-12 (○—○); vasostatin plus murine IL-12 (▵—▵).
Treatment of malignant tumors with vasostatin, IL-12, and vasostatin plus IL-12.
All treatments were given by means of daily (6 days per week) sc injections of either buffer alone, vasostatin (100 μg per mouse), murine IL-12 (100 ng per mouse), or vasostatin (100 μg per mouse) plus murine IL-12 (100 ng per mouse) at a site proximal to the tumor. Tumor size was estimated as the product of 2-dimensional caliper measurements. Mean tumor size is shown for each time point. Mice were killed and autopsied; tumors were removed in toto, weighed, and formalin fixed. Mean tumor weight and standard deviations are shown for each group. Gross morphology of representative tumors (hematoxylin and eosin stain) from each group is shown. CA46 Burkitt lymphoma cell line was inoculated subcutaneously into athymic mice (8 × 106cells per mouse), and 5 days later, tumor treatment was initiated; there were 18 mice in the group treated with vasostatin plus IL-12; 11 mice in each of the other groups. SW620 colon carcinoma cell line was inoculated subcutaneously into athymic mice (107 cells per mouse); treatment with buffer alone and vasostatin alone was started 1 day after cell injection, and IL-12 was started 1 day later. There were 11 mice in each group. OVCAR ovarian carcinoma cell line was inoculated subcutaneously into athymic mice (8 × 106 cells per mouse); all treatment was started 8 hours after cell injection. There were 9 mice in the buffer-treated group, and 10 mice in each of the other groups. Buffer (●—●); vasostatin (⋄—⋄); murine IL-12 (○—○); vasostatin plus murine IL-12 (▵—▵).
Treatment with vasostatin and muIL-12 in combination was also very effective at reducing the growth of the colon carcinoma SW620 and the ovarian carcinoma OVCAR cell lines in nude mice (Figure 1). In both experiments, vasostatin (100 μg/d; 6 days per week) and muIL-12 (100 ng/d; 6 days per week) were injected sc beginning 1 day and 2 days, respectively, after cell inoculation. The growth rate and weight of colon carcinoma tumors were significantly reduced by vasostatin, muIL-12, and the combination of vasostatin and muIL-12, compared with the control group (P < .05, all comparisons). Furthermore, the combination treatment of vasostatin plus muIL-12 was significantly more effective at reducing tumor size and weight than each drug alone (P < .05, both comparisons).
With ovarian carcinoma (Figure 1), muIL-12 alone or in combination with vasostatin significantly reduced tumor growth rate and weight compared with buffer alone (P < .05 both comparisons). As expected, the effect of vasostatin alone did not reach statistical significance (P > .05). In combination, muIL-12 plus vasostatin appeared more effective than vasostatin alone and muIL-12 alone at reducing tumor weight. The effect was significant when the combination treatment was compared with vasostatin alone (P < .05) and approached significance when compared with muIL-12 alone (P = .065). Thus, treatment with 2 distinct inhibitors of angiogenesis, vasostatin and IL-12, was very effective at reducing experimental tumor growth, and the combined effect, in most cases, was significantly more effective than each agent used alone.
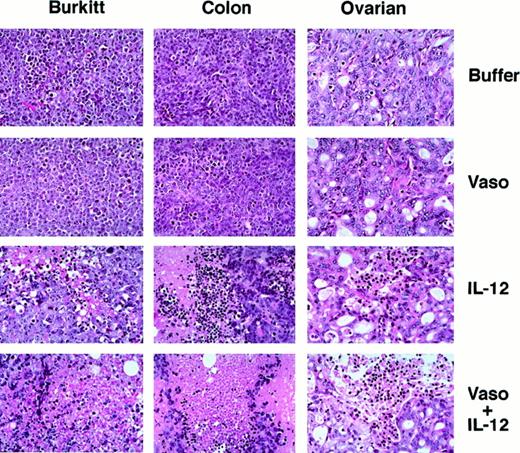
Histologically, the Burkitt tumor tissue from mice treated with formulation buffer alone or vasostatin alone (Figure2) consisted of a mostly viable monomorphic population of large lymphoid cells with prominent nucleoli and numerous mitoses. There was minimal tumor tissue necrosis and inflammation. Tumors treated with IL-12 alone displayed massive zonal tissue necrosis interspersed with islands of viable tumor. Infiltration by lymphocytes and macrophages was noted within the necrotic tumor tissue. Mice treated with vasostatin plus mIL-12 displayed only a few small foci of viable Burkitt tumor cells and residual foci of necrotic tumor. The surrounding soft tissue was edematous with infiltration by macrophages and lymphocytes.
Histopathology of malignant tumors treated with vasostatin, murine IL-12, or vasostatin plus murine IL-12.
Tissues from representative Burkitt lymphoma, colon carcinoma, and ovarian carcinoma tumors from experiments displayed in Figure 1 are shown. Hematoxylin and eosin stain; original magnification ×40.
Histopathology of malignant tumors treated with vasostatin, murine IL-12, or vasostatin plus murine IL-12.
Tissues from representative Burkitt lymphoma, colon carcinoma, and ovarian carcinoma tumors from experiments displayed in Figure 1 are shown. Hematoxylin and eosin stain; original magnification ×40.
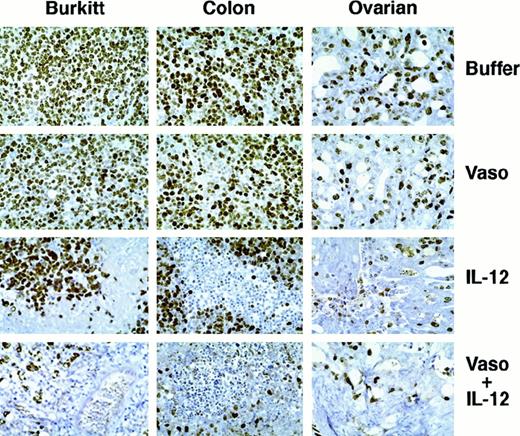
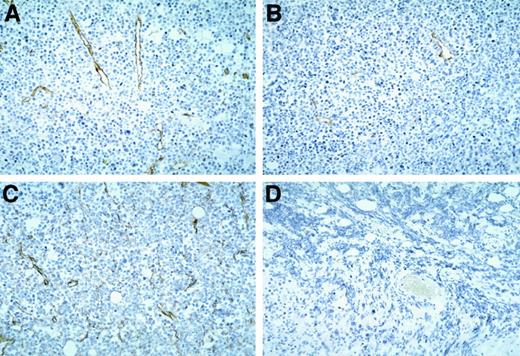
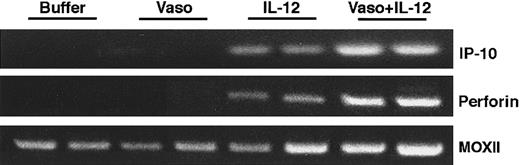
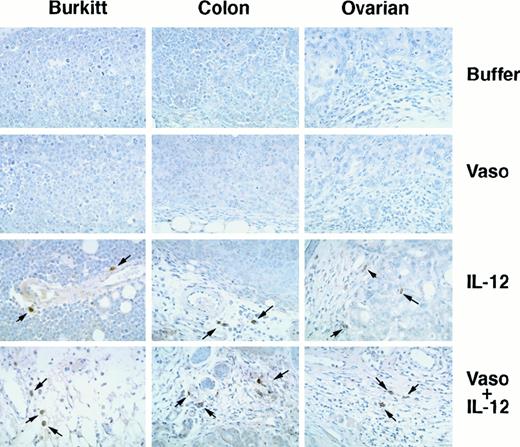
Staining these Burkitt tissues for human Ki-67 nuclear antigen revealed that the proportion of proliferating cells within the viable tumor was similar in control, vasostatin-, or IL-12–treated animals (Figure 3). Because of the extensive tumor tissue necrosis, Ki-67 staining was restricted to the islands of viable tumor tissue in IL-12–treated animals. Ki-67 positive cells were rare in tumors treated with both drugs together, presumably because of the paucity of residual viable tumor cells. The proportion of apoptotic cells in Burkitt tumor tissues, estimated by TUNEL, was on the average, 28 positive cells per low power (40×) field in the control, as opposed to 49 in the vasostatin group. Large areas of confluent tumor cells and numerous scattered individual cells (92 per field) were TUNEL-positive in the IL-12–treated group. There were numerous TUNEL-positive cells confined to small areas of residual tumor tissues from the group that received vasostatin plus IL-12. The tumor vasculature, assessed by staining for the murine endothelial cell antigen CD31, was recognized throughout Burkitt tumor tissues treated with buffer alone (Figure 4A). By contrast, CD31-positive structures and cells were noted infrequently in vasostatin-treated tumors (Figure 4B). Tumors treated with IL-12 displayed abundant and disorganized proliferation CD31-positive cells not resulting in the formation of distinct vascular channels (Figure4C). There were virtually no CD31 positive vascular structures or cells within tumor tissues from animals treated with IL-12 plus vasostatin (Figure 4D). By semiquantitative reverse transcriptase (RT)-PCR analysis, expression of the murine IFNγ-inducible chemokine IP-10 was significantly higher in tumor tissues from IL-12 and IL-12 plus vasostatin-treated animals compared with control and vasostatin only-treated animals (Figure 5). Expression of perforin, an enzyme expressed by activated NK cells, which mediates NK-cell mediated killing, was also significantly increased in IL-12 and IL-12 plus vasostatin-treated animals, compared with control and vasostatin-only treated animals (Figure 5). Also, immunohistochemical staining identified perforin-positive cells in IL-12–treated tumors with or without vasostatin, but not in buffer and vasostatin only-treated tumors (Figure6).
Cell proliferation in human tumors treated with vasostatin, murine IL-12, or vasostatin plus murine IL-12 detected by immunohistochemical analysis of Ki-67 antigen.
Hematoxylin counterstain, original magnification ×40.
Cell proliferation in human tumors treated with vasostatin, murine IL-12, or vasostatin plus murine IL-12 detected by immunohistochemical analysis of Ki-67 antigen.
Hematoxylin counterstain, original magnification ×40.
Vascular structures in Burkitt tumors treated with vasostatin, murine IL-12, or vasostatin plus murine IL-12 detected by immunohistochemical staining of murine CD31.
Counterstained with hematoxylin, original magnification ×20. Tumor tissue from animals treated with (A) buffer alone; (B) vasostatin alone; (C) murine IL-12 alone; and (D) vasostatin plus murine IL-12.
Vascular structures in Burkitt tumors treated with vasostatin, murine IL-12, or vasostatin plus murine IL-12 detected by immunohistochemical staining of murine CD31.
Counterstained with hematoxylin, original magnification ×20. Tumor tissue from animals treated with (A) buffer alone; (B) vasostatin alone; (C) murine IL-12 alone; and (D) vasostatin plus murine IL-12.
Murine IP-10 and perforin expression in Burkitt tumors treated with vasostatin, murine IL-12, or vasostatin plus murine IL-12.
Semiquantitative RT-PCR analysis of total cellular RNA using appropriately designed primers.
Murine IP-10 and perforin expression in Burkitt tumors treated with vasostatin, murine IL-12, or vasostatin plus murine IL-12.
Semiquantitative RT-PCR analysis of total cellular RNA using appropriately designed primers.
NK cells in tumor tissues detected by immunocytochemical staining with antiperforin antibody.
Burkitt, colon carcinoma, and ovarian carcinoma tumors were treated with buffer alone, vasostatin, IL-12, or vasostatin plus IL-12. Hematoxylin counterstain, original magnification ×40.
NK cells in tumor tissues detected by immunocytochemical staining with antiperforin antibody.
Burkitt, colon carcinoma, and ovarian carcinoma tumors were treated with buffer alone, vasostatin, IL-12, or vasostatin plus IL-12. Hematoxylin counterstain, original magnification ×40.
The microscopic features of colon and ovarian carcinomas treated with vasostatin, muIL-12, or vasostatin plus muIL-12 mimicked closely the patterns noted with Burkitt tumors. From the control and vasostatin-treated groups, colon carcinomas were composed of confluent sheets of poorly differentiated epithelial cells with occasional small foci of dead tumor cells, whereas ovarian carcinomas were composed mostly of glandular structures with a modest fibrovascular core (Figure2). By contrast, colon and ovarian carcinomas treated with IL-12 alone displayed large areas of zonal necrotic tumor tissue mixed with viable tumor tissue (Figure 2). After treatment with IL-12 plus vasostatin, only small islands of viable tumor remained in colon carcinomas, whereas in ovarian carcinomas, a higher proportion of viable tumor was noted (Figure 2). Staining for human Ki-67 nuclear antigen revealed that the proportion of proliferating cells was similar in control and vasostatin-treated colon and ovarian carcinomas (Figure 3). However, in tumors treated with IL-12 with or without vasostatin, Ki-67 positive cells were confined to viable areas of the tumor. In the viable tumor, which was infrequent in animals bearing colon carcinomas treated with IL-12 plus vasostatin, the proportion of proliferating cells was similar to the controls (Figure 3). By TUNEL, apoptotic cells were more numerous in vasostatin than in buffer-treated colon and ovarian carcinomas (on the average, 92 as opposed to 52 positive cells per field [40×] in colon carcinomas, and 58 as opposed to 17 in ovarian carcinomas, respectively). Large areas of nonviable tissue from colon and ovarian carcinoma tumors treated with IL-12 stained positive for TUNEL. Within residual viable ovarian carcinoma tissues, the number of apoptotic cells was similar in the IL-12 plus vasostatin-treated group (66 and 58 positive cells per field, respectively). In colon carcinomas, accurate determinations of TUNEL-positive cells in tumors treated with IL-12 plus vasostatin could not be made because of the extensive tissue necrosis. Perforin-positive cells were identified in IL-12–treated colon (Figure 6) and ovarian (Figure 6) tumors with or without vasostatin, but rarely in buffer or vasostatin only-treated tumors.
Discussion
These studies reveal that combination therapy with distinct angiogenesis inhibitors is extremely effective at reducing experimental tumor growth. Although human Burkitt lymphoma, colon carcinoma, and ovarian carcinoma cell lines grow rapidly as sc tumors in nude mice, vasostatin and IL-12 in combination essentially halted their growth. Vasostatin is a direct and specific inhibitor of new vessel formation that suppresses endothelial cell growth.27,28 IL-12 targets the tumor vasculature, acting indirectly through the downstream IFN-γ–inducible mediators, IP-10 and Mig.33-35,40 The antitumor effects of IL-12 in T-cell immunodeficient mice may be the result of its antiangiogenic activities as well as other less characterized functions.40
Burkitt, colon, and ovarian tumors from mice treated with vasostatin alone differed from the controls for their smaller size but were otherwise indistinguishable from controls, macroscopically and microscopically. The only noted differences included a small increase in the frequency of tumor cell death detected by TUNEL and an overall reduction of CD31-positive tumor vasculature in the vasostatin as opposed to the control groups. This pattern would be expected from a pure inhibitor of new vessel formation that blocks endothelial cell growth, leaving quiescent blood vessels intact. In the absence of neovascularization, a tumor should not grow or grow only to the extent to which the existing vasculature or the formation of nonendothelial blood channels would permit it. Because tumor cells generally die by apoptosis when deprived of nutrients,41 tumor size would be the result of a balance between tumor cell growth and subsequent cell death.
In contrast to tumors from mice treated with vasostatin alone, tumors from mice treated with IL-12 alone were remarkable, not only for their reduced size compared with the controls but also for the extensive zonal tissue necrosis involving all parts of the tumor. The substantial amount of tumor tissue necrosis indicates that the tumor grew and subsequently died. The presence of abundant CD31-positive cells that did not form distinct vascular structures and the zonal distribution of tissue necrosis point toward a drug effect on tumor vessels, resulting in the starvation of the tumor zones fed by those vessels. In this system, murine IL-12 is not expected to have a direct or IFNγ-mediated effect on the human tumor cells because of the strict species specificity of these molecules.42 Also, murine IL-12 is not expected to cause Burkitt cell killing by activated murine NK cells because Burkitt tumor cells are resistant to NK cell killing.43 Rather, many studies in mice support a critical role for IP-10 and Mig in mediating the antitumor effects of IL-12.44,45 These are chemokines induced by IL-12 through IFNγ, which can attract activated T and NK cells and can inhibit angiogenesis.46 Other, less defined, biologic activities of IL-12 may also contribute to the cytokine antitumor effects in T-cell immunodeficient hosts.40
The mechanism by which IP-10 and Mig target the endothelium is controversial, in part because of conflicting reports as to the expression of the CXCR3 IP-10 and Mig receptor on endothelial cells, and the possibility that their antiangiogenic effect may be indirect.47-49 We have previously shown that NK cells play a critical role as mediators of the antiangiogenic activities of IL-12 in nude mice.33 Because NK cells activated by IL-12 are strongly cytotoxic for primary cultures of syngeneic endothelial cells, we have proposed that NK-cell–mediated cytotoxicity of endothelial cells contributes to inhibition of angiogenesis by IL-12. In this scenario, NK cells would play a dual role as a source of muIFNγ for induction of IP-10 and Mig, and as effectors of endothelial cell killing. Yet, IL-12–treated mice do not display broad toxicity to the normal vasculature.45 Therefore, one would propose that the tumor vasculature is preferentially targeted in IL-12–treated mice. There is evidence that tumor vessels differ from normal vessels by a variety of criteria, such as abnormal morphologies, decreased periendothelial support, and rapid proliferative rates.14-16 These differences may render tumor vessels more susceptible to the effects of IL-12 and other agents, but this possibility needs testing.
The results of dual therapy with vasostatin and IL-12 suggest that this novel approach to cancer treatment can be very effective. Tumors from mice treated with vasostatin and IL-12 in combination were in most cases significantly smaller than tumors from mice treated with each agent alone. Within the small residual tumor tissue, there was tissue necrosis often mixed in with foci of viable tumor tissue or just clusters of tumor cells. Few, if any, vessels or cells staining for CD31 were identified within or immediately surrounding the small residual tumor tissue. Both the size and the morphology of tumors from mice treated with vasostatin plus IL-12 could be explained as the result of antiangiogenic agents acting independently. By inhibiting new vessel formation, vasostatin would reduce progressive tumor growth. By targeting unique characteristics of the tumor vasculature, IL-12 would compromise tumor angiogenesis occurring despite inhibition by vasostatin. As a result, there would be tissue necrosis within already small tumors.
There is considerable new evidence that angiogenesis is essential to tumor growth.6 The encouraging results of therapy that combines distinct inhibitors of angiogenesis supports the view that antiangiogenic therapy can be an effective approach to cancer treatment. Should these angiogenesis inhibitors be combined with treatment modalities that target directly the tumor cells such as chemotherapy and radiation therapy, perhaps complete eradication of cancer might be possible.
Acknowledgments
We thank Drs Josh Farber, Parris Burd, Hynda Kleinman, Robert Yarchoan, and Hira Nakhasi for their contributions to different aspects of this work, and Genetics Institute, Inc, for donating muIL-12.
Supported in part by a National Cancer Institute intramural grant (IRA AWD-7).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Giovanna Tosato, NIH Bethesda Campus, Bldg 10, Rm 12N226, 8800 Rockville Pike, Bethesda, MD 20892; e-mail:tosatog@mail.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal