Abstract
During acute graft-versus-host disease (GVHD) the activation of macrophages (Mφ) is mediated by 2 signals, interferon (IFN)-γ and bacteria-derived lipopolysaccharide (LPS). A cascade of inflammatory responses that includes the release of mediators of tissue injury follows Mφ activation. Among the tissues characteristically targeted during acute GVHD are epithelial tissues of the skin and gastrointestinal tract that normally undergo continuous proliferation and are therefore sensitive to cytostatic processes. We have investigated whether Mφ can mediate cytostatic mechanisms capable of interrupting cell proliferation during acute GVHD. GVHD was induced in nonirradiated C57BL/6XAF1 (B6AF1) mice by the injection of 60 × 106 (acute GVHD) or 30 × 106 (nonlethal GVHD) C57BL/6 (B6) lymphoid cells. Mφ from animals undergoing acute GVHD could be triggered by normally insignificant quantities of LPS to mediate a cytostatic effect on target cells, resulting in the complete shutdown of cellular proliferation. The same amounts of LPS had no effect on Mφ from normal or syngeneically transplanted animals. Mφ mediated the release of significant quantities of intracellular iron from target cells undergoing cytostasis. Reversal of cytostasis occurred following inhibition of nitric oxide (NO) production by NG-monomethyl-L-arginine (NMMA). Production of NO by LPS-triggered Mφ reflected the severity of GVHD. NO release increased significantly during acute GVHD but was only transiently increased during nonlethal GVHD. The results provide evidence that, as a result of activation during acute GVHD, Mφ produce NO and induce the release of iron from target cells, resulting in a potent cytostatic effect that inhibits cellular proliferation.
Introduction
Graft-versus-host disease (GVHD) is initiated by the interaction of donor T lymphocytes with alloantigens on host tissues and is a frequent complication of allogeneic bone marrow transplantation. In acute GVHD, initiation of the afferent phase of the disease process is followed by a cascade of cellular and cytokine responses, release of inflammatory mediators, the onset of T- and B-cell immunosuppression, and tissue injury.1-4 In contrast to the suppressed state of T and B cells, nonspecific Mφ inflammatory mechanisms become activated. Indeed, a key step in the efferent phase of acute GVHD is the progressive activation or priming of Mφ that results in inflammatory tissue injury, weight loss, and death.4-7
Normal Mφ are primed and release nitric oxide (NO) when exposed to interferon (IFN)-γ. Further activation or triggering of the primed cells by bacterial endotoxin—that is, lipopolysaccharide (LPS)—greatly increases NO production8 and triggers the release of tumor necrosis factor (TNF)-α.9 During the development of acute GVHD, increased production of IFN-γ primes Mφ and initiates the inflammatory reaction of the efferent phase.10,11 LPS entering through the injured intestinal epithelium rapidly intensifies the inflammatory cascade as it comes into contact with and triggers primed Mφ to a fully activated state.4,5 12-14
Sale has proposed that during GVHD, Mφ and/or T cells specifically target proliferating stem cells within the epithelial tissues of the skin and gut.15 Epithelial stem cells within these characteristic target organs appear to be preferentially damaged by unidentified mechanisms during acute GVHD.16-18Proliferating subpopulations of stem cells maintain the integrity of the organs by continual replacement of cells that are shed or sloughed off. Although epithelial damage may occur as a direct result of cytotoxicity, Mφ-mediated cytostatic mechanisms that target self-renewing stem cells would also cause epithelial lesions by preventing adequate cell replacement.
Mφ-mediated cytotoxicity preferentially acts on neoplastic cells,19,20 whereas cytostasis targets processes found in all proliferating cells.21-23 NO produced by activated Mφ mediates cytostasis by inhibiting ribonucleotide reductase, a nonheme iron-containing enzyme essential for DNA synthesis.21-24 It also causes the release of intracellular iron from nonheme iron-containing enzymes that are essential for cellular division and mitochondrial respiration.25-27 In experimental GVHD, treatment with NG-monomethyl-L-arginine (NMMA), a competitive inhibitor of NO synthase (NOS) activity, prevents intestinal pathology28and reverses suppression of splenic lymphocyte mitogen responses.29 30 These findings indicate that NO contributes to intestinal epithelial injury and immunosuppression, which are both characteristic hallmarks of acute GVHD.
To determine whether Mφ act as cytostatic effector cells during acute GVHD, we have examined Mφ isolated from nonirradiated F1 hybrid mice transplanted with parental strain lymphoid cells. The results indicate that during acute GVHD, Mφ mediate a potent cytostatic effect when triggered by normally insignificant amounts of LPS and that cytostasis is accompanied by Mφ-mediated release of intracellular iron from the target cells. Mφ-mediated cytostatic function and NO release during acute GVHD were both blocked by NMMA, an inhibitor of NO synthesis. Levels of NO produced by Mφ, in response to LPS, increased during acute GVHD—in marked contrast to the transient increase observed during the course of nonlethal GVHD. Our results demonstrate that, as a result of priming during acute GVHD, Mφ are triggered by LPS to act as cytostatic effector cells through the production and release of NO.
Materials and methods
Mice
Male C57BL/6 (B6) and C57BL/6xAF1 (B6AF1) mice were bred and maintained under conventional conditions in our laboratory and used at 12 to 18 weeks of age.
Reagents and media
Monoclonal antimurine IFN-γ was prepared by ammonium sulfate precipitation of R46A2 supernatants. NMMA and LPS (Escherichia coli 0111:B4) were from Calbiochem Corp (La Jolla, CA). LPS in phosphate-buffered saline (PBS) was sterilized by irradiation (15 000 rad). Sulfanilamide, naphthylethylenediamine dihydrochloride, and sodium nitrite were from Sigma Chemical Co (St Louis, MO), phosphoric acid from BDH Chemicals (Montreal, Quebec, Canada), and concanavalin A (ConA) from Pharmacia (Uppsala, Sweden). RPMI 1640, RPMI 1640-Select-Amine kit, Hank's balanced salt solution (HBSS), PBS (GIBCO, Grand Island, NY) and endotoxin-free fetal calf serum (FCS) (Sterile Systems, Logan, UT) contained less than 50 pg/mL endotoxin as quantified by the Limulus amoebocyte lysate assay (Associates of Cape Cod, Woods Hole, MA). All glassware was heated at 180°C for 4 hours. Recombinant murine TNF-α (4 × 104 U/μg) was from Genzyme (Boston, MA). Rat antimurine TNF-α was purchased from UBI (Lake Placid, NY).
Induction of GVHD
Single-cell suspensions of donor spleen and lymph nodes were prepared in HBSS as previously described.4,5 Recipient B6AF1 mice were injected intravenously with 30 × 106 B6 (nonlethal GVHD) or 60 × 106B6 (acute GVHD) or 60 × 106 B6AF1 (syngeneic transplant) lymphoid cells. GVHD induction was monitored by assaying for suppression of the plaque-forming cell response to sheep red blood cells (SRBCs) as previously described.1 2
Cell lines
P815, a DBA/2-derived mastocytoma cell line; L5178Y, a DBA/2-derived lymphoma; MDW4, a DBA/2-derived leukemia cell line; and 3T6, a Swiss mouse embryo fibroblast line, were maintained in RPMI 1640 plus 5%-to-10% FCS (37°C, 5% CO2/air). Cell lines tested mycoplasma-free using indicator 3T6 cells and 6-mercaptopurine deoxyribose (BRL, Gaithersburg, MD).
Interferon
Mouse ConA supernatant was prepared as a source of IFN-γ by culturing 3 × 106 B6AF1 spleen cells/mL for 48 hours (37°C, 5% CO2/air) in RPMI 1640, 10% FCS, 5 × 10−5-mol/L 2-mercaptoethanol, and 5 μg/mL ConA. Treatment of supernatants with R46A2 antimurine IFN-γ completely inhibited activation of normal Mφ as previously described.4
Charging of apotransferrin with 59Fe
Human apotransferrin (iron-free transferrin, Behringwerke AG, Marburg, Germany) was charged with 59Fe as previously described.31 Briefly, 20 mol of sodium citrate per 1 mol of iron was added to [59Fe]ferric chloride (209 MBq/mL; specific activity, 370- to 925-MBq/mg Fe; NEN, Lachine, Quebec, Canada). The [59Fe]ferric citrate was added to apotransferrin at a ratio of 2.2 mol Fe:1 mol transferrin and the volume adjusted to a final concentration of 250 μmol/L transferrin in 0.6 mol/L NaHCO3−. After 3 hours at room temperature, the solution was extensively dialyzed against normal saline and then PBS. The 59Fe-transferrin was filter-sterilized and stored at 4°C.
Mφ monolayer preparation
Mφ monolayers were prepared for NO, iron-release, cytostasis, and cytotoxicity assays as previously described.4 5 Briefly, peritoneal cells collected 3 days after intraperitoneal injection of 1 mL of aged, sterile Brewer's thioglycollate medium (10% wt/vol) (Difco Labs, Detroit, MI) were washed twice, adjusted to 2 × 106 cells/mL in cold HBSS, and 100-μL aliquots plated into 96-well flat-bottom plates (Costar #3596, Rochester Scientific, Rochester, NY). After 1.5 hours at 37°C (5% CO2/air), cultures were washed vigorously 4 times with warm HBSS and appropriate assay medium added. Monolayers consisted of more than 95% Mφ as determined by morphology, Diffquick staining, and phagocytosis of latex beads (Sigma).
Nitrite assay
Mφ monolayers were cultured in 200 μL of nitrite assay medium (phenol red–free RPMI 1640 containing 1 mmol/L L-arginine, 10% FCS, 10 U/mL penicillin, and 100 μg/mL streptomycin) or nitrite assay medium supplemented as outlined in “Results.” After 48 hours at 37°C (5% CO2/air), 100 μL of supernatant was collected from triplicate cultures and nitrite measured as previously described.5 32 Briefly, 100 μL of supernatant was added to 100 μL of Griess reagent (1% sulfanilamide in 5% phosphoric acid mixed 1:1 vol/vol with 0.1% naphthylethylenediamine dihydrochloride) at room temperature and the absorbance read at 550 nm on an enzyme-linked immuosorbent assay plate reader (SLT Labinstruments, Salzburg, Austria). Samples were blanked against supernatant from wells containing the identical reagents without Mφ. Nitrite concentrations were determined using a sodium nitrite standard curve.
Mφ cytotoxicity assay
Mφ cytotoxic activity was determined as previously described.4,5 33 Briefly, Mφ monolayers were incubated in 100 μL of assay medium either alone or with additional reagents for 4 hours (37°C, 5% CO2/air). Target cells were labeled with 111In by adding 370 kBq of indium [111In]oxine (37 MBq/mL; specific activity, 370 MBq/μg In; Amersham, Oakville, Ontario, Canada) to 7.5 × 106 cells in 0.5 mL of RPMI 1640 plus 10% FCS at room temperature for 10 minutes. The cells were washed 3 times in HBSS, resuspended in assay medium (RPMI 1640 plus 10% FCS, 10 U/mL penicillin, and 100 μg/mL streptomycin), and 104111In-labeled cells in 100 μL of assay medium plated. After 48 hours (37°C, 5% CO2), plates were centrifuged (5 minutes at 500g) and 100 μL of supernatant counted in a gamma counter (LKB, Turku, Finland). The percent specific radioisotope release was calculated from 6 replicates as follows: [(test cpm − spontaneous cpm)/(total cpm − spontaneous cpm)] × 100. Spontaneous release cultures contained normal Mφ and targets in assay medium. Total cpm (counts per minute) was determined from cultures of 104 labeled target cells in 100 μL of assay medium resuspended with 100 μL of 4% Nonidet P-40 (BDH Chemicals).
Mφ-mediated release of iron from dual-labeled target cells
MDW4 target cells were dual-labeled with 59Fe and51Cr by adding 2.5 × 106 cells in exponential growth phase to 10 mL of RPMI 1640 containing 5% FCS and 20 μmol/L 59Fe-transferrin and culturing for 36 hours.59Fe-labeled cells were washed and labeled with51Cr by incubating 2 × 106 cells for 1 hour at 37°C with 3.7 MBq [51Cr]sodium chromate (37 MBq/mL; specific activity, 9.25- to 18.5 GBq/mg Cr; NEN). The cells were washed in HBSS and resuspended in assay medium (RPMI 1640, 10% FCS, 10 U/mL penicillin, and 100 μg/mL streptomycin). Mφ monolayers were incubated for 4 hours (37°C, 5% CO2/air) in 100 μL of assay medium or assay medium supplemented as outlined in “Results,” and 10459Fe, 51Cr dual-labeled tumor cells were added in 100 μL of assay medium. After 18 hours (37°C, 5% CO2), 100-μL aliquots of supernatant were counted in a gamma counter using nonoverlapping channels that independently detect the emission spectra of 59Fe and51Cr. The percent specific release of each isotope was calculated from 4 to 6 replicates as in the Mφ cytotoxicity assay.
Mφ cytostasis assay
Mφ monolayers were incubated 4 hours in 100 μL of assay medium (RPMI 1640, 10% FCS, 10 U/mL penicillin, and 100 μg/mL streptomycin) or medium containing additional reagents as indicated in “Results” and 104 MDW4 target cells added in 100 μL of assay medium. Separate Mφ and target cell cultures were also prepared. After 36 hours at 37°C (5% CO2/air), cultures were pulsed with 37 kBq/well of [methyl-3H]thymidine (TdR) (NEN), incubated for 12 hours, harvested, and counted on a beta counter (LKB). Mφ-mediated cytostasis was determined from the [3H]TdR incorporation in 6 replicate cultures each of Mφ plus target cells, Mφ alone, and targets alone as follows: [(cpm Mφ + target)/[(cpm Mφ) + (cpm target)]] × 100.
In all cases, more than 98% of [(cpm Mφ) + (cpm target)] was accounted for by (cpm target).
Results
Cytotoxic activity of LPS-triggered Mφ during acute GVHD
Acute GVHD occurred following the transplantation of 60 × 106 B6 cells into nonirradiated B6AF1mice, a transplant combination that differs across the entire major histocompatibility complex. The induction of GVHD in transplanted animals was confirmed by the complete suppression of the antibody response to SRBC, a T-cell– dependent antigen (data not shown), as previously demonstrated.1,2,4 5 Mortality was first observed 16 to 18 days posttransplantation and ranged from 70% to 96% by day 25.
During acute GVHD, Mφ are primed and therefore can be triggered by extremely low concentrations of LPS to release TNF-α and NO and to express cytotoxic activity against TNF-α–sensitive target cells.4,5 To determine whether Mφ primed during acute GVHD express alternate mechanisms of cell injury, including NO-mediated killing, we used 3 target cell lines that differ in their sensitivity to Mφ-mediated effector mechanisms. L5178Y cells are killed by activated Mφ through a TNF-α–mediated mechanism,4whereas P815 cells are sensitive to Mφ cytotoxic activity mediated by NO.34 In contrast, MDW4 cells are resistant to Mφ-mediated cytotoxicity.33
Mφ isolated from acute GVHD animals 12 to 14 days posttransplantation and then triggered by LPS were able to kill P815 and L5178Y target cells (Table 1). Addition of LPS did not trigger Mφ-mediated lysis of MDW4 targets. Although MDW4 cells were not killed, microscopic examination of the assay wells indicated that the GVHD Mφ appeared to be mediating a cytostatic effect. Unlike the other 2 targets, MDW4 cells were still present on top of monolayers of acute GVHD Mφ triggered with LPS, but they had not proliferated over the 48-hour assay period (data not shown). In contrast, confluent growth of all 3 target cell lines, including MDW4, occurred on LPS-treated normal Mφ as well as on untreated monolayers of acute GVHD Mφ.
Mφ cytotoxic activity triggered by LPS during acute GVHD
| Treatment in vitro . | Mφ-mediated specific cytotoxicity, % . | |||||
|---|---|---|---|---|---|---|
| MDW4 . | P815 (NO sensitive) . | L5178Y (TNF-α sensitive) . | ||||
| Normal . | Acute GVHD . | Normal . | Acute GVHD . | Normal . | Acute GVHD . | |
| Medium | 0.3 ± 1.2 | 0.4 ± 1.9 | −0.1 ± 0.5 | 3.6 ± 1.2 | 0.2 ± 0.6 | −2.2 ± 1.4 |
| LPS 2.5 ng/mL | 0.6 ± 0.6 | 2.2 ± 2.7 | 2.8 ± 4.3 | 21.2 ± 6.0 | −2.8 ± 1.1 | 29.2 ± 6.6 |
| LPS 50 ng/mL | 1.2 ± 0.7 | 3.5 ± 4.4 | 4.9 ± 0.9 | 33.2 ± 8.2 | −1.3 ± 1.6 | 40.3 ± 3.3 |
| Treatment in vitro . | Mφ-mediated specific cytotoxicity, % . | |||||
|---|---|---|---|---|---|---|
| MDW4 . | P815 (NO sensitive) . | L5178Y (TNF-α sensitive) . | ||||
| Normal . | Acute GVHD . | Normal . | Acute GVHD . | Normal . | Acute GVHD . | |
| Medium | 0.3 ± 1.2 | 0.4 ± 1.9 | −0.1 ± 0.5 | 3.6 ± 1.2 | 0.2 ± 0.6 | −2.2 ± 1.4 |
| LPS 2.5 ng/mL | 0.6 ± 0.6 | 2.2 ± 2.7 | 2.8 ± 4.3 | 21.2 ± 6.0 | −2.8 ± 1.1 | 29.2 ± 6.6 |
| LPS 50 ng/mL | 1.2 ± 0.7 | 3.5 ± 4.4 | 4.9 ± 0.9 | 33.2 ± 8.2 | −1.3 ± 1.6 | 40.3 ± 3.3 |
B6AF1 animals were transplanted with 60 × 106 B6 cells, and their Mφ were isolated 12 to 14 days posttransplantation. Mφ-mediated cytotoxicity was determined against111ln-labeled targets in a 48-hour assay as described in “Materials and methods.” Results are expressed as the mean ± SEM of 3 experiments.
LPS-triggered Mφ mediate cytostasis during acute GVHD
Normal Mφ activated with IFN-γ and LPS induce cytostasis in target cells by inhibiting ribonucleotide reductase, a rate-limiting enzyme in DNA replication that contains nonheme iron.21-24 We investigated whether Mφ that are primed during acute GVHD can be triggered by low concentrations of LPS to mediate a cytostatic effect. On day 14 after transplantation, addition of 2.5 ng/mL LPS to acute GVHD Mφ triggered a potent cytostatic effector mechanism, resulting in the complete inhibition of [3H]TdR uptake by MDW4 target cells (Table2). Mφ isolated from normal B6AF1 or from B6AF1 mice that had received a syngeneic transplant of 60 × 106 B6AF1 cells did not show any evidence of priming—that is, they could not be triggered by LPS. Cytostatic function could not be triggered in these Mφ even when 50 ng/mL LPS was added (data not shown). Expression of cytostatic activity by Mφ from normal or syngeneic transplant recipients was observed only following activation with both IFN-γ and LPS and was effectively inhibited in the presence of anti–IFN-γ. In contrast, LPS-triggered cytostatic activity mediated by acute GVHD-primed Mφ was not reduced by anti–IFN-γ, indicating that the cells had been previously exposed to the initial priming signal.
Mφ cytostatic activity triggered by LPS during acute GVHD
| Treatment in vitro . | Proliferation of MDW4 targets cocultured with Mφ, % . | ||
|---|---|---|---|
| Normal . | Acute GVHD . | Syngeneic . | |
| Medium | 83.1 ± 1.3 | 82.4 ± 5.0 | 86.4 ± 1.2 |
| LPS 2.5 ng/mL | 80.9 ± 3.1 | 0.5 ± 0.1 | 82.9 ± 3.1 |
| LPS 2.5 ng/mL + IFN-γ | 1.7 ± 0.1 | 0.6 ± 0.3 | 1.3 ± 0.9 |
| LPS 2.5 ng/mL + IFN-γ + anti–IFN-γ | 83.0 ± 8.6 | 3.8 ± 0.9 | 87.1 ± 4.2 |
| LPS 2.5 ng/mL + anti–IFN-γ | 89.4 ± 4.9 | 5.6 ± 3.4 | 82.3 ± 3.7 |
| Treatment in vitro . | Proliferation of MDW4 targets cocultured with Mφ, % . | ||
|---|---|---|---|
| Normal . | Acute GVHD . | Syngeneic . | |
| Medium | 83.1 ± 1.3 | 82.4 ± 5.0 | 86.4 ± 1.2 |
| LPS 2.5 ng/mL | 80.9 ± 3.1 | 0.5 ± 0.1 | 82.9 ± 3.1 |
| LPS 2.5 ng/mL + IFN-γ | 1.7 ± 0.1 | 0.6 ± 0.3 | 1.3 ± 0.9 |
| LPS 2.5 ng/mL + IFN-γ + anti–IFN-γ | 83.0 ± 8.6 | 3.8 ± 0.9 | 87.1 ± 4.2 |
| LPS 2.5 ng/mL + anti–IFN-γ | 89.4 ± 4.9 | 5.6 ± 3.4 | 82.3 ± 3.7 |
Proliferation was calculated from 48-hour cultures of Mφ plus targets, Mφ alone, or target cells alone and expressed as the percent incorporation of [3H]TdR as follows: [(cpm Mφ + target)/[(cpm Mφ) + (cpm target)]] × 100. Acute GVHD animals were transplanted with 60 × 106 B6 cells, and syngeneic animals were transplanted with 60 × 106B6AF1 cells. Mφ were isolated on day 14 posttransplantation. Data are presented as mean ± SEM of 3 experiments. The cpm of target cells cultured in medium or medium containing 2.5 ng/mL LPS was 355 ± 19 and 359 ± 13 (×10−3), respectively.
Our observation of cytostasis could be interpreted as resulting from the inhibitory effect of activating agents, such as LPS, on target cell growth. However, [3H]TdR incorporation by target cells in the absence of Mφ was the same whether the cells were grown in medium or medium containing 2.5 ng/mL LPS (Table 2). Although Mφ are able to secrete thymidine,35 which could potentially block the cell cycle or competitively inhibit radiolabeled thymidine uptake, we found that [3H]TdR incorporation was identical for target cells grown in supernatants from cultures of GVHD Mφ grown in either medium alone or in medium containing 2.5 ng/mL LPS (data not shown).
LPS-triggered Mφ production of NO is related to the severity of GVHD and time posttransplantation
Transplantation of either 30 × 106 or 60 × 106 B6 lymphoid cells into B6AF1recipients causes tissue injury and immunosuppression of T- and B-cell function.2,4,7 When housed in a conventional environment, animals that receive 60 × 106 B6 cells start dying approximately day 16 to 18 posttransplantation, and most die of acute GVHD by day 25. Transplantation of 30 × 106 B6 cells results in nonlethal GVHD from which the animals eventually recover. We therefore compared mice undergoing acute and nonlethal GVHD on days 7 and 14 to determine whether the levels of NO produced correspond to the severity of GVHD. Mφ production of NO was examined by measuring NO2− (nitrite), the oxidized by-product of NO. NO production could be detected in the culture supernatants of Mφ isolated from either acute or nonlethal GVHD animals after incubation of the cells with 2.5 ng/mL LPS (Table3). The levels of NO were equivalent in the 2 transplant groups on day 7 after transplantation. By day 14, NO levels were reduced in the nonlethal GVHD group but had more than doubled in the acute GVHD group. Mφ from normal animals released NO only after activation with both IFN-γ and LPS. Incubation of acute GVHD Mφ with LPS and anti–IFN-γ as compared with LPS alone did not result in a significant reduction in the amount of NO. Detection of NO2− in culture supernatants was dependent on the presence of L-arginine, indicating that the measured NO2− production resulted from the oxidation of L-arginine, a process involving NO as an intermediate.36
LPS-triggered Mφ production of NO during acute and nonlethal GVHD
| Treatment in vitro . | Normal B6AF1 . | Nitrite in Mφ culture supernatants (μmol/L) . | |||
|---|---|---|---|---|---|
| Nonlethal GVHD B6AF1 . | Acute GVHD B6AF1 . | ||||
| Day 7 . | Day 14 . | Day 7 . | Day 14 . | ||
| Medium | < 1 | < 1 | < 1 | < 1 | < 1 |
| LPS 2.5 ng/mL | 1.9 ± 0.5 | 14.9 ± 5.8 | 5.4 ± 1.4 | 18.1 ± 2.9 | 45.0 ± 4.3 |
| LPS 2.5 ng/mL + IFN-γ | 50.5 ± 5.1 | 42.8 ± 5.1 | 54.4 ± 3.8 | 31.7 ± 3.0 | 58.1 ± 7.1 |
| LPS 2.5 ng/mL + anti–IFN-γ | < 1 | 14.3 ± 1.4 | 2.4 ± 0.5 | 12.4 ± 1.1 | 35.5 ± 3.1 |
| LPS 2.5 ng/mL + IFN-γ + anti–IFN-γ | 4.7 ± 1.4 | 14.1 ± 5.5 | 7.0 ± 2.0 | 9.3 ± 5.4 | 30.5 ± 3.6 |
| LPS 2.5 ng/mL L-arginine-free medium | < 1 | < 1 | < 1 | < 1 | < 1 |
| Treatment in vitro . | Normal B6AF1 . | Nitrite in Mφ culture supernatants (μmol/L) . | |||
|---|---|---|---|---|---|
| Nonlethal GVHD B6AF1 . | Acute GVHD B6AF1 . | ||||
| Day 7 . | Day 14 . | Day 7 . | Day 14 . | ||
| Medium | < 1 | < 1 | < 1 | < 1 | < 1 |
| LPS 2.5 ng/mL | 1.9 ± 0.5 | 14.9 ± 5.8 | 5.4 ± 1.4 | 18.1 ± 2.9 | 45.0 ± 4.3 |
| LPS 2.5 ng/mL + IFN-γ | 50.5 ± 5.1 | 42.8 ± 5.1 | 54.4 ± 3.8 | 31.7 ± 3.0 | 58.1 ± 7.1 |
| LPS 2.5 ng/mL + anti–IFN-γ | < 1 | 14.3 ± 1.4 | 2.4 ± 0.5 | 12.4 ± 1.1 | 35.5 ± 3.1 |
| LPS 2.5 ng/mL + IFN-γ + anti–IFN-γ | 4.7 ± 1.4 | 14.1 ± 5.5 | 7.0 ± 2.0 | 9.3 ± 5.4 | 30.5 ± 3.6 |
| LPS 2.5 ng/mL L-arginine-free medium | < 1 | < 1 | < 1 | < 1 | < 1 |
Mφ were collected on the day indicated, incubated for 48 hours, and the concentration of nitrite in culture supernatants was determined. Nonlethal GVHD received 30 × 106, and acute GVHD received 60 × 106 B6 cells. Results are the mean ± SEM of 3 experiments. For treatment with 2.5-ng/mL LPS, P < .001 on day 7 compared with day 14 for acute GVHD, and P < .001 for nonlethal GVHD compared with acute GVHD on day 14.
LPS triggers Mφ-mediated release of iron from target cells during acute GVHD
Normal Mφ that are activated in vitro mediate the release of intracellular iron from nonheme iron-containing enzymes in targets cells, resulting in cytostasis.25-27,37 The release or loss of iron from targets can also be reproduced by authentic NO.38 Mφ primed during acute GVHD produced and released NO when triggered by the same low concentrations of LPS that were found to trigger Mφ cytostatic activity (Tables 2 and 3). We therefore examined whether exposure to similarly low levels of LPS during acute GVHD could trigger Mφ-mediated release of iron from target cells undergoing cytostasis.
Dual labeling with 59Fe and 51Cr was used to distinguish between cytostatic mechanisms that selectively mediate the loss of intracellular iron and cytotoxic effects that result in the nonspecific release of 51Cr-labeled cytoplasmic proteins. Target cells were physiologically labeled by growing them in medium containing [59Fe]transferrin, thereby incorporating59Fe into nonheme iron-containing enzymes. This was followed by nonspecific labeling with [51Cr]sodium chromate.
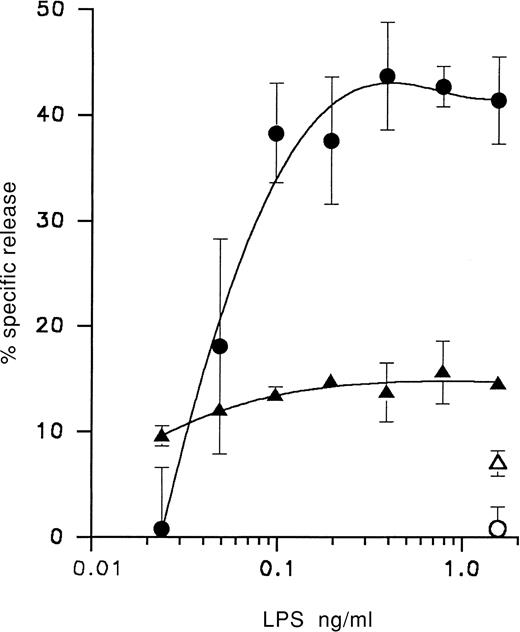
Mφ isolated from acute GVHD animals 14 days posttransplantation could be triggered with 2.5 ng/mL LPS to selectively release59Fe from dual-labeled target cells without an equivalent release of 51Cr (Table 4). Mφ from normal animals did not selectively cause 59Fe release from targets unless activated with both IFN-γ and LPS and the activity was inhibited by the addition of anti–IFN-γ (Table 4). In contrast, iron release mediated by Mφ primed in vivo during GVHD and then incubated with LPS was not significantly reduced by anti–IFN-γ. Target cells single-labeled with 59Fe and cultured for up to 48 hours in the presence of recombinant TNF-α, without any Mφ, did not release iron (Table 4). During acute GVHD, Mφ were triggered by LPS in a dose-dependent manner to induce iron loss from target cells (Figure 1). Only minor variations in release of 51Cr occurred over the same LPS concentration range that triggered 59Fe release, indicating that loss of iron was independent of 51Cr release. As indicated, release of intracellular 59Fe increased steadily over an LPS concentration range of 0.02 to 0.3 ng/mL.
Release of intracellular iron mediated by LPS-triggered Mφ during acute GVHD
| Treatment in vitro . | Specific release of59Fe and 51Cr from dual-labeled MDW4 target cells, % . | |||
|---|---|---|---|---|
| Normal . | Acute GVHD . | |||
| 59Fe . | 51Cr . | 59Fe . | 51Cr . | |
| Medium | −0.1 ± 0.1 | 0.1 ± 0.1 | −0.7 ± 1.9 | −0.4 ± 1.6 |
| LPS 2.5 ng/mL | −0.7 ± 2.1 | −1.6 ± 1.8 | 41.4 ± 5.3 | 10.9 ± 2.0 |
| LPS 2.5 ng/mL + IFN-γ | 38.0 ± 10.2 | 9.0 ± 3.1 | 41.5 ± 7.8 | 9.5 ± 2.1 |
| LPS 2.5 ng/mL + anti–IFN-γ | 3.1 ± 2.5 | −0.3 ± 0.6 | 34.3 ± 12.1 | 3.0 ± 4.8 |
| LPS 2.5 ng/mL + IFN-γ + anti–IFN-γ | 4.9 ± 3.3 | −2.0 ± 1.8 | 35.2 ± 8.8 | 12.0 ± 4.8 |
| Treatment in vitro . | Specific release of59Fe and 51Cr from dual-labeled MDW4 target cells, % . | |||
|---|---|---|---|---|
| Normal . | Acute GVHD . | |||
| 59Fe . | 51Cr . | 59Fe . | 51Cr . | |
| Medium | −0.1 ± 0.1 | 0.1 ± 0.1 | −0.7 ± 1.9 | −0.4 ± 1.6 |
| LPS 2.5 ng/mL | −0.7 ± 2.1 | −1.6 ± 1.8 | 41.4 ± 5.3 | 10.9 ± 2.0 |
| LPS 2.5 ng/mL + IFN-γ | 38.0 ± 10.2 | 9.0 ± 3.1 | 41.5 ± 7.8 | 9.5 ± 2.1 |
| LPS 2.5 ng/mL + anti–IFN-γ | 3.1 ± 2.5 | −0.3 ± 0.6 | 34.3 ± 12.1 | 3.0 ± 4.8 |
| LPS 2.5 ng/mL + IFN-γ + anti–IFN-γ | 4.9 ± 3.3 | −2.0 ± 1.8 | 35.2 ± 8.8 | 12.0 ± 4.8 |
Mφ-mediated percent specific release from dual-labeled MDW4 target cells was determined in an 18-hour assay. Acute GVHD animals received 60 × 106 B6 cells. Mφ were isolated on day 14. Results are the mean ± SEM of 3 experiments. In the absence of Mφ, the percent specific release of 59Fe from cells single-labeled with 59Fe and incubated for 48 hours with 500 U/mL recombinant TNF-α equaled 0.1% ± 1.5% and −0.6% ± 1.5% for incubation with 500 U/mL recombinant TNF-α plus 1 μg/mL actinomycin D.
LPS dose response for Mφ-mediated release of intracellular iron from target cells during acute GVH.
Target cell release of 59Fe (○) and 51Cr (▵) mediated by Mφ from normal B6AF1 mice and release of 59Fe (●) and 51Cr (▴) mediated by Mφ from acute GVHD B6AF1 mice transplanted 14 days previously with 60 × 106 B6 cells. Mφ-mediated radioisotope release was determined using 59Fe,51Cr dual-labeled MDW4 cells in an 18-hour assay as described in “Materials and methods.” Each value represents the mean ± SD of 4 replicates of pooled Mφ effector cells. Similar results were obtained in 2 separate experiments.
LPS dose response for Mφ-mediated release of intracellular iron from target cells during acute GVH.
Target cell release of 59Fe (○) and 51Cr (▵) mediated by Mφ from normal B6AF1 mice and release of 59Fe (●) and 51Cr (▴) mediated by Mφ from acute GVHD B6AF1 mice transplanted 14 days previously with 60 × 106 B6 cells. Mφ-mediated radioisotope release was determined using 59Fe,51Cr dual-labeled MDW4 cells in an 18-hour assay as described in “Materials and methods.” Each value represents the mean ± SD of 4 replicates of pooled Mφ effector cells. Similar results were obtained in 2 separate experiments.
NO mediates LPS-triggered Mφ cytostatic activity during acute GVHD
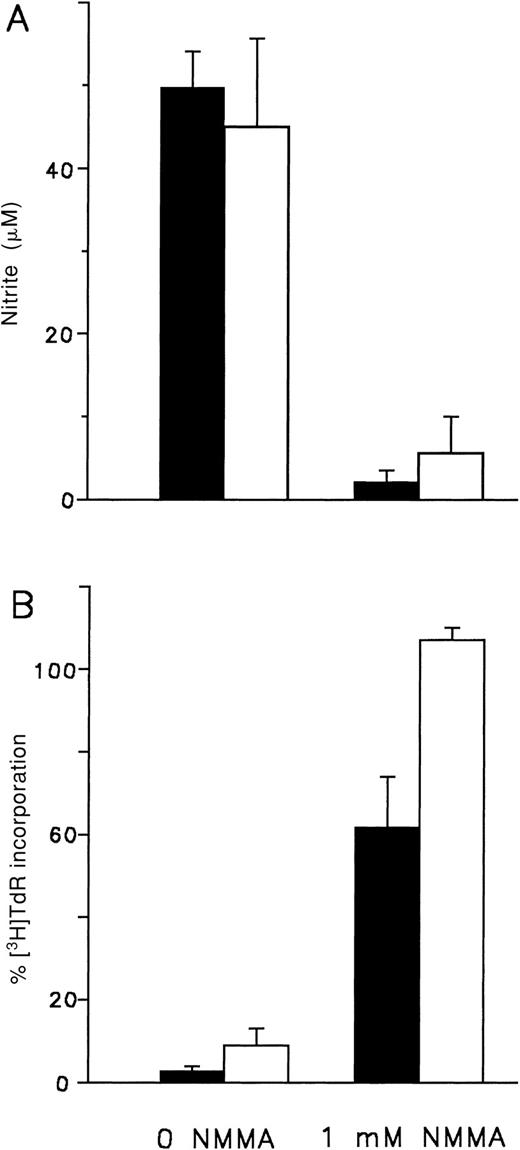
To determine whether acute GVHD Mφ mediate their cytostatic activity as a result of NO production and release, we studied the effect of inhibiting Mφ synthesis of NO. Accumulation of NO in culture supernatants of LPS-triggered day 14 acute GVHD Mφ was inhibited by the addition of NMMA, a competitive inhibitor of inducible NOS (iNOS) (Figure 2A). Inhibition of the cytostatic effect mediated by LPS-triggered acute GVHD Mφ was also observed in the presence of NMMA. Addition of the inhibitor restored target cell proliferation to approximately 60% (Figure 2B).
NMMA inhibits NO production and cytostatic activity mediated by acute GVHD-primed, LPS-triggered Mφ.
Acute GVHD Mφ (▪) from B6AF1 animals transplanted 14 days previously with 60 × 106 B6 cells were activated with 2.5 ng/mL LPS, and normal Mφ (■) from B6AF1animals were activated with 2.5 ng/mL LPS and IFN-γ. (A) The concentration of NO in 48-hour culture supernatants was determined as described in “Materials and methods.” Mφ were cultured in phenol red–free RPMI 1640 containing 1.0 mmol/L L-arginine plus 10% FCS with or without NMMA as indicated. Results represent the mean ± SD of triplicates. Similar results were obtained in 2 separate experiments. (B) Mφ-mediated cytostasis of MDW4 cells was determined as described in “Materials and methods.” Mφ and targets were cultured for 48 hours in RPMI 1640 plus 10% FCS containing 1.0 mmol/L L-arginine either with or without NMMA as indicated. Results represent the mean ± SEM of 3 experiments.
NMMA inhibits NO production and cytostatic activity mediated by acute GVHD-primed, LPS-triggered Mφ.
Acute GVHD Mφ (▪) from B6AF1 animals transplanted 14 days previously with 60 × 106 B6 cells were activated with 2.5 ng/mL LPS, and normal Mφ (■) from B6AF1animals were activated with 2.5 ng/mL LPS and IFN-γ. (A) The concentration of NO in 48-hour culture supernatants was determined as described in “Materials and methods.” Mφ were cultured in phenol red–free RPMI 1640 containing 1.0 mmol/L L-arginine plus 10% FCS with or without NMMA as indicated. Results represent the mean ± SD of triplicates. Similar results were obtained in 2 separate experiments. (B) Mφ-mediated cytostasis of MDW4 cells was determined as described in “Materials and methods.” Mφ and targets were cultured for 48 hours in RPMI 1640 plus 10% FCS containing 1.0 mmol/L L-arginine either with or without NMMA as indicated. Results represent the mean ± SEM of 3 experiments.
Discussion
In this study, we have examined the cytostatic function of Mφ during the development of GVHD. Our results demonstrate that, as a result of priming during acute GVHD, Mφ mediate a strong cytostatic effect when triggered by normally insignificant amounts of LPS. Cytostasis of target cells, which is accompanied by the release of intracellular iron, can be reversed by inhibition of macrophage NO production. Furthermore, Mφ production of NO in response to LPS reflects the severity of GVHD. During nonlethal GVHD, NO production is transiently increased in contrast to the steadily increased production that occurs during acute GVHD.
During the development of acute GVHD, increased production of IFN-γ,10,11 combined with entry and accumulation of bacteria-derived LPS,4,7,12-14 results in Mφ activation and release of inflammatory products including TNF-α, NO, and interleukin (IL)-1.4,5,39 In acute GVHD, injury to the proliferating intestinal epithelium and suppression of lymphocyte proliferation are prevented by inhibitors of NO.28-30Suppression of ConA-induced lymphocyte proliferation in cocultures of GVHD plus normal splenic lymphocytes can be reversed by depletion of L-leucine methyl ester–sensitive cells expressing Mφ surface markers and intracellular iNOS.40 In human and experimental animal transplant recipients, the symptoms of GVHD are preceded by an increase in serum levels of NO oxidation products—that is, NO2−and NO3−.41 42
Authentic NO directly mediates target cell cytostasis by inhibiting a nonheme iron-containing enzyme, ribonucleotide reductase.24 Similarly, normal Mφ activated with both IFN-γ and LPS produce NO and mediate cytostasis by inhibiting target cell ribonucleotide reductase.21,24 Exposure to increasing amounts of IFN-γ results in a significant reduction in the amount of LPS needed to trigger Mφ synthesis of inflammatory products.8,9 As a result of IFN-γ production during the development of acute GVHD, Mφ become primed and, therefore, normally insignificant quantities of LPS trigger production of NO and TNF-α.4,5 The cytostatic activity of Mφ during acute GVHD was measured using MDW4, a target cell line that is resistant to Mφ-mediated killing33 (Table 1). Target cell proliferation was completely inhibited by acute GVHD Mφ, and cytostasis could be triggered by concentrations of LPS as low as 2.5 ng/mL (Table 2). Addition of NMMA effectively inhibited NO production by IFN-γ plus LPS-activated normal Mφ and by LPS-triggered GVHD Mφ (Figure 2A). Cytostatic function was also inhibited by NMMA, although cytostasis mediated by GVHD Mφ was not completely reversed (Figure 2B). NO-independent mechanisms could also contribute to the loss of proliferation.43 Activation of additional cytostatic mechanisms appears to occur during the prolonged in vivo priming period that GVHD Mφ undergo prior to being triggered by LPS. Target organs injured during acute GVHD, including the gut and skin, contain subpopulations of proliferating stem cells that may be particularly sensitive targets of the direct cytostatic effector mechanism(s) mediated by activated Mφ.15-18
We previously demonstrated that the severity of GVHD is directly related to the level of Mφ priming.4 Sensitivity to LPS is much greater during acute than nonlethal GVHD, as shown by the large reduction in LPS needed to induce lethal endotoxic shock and TNF-α production.4 A similar relationship was observed for LPS-triggered NO production. In nonlethal GVHD, LPS-triggered Mφ production of NO was observed 7 days after transplantation but was greatly reduced by 14 days (Table 3). In contrast, in acute GVHD, NO production by LPS-triggered Mφ increased between 7 and 14 days posttransplantation. Transient priming of Mφ during nonlethal GVHD probably reflects a decreased level of IFN-γ production because, without continual priming, the ability of Mφ to be triggered by LPS decays with time.5 During acute GVHD, Mφ generation of NO may be further augmented through an autocrine, positive feedback effect of Mφ-derived TNF-α.8 However, addition of neutralizing rat monoclonal or rabbit polyclonal antimurine TNF-α to acute GVHD Mφ did not significantly inhibit LPS-triggered NO generation or Mφ-mediated cytostasis (data not shown).
Increased IFN-γ production and priming of Mφ are key steps in the onset and progression of acute GVHD. Clinical bone marrow transplant recipients have more IFN-γ–producing cells, and the symptoms of acute GVHD are preceded by a marked increase in serum IFN-γ levels and production of neopterin, a cofactor in NO metabolism.10,44 Levels of iNOS expression and NO production in human macrophage lineage cells do not reach the high levels observed in murine macrophages. However, recent studies on human Mφ have convincingly documented iNOS protein and messenger RNA (mRNA) expression as well as NO production under a variety of activation conditions and in several disease states.45 In experimental acute GVHD, the percentage of lymphoid cells expressing IFN-γ mRNA is significantly increased.46Transplantations using IFN-γ knockout donors lead to longer survival times.47 A shift in donor T-cell populations from Th1 to Th2 reduces the number of IFN-γ–producing cells, inhibits ConA-induced NO production by splenocytes, reduces sensitivity to the lethal effects of LPS, and prevents acute GVHD.30,48,49 In acute GVHD, both IFN-γ and NO mRNA are expressed within target organs, and augmented IFN-γ expression persists despite the onset of T-cell immunosuppression.5 IL-12 p40 mRNA is also detectable in target organs and in Mφ during acute GVHD, and anti–IL-12 treatment polarizes the recipient cytokine profile to a Th2 type and can prevent acute GVHD.5,50 Activation of effector cells, including natural killer (NK) cells and Mφ, thus appears to be mediated by IL-12–induced IFN-γ production, allowing for continual priming of Mφ during acute GVHD.5
Mφ-derived and authentic NO inactivate nonheme iron-containing enzymes via the action of NO on iron centers in the molecules. Activated Mφ mediate target cell release of intracellular iron,25-27 losses of iron from nonheme iron-containing mitochondrial enzymes,26,27 and formation of iron-nitrosyl compounds in mitochondria.51 Loss of intracellular iron is directly mediated by NO.38 During acute GVHD, LPS-triggered Mφ mediated the selective release of approximately 40% of intracellular iron from target cells over an 18-hour period (Table4, Figure 1). Although NO is recognized as a mediator of iron release from target cells, additional Mφ mechanisms may also contribute to release of iron.52 In human transplant recipients, the appearance of bleomycin-reactive, that is, free, nontransferrin-bound plasma iron, is associated with the development of acute GVHD.53 Chemically reactive iron released from target cells can act as a catalyst in the Fenton reaction between hydrogen peroxide and superoxide anions produced by Mφ, resulting in hydroxyl radical formation, and thus could significantly contribute to tissue damage.54
Large numbers of Mφ within the gastrointestinal tract, splenic red pulp, and liver (ie, Kupffer cells) intercept bacteria that enter from the external environment via translocation through the intestinal epithelium.55,56 During the development of GVHD, initial damage to the intestinal epithelium is mediated directly by IFN-γ57,58 or indirectly via activation of NK or NK-like effector cells.2,59,60 As a result, increased translocation of gram-negative bacteria or bacteria-derived LPS triggers IFN-γ–primed Mφ to produce NO and TNF-α, leading to further epithelial injury.4,5,12,13 The barrier function of epithelial tissues can be further compromised by the NO-mediated cytostatic effect of activated Mφ on rapidly proliferating epithelial stem cells in the skin, gut, and liver. Despite the immunosuppression that accompanies GVHD, entry of live gram-negative bacteria can initially be well tolerated and infections avoided as a result of IFN-γ–mediated activation of Mφ bactericidal function.11 Nevertheless, the end result is a progressive accumulation of LPS in the liver and spleen.13 These events initiate an inflammatory cascade of acute-phase secretory products, including NO, TNF-α, and IL-1, that participate in pathologic tissue injury.4-7,39 The inflammatory cascade is magnified as the capacity of the liver to bind, detoxify, and excrete LPS becomes saturated and LPS begins to appear in the serum.4 13 LPS entry into the circulation and delivery to target organs further escalates release of inflammatory mediators because of the triggering effect of LPS at sites throughout the body where primed Mφ are found and finally results in septic shock and death.
Acknowledgments
We are grateful to Michel Emond, Ailsa Lee Loy, and Rosmarie Siegrist-Johnstone for their expert technical assistance, and we thank Ania Wilczynska and Jane Barraclough for assistance in the preparation of radiolabeled transferrin. We gratefully acknowledge Dr John Hibbs Jr for critical review of the manuscript.
Supported by grants from the Medical Research Council of Canada.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Frederick Nestel, Department of Physiology, McGill University, McIntyre Medical Sciences Bldg, 3655 Drummond St, Montreal, Quebec, Canada, H3G 1Y6; e-mail:fnestel@med.mcgill.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal