Abstract
Accumulating evidence suggests that successful metastatic spread may depend on the ability of tumor cells to undergo extensive interactions with platelets. However, the mechanisms mediating tumor cell adhesion to platelets under conditions of flow remain largely unknown. Therefore, this study was designed to analyze the ability of 3 human colon carcinoma cell lines (LS174T, COLO205, and HCT-8) to bind to surface-anchored platelets under flow and to identify the receptors involved in these processes. Immobilized platelets support LS174T cell adhesion at wall shear stresses up to 1.4 dyn/cm2. Our data suggest that platelets primarily recruit LS174T cells through a 2-step, sequential process of adhesive interactions that shares common features but is distinct from that elaborated for neutrophils. Platelet P-selectin mediates LS174T cell tethering and rolling in a PSGL-1- and CD24-independent manner. Moreover, platelet αIIbβ3-integrins appear to be capable of directly capturing LS174T cells from the fluid stream, and also convert instantaneously transient tethers initiated by P-selectin into stable adhesion. This step is at least partially mediated by von Willebrand factor, but not fibrinogen or fibronectin, that bridges platelet αIIbβ3 with a yet unidentified receptor on the LS174T cell surface via an RGD-dependent mechanism. The sequential engagement of platelet P-selectin and αIIbβ3 is also requisite for the optimal adhesion of COLO205. Furthermore, HCT-8 cells, which fail to interact with P-selectin, tether minimally to surface-anchored platelets under flow, despite their extensive adhesive interactions under static conditions. This cascade of events depicts an efficacious process for colon carcinoma arrest at sites of vascular injury.
Introduction
Blood-borne metastasis is a highly regulated and dynamic process in which cancerous cells separate from a primary tumor, migrate across blood vessel walls into the bloodstream, and disperse throughout the body to generate new colonies. During their transit into the circulatory system, tumor cells are exposed to fluid mechanical forces, plasma proteins, and vascular cells such as platelets and neutrophils, all of which may affect their survival and extravasation from the vasculature.
Several lines of evidence suggest that platelets facilitate hematogenous dissemination of tumor cells. The most convincing evidence is the inhibition of metastasis by experimental thrombocytopenia and the restoration of metastatic potential by platelet infusion.1,2 Morphologic observations of tumor cells arrested in capillaries have documented the close association of tumor cells with activated platelets.3 However, the mechanisms by which platelets assist tumor metastasis are not clearly understood. Some studies suggest that platelets, by adhering to tumor cells, provide a protective shield that masks them from the cytotoxic activity of natural killer cells.4,5 Alternatively, platelets may facilitate tumor extravasation by potentiating tumor cell adhesive interactions with extracellular matrix proteins of the vessel wall,4,6,7 thereby assisting tumor cells to escape from the circulation into the interstitium and form a distinct secondary colony. Moreover, activated platelets may release a number of growth factors such as platelet-derived growth factor that has been reported to stimulate the growth of highly metastatic colon carcinoma cells.4 Along with these lines, it has recently been hypothesized that platelets may contribute to tumor-induced angiogenesis.8 Therefore, elucidating the molecular mechanisms of tumor cell-platelet adhesion may eventually provide a rational basis for the development of novel therapeutic strategies to combat metastasis.
Platelets have been reported to bind to certain melanoma, breast, and colon cancer cells through the integrin receptor αIIbβ3 on the platelet surface.4,7,9-11 The tumor cells that interact with platelets in an αIIbβ3-dependent manner may actually themselves express an αIIbβ3-like complex,4,10,11 and their interaction with platelets may therefore be mechanistically similar to that of homotypic platelet aggregation.12 Alternatively, platelets may attach to small-cell lung and colon carcinoma via P-selectin in an αIIbβ3-independent mechanism.13-16 It is currently unknown whether platelet P-selectin and αIIbβ3 cooperate to mediate adhesive interactions between platelets and certain types of tumor cells.
Most of the prior work on tumor cell adhesion to platelets was carried out in vitro mainly under static conditions.9,14-16However, these interactions typically occur in the bloodstream, in the presence of shear flow. As has been argued in the literature, data obtained in vitro using static assays may not be relevant to the fluid dynamic environment of the vasculature.17 For instance, melanoma cells fail to adhere directly to collagen I extracellular matrix under flow, despite their ability to interact extensively with this matrix under static conditions.6Furthermore, previous studies on leukocyte-endothelial cell adhesion as well as on platelet binding to extracellular matrix proteins have clearly established that the shear stress generated by blood flow critically affects cell adhesive interactions.12,18,19Consequently, the present study was undertaken to investigate the molecular mechanisms of tumor cell attachment to surface-bound platelet layers under flow conditions. Prior work has shown that platelets adherent to thrombogenic surfaces support neutrophil recruitment through a multistep, sequential process of adhesive interactions. P-selectin expressed on the surface of immobilized, activated platelets mediates the initial tethering and rolling of neutrophils in shear flow.20,21 Activation-dependent attachments of β2-integrin receptors on neutrophils to platelet-associated fibrinogen22,23 and intracellular adhesion molecule (ICAM)-223 convert these transient rolling interactions into stable neutrophil adhesion. We therefore hypothesized that tumor cell adhesion to surface-bound platelets may follow a similar cascade of adhesive interactions. To test this hypothesis we chose a human colon carcinoma cell model, because colon cancer is prevalent in the United States. The LS174T human colon adenocarcinoma cell line was chosen because it has been extensively characterized and widely used in a number of diverse assays ranging from characterization of surface adhesion molecules to cell-substrate and cell-cell interaction studies.14,16,24-26 For comparison purposes, we also looked at sLex-bearing (COLO205) and sLex-negative (HCT-8) colon adenocarcinoma cell lines.9,10 14
The present study demonstrates that surface-adherent platelets support extensive adhesive interactions of sLex-bearing colon cancer cells, L5174T and COLD205, under flow conditions simulated in vitro using a parallel-plate flow chamber. Our results indicate that tumor cell tethering and rolling involve binding of a sialylated molecule on the tumor cell surface to platelet P-selectin that is distinct from P-selection glycoprotein ligand-1 (PSGL-1) and CD24. Furthermore, platelet αIIbβ3 can directly capture tumor cells from the fluid stream and also stabilize transient tethering interactions mediated by P-selectin.
Materials and methods
Monoclonal antibodies
The monoclonal antibodies (mAbs) AK4 (blocking anti-P-selectin),27 HIP8 (blocking anti-αIIbβ3), HIP1 (blocking anti-CD42b), VBP36.7 (blocking anti-β2-integrin), 2H5 (anti-CD15s), and ML5 (nonblocking anti-CD24) were from Pharmingen, San Diego, CA. The blocking mAbs PL1 (anti-PSGL-1), P2 (anti-αIIb), SZ21 (anti-β3), FA6-152 (anti-GPIV), and DIG10VL2 (antifibrinogen) were purchased from Immunotech, Westbrook, ME. A mAb NYB-4.2 (blocking antifibrinogen) was obtained from Accurate, Westbury, NY. The blocking mAbs FB12 (anti-α1), P1E6 (anti-α2), ASC-1 (anti-α3), P1H4 (anti-α4), NKI-GoH3 (anti-α6), NKI-M9 (anti-αv), ASC-3 (anti-β4), LM609 (anti-αvβ3), and B3A (anti-β3) were from Chemicon, Temecula, CA. A blocking anti-β1 mAb 4B4 was purchased from Coulter, Miami, FL. GRGDSP and GRGESP synthetic peptides and mAb P1D6 (blocking anti-α5) were from Life Technologies, Gaithersburg, MD. A nonblocking mAb to P-selectin (AC1.2) was obtained from Becton Dickinson, San Jose, CA. A blocking antifibronectin mAb type 228 was purchased from Calbiochem, San Diego, CA. Isotype-matched IgG, IgM mAbs and a polyclonal anti-von Willebrand factor (vWf) were purchased from Sigma, St Louis, MO. Rb6096, a rabbit polyclonal anti-L1 antibody, and EP5C7, an anti-P-/E-selectin mAb, were generously provided by Drs John Hemperly (Becton Dickinson) and Ellen L. Berg (Protein Labs), respectively. XV454 is a nonpeptide small-molecule platelet αIIbβ3 antagonist with comparable platelet αIIbβ3-binding kinetics to c7E3.29
Cell lines and culture
The LS174T, COLO205, and HCT-8 human colon adenocarcinoma cells were obtained from the American Type Culture Collection, and cultured in the recommended medium. COLO205 were harvested with a nonenzymatic cell dissociation solution (Specialty Media, Lavallette, NJ).30 In contrast, LS174T and HCT-8 cells were detached from culture flasks by mild trypsinization (0.25% trypsin/EDTA for 2 minutes at 37°C; Life Technologies), and subsequently incubated at 37°C for 2 hours to regenerate surface glycoproteins, as previously described.14 Earlier work has shown that this treatment does not affect colon cancer cell ability to interact with E-, L-, and P-selectin.14 Subsequently, tumor cells were washed once, resuspended in serum-free media containing 0.1% bovine serum albumin (BSA) at a concentration of 1 × 106 cells/mL, and stored at 4°C for no longer than 5 hours before use in adhesion assays or flow cytometry.
Enzyme treatment
To remove terminal cell surface sialic acid residues, tumor cells (107/mL) were incubated with 0.1 U/mL Vibrio cholerae neuraminidase (Boehringer Mannheim, Indianapolis, IN) for 30 minutes at room temperature.31,32 In independent experiments, tumor cells were treated with 1 U/mL PI-PLC (Sigma, Glyko, Novato, CA) for 1 hour at 37°C to cleave GPI-linked molecules such as CD24.33 Following enzyme treatment, tumor cells were washed once and analyzed by flow cytometry or infused into the flow chamber for adhesion assays.
Generation of P-selectin–coated surfaces
Recombinant P-selectin (R&D Systems, Minneapolis, MN) was diluted with D-phosphate-buffered saline (D-PBS) containing Ca++/Mg++ to a final concentration of 4 μg/mL, layered on a 35-mm tissue culture dish (Corning, Corning, NY) and allowed to coat overnight at 4°C. Subsequently the dishes were washed 3 times with D-PBS before incubation for 2 hours with D-PBS/1% BSA at 4°C to eliminate nonspecific interactions.
Immobilization of platelet layers on glass slides
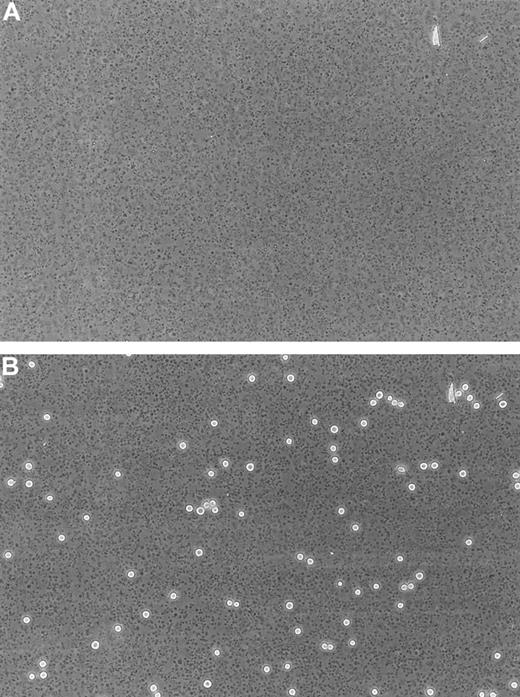
To provide a substrate that readily binds platelets, glass slides (24 × 50 mm) were coated with 3-aminopropyltriethoxysilane (APES; Sigma).20 Human blood was drawn by venipuncture from healthy volunteers and a patient with Glanzmann thrombasthenia (GT)34 into sodium citrate (0.38% wt/vol) anticoagulant. Platelet-rich plasma (PRP) was prepared by centrifugation of whole blood at 160g for 15 minutes. The PRP count was adjusted to 2 × 108/mL before being bound to APES-treated glass slides for 60 minutes. Nonspecific binding was blocked with 0.1% BSA for 10 minutes at 37°C. Under these conditions, a confluent layer of platelets was formed as evaluated by light microscopy for each experiment (Figure 1A). The density (17 580 ± 971/mm2) and confluency of platelet layers were not affected during the flow experiment.
Immobilized human platelets.
Phase-contrast photomicrographs (× 20) of a confluent layer of immobilized human platelets (17 580 ± 971/mm2, n = 5) bound to an APES-treated glass slide before (A) and after (B) the perfusion of LS174T cells for 10 minutes at a shear stress level of 0.8 dyn/cm2.
Immobilized human platelets.
Phase-contrast photomicrographs (× 20) of a confluent layer of immobilized human platelets (17 580 ± 971/mm2, n = 5) bound to an APES-treated glass slide before (A) and after (B) the perfusion of LS174T cells for 10 minutes at a shear stress level of 0.8 dyn/cm2.
Flow adhesion assays
Tumor cell adhesion to immobilized platelets (and purified P-selectin) was quantitated under flow conditions using a parallel-plate flow chamber. A platelet-coated glass slide was assembled to a flow chamber (150-μm channel depth, 1.26-cm channel width) and mounted on the stage of an inverted microscope (Nikon TE300) equipped with × 10 and × 20 phase objectives (Nikon, Melville, NY), a × 0.55 projection lens (Nikon) and a CCD100 camera (Dage-MTI, Michigan City, IN) connected to a VCR and a TV monitor. Surface-adherent platelets were then incubated with either 1 U/mL thrombin (only in experiments involving LS174T cells) or D-PBS/0.1% BSA for 10 minutes at 37°C. After washing the platelet layer with D-PBS/0.1% BSA for approximately 2 minutes, tumor cells were perfused through the chamber for 10 minutes at the appropriate flow rates to obtain wall shear stresses of 0.6 to 1.4 dyn/cm2, thereby mimicking the fluid mechanical environment of the microcirculation and postcapillary venules. The flow system was maintained at 37°C in an air curtain incubator. Interactions between tumor cells and surface-adherent platelets were visualized in real-time by phase-contrast videomicroscopy. A single field of view (× 20; 0.14 mm2) was monitored during the 10 minutes of the experiment, and at the end 5 fields of view (× 10; 0.55 mm2) were monitored for 15 seconds each. Three parameters were quantified in the analysis: (1) the number of total interacting cells during the entire 10-minute experiment; (2) the number of firmly adherent cells after 10 minutes of shear flow; and (3) the average rolling velocity. Interacting cells were defined as those that interacted with the platelet layer for at least 2 seconds, and included both immediately arrested and fast rolling or skipping cells that interacted intermittently with the monolayer. Their number was determined manually by reviewing the videotapes. Firmly adherent cells were considered as those that remained stationary for at least 10 seconds at the end of the 10-minute run. To quantify their number, images were digitized from the videotape recorder using a Scion frame grabber and a personal computer and processed with OPTIMAS 6.1 software package (Argis-Schoen Vision Systems Inc, Alexandria, VA). Rolling velocity was computed as the distance traveled by the centroid of the translating cell divided by the time interval, using OPTIMAS 6.1 software.
For some inhibition studies, tumor cells were pretreated for 10 minutes with mAbs (20 μg/mL, unless otherwise stated), which were kept present during the flow assays. For others, surface-adherent platelets were preincubated with mAbs (20 μg/mL), XV454 (150 nmol/L), GRGDSP or GRGESP (500 μmol/L), or anti-vWf (1:250 dilution) for 10 minutes during the thrombin or buffer incubation. Saturating concentrations were also maintained in the flow buffer only for the GRGDSP and GRGESP peptides, anti-vWf, antifibronectin, and antifibrinogen antibodies.
The extent of LS174T cell attachment to surface-anchored platelets was unaffected by platelet exposure to thrombin before tumor cell perfusion (data not shown), indicating a high degree of platelet activation had occurred as previously shown.35 Furthermore, LS174T cell adhesion to immobilized platelets was unaltered by the presence or absence of an IgG control mAb (untreated samples: 249 ± 54 firmly adherent cells/mm2 versus nonspecific IgG-treated samples: 247 ± 39 firmly adherent cells/mm2; n = 3). Similar results were also observed for COLO205 and HCT-8 cells (data not shown).
Flow cytometry
Indirect single-color immunofluorescence assays were performed to determine tumor cell surface glycoprotein expression.31Tumor cells were incubated with saturating concentrations of each mAb for 30 minutes on ice, and then washed with D-PBS/0.1% BSA. After an additional 30 minutes of incubation with 15 μg/mL phycoerythrin (PE)-labeled IgG or IgM antibody (Vector Laboratories, Burlingame, CA), the specimens were washed again, fixed with 1% formaldehyde, and analyzed in a FACScan flow cytometer (Becton Dickinson). Tumor cells were distinguished from debris on the basis of their characteristic forward- and side-scatter profiles, and the geometric mean PE fluorescence of each specimen was recorded. Appropriate isotype-matched IgG or IgM mAbs were also included for background fluorescence determination.
Statistics
Data are expressed as the mean ± SEM. Statistical significance of differences between means was determined by ANOVA. If means were shown to be significantly different, multiple comparisons by pairs were performed by the Tukey test. Probability values ofP < .05 were selected to be statistically significant.
Results
Surface-adherent platelets support LS174T cell adhesion under flow
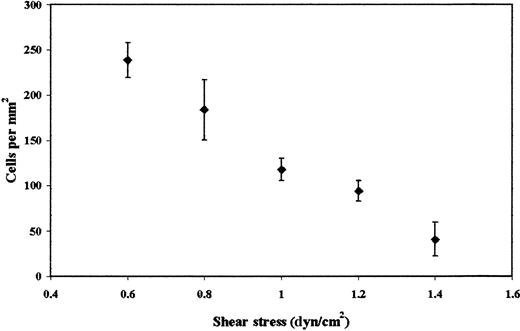
Previous studies have shown that platelets interact with a number of cell lines derived from colon carcinomas, including LS174T, but cell adhesion was studied exclusively under static conditions.14,16 To determine whether LS174T cells also adhere to platelets under flow, tumor cells were perfused through a parallel-plate flow chamber whose lower plate was coated with a layer of platelets (Figure 1). Figure 2 shows that immobilized platelets supported extensive LS174T cell adhesion under flow in a shear stress-dependent manner. A progressive decrease in the extent of adhesion was detected between 0.6 dyn/cm2and 1.4 dyn/cm2. No interaction was observed at higher stress levels. In distinct contrast, freshly isolated neutrophils tethered to platelets even at shear stress levels in the excess of 3.0 dyn/cm2 (data not shown), an observation that is in agreement with previously published work.21 LS174T cells that tethered from the fluid stream to the platelet layer became firmly adherent instantaneously (Figure 1B). Under these control settings, rolling or irregular translocation of LS174T cells along the platelet surface were rather rare events (firmly adherent cells: 184 ± 18 cells/mm2 versus total interacting cells: 208 ± 11 cells/mm2 at 0.8 dyn/cm2).
Effect of wall shear stress on LS174T cell adhesion to surface adherent platelets.
Confluent layers of platelets were incubated with thrombin (1 U/mL) for 10 minutes. LS174T cells (106/mL) were then perfused over the platelet layer for 10 minutes, at which time the number of adherent tumor cells were counted (diamonds). Values are mean ± SEM of 4 experiments.
Effect of wall shear stress on LS174T cell adhesion to surface adherent platelets.
Confluent layers of platelets were incubated with thrombin (1 U/mL) for 10 minutes. LS174T cells (106/mL) were then perfused over the platelet layer for 10 minutes, at which time the number of adherent tumor cells were counted (diamonds). Values are mean ± SEM of 4 experiments.
Roles of platelet P-selectin and αIIbβ3-integrins in LS174T cell adhesion to immobilized platelets under flow
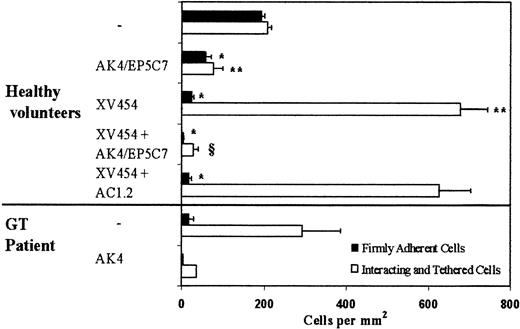
Ensuing experiments focused on the elucidation of the molecular pathways involved in the adhesive interactions between LS174T cells and surface-anchored platelets at a wall shear stress of 0.8 dyn/cm2. Prior work using static assays showed that binding of activated platelets to LS174T cells is P-selectin dependent, whereas the extent of this heterotypic interaction was not affected by a blocking antibody specific for αIIbβ3.14 Hence, as a first step, we examined the potential role of platelet P-selectin in these adhesive interactions under dynamic flow conditions. The results indicate that incubation of the platelet layer with a function-blocking anti-P-selectin antibody alone resulted in an approximately 70% to 75% reduction of adhesion (Figure 3).
Effects of platelet P-selectin and αIIbβ3 antagonists on LS174T cell adhesion to surface-adherent platelets under conditions of flow (0.8 dyn/cm2).
Platelets were isolated either from healthy volunteers or a patient with Glanzmann thrombasthenia (GT). Closed bars represent stable adherent cells (stationary for > 10 seconds) counted at the end of the 10-minute experiment, whereas open bars represent the total interacting cells counted throughout the entire 10-minute experiment. Immobilized platelets were treated with agents specific for P-selectin and αIIbβ3 during the 10-minute thrombin incubation. XV454 (blocking αIIbβ3function); AC1.2 (nonblocking anti-P-selectin mAb); AK4 or EP5C7 (blocking P-selectin function). *, **P < .05 with respect to no-treatment control. § P < .05 with respect to XV454-treated specimens. Values are mean ± SEM (n = 5-31 healthy volunteers; n = 1 GT patient performed in triplicate).
Effects of platelet P-selectin and αIIbβ3 antagonists on LS174T cell adhesion to surface-adherent platelets under conditions of flow (0.8 dyn/cm2).
Platelets were isolated either from healthy volunteers or a patient with Glanzmann thrombasthenia (GT). Closed bars represent stable adherent cells (stationary for > 10 seconds) counted at the end of the 10-minute experiment, whereas open bars represent the total interacting cells counted throughout the entire 10-minute experiment. Immobilized platelets were treated with agents specific for P-selectin and αIIbβ3 during the 10-minute thrombin incubation. XV454 (blocking αIIbβ3function); AC1.2 (nonblocking anti-P-selectin mAb); AK4 or EP5C7 (blocking P-selectin function). *, **P < .05 with respect to no-treatment control. § P < .05 with respect to XV454-treated specimens. Values are mean ± SEM (n = 5-31 healthy volunteers; n = 1 GT patient performed in triplicate).
Subsequent experiments aimed to identify the platelet receptor(s) responsible for the residual LS174T cell attachment to immobilized platelets in the presence of an anti-P-selectin antibody. The integrin αIIbβ3 is the most abundant receptor on the platelet surface (60 000-80 000 copies per activated platelet12,36), and has previously been implicated in the interactions of platelets with a variety of melanoma and colon (other than LS174T and COLO205) cancer cells.4,7 9-11 To this end, surface-adherent platelets were treated concurrently with specific antagonists of P-selectin and αIIbβ3-integrins during the 10-minute thrombin incubation and before LS174T cell perfusion. Figure 3 shows that simultaneous blockade of platelet P-selectin and αIIbβ3-integrins essentially eliminated LS174T cell adhesion to immobilized platelets.
To further assess the role of platelet αIIbβ3 in these heterotypic interactions, platelet layers were incubated with agents that specifically block αIIbβ3-integrin alone. Use of either XV454 or a function-blocking anti-αIIbβ3 mAb, HIP8, yielded similar results (Figures 3 and 4). The data indicate that platelet αIIbβ3 blockade essentially abolished LS174T firm adhesion while dramatically (P < .05) increasing the number of tethered cells (Figure3). This abrupt increase in the number of total interacting cells is due to the fact that LS174T cells that tethered upstream of our field of view failed to develop firm adhesion (as did the control specimens) and continued to translocate along the platelet layer eventually entering the field of observation.32,37 38 This LS174T cell tethering was mediated by P-selectin as evidenced by the absence of adhesive interactions on platelet treatment with anti-P-selectin mAb in concert with an αIIbβ3-platelet blocker. In contrast, the nonblocking anti-P-selectin mAb did not affect the extent of cell tethering (Figure 3). Cumulatively, our data suggest that platelet P-selectin is primarily involved in LS174T cell tethering and rolling, whereas platelet αIIbβ3mediates stable tumor cell attachment under flow.
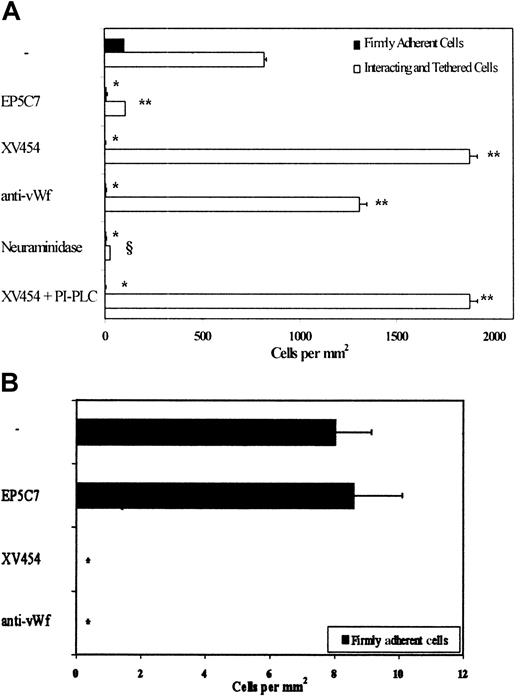
LS174T cell adhesion to surface-adherent platelets under conditions of flow (0.8 dyn/cm2)—effects of antibodies and peptides.
Closed bars represent the stable adherent cells (stationary for > 10 seconds) counted at the end of the 10-minute experiment, whereas open bars represent the total interacting cells counted throughout the entire 10-minute experiment. Immobilized platelets were treated with antiplatelet agents during the 10-minute thrombin incubation. Saturating concentrations were also maintained in the flow buffer only for the GRGDSP peptide, anti-vWf polyclonal antibody, and antifibrinogen (NYB-4.2) mAb. HIP8 (blocking αIIbβ3 function); P2 (blocking anti-αIIb mAb); SZ21 (blocking anti-β3mAb).*, § P < .05 with respect to no-treatment control. Values are mean ± SEM of 3-31 experiments.
LS174T cell adhesion to surface-adherent platelets under conditions of flow (0.8 dyn/cm2)—effects of antibodies and peptides.
Closed bars represent the stable adherent cells (stationary for > 10 seconds) counted at the end of the 10-minute experiment, whereas open bars represent the total interacting cells counted throughout the entire 10-minute experiment. Immobilized platelets were treated with antiplatelet agents during the 10-minute thrombin incubation. Saturating concentrations were also maintained in the flow buffer only for the GRGDSP peptide, anti-vWf polyclonal antibody, and antifibrinogen (NYB-4.2) mAb. HIP8 (blocking αIIbβ3 function); P2 (blocking anti-αIIb mAb); SZ21 (blocking anti-β3mAb).*, § P < .05 with respect to no-treatment control. Values are mean ± SEM of 3-31 experiments.
To further validate these findings, we used blood from a patient with GT whose platelets are devoid of αIIbβ3.34 The results indicate that LS174T cells tethered extensively on the GT platelet surface, but failed to firmly attach to it (Figure 3). The tethering events were attributed to platelet P-selectin as suggested by the elimination of interaction on GT platelet incubation with a function-blocking anti-P-selectin mAb (Figure 3). Our data using GT platelets are similar to those using platelets from normal volunteers pretreated with an αIIbβ3-blocker, and provide clear evidence for the functional roles of both P-selectin and αIIbβ3 in the LS174T-platelet adhesion process.
Dependence of LS174T cell adhesion on platelet αIIb-integrin subunit, vWf, and divalent cations
We next wished to localize the binding site on the platelet αIIbβ3-integrin that supports LS174T cell firm adhesion using function-blocking mAbs directed against αIIb and β3 subunits. Figure4 illustrates that an anti-β3 antibody, SZ21, did not significantly affect the extent and pattern of these adhesive interactions. Similar results were also obtained using another anti-β3-blocking mAb, B3A (control samples: 327 ± 35 firmly adherent cells/mm2versus anti-β3-treated samples: 307 ± 51 firmly adherent cells/mm2; n = 3). In contrast, an anti-αIIb mAb, P2,39 dramatically inhibited the number of firmly adherent LS174T cells to the platelet layers, an effect that was accompanied by a marked increase in the number of tethered cells (Figure 4). Similar results were also obtained using the RGD-containing peptide GRGDSP (Figure 4). As expected, a GRGESP peptide did not alter the extent of LS174T cell attachment to immobilized platelets (control samples: 327 ± 35 firmly adherent cells/mm2 versus GRGESP-treated samples: 285 ± 33 firmly adherent cells/mm2; n = 3). Taken together, these data suggest that the platelet αIIbβ3-integrin is involved in the formation of stable adhesive interactions between LS174T cells and surface-adherent platelets via an RGD-dependent mechanism. Although an intact αIIbβ3-complex is required in this process, it is likely that the ligand binding site resides in the αIIb subunit.
We next explored the potential involvement in this process of adhesive proteins such as vWf, fibrinogen, and fibronectin, which are anchored on the surface of activated platelets predominantly via αIIbβ3-integrins. At first, we confirmed the localization of vWf on the platelet substrates. Immunofluorescent staining of platelet layers using a polyclonal antibody to vWf showed clear uniform fluorescence (data not shown). Previous work has shown that this antibody can effectively block platelet-endothelial cell adhesive interactions mediated, in part, by vWf.28Treatment of immobilized platelets with this anti-vWf polyclonal antibody resulted in a significant inhibition (53% ± 8%;P < .05) in the extent of firmly adherent LS174T cells and a concomitant increase in the number of total interacting cells (Figure 4). Immunofluorescence studies also demonstrated the surface expression of fibrinogen on immobilized platelet layers. However, blockade of fibrinogen with a mAb failed to substantially affect the extent of LS174T cell-platelet adhesive interactions. Use of another mAb (DIG10VL2) against fibrinogen, which has previously been shown to block platelet attachment to endothelial cells,28 yielded very similar results (control samples: 246 ± 22 firmly adherent cells/mm2 versus antifibrinogen-treated samples: 267 ± 57 cells/mm2; n = 2; mean ± range). Furthermore, a blocking antifibronectin mAb type 228 did not affect the extent of LS174T cell attachment to immobilized platelets (control samples: 356 ± 30 firmly adherent cells/mm2 versus antifibronectin-treated samples: 348 ± 43 cells/mm2; n = 2; mean ± range). These observations suggest that platelet-associated vWf, but not fibrinogen or fibronectin, contributes to stable adhesion of LS174T cells under dynamic flow conditions.
Experiments were performed in the presence of 5 mmol/L EDTA to assess the divalent cation requirements. These ions are necessary for selectin- and integrin-mediated adhesion.12 When EDTA was added to the perfusion buffer, LS174T (Figure 4), cell interactions with immobilized platelets were totally abrogated, a finding that is consistent with the platelet P-selectin and αIIbβ3integrin involvement in this adhesion process.
Characterization of LS174T counter-receptors involved in adhesive interactions with platelets
We next aimed to identify the counter-receptor for platelet P-selectin on the LS174T cell surface. Prior studies have demonstrated that the major ligand on leukocytes involved in the recruitment to P-selectin is PSGL-1.40,41 Therefore, its contribution to LS174T binding to surface-adherent platelets was examined. Flow cytometric analysis of LS174T adhesion receptor expression suggests that PSGL-1 may be marginally present on the tumor cell surface (Table1). Blocking PSGL-1 function with a mAb failed to substantially reduce the extent of LS174T cell tethering to αIIbβ3-blocked platelet surfaces (Figure5A). In contrast, this anti-PSGL-1 antibody was effective in inhibiting neutrophil binding to purified P-selectin (data not shown). These results are in agreement with recent reports that suggest that a variety of cell lines from breast and colon carcinomas interact with P-selectin substrates in a PSGL-1–independent manner.30 42
Flow cytometric analysis of adhesion receptor expression on LS174T, COLO205, and HCT-8 colon adenocarcinoma cell lines
| Receptor . | Geometric mean fluorescence . | ||
|---|---|---|---|
| LS174T . | COLO205 . | HCT-8 . | |
| Control IgG | 5.3 ± 0.3 | 5.1 ± 0.3 | 4.2 ± 0.3 |
| PSGL-1 | 11.7 ± 2.9 | 7.3 ± 0.7 | 4.2 ± 0.3 |
| CD24 | 264 ± 8.8 | 300 ± 27 | 4.5 ± 0.2 |
| CD24 + PI-PLC | 5.9 ± 0.9 | 27.9 ± 6.5 | nd |
| sLex | 722 ± 136 | 1204 ± 386 | 7.8 ± 1.0 |
| sLex + Neuraminidase | 215 ± 90 | 32 ± 18 | nd |
| αIIbB3 | 5.7 ± 0.2 | 5.2 ± 0.8 | nd |
| GPIb | 5.7 ± 0.3 | 5.6 ± 0.5 | nd |
| αvB3 | 8.3 ± 0.9 | 9.0 ± 1.1 | 8.4 ± 0.5 |
| αv | 35.6 ± 3.3 | nd | nd |
| PECAM-1 | 4.9 ± 0.2 | nd | nd |
| GPIV | 9.5 ± 0.2 | nd | nd |
| L1 | 21.1 ± 1.8 | 18.5 ± 2.4 | nd |
| Receptor . | Geometric mean fluorescence . | ||
|---|---|---|---|
| LS174T . | COLO205 . | HCT-8 . | |
| Control IgG | 5.3 ± 0.3 | 5.1 ± 0.3 | 4.2 ± 0.3 |
| PSGL-1 | 11.7 ± 2.9 | 7.3 ± 0.7 | 4.2 ± 0.3 |
| CD24 | 264 ± 8.8 | 300 ± 27 | 4.5 ± 0.2 |
| CD24 + PI-PLC | 5.9 ± 0.9 | 27.9 ± 6.5 | nd |
| sLex | 722 ± 136 | 1204 ± 386 | 7.8 ± 1.0 |
| sLex + Neuraminidase | 215 ± 90 | 32 ± 18 | nd |
| αIIbB3 | 5.7 ± 0.2 | 5.2 ± 0.8 | nd |
| GPIb | 5.7 ± 0.3 | 5.6 ± 0.5 | nd |
| αvB3 | 8.3 ± 0.9 | 9.0 ± 1.1 | 8.4 ± 0.5 |
| αv | 35.6 ± 3.3 | nd | nd |
| PECAM-1 | 4.9 ± 0.2 | nd | nd |
| GPIV | 9.5 ± 0.2 | nd | nd |
| L1 | 21.1 ± 1.8 | 18.5 ± 2.4 | nd |
Values are geometric mean fluorescence intensities ± SEM of n = 3-7 experiments using different batches of cells each time. nd, not done.
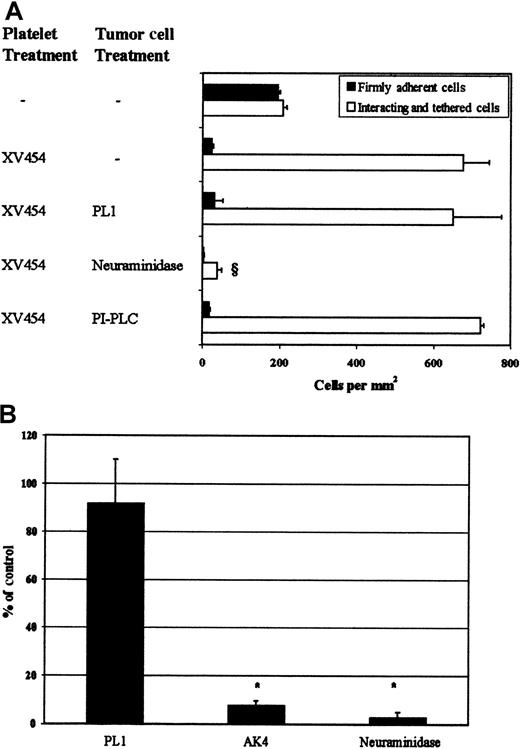
LS174T cell tethering.
(A) LS174T cell adhesion to surface-adherent platelets under conditions of flow (0.8 dyn/cm2)—effects of mAbs and enzymes. Closed bars represent the stable adherent cells (stationary for > 10 seconds) counted at the end of the 10-minute experiment, whereas open bars represent the total interacting cells counted throughout the entire 10-minute experiment. § P < .05 with respect to anti-αIIbβ3 sample. Values are mean ± SEM of 3-31 experiments. (B) LS174T cell rolling over purified P-selectin at a wall shear stress of 0.8 dyn/cm2. Data are expressed as percentage of untreated (control) LS174T cells that interacted with recombinant P-selectin throughout the 10-minute experiment. *P < .05 with respect to no-treatment control. Values are mean ± SEM of 4-5 experiments. Immobilized platelets were treated with XV454 (blocking αIIbβ3 function) during the 10-minute thrombin incubation. LS174T cells were incubated with PL1 (blocking anti-PSGL-1; 10 μg/mL) for 10 minutes before their perfusion over platelet layers. PL1 saturating concentrations were also maintained in the perfusion buffer. Alternatively, LS174T cells were treated with PI-PLC (1 U/mL for 1 hour at 37°C) or neuraminidase (0.1 U/mL for 30 minutes at room temperature) before infusion to the flow chamber. Purified P-selectin was incubated with an anti-P-selectin mAb (AK4) for 10 minutes before LS174T cell perfusion.
LS174T cell tethering.
(A) LS174T cell adhesion to surface-adherent platelets under conditions of flow (0.8 dyn/cm2)—effects of mAbs and enzymes. Closed bars represent the stable adherent cells (stationary for > 10 seconds) counted at the end of the 10-minute experiment, whereas open bars represent the total interacting cells counted throughout the entire 10-minute experiment. § P < .05 with respect to anti-αIIbβ3 sample. Values are mean ± SEM of 3-31 experiments. (B) LS174T cell rolling over purified P-selectin at a wall shear stress of 0.8 dyn/cm2. Data are expressed as percentage of untreated (control) LS174T cells that interacted with recombinant P-selectin throughout the 10-minute experiment. *P < .05 with respect to no-treatment control. Values are mean ± SEM of 4-5 experiments. Immobilized platelets were treated with XV454 (blocking αIIbβ3 function) during the 10-minute thrombin incubation. LS174T cells were incubated with PL1 (blocking anti-PSGL-1; 10 μg/mL) for 10 minutes before their perfusion over platelet layers. PL1 saturating concentrations were also maintained in the perfusion buffer. Alternatively, LS174T cells were treated with PI-PLC (1 U/mL for 1 hour at 37°C) or neuraminidase (0.1 U/mL for 30 minutes at room temperature) before infusion to the flow chamber. Purified P-selectin was incubated with an anti-P-selectin mAb (AK4) for 10 minutes before LS174T cell perfusion.
Treatment of LS174T cells with neuraminidase, an enzyme that cleaves sialic acid residues from cell surfaces (Table1),31 abolished their tethering to immobilized αIIbβ3-blocked platelets (Figure 5A), suggesting that the tumor cell P-selectin ligand is sialylated. Recent studies have revealed a role for the mucin-like glycoprotein CD24 in PSGL-1-negative tumor cell lines as a counter-receptor for P-selectin.33,42 Flow cytometric measurements indicate that CD24 is expressed on the LS174T cell surface (Table 1). To examine whether it is required for LS174T cell tethering under flow, tumor cells were treated with PI-PLC, an enzyme that specifically removes GPI-linked molecules such as CD24.33 42 Treatment of LS174T cells with PI-PLC abrogated CD24 cell surface expression (Table1), but failed to reduce their tethering to αIIbβ3-blocked platelet layers (Figure 5A). Taken together, these data suggest that the P-selectin ligand on the LS174T cell surface is a sialylated molecule that is distinct from PSGL-1 and CD24.
In an effort to demonstrate that P-selectin is sufficient to support LS174T cell tethering, in the absence of any other adhesion receptor(s) present on the surface of immobilized αIIbβ3-blocked platelets, flow experiments were carried out using purified P-selectin as a substrate. Our data show that purified P-selectin mediated extensive LS174T cell tethering (1569 ± 550 cells/mm2) at a wall shear stress of 0.8 dyn/cm2, which was essentially abolished with a function-blocking anti-P-selectin mAb (Figure 5B). Treatment of tumor cells with either an anti-PSGL-1 mAb or neuraminidase before infusion over purified P-selectin substrates yielded results similar to those obtained with αIIbβ3-blocked platelet layers (Figure 5B).
We finally aimed to identify the LS174T cell counter-receptor for platelet αIIbβ3. Previous work has suggested that a variety of tumor cell lines express platelet immunorelated glycoproteins such as GPIb-like and αIIbβ3-like integrin, that are presumably involved in tumor cell-platelet adhesion. The most obvious candidates on the tumor cell surface, capable of binding vWf, are GPIb, αvβ3, and αIIbβ3. Using a panel of mAbs against GPIb, αvβ3, αIIbβ3, as well as GPIV and platelet-endothelial cell adhesion molecule-1 (PECAM)-1, we were unable to detect expression of these adhesion molecules on the surface of LS174 T cells (Table 1). Furthermore, simultaneous blockade of the α1, α2, α3, α4, α5, and α6 integrins or β1 and β4integrins (β2 and β3 are not present on the LS174T cell surface as monitored by flow cytometry) failed to inhibit the extent of firm adhesion (data not shown). Similarly, αv integrins, although present on the LS174T cell surface, do not seem to be involved in the adhesion process as evidenced by the inability of function-blocking anti-αvmAbs to reduce LS174T cell attachment to immobilized platelets (control samples: 198 ± 26 firmly adherent cells/mm2 versus anti-αv-treated LS174T cells: 177 ± 46 cells/mm2; n = 2; mean ± range). These data suggest that a novel receptor may be involved in the binding of LS174T cells to platelet αIIbβ3.
COLO205 and HCT-8 cell adhesion to immobilized platelets under flow
To validate that immobilized platelets are capable of supporting tethering, rolling, and firm adhesion of colon cancer cells other than LS174T, we chose to look at sLex-bearing (COLO205) and sLex-negative (HCT-8) cell lines (Table1).9,10 14 Our results indicate that COLO205 tethered and rolled extensively on immobilized platelets at a wall shear stress of 0.8 dyn/cm2 (Figure 6A). A significant number of interacting COLO205 cells became firmly adherent within a 1- to 5-second interval after tethering to the platelet substrate. Blockade of platelet P-selectin or treatment of COLO205 cells with neuraminidase but not PI-PLC nearly eliminated any cell tethering/adhesive interactions. Furthermore, blockade of either platelet αIIbβ3 or platelet-bound vWf abrogated COLO205 firm adhesion while dramatically increasing the number of tethered cells (Figure 6A). Taken together, these data suggest that platelet P-selectin initiates COLO205 tethering and rolling to platelet layers in shear flow. In accordance with the LS174T data, the P-selectin ligand on COLO205 cells appears to be a sialylated molecule that is distinct from PSGL-1 (Table 1) and CD24 (Figure 6A). COLO205 tethering and rolling events are occasionally followed by stable adhesion that is totally dependent on platelet αIIbβ3 and platelet-bound vWf. It is worth noting that HCT-8 cells, which are devoid of sLex residues (Table 1), tether minimally to surface-anchored platelets in shear flow (Figure 6B), despite their extensive adhesive interactions under static conditions (data not shown). The very few HCT-8 cells that tethered from the fluid stream to the platelet layer became firmly adherent instantaneously with no obvious period of rolling in a αIIbβ3- and vWf-dependent manner (Figure 6B).
COLO205 and HCT-8 cell adhesion to immobilized platelets.
(A) COLO205 cell adhesion to surface-adherent platelets under conditions of flow (0.8 dyn/cm2)—effects of mAbs and enzymes. Closed bars represent the stable adherent cells (stationary for > 10 seconds) counted at the end of the 10-minute experiment, whereas open bars represent the total interacting cells counted throughout the entire 10-minute experiment. *, **P < .05 with respect to no-treatment control. §P < .05 with respect to XV454-treated specimens. Values are mean ± SEM (n = 3-5). (B) HCT-8 cell firm adhesion to surface-adherent platelets under conditions of flow (0.8 dyn/cm2)—effects of mAbs. *P < .05 with respect to no-treatment control. Values are mean ± SEM (n = 3). Immobilized platelets were treated with agents specific for P-selectin, αIIbβ3, and vWf for 10 minutes before the perfusion of tumor cells. Saturating concentrations were maintained in the flow buffer only for the anti-vWf polyclonal antibody. Alternatively, COLO205 cells were treated with neuraminidase (0.1 U/mL for 30 minutes at room temperature) or PI-PLC (1 U/mL for 1-hour at 37°C) before infusion to the flow chamber. XV454 (blocking αIIbβ3 function); EP5C7 (blocking P-selectin function).
COLO205 and HCT-8 cell adhesion to immobilized platelets.
(A) COLO205 cell adhesion to surface-adherent platelets under conditions of flow (0.8 dyn/cm2)—effects of mAbs and enzymes. Closed bars represent the stable adherent cells (stationary for > 10 seconds) counted at the end of the 10-minute experiment, whereas open bars represent the total interacting cells counted throughout the entire 10-minute experiment. *, **P < .05 with respect to no-treatment control. §P < .05 with respect to XV454-treated specimens. Values are mean ± SEM (n = 3-5). (B) HCT-8 cell firm adhesion to surface-adherent platelets under conditions of flow (0.8 dyn/cm2)—effects of mAbs. *P < .05 with respect to no-treatment control. Values are mean ± SEM (n = 3). Immobilized platelets were treated with agents specific for P-selectin, αIIbβ3, and vWf for 10 minutes before the perfusion of tumor cells. Saturating concentrations were maintained in the flow buffer only for the anti-vWf polyclonal antibody. Alternatively, COLO205 cells were treated with neuraminidase (0.1 U/mL for 30 minutes at room temperature) or PI-PLC (1 U/mL for 1-hour at 37°C) before infusion to the flow chamber. XV454 (blocking αIIbβ3 function); EP5C7 (blocking P-selectin function).
Pattern of interactions/rolling velocities
We observed substantial heterogeneity in the qualitative binding patterns of colon cancer cells to platelet P-selectin. Some LS174T or COLO205 cells that tethered from the fluid stream rolled along the P-selectin surface for some distance, which was variable but sometimes quite extended (the entire path of a field of view) at velocities significantly lower than the critical velocity (Table 2). The latter was experimentally calculated to be approximately 700 μm/s at a wall shear stress of 0.8 dyn/cm2 for noninteracting tumor cells traveling adjacent to the lower wall of the flow chamber.42 Other LS174T or COLO205 cells formed only brief adhesive interactions after which they skipped to velocities near the critical value before they tethered again to the substrate downstream to the site of initial contact. In general, COLO205 cells exhibited a rolling velocity that was significantly lower than that of LS174T cells (Table 2), a finding that correlates inversely with the sLex content of these cell lines.
Rolling velocities of LS174T and COLO205 cells and neutrophils over platelet P-selectin under flow
| LS174T over platelet P-selectin | 124 ± 9.3 μm/s (n = 27) |
| COLO205 over platelet P-selectin | 56 ± 4.1 μm/s (n = 24) |
| Neutrophils over platelet P-selectin | 4.1 ± 0.9 μm/s (n = 18) |
| LS174T over platelet P-selectin | 124 ± 9.3 μm/s (n = 27) |
| COLO205 over platelet P-selectin | 56 ± 4.1 μm/s (n = 24) |
| Neutrophils over platelet P-selectin | 4.1 ± 0.9 μm/s (n = 18) |
LS174T, COLO205 cells and neutrophils (106/mL) were perfused over a platelet P-selectin substrate (αIIbβ3-blocked platelets) at wall shear stresses of 0.8 dyn/cm2 (LS174T and COLO205 cells) and 1.0 dyn/cm2 (neutrophils). The critical velocity of free-flowing tumor cells adjacent to the wall was experimentally calculated to be about 700 μm/s.42
Both the pattern of interaction and magnitude of rolling velocities of LS174T and COLO205 cells are remarkably different from those of neutrophils isolated with standard procedures.43 The latter rolled stably along the P-selectin substrates with an average velocity of approximately 4.0 μm/s, which is substantially lower than that of tumor cells (approximately 50-100 μm/s; Table2).30 These data provide evidence that the P-selectin ligand activity on the colon cancer cells appears to be less efficient and more sensitive to shear stress than the PSGL-1 on neutrophils at mediating rolling interactions with P-selectin substrates.
Discussion
To the best of our knowledge, this is the first study to demonstrate that surface-bound platelets are capable of mediating colon cancer cell tethering, rolling, and firm adhesion in shear flow. These observations suggest that tumor cell recruitment to sites of platelet deposition may follow a multistep sequential process of adhesive interactions similar to that outlined for neutrophils.20-23 However, a detailed analysis of LS174T cell binding to immobilized platelets has revealed several similarities and disparities to the “neutrophil model.” In concert with this model, platelet P-selectin is required for the efficient capture of free-flowing LS174T cells (Figure 7). Its role was established by the ability of function-blocking anti-P-selectin mAbs to dramatically reduce tumor cell binding to surface-anchored platelets under flow conditions.
Proposed model of adhesion of LS174T cells to surface-immobilized platelets under dynamic flow conditions via 2 distinct pathways.
(1a) Platelet P-selectin mediates LS174T cell tethering/rolling, and (1b) subsequent platelet αIIbβ3 involvement converts these transient interactions into stable adhesion. (2) Platelet αIIbβ3-integrins can directly capture and instantaneously stabilize free-flowing LS174T cells at low shear stress conditions. The sLex-bearing COLO205 cells follow the sequential cascade of adhesive interactions outlined in pathway 1, whereas the attachment of sLex-negative HCT-8 cells to platelets is described by pathway 2.
Proposed model of adhesion of LS174T cells to surface-immobilized platelets under dynamic flow conditions via 2 distinct pathways.
(1a) Platelet P-selectin mediates LS174T cell tethering/rolling, and (1b) subsequent platelet αIIbβ3 involvement converts these transient interactions into stable adhesion. (2) Platelet αIIbβ3-integrins can directly capture and instantaneously stabilize free-flowing LS174T cells at low shear stress conditions. The sLex-bearing COLO205 cells follow the sequential cascade of adhesive interactions outlined in pathway 1, whereas the attachment of sLex-negative HCT-8 cells to platelets is described by pathway 2.
Previous work has demonstrated that P-selectin, expressed either on the surface of activated endothelial cells or platelets, mediates tethering and rolling of leukocytes.12,18,19 However, LS174T cells that tethered to immobilized platelets were instantly arrested with no obvious period of rolling. We therefore hypothesized that platelet P-selectin may cooperate with another receptor to stabilize LS174T cell-platelet interactions. The αIIbβ3-integrin is the most abundant receptor on the platelet surface and has been reported to promote immediate platelet arrest in the absence of any rolling interaction onto immobilized fibrinogen.44 Furthermore, platelet αIIbβ3-integrin has been shown to mediate adhesive interactions between a variety of human tumor cell lines and activated platelets under stationary conditions.4 9-11 The role of αIIbβ3 in firm adhesion of LS174T cells to platelets was established by using specific αIIbβ3-antagonists as well as αIIbβ3-deficient platelets from a patient with GT. Under these conditions, LS174T cells tethered extensively and rolled along the platelet layer at velocities below the critical value, without firmly attaching to it. In either case (αIIbβ3-blocked platelets or GT platelets), the transient adhesive interactions between LS174T and platelets were abolished after incubation of the monolayer with a function-blocking anti-P-selectin mAb. Together, our data suggest that optimal LS174T cell adhesion to immobilized platelets under shear flow conditions requires the sequential action of platelet P-selectin and αIIbβ3 integrin (Figure 7). In particular, P-selectin binds free-flowing tumor cells, and subsequent platelet αIIbβ3 involvement contributes to conjugate stabilization. However, platelet αIIbβ3appears to directly capture LS174T cells at low shear stresses (0.8 dyn/cm2), presumably due to its abundant expression on the platelet surface (Figure 7).
The αIIbβ3-integrin is a heterodimeric complex composed of the noncovalent association of 2 integral membrane proteins, αIIb and β3. Using function-blocking mAbs specific for either the αIIb or β3-integrin subunits, we attempted to localize the epitope on the αIIbβ3 complex that mediates LS174T cell firm adhesion. Our data suggest that this epitope is likely to reside on the αIIb rather than β3subunit. This observation may be favored by a recent study showing that a point mutation in the platelet αIIb subunit disrupts the structural conformation and the ligand binding properties of the heterodimeric complex.45
We next explored whether LS174T cell adhesion to immobilized platelets involves a direct interaction between the 2 cell types or whether bridging ligands are required. We hypothesized that the platelet integrin αIIbβ3 and its counter-receptor on LS174T cells interact via RGD-containing plasma proteins, such as fibrinogen, fibronectin, and vWf.9 Along with these lines, antibody blockade of vWf, but not fibrinogen or fibronectin, significantly reduced the extent of LS174T cell firm adhesion to platelet layers. Although vWf is anchored on the surface of activated platelets predominantly via αIIbβ3, other receptors such as αvβ3 or GPIb also bind vWf.12 However, blockade of all platelet integrins other than αIIbβ3 (eg, α1 through α6, β1 through β4, αv and αvβ3), either alone or simultaneously, failed to reduce LS174T cell attachment to platelets under flow. Similarly, a function-blocking anti-GPIb mAb did not affect these adhesive interactions (data not shown). Taken together, our data suggest that αIIbβ3-bound vWf is a ligand involved in the adhesion of LS174T cells to immobilized platelets in shear flow. The possibility that plasma vWf, not associated with platelets but instead directly attached to APES-treated slides, may be involved in the LS174T cell adhesion can be ruled out for the following reasons: (1) blocking both platelet P-selectin and αIIbβ3 abolished LS174T cell attachment to immobilized platelets, indicating that only platelets and platelet-associated proteins are involved in the adhesion process, and (2) the extent of LS174T cell adhesion to immobilized washed platelets was at least equal to that observed with PRP (washed platelets: 364 ± 74 firmly adherent cells/mm2 versus PRP: 223 ± 51 firmly adherent cells/mm2; n = 4). Furthermore, given the high platelet density and the relative size of platelets (μm) and plasma factors (nm), it is unlikely that nonplatelet-bound vWf is involved in the adhesion process.
We also aimed to identify the P-selectin ligand on the LS174T cell surface. Prior work indicates that PSGL-1, expressed on the neutrophil surface, is the primary ligand for platelet P-selectin under hydrodynamic flow conditions.43 Using flow cytometry, we found that PSGL-1 may be marginally expressed on the LS174T cell surface. Blocking its function with a mAb failed to inhibit the extent of LS174T cell tethering on the αIIbβ3-blocked platelets expressing P-selectin, indicating that PSGL-1 is not involved in this process. The ability of LS174T cells to interact with platelets in a PSGL-1-independent manner was also confirmed using purified P-selectin substrates. These data are in accord with previously published reports demonstrating that a variety of tumor cell lines bind to P-selectin through novel glycoprotein ligands that are structurally and functionally distinct from PSGL-1.30,33,42 The glycoprotein GPIb-IX complex was recently identified as a counter-receptor for P-selectin.46 Published data show that certain tumor cell lines express an authentic GPIb-IX complex on their surfaces.4 11 However, flow cytometric analysis of LS174T cells revealed the absence of GPIb-IX complex from the cell surface, thereby eliminating its potential involvement from these adhesive interactions.
Recent studies have identified CD24, a mucin-type GPI-linked cell surface glycoprotein, as a ligand for P-selectin.33 Its physiologic relevance became evident by flow experiments showing that CD24 mediates rolling of PSGL-1-negative breast tumor cells to purified P-selectin under flow.42 Using flow cytometry, we documented the presence of CD24 on the LS174T cell surface. To assess its involvement in tumor cell binding to platelet P-selectin, and in the absence of any blocking anti-CD24 mAbs, LS174T cells were treated with PI-PLC, an enzyme that specifically cleaves GPI-anchored molecules from the cell surface. Although this enzyme treatment abolished CD24 expression from the LS174T cell surface, it did not affect tumor cell tethering/rolling on platelet P-selectin. In contrast, treatment of LS174T cells with neuraminidase abrogated the divalent cation-dependent tethering/rolling events. These data collectively suggest that the P-selectin ligand on the LS174T cell surface is a sialylated molecule that is distinct from PSGL-1 and CD24.
We next sought to identify a potential counter-receptor on LS174T cells for the platelet integrin subunit αIIbβ3. We reasoned that platelet αIIbβ3 binds to a related tumor cell receptor using a mechanism similar to that which governs platelet-platelet cohesion under hydrodynamic shear conditions.12 This hypothesis was supported by several studies that have demonstrated the presence of αIIbβ3-like and αvβ3-like molecules on the surface of certain tumor cells.4,7,9-11 However, LS174T cells failed to bind a panel of mAbs directed against αIIbβ3, αvβ3, GPIb, and GPIV, thereby ruling out any potential role for these molecules. A recent study suggested that platelet αIIbβ3 may interact with αv-integrins present on the surface of melanoma cells to mediate platelet-tumor cell adhesion under conditions of flow.7 Using flow cytometry, we were able to detect expression of αv-integrins on the LS174T cell surface. However, blocking its function failed to significantly reduce the extent of LS174T cell attachment to platelets. These data coupled with the inability of function-blocking mAbs specific for α1, α2, α3, α4, α5, and α6 integrins or β1, β2, β3, and β4 to inhibit LS174T cell firm adhesion to immobilized platelets suggest the involvement of a novel receptor in these stable adhesive interactions. A plausible candidate might be the neural cell adhesion molecule L1 that was recently shown to bind to platelet integrin αIIbβ3.47 Using indirect one-color immunofluorescence assays and an anti-L1 polyclonal antibody, we detected the presence of L1 on the LS174T cell surface. Because no function-blocking antibodies against human L1 have yet been described, we were unable to demonstrate its requirement in LS174T cell adhesion to surface-adherent platelets.
As summarized in Figure 7, our data provide evidence that LS174T cell recruitment to sites of platelet deposition follows a cascade of events that shares common features but is distinct from that outlined for neutrophils.20-23 Platelet P-selectin supports LS174T cell tethering/rolling by binding to a sialylated molecule that is distinct from PSGL-1 and CD24. Subsequent platelet αIIbβ3 involvement contributes to conjugate stabilization by converting LS174T cell tethering instantly to firm arrest. Furthermore, platelet αIIbβ3integrins are also capable of directly capturing free-flowing LS174T cells at low shear stresses. To extend our findings, we also analyzed the adhesive interactions of 2 other commonly used colon carcinoma cell lines with immobilized platelets under dynamic flow conditions. In accordance with the proposed LS174T model, platelets supported extensive COLO205 cell tethering and rolling in a P-selectin-dependent manner. The P-selectin ligand on COLO205 cells appears to be a sialylated molecule that is different from PSGL-1 and CD24. Furthermore, a substantial number of transiently interacting COLO205 cells became firmly adherent within a 1- to 5-second interval after tethering to the platelet surface, a process that was dependent on the availability of platelet αIIbβ3. Along with these lines, the sLex-negative HCT-8 cells tethered minimally to platelet layers under flow despite their extensive and shear-resistant binding under stationary conditions. The very few tethered HCT-8 cells were instantaneously arrested with no obvious period of rolling in a αIIbβ3-dependent manner. In contrast to the neutrophil model in which fibrinogen appears to play a critical role in neutrophil attachment to platelet layers, vWf acts to bridge platelet αIIbβ3 with a yet unidentified receptor on the colon cancer cell surface to mediate the firm adhesion step. These findings enhance our understanding of the molecular mechanisms of tumor-platelet adhesion and may provide insights for the rational development of novel therapeutic strategies aimed to alter these adhesive interactions.
Acknowledgments
The authors wish to thank Dr William R. Bell (Johns Hopkins University, Baltimore) and his patient for providing us with a blood specimen; Dr John Hemperly (Becton Dickinson, Research Triangle, NC) for supplying us with an anti-L1 polyclonal antibody; Dr Ellen L. Berg (Protein Design Labs, Mountain View, CA) for her generous donation of EP5C7; Dr Bruce S. Bochner (Johns Hopkins University, Baltimore) for insightful comments and reagent donation; and Dr Ronald L. Schnaar (Johns Hopkins University, Baltimore) for helpful discussions.
Supported by a Whitaker Foundation grant (K.K.), a DuPont Young Professor grant (K.K.), and a National Institutes of Health grant HL58564 (P.F.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Konstantinos Konstantopoulos, Department of Chemical Engineering, Johns Hopkins University, 3400 North Charles St, Baltimore, MD 21218-2694; e-mail: konst_k@jhu.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal