Abstract
Stem cell factor (SCF) has been suggested as essential for optimal production of various hematopoietic lineages mainly because of its apoptosis prevention function when it costimulates with other cytokines. However, the underlying mechanism of this synergism of apoptosis prevention is largely unknown. The present study examined the expression of some Bcl-2 family members, including Bcl-2, Bcl-XL, Mcl-1, and Bax, in response to cytokine stimulation in TF-1 and JYTF-1 cells in which SCF costimulation is differentially required for optimal proliferation. The results revealed that only the expression of Mcl-1 highly correlated with the antiapoptotic activity of interleukin-5 (IL-5) and the synergistic effect of SCF. In TF-1 cells, the defect of IL-5 in apoptosis suppression and Mcl-1 induction was associated with the incapability to highly phosphorylate Janus kinases (JAK1, JAK2), signal transducer and activator of transcription-5 (STAT5), mitogen-activated protein kinase (MAPK), and Akt/PKB, whereas SCF costimulation restored the potent phosphorylation of MAPK and Akt/PKB, but not STAT5. The importance of MAPK and Akt/PKB signaling pathways in regulating the expression of Mcl-1 and cell survival was further supported by the observation that inhibition of MEK by PD98059 or phosphatidylinositol-3 kinase (PI-3K) by LY294002 independently resulted in the reduction of Mcl-1 expression and loss of cell viability. Therefore, the data suggest that Mcl-1 is a common antiapoptotic target of both early-stage cytokine SCF and late-stage cytokine IL-5. Both MEK/MAPK and PI-3K/Akt signaling pathways are essential in the regulation of Mcl-1 expression and apoptosis prevention.
Introduction
The development of different cell lineages in hematopoiesis depends on direct cell–cell contact and the release of soluble factors. Among these external soluble factors, cytokines play important roles. Stem cell factor (SCF) is an early acting cytokine produced by marrow stromal cells. Although SCF alone does not stimulate myelopoiesis, when combined with other cytokines, SCF increases the cloning efficacy of hematopoietic progenitor cells from all lineages.1 The maintenance of high levels of myelopoiesis and erythropoiesis in vitro by SCF was shown to be due to the suppression of apoptosis.2,3 By contrast, interleukin (IL)-5 belongs to a group of late-acting cytokines with a restricted set of targets in hematopoiesis, because the major role of IL-5 is limited to the eosinophilic/basophilic lineage in humans1and to B-cell development additionally in mice.4-6Recently, IL-5 was shown to be able to promote the eosinophilic outgrowth from bone marrow stem and progenitor cells. However, according to an in vitro assay,7 the maximal production of eosinophils from stem cell population requires the costimulation of SCF.
Binding of IL-5 to its surface high-affinity receptor complexes, which are composed of an α chain (IL-5Rα) and a common β chain (βc),8,9 results in rapid tyrosine phosphorylation of βc and activation of several important signal components including Ras, phosphatidylinosital-3 (PI-3) kinase, Shc, p70S6K, Raf, mitogen-activated protein kinase (MAPK),10,11 Vav, HS1,12 Btk, signal transducer and activator of transcription (STAT)5a, STAT5b, STAT1, and STAT3.13-17These events are believed to be triggered via preassociated nonreceptor tyrosine kinases JAK1 and JAK2 on the receptor molecules.18,19 These signaling pathways eventually reach the nucleus and result in the induction of specific genes, including transcription factors c-fos, c-jun, junB, c-myc,10,11 and pim-110 and genes important for cell cycle regulation, including various cyclins and cyclin-dependent kinases.20Similarly, binding of SCF to membrane-bound c-Kit receptors activates phosphorylation of a similar, but not identical, set of cellular proteins via the kinase domain of the c-Kit receptor cytoplasmic tail.21-28
The involvement of several signaling pathways in the regulation of apoptosis has been extensively investigated, and gene products including Ras, Raf, MAPK,29 PI-3 kinase, and PKB/Akt30 have been suggested as having an important role in transducing the antiapoptotic function. However, the target of these signaling molecules is largely unclear. Recently, the Bcl-2gene family, which is composed of 14 members, was shown to compose the central players of apoptosis regulation (for review, see reference 31). Some members of this family, such as Bcl-2, Bcl-XL, A1, and Mcl-1, inhibited apoptosis whereas other members, such as Bax, Bad, and Bak, accelerated apoptosis. Among Bcl-2 family members, Bcl-2, Bcl-XL, and Mcl-1 all have been shown to be up-regulated by IL-3 in various IL-3–dependent cell lines.30 32
TF-1 is a human hematopoietic progenitor cell line derived from the bone marrow of an erythroleukemic patient.34 TF-1 cells, like normal progenitor cells, express high levels of granulocyte-macrophage colony-stimulating factor (GM-CSF) receptor α chain, but very low levels of IL-5Rα.35 In the presence of IL-5, TF-1 cells can replicate DNA and proliferate, but cannot keep all progeny cells alive35 because of a defect in apoptosis suppression. Increased expression of IL-5Rα in TF-1 cells by genetic selection (as in JYTF-1 cells) or by the retroviral infection method34 resumes optimal proliferation of these cells in IL-5, a result caused by correction of the antiapoptotic defect. SCF synergism in TF-1 cells was observed only when the signal of IL-5 was handicapped with a lack of antiapoptosis ability.34 The strong apoptosis prevention activity of SCF cooperated with the mitogenic activity of IL-5 to achieve the maximal proliferation-promoting effect. However, once the expression of IL-5Rα increased in JYTF-1 cells, the antiapoptotic activity of IL-5 was restored and SCF costimulation became dispensable for optimal proliferation.
Our previous study indicated that Mcl-1 plays a role in the viability response of GM-CSF.33 When GM-CSF was withdrawn from the culture medium of human GM-CSF–dependent cell line TF-1, the level of endogenous Mcl-1 decreased rapidly down to the background level in 6 hours. On restimulation of these starved cells with GM-CSF,mcl-1 is one of the immediate early genes induced and its messenger RNA (mRNA) peaked at 30 minutes and its protein product was accordingly resynthesized.33 Ectopic overexpression of Mcl-1 in TF-1 cells significantly delayed cell death triggered by GM-CSF deprivation and reduced expression of endogenous Mcl-1 by an antisense construct decreased the viability of cells cultured in the presence of cytokine.33 In the present study, we attempted to further identify the candidate Bcl-2 gene family members through which the antiapoptotic signals of IL-5 and SCF merge and to delineate the signaling pathways that lead to activation of Bcl-2 family proteins from both IL-5 and SCF receptors. We demonstrate that the Mcl-1, but not Bcl-2, Bcl-XL or Bax, is one of the common targets of SCF and IL-5 for their antiapoptosis function and that both MEK/MAPK and PI-3K/Akt pathways are essential for the survival functions of these 2 cytokine receptors.
Materials and methods
Cell lines, cytokines, and chemical reagents
Both TF-1 and JYTF-135 cell lines were maintained in serum-medium supplemented with 2 ng/mL GM-CSF as previously described. When other cytokines were used to cultivate these 2 cell lines, 5 ng/mL of IL-5 or 50 ng/mL of SCF was supplemented. Recombinant human GM-CSF, IL-5, and SCF were purchased from R & D Systems (Minneapolis, MN). The MEK inhibitor PD98059 (catalogue no. 513000-S) and the PI-3K inhibitor LY294002 (catalogue no. 440202-S) were purchased from Calbiochem-Novabiochem (San Diego, CA).
Cell proliferation assay
Accumulation of viable cells in culture was used to demonstrate active proliferation and was monitored by trypan blue exclusion assay.35
Nucleosome-releasing assay and DNA fragmentation analysis
Quantitative measurement of the apoptosis activity in cultured cells was performed by nucleosome-releasing assay (Boehringer Mannheim, Mannheim, Germany; catalogue no. 1774425) and qualitative demonstration of apoptosis was performed by DNA fragmentation assay.35
Immunoprecipitation and Western blot analysis
Cells were washed and starved in 0.5% fetal bovine serum (FBS) medium for 20 to 24 hours, then stimulated with 10 ng/mL GM-CSF or IL-5, or 50 ng/mL SCF (R&D Systems). For protein tyrosine kinase activation analyses, cells were stimulated with various cytokines for 5 minutes. For measurement of the expression of Bcl-2 family proteins, cells were incubated for 1 hour. After washing with phosphate-buffered saline (PBS), cells were lysed at 4°C in 0.5 mL of lysis buffer (1% NP-40, 50 mmol/L Tris-HCl, pH 7.4, 0.25% sodium deoxycholate, 150 mmol/L NaCl, 1 mmol/L EGTA, 1 mmol/L PMSF, 1 μg/mL leupeptin, 1 mmol/L Na3VO4, 1 mmol/L NaF) and the unsolubilized material was removed by centrifugation for 10 minutes at 14 000 rpm. The lysates were precleared with protein A-Sepharose beads and then reacted with the indicated primary antibody overnight at 4°C. The antigen-antibody complexes were then precipitated with protein A-Sepharose beads for 2 hours at 4°C, washed 3 times with lysis buffer, and then boiled for 5 minutes with 2 × sodium dodecyl sulfate (SDS) sample buffer. The boiled supernatants were electrophoresed on a SDS-polyacrylamide gel electrophoresis (PAGE) and analyzed with Western blotting. After binding with horseradish peroxidase-conjugated secondary antibodies, blots were visualized with an enhanced chemiluminescence (ECL) detection system (Amersham, Little Chalfont, Buckinghamshire, UK). The antibodies against Bcl-2 (N-19), Mcl-1 (S-19), Bax (N-20), Bcl-XL (M-125), human βc chain (S-16), JAK1 (HR-785), JAK2 (HR-758), and STAT5 (C-17) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antiphosphotyrosine monoclonal antibody 4G10 was purchased from UBI (Lake Placid, NY). Antibodies against phospho-MAPK(Thr202/Tyr204), MAPK, phospho-Akt(Ser473), and Akt/PKB were all purchased from New England BioLabs (Beverly, MA).
In vitro kinase assay
The MAPK in vitro kinase assay was performed using the p44/42 MAP kinase assay kit (New England BioLabs). Briefly, cells were lysed in MAPK lysis buffer (20 mmol/L Tris-HCl, pH7.5, 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1% Triton X-100, 2.5 mmol/L sodium pyrophosphate, 1 mmol/L β-glycerolphosphate, 1 mmol/L Na3VO4, 1 μg/mL leupeptin, 1 mmol/L PMSF), and MAP kinases were immunoprecipitated with the immobilized phospho-p44/42 MAPK monoclonal antibody. After washing twice with lysis buffer and twice with kinase buffer (25 mmol/L Tris-HCl, pH7.5, 5 mmol/L β-glycerolphosphate, 2 mmol/L DTT, 0.1 mmol/L Na3VO4, 10 mmol/L MgCl2), the immunoprecipitates were assayed for MAP kinase activity in kinase buffer with 200 μmol/L adenosine triphosphate (ATP) and 2 μg Elk-1 fusion protein per reaction. The reaction was stopped with SDS sample buffer and analyzed by Western blotting with specific antiphospho-Elk-1 antibody.
The Akt in vitro kinase assays were performed as described by Franke and colleagues.36 Cells were lysed in Akt lysis buffer (20 mmol/L Tris-HCl, pH7.5, 150 mmol/L NaCl, 10% glycerol, 1% NP-40, 10 mmol/L NaF, 1 mmol/L Na3VO4, 1 mmol/L Na4P2O7, 2 μmol/L leupeptin, 2 μmol/L aprotinin, 1 mmol/L PMSF) and the Akt protein was immunoprecipitated with anti-Akt antibody (New England BioLabs). The immune complexes were collected with protein A-Sepharose and washed 3 times with lysis buffer, once with cold water, and once with kinase buffer (20 mmol/L HEPES, pH7.4, 1 mmol/L DTT, 10 mmol/L MnCl2, 10 mmol/L MgCl2). The Akt kinase activity was then assayed in kinase buffer with 5 μmol/L ATP, 3 μg histone 2B, and 20 μCi of [γ-32P]ATP per reaction. Akt kinase reaction was stopped with SDS sample buffer and the products were separated by SDS-PAGE for autoradiography.
Results
Mcl-1 expression is closely associated with apoptosis prevention ability of cytokines
In our previous study,34 we demonstrated that either costimulation with SCF or increased expression of IL-5Rα allowed hematopoietic progenitor cells to grow optimally in medium containing IL-5. In the present study, we explored whether these 2 treatments lead to apoptosis prevention by a similar mechanism. When healthy TF-1 cells were transferred from medium containing GM-CSF (Figure1A, lane 2) into medium containing IL-5, cell death was detectable by DNA ladder analysis after 12 hours of incubation and became very obvious within 24 hours (Figure 1A, lanes 5-7). However, IL-5 was capable of suppressing apoptosis in JYTF-1 cells in which the expression of IL-5Rα is increased and the DNA ladder was not detectable throughout the experimental period (Figure1A, lanes 11-14). We previously reported that overexpression of Bcl-2 or Mcl-1 protein in TF-1 cells strongly suppresses cytokine withdrawal-induced apoptosis and that Mcl-1 is one component of both GM-CSF and IL-3 survival responses in cytokine-dependent cell lines.33,34 We were curious whether Mcl-1 also plays an important role in the survival effect of IL-5 and SCF in the hematopoietic progenitor cell line. To address this issue, cell lysates were prepared from cells grown in various culture conditions and then subjected to Western blot analysis for the protein expression of several Bcl-2 family members, including Bcl-2, Bcl-XL, Mcl-1, and Bax. TF-1 and JYTF-1 cells cultured in GM-CSF were shifted to medium containing IL-5 for various time periods. As shown in Figure1B, the expression level of Mcl-1 in both cell lines was 3- to 4-fold higher in medium containing GM-CSF than in cytokine-free medium (Figure1B, lanes 1, 7, 8, and 14). IL-5 was not able to sustain the expression of Mcl-1 after transferring TF-1 cells from GM-CSF-containing into IL-5-containing medium, and Mcl-1 expression reduced to basal level within 18 to 24 hours (Figure 1B, lanes 5-7). The decrease in Mcl-1 expression correlated well with the appearance of the DNA ladder (Figure 1A, lanes 6 and 7). On the other hand, IL-5 was capable of maintaining the expression of Mcl-1 in JYTF-1 cells at a level similar to the effect of GM-CSF within 24 hours (Figure 1B, lanes 10-13). Under the same conditions, the expression levels of 3 other Bcl-2 family members (Bcl-2, Bcl-XL, and Bax) remained the same throughout the experimental period (Figure 1B). Next, we examined whether cytokines (GM-CSF, IL-5, and SCF) capable of sustaining the survival of JYTF-1 cells stimulate Mcl-1 expression. The result shown in Figure 1C indicates that this is indeed the case, whereas the expression levels of Bcl-XL, a Bcl-2 family member known to be involved in the survival response of IL-3 in certain cell systems,31 are not stimulated by any of these 3 cytokines.
IL-5 is unable to suppress apoptosis or induce Mcl-1 in TF-1 cells.
(A) IL-5 suppressed apoptosis in JYTF-1, but not in TF-1 cells. TF-1 (lanes 2-8) and JYTF-1 (lanes 9-15) cells were transferred from medium containing GM-CSF (lanes 2 and 9) into medium containing IL-5 for various time periods (lanes 3-7 and 10-14) and were subjected to DNA ladder analysis. Two DNA samples from cells kept in 0.5% FBS cytokine-free medium for 24 hours were used as controls (lanes 8 and 15). DNA samples were fractionated in 1.5% agarose gel. Mr indicates molecular weight marker. (B) IL-5 sustained Mcl-1 expression in JYTF-1, but not in TF-1 cells. TF-1 (lanes 1-7) and JYTF-1 (lanes 8-14) cells were treated as described in panel A. Cell lysates were prepared from cells at each time point and subjected to Western blot analysis for the protein expression of Mcl-1, Bcl-2, Bcl-XL, Bax, and α-tubulin probing with antibodies specific for each antigen. The immunoblots were visualized using an ECL system. Experiments were repeated 3 times and a representative result is shown in this figure. (C) Expression of Mcl-1, but not of Bcl-XL, is stimulated by GM-CSF, IL-5, or SCF in JYTF-1 cells. JYTF-1 cells were deprived of cytokines for 24 hours (lane 1) and restimulated with GM-CSF (lane 2), IL-5 (lane 3), or SCF (lane 4) for 1 hour. Cell lysates were analyzed as described in panel B, except that only Mcl-1, Bcl-XL, or α-tubulin levels were probed with specific antibodies.
IL-5 is unable to suppress apoptosis or induce Mcl-1 in TF-1 cells.
(A) IL-5 suppressed apoptosis in JYTF-1, but not in TF-1 cells. TF-1 (lanes 2-8) and JYTF-1 (lanes 9-15) cells were transferred from medium containing GM-CSF (lanes 2 and 9) into medium containing IL-5 for various time periods (lanes 3-7 and 10-14) and were subjected to DNA ladder analysis. Two DNA samples from cells kept in 0.5% FBS cytokine-free medium for 24 hours were used as controls (lanes 8 and 15). DNA samples were fractionated in 1.5% agarose gel. Mr indicates molecular weight marker. (B) IL-5 sustained Mcl-1 expression in JYTF-1, but not in TF-1 cells. TF-1 (lanes 1-7) and JYTF-1 (lanes 8-14) cells were treated as described in panel A. Cell lysates were prepared from cells at each time point and subjected to Western blot analysis for the protein expression of Mcl-1, Bcl-2, Bcl-XL, Bax, and α-tubulin probing with antibodies specific for each antigen. The immunoblots were visualized using an ECL system. Experiments were repeated 3 times and a representative result is shown in this figure. (C) Expression of Mcl-1, but not of Bcl-XL, is stimulated by GM-CSF, IL-5, or SCF in JYTF-1 cells. JYTF-1 cells were deprived of cytokines for 24 hours (lane 1) and restimulated with GM-CSF (lane 2), IL-5 (lane 3), or SCF (lane 4) for 1 hour. Cell lysates were analyzed as described in panel B, except that only Mcl-1, Bcl-XL, or α-tubulin levels were probed with specific antibodies.
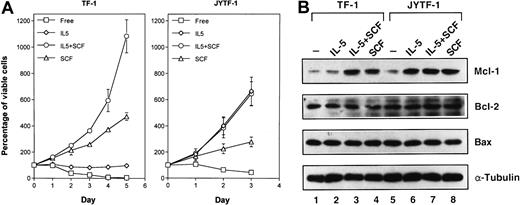
The correlation between the induction of the Mcl-1 protein and the apoptosis prevention ability of cytokine was further strengthened by studying Mcl-1 induction by SCF costimulation. Although the optimal proliferation of TF-1 cells required IL-5 and SCF costimulation (Figure2A, left panel), as previously demonstrated,34 IL-5 was able to promote the optimal growth of JYTF-1 cells in an SCF-independent manner (Figure 2A, right panel). These data were consistent with the responses previously observed in an IL-5Rα-overexpressing TF-1 derivative, TFα8.34 SCF alone induced the expression of Mcl-1 in both TF-1 and JYTF-1 cell lines (Figure 2B, lanes 3, 4, 7, and 8). SCF also complemented the defect of IL-5 in Mcl-1 induction in TF-1 cells and supported a higher level of expression when costimulated with IL-5 (Figure 2B, lanes 2 and 3). The results obtained from JYTF-1 cells showed no additional effect on the induction of Mcl-1 when the cells were incubated in IL-5 alone or together with SCF. These data further suggest that Mcl-1 expression is closely associated with the apoptosis prevention ability of cytokines and may be a common target of both IL-5 and SCF signaling pathways.
Proliferation synergism of SCF correlates with Mcl-1 inducibility.
(A) Requirement of SCF costimulation for optimal proliferation of TF-1 cell line. Stationary TF-1 and JYTF-1 cells were transferred into fresh culture media containing IL-5 (⋄), SCF (▵), IL-5 plus SCF (○), or no cytokine (■) and the viable cell numbers were measured every 24 hours by trypan blue dye exclusion assay. Left panel is for TF-1 cells and right panel is for JYTF-1 cells. Each number is the average of 3 independent measurements and SDs are shown as error bars. (B) SCF costimulation induced Mcl-1 expression in TF-1 cells. TF-1 (lanes 1-4) and JYTF-1 (lanes 5-8) cells were starved in cytokine-free medium for 24 hours (lanes 1 and 5) and then treated with IL-5 (lanes 2 and 6), SCF (lanes 4 and 8), or IL-5 plus SCF (lanes 3 and 7) for 1 hour before harvesting for lysate preparation. Western blot analysis was performed for the expression of Mcl-1, Bcl-2, Bax, and α-tubulin as described in Figure 1B. Experiments were repeated twice and a representative result is shown in this figure.
Proliferation synergism of SCF correlates with Mcl-1 inducibility.
(A) Requirement of SCF costimulation for optimal proliferation of TF-1 cell line. Stationary TF-1 and JYTF-1 cells were transferred into fresh culture media containing IL-5 (⋄), SCF (▵), IL-5 plus SCF (○), or no cytokine (■) and the viable cell numbers were measured every 24 hours by trypan blue dye exclusion assay. Left panel is for TF-1 cells and right panel is for JYTF-1 cells. Each number is the average of 3 independent measurements and SDs are shown as error bars. (B) SCF costimulation induced Mcl-1 expression in TF-1 cells. TF-1 (lanes 1-4) and JYTF-1 (lanes 5-8) cells were starved in cytokine-free medium for 24 hours (lanes 1 and 5) and then treated with IL-5 (lanes 2 and 6), SCF (lanes 4 and 8), or IL-5 plus SCF (lanes 3 and 7) for 1 hour before harvesting for lysate preparation. Western blot analysis was performed for the expression of Mcl-1, Bcl-2, Bax, and α-tubulin as described in Figure 1B. Experiments were repeated twice and a representative result is shown in this figure.
Differential activation of signaling components in TF-1 and JYTF-1 cells by IL-5
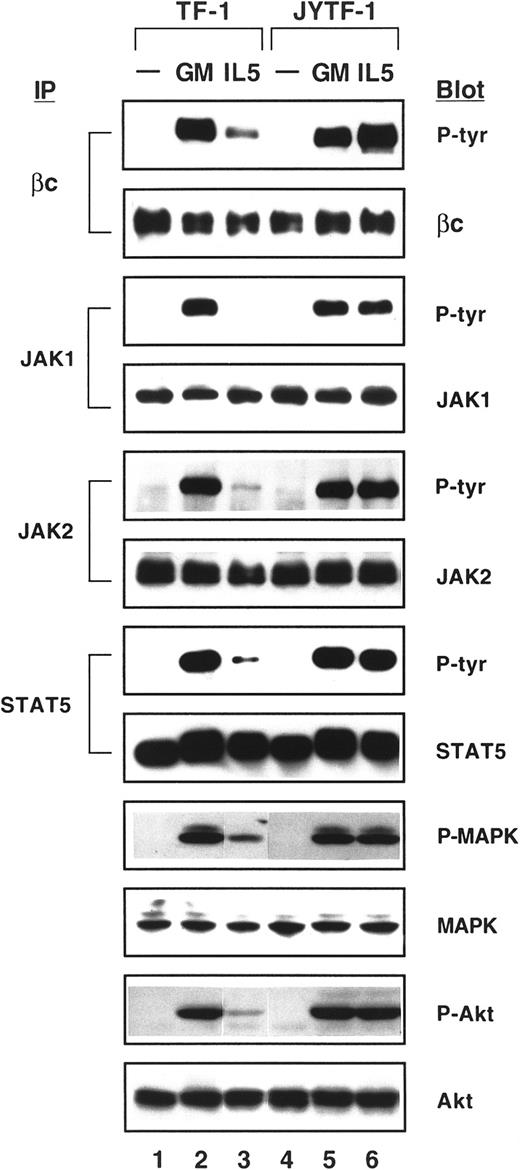
In response to IL-5, extensive tyrosine phosphorylation of intracellular proteins and activation of various kinases have been reported.17,37 38 However, the specific role of each individual signaling pathway in IL-5–dependent antiapoptotic activity and in Mcl-1 induction remains unclear. We next investigated how signaling molecules known to be activated by IL-5 are differentially affected in TF-1 cells when the cell's antiapoptotic function is lacking. As shown in Figure 3, βc, JAK1, and JAK2 were tyrosine phosphorylated after stimulation by GM-CSF in both TF-1 and JYTF-1 cell lines (Figure 3, lanes 2 and 5). However, in TF-1 cells, the phosphorylation of JAK1 was not detectable (Figure3, lane 3) and βc and JAK2 were only weakly phosphorylated by IL-5 (Figure 3, lane 3). The tyrosine phosphorylation of JAK2 in TF-1 cells, although weak, was highly reproducible. However, we were not able to detect the signal of JAK1 phosphorylation even when 5 mg of protein lysate was used in an immunoprecipitation reaction and the blots were exposed for up to 1 hour after ECL reaction (data not shown). This defect in JAK1 phosphorylation by IL-5 was restored and the phosphorylation of βc and JAK2 was also greatly improved in JYTF-1 cells (Figure 3, lane 6) as well as in 2 of the cell lines overexpressing IL-5Rα, TFα1, and TFα8 (data not shown). Phosphorylation of JAK3 and Tyk2 was not detectable by GM-CSF and IL-5 in TF-1 and JYTF-1 cell lines (data not shown). Furthermore, GM-CSF and IL-5 both were able to stimulate the phosphorylation of STAT5 in TF-1 and JYTF-1 cell lines (Figure 3, lanes 2, 3, 5, and 6), although the level of phosphorylation was much weaker in TF-1 cells stimulated by IL-5 (lane 3). Among other STAT family members, STAT3 was slightly tyrosine phosphorylated on stimulation by GM-CSF, but not IL-5, in both cell lines (data not shown).
Differential phosphorylation of signaling components by hIL-5 in TF-1 and JYTF-1 cells.
Both TF-1 and JYTF-1 cells were cytokine-depleted for 24 hours (lanes 1 and 4) before stimulation with GM-CSF (lanes 2 and 5) and IL-5 (lanes 3 and 6) (as indicated above the picture) for 5 minutes. Cell lysates were prepared and subjected to immunoprecipitation (IP)-Western (Blot) (for βc, JAK1, JAK2, and STAT5) or direct Western (for MAPK and Akt) blot analysis. For IP-Blot analysis, cell lysates were immunoprecipitated, fractionated, and transferred by Western blotting and the membrane was probed with antiphosphotyrosine antibody (indicated as P-tyr on the right-hand side, under Blot). This membrane was stripped as described in Materials and methods and then reprobed with anti-βc, -JAK1, -JAK2, or -STAT5 antibody (as indicated on the right-hand side, under Blot). For direct Western blot analysis, 150 μg of whole cell lysates was fractionated directly in a 10% SDS-PAGE and the Western blot was then probed with antibodies against phospho-MAPK (MAPK = ERK1p44 + ERK2p42) and phospho-Akt. These 2 blots were then reprobed with anti-MAPK and anti-Akt antibodies. The Western blot signals were visualized by ECL reaction and exposed onto x-ray film.
Differential phosphorylation of signaling components by hIL-5 in TF-1 and JYTF-1 cells.
Both TF-1 and JYTF-1 cells were cytokine-depleted for 24 hours (lanes 1 and 4) before stimulation with GM-CSF (lanes 2 and 5) and IL-5 (lanes 3 and 6) (as indicated above the picture) for 5 minutes. Cell lysates were prepared and subjected to immunoprecipitation (IP)-Western (Blot) (for βc, JAK1, JAK2, and STAT5) or direct Western (for MAPK and Akt) blot analysis. For IP-Blot analysis, cell lysates were immunoprecipitated, fractionated, and transferred by Western blotting and the membrane was probed with antiphosphotyrosine antibody (indicated as P-tyr on the right-hand side, under Blot). This membrane was stripped as described in Materials and methods and then reprobed with anti-βc, -JAK1, -JAK2, or -STAT5 antibody (as indicated on the right-hand side, under Blot). For direct Western blot analysis, 150 μg of whole cell lysates was fractionated directly in a 10% SDS-PAGE and the Western blot was then probed with antibodies against phospho-MAPK (MAPK = ERK1p44 + ERK2p42) and phospho-Akt. These 2 blots were then reprobed with anti-MAPK and anti-Akt antibodies. The Western blot signals were visualized by ECL reaction and exposed onto x-ray film.
As demonstrated by Kinoshita and colleagues,20 a mutant βc unable to activate the Ras signaling pathway failed to suppress apoptosis. Therefore, MAPK and Akt/PKB, 2 prominent targets of Ras signaling, were analyzed for possible correlation with the activation of apoptosis prevention. In these experiments, the specific phosphopeptide antibodies for MAPK and Akt/PKB were used in Western blot analysis to indicate the level of activation of these kinases. MAPK proteins, including ERK1p44 and ERK2p42, were phosphorylated at threonine202 (T202) and tyrosine204(Y204) (numbered according to ERK1) not only by the stimulation of GM-CSF (Figure 3, lanes 2 and 5), but also by IL-5 in both TF-1 and JYTF-1 cells (Figure 3, lanes 3 and 6). However, the phosphorylation level of MAPK stimulated by IL-5 was reduced by 5-fold in TF-1 cells (lane 3) compared to JYTF-1 cells (lane 6). Akt/PKB phosphorylation showed similar results. In JYTF-1 cells, both GM-CSF and IL-5 dramatically induced serine 473 (S473) phosphorylation of Akt (Figure 3, lanes 5 and 6), whereas in TF-1 cells, IL-5 could not efficiently induce Akt phosphorylation (Figure 3, lane 3). These data strongly suggest that STAT, MAPK, and Akt/PKB pathways may all be important for transducing the apoptosis prevention signals of the cytokine receptors.
Both MAPK and Akt/PKB pathways are activated by SCF
To further explore the commitment of each signaling pathway in apoptosis prevention, we costimulated SCF with IL-5 and checked the activation of STAT5, MAPK, and Akt/PKB by Western blotting as described in Figure 3. As shown in Figure 4, SCF activated MAPK and Akt/PKB, but did not activate STAT5 in either TF-1 or JYTF-1 cell lines (Figure 4, lanes 4 and 8). When costimulated with IL-5, SCF complemented the defect in MAPK and Akt/PKB activation of IL-5, but did not complement the defect in STAT5 activation (Figure 4, lane 3). Therefore, activation of MAPK and Akt/PKB pathways seemed to be more closely correlated with antiapoptosis ability. When the proliferation of JYTF-1 cell line was optimally activated by IL-5, the levels of phosphorylation of MAPK at Thr202/Tyr204 and Akt/PKB at Ser473were significantly less than when stimulated by the optimizing concentration of SCF (Figure 4, lanes 6 versus 8). The signal intensity of phospho-MAPK and phospho-Akt in SCF-treated samples was about 2- to 3-fold higher than that in IL-5–treated JYTF-1 samples. However, the proliferation response of JYTF-1 cells to IL-5 was constantly greater than the effect of SCF (Figure 2A), suggesting that the STAT5 pathway or other unidentified pathways may also play important roles in supporting the proliferation of hematopoietic cells.
Complementation effect of SCF costimulation on various signaling pathways.
TF-1 and JYTF-1 cells were treated as described in Figure 2B, except that cytokine stimulation was for only 5 minutes, and cell lysates were analyzed for the activation of STAT5, MAPK, and Akt molecules as described in Figure 3.
Complementation effect of SCF costimulation on various signaling pathways.
TF-1 and JYTF-1 cells were treated as described in Figure 2B, except that cytokine stimulation was for only 5 minutes, and cell lysates were analyzed for the activation of STAT5, MAPK, and Akt molecules as described in Figure 3.
Both MEK/MAPK and PI-3K/Akt activities are essential for apoptosis prevention and Mcl-1 induction
To further confirm the roles of the MAPK and Akt/PKB pathways in apoptosis prevention, we applied specific inhibitors to abrogate each pathway and explored the possible interference of the growth property. PD98059, a specific MEK inhibitor, and LY294002, an inhibitor of PI-3 kinase, were used to treat JYTF-1 cells, and a time course study on the viable cell numbers was performed. As shown in Figure5A, both PD98059 (100 μmol/L) or LY294002 (40 μmol/L) effectively suppressed growth of JYTF-1 cells in both IL-5– and SCF-containing medium. Both PD98059 and LY294002 completely inhibited the mitogenic activity of SCF and IL-5 (data not shown), whereas they showed differential inhibition of the antiapoptotic effect of cytokines (Figure 5B). As revealed by a nucleosome releasing assay, LY294002 abrogated the antiapoptotic effect of IL-5 slightly better than did PD98059 (Figure 5B, IL5+LY versus IL-5+PD). In contrast, both inhibitors completely inhibited the survival effect of SCF (Figure 5B, SCF+PD and SCF+LY).
Effect of kinase inhibitors on the growth activity of IL-5 and SCF in JYTF-1 cells.
(A) Differential growth properties to inhibitors in cells cultured in IL-5 and SCF. JYTF-1 cells were seeded into media containing IL-5 or SCF with or without PD98059 (100 μmol/L) or LY294002 (40 μmol/L). After 24 and 48 hours of incubation, viable cell numbers were measured in each sample and the values are presented as the percentage of starting cell number in each culture condition. Each number represents the average of 3 independent experiments performed in duplication. Free (■), IL-5 (⋄), IL-5 plus 100PD (○), IL-5 plus 40LY (▵), SCF (♦), SCF plus 100PD (●), SCF plus 40LY (▴). (B) Differential effect on antiapoptotic activity in IL-5– and SCF-cultured cells. JYTF-1 cells were treated as described in panel A and the extent of nucleosome released from apoptotic cells was measured after 24 hours of incubation. Each value is the average of 3 independent quadruplet experiments, and the SEs are shown as error bars.
Effect of kinase inhibitors on the growth activity of IL-5 and SCF in JYTF-1 cells.
(A) Differential growth properties to inhibitors in cells cultured in IL-5 and SCF. JYTF-1 cells were seeded into media containing IL-5 or SCF with or without PD98059 (100 μmol/L) or LY294002 (40 μmol/L). After 24 and 48 hours of incubation, viable cell numbers were measured in each sample and the values are presented as the percentage of starting cell number in each culture condition. Each number represents the average of 3 independent experiments performed in duplication. Free (■), IL-5 (⋄), IL-5 plus 100PD (○), IL-5 plus 40LY (▵), SCF (♦), SCF plus 100PD (●), SCF plus 40LY (▴). (B) Differential effect on antiapoptotic activity in IL-5– and SCF-cultured cells. JYTF-1 cells were treated as described in panel A and the extent of nucleosome released from apoptotic cells was measured after 24 hours of incubation. Each value is the average of 3 independent quadruplet experiments, and the SEs are shown as error bars.
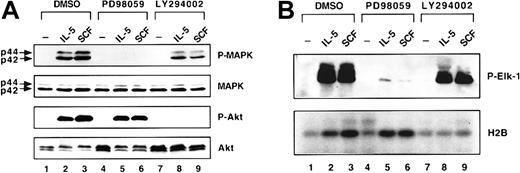
To confirm the specificity of these kinase inhibitors, the activities of MAPK and Akt/PKB kinases after treating with inhibitors were measured. Results from Western blot analyses with phospho-MAPK (P-MAPK) and phospho-Akt (P-Akt) specific antibodies revealed that the phosphorylation of MAPK and Akt by their upstream kinases were dramatically inhibited by PD98059 and LY294002, respectively (Figure6A). Furthermore, the kinase activities for both MAPK and Akt were also measured by in vitro kinase assay (Figure 6B). A known MAPK substrate, Elk-1, was included in the MAPK kinase reaction and its phosphorylation was detected with phospho-Elk specific antibody. In contrast, histone H2B was used as a pseudosubstrate of Akt and the phosphorylation of H2B was detected by autoradiography after labeling with 32P-ATP by kinase. The results of the in vitro kinase reaction suggested that both PD98059 and LY294002 have a very potent inhibition effect to their target kinases (Figure 6B). Intriguingly, LY294002 also showed a partial inhibitory effect on the activity of MAPK in both the phosphorylation assay (Figure 6A) and in the in vitro kinase assay (Figure 6B). Additionally, LY294002 inhibited the phosphorylation of MEK, an upstream kinase of MAPK, by 50% (data not shown).
Inhibition of cytokine-induced activation of MAPK and Akt by kinase inhibitors.
(A) Inhibition of phosphorylation of MAPK and Akt by kinase inhibitors. JYTF-1 cells were cytokine starved for 24 hours before treatment with PD98059 (lanes 4-6) or LY294002 (lanes 7-9). After 1-hour pretreatment with drugs, cytokines were added to the culture media for 5 minutes before cells were harvested. Cell lysates were prepared and subjected to Western blot analysis for the activation of MAPK and Akt as described in Figure 4. DMSO was used as the solvent and was included as a negative control (lanes 1-3). The same experiment was repeated twice and a representative result is shown here. (B) Inhibition of kinase activities of MAPK and Akt by inhibitors. JYTF-1 cells were treated as described in panel A and cell lysates were immunoprecipitated with anti-P-MAPK or Akt antibody. The immunoprecipitates were then subjected to in vitro kinase assay for both MAPK and Akt, as described in “Materials and methods.” The results of MAPK activity were visualized using an ECL system and the results of Akt activity are shown as an autoradiograph. P-Elk1 is the product of the kinase reaction of MAP kinase and H2B is the substrate of Akt kinase.
Inhibition of cytokine-induced activation of MAPK and Akt by kinase inhibitors.
(A) Inhibition of phosphorylation of MAPK and Akt by kinase inhibitors. JYTF-1 cells were cytokine starved for 24 hours before treatment with PD98059 (lanes 4-6) or LY294002 (lanes 7-9). After 1-hour pretreatment with drugs, cytokines were added to the culture media for 5 minutes before cells were harvested. Cell lysates were prepared and subjected to Western blot analysis for the activation of MAPK and Akt as described in Figure 4. DMSO was used as the solvent and was included as a negative control (lanes 1-3). The same experiment was repeated twice and a representative result is shown here. (B) Inhibition of kinase activities of MAPK and Akt by inhibitors. JYTF-1 cells were treated as described in panel A and cell lysates were immunoprecipitated with anti-P-MAPK or Akt antibody. The immunoprecipitates were then subjected to in vitro kinase assay for both MAPK and Akt, as described in “Materials and methods.” The results of MAPK activity were visualized using an ECL system and the results of Akt activity are shown as an autoradiograph. P-Elk1 is the product of the kinase reaction of MAP kinase and H2B is the substrate of Akt kinase.
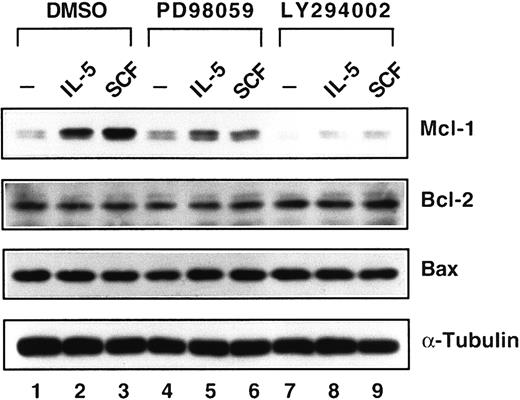
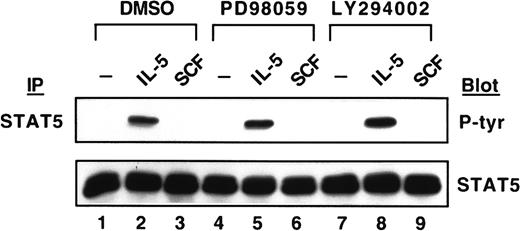
After demonstrating the inhibition of the MEK/MAPK signaling pathway by PD98059 and PI-3K/Akt signaling pathway by LY294002, we next investigated effects of these inhibitors on the induction of Mcl-1 protein. LY294002 completely suppressed the expression of Mcl-1 induced by either IL-5 or SCF (Figure 7, lanes 8 and 9). However, PD98059 only reduced Mcl-1 expression 36% by IL-5 induction (Figure 7, lane 5 versus lane 2) and 77% by SCF induction (Figure 7, lane 6 versus lane 3). Meanwhile, the expression of Bcl-2 and Bax proteins was not affected at all when cells were treated with either kinase inhibitor (Figure 7). It was previously demonstrated that the phosphorylation of STAT5 protein by erythropoietin was not inhibited by PI-3K inhibitor and PD98059 in primary erythroid colony-forming cells.39 We, therefore, investigated the tyrosine phosphorylation of the STAT5 protein. As shown in Figure8, the induction of tyrosine phosphorylation of the STAT5 protein was not affected by either kinase inhibitor in medium containing IL-5 (Figure 8, lanes 2, 5, and 8), and SCF did not activate STAT5 tyrosine phosphorylation, the same result as shown in Figure 4.
Down-regulation of Mcl-1 expression by kinase inhibitors.
JYTF-1 cells were treated as described in Figure 6A and cell lysates were analyzed for the expression levels of Mcl-1, Bcl-2, and Bax, as described in Figure 1B. The expression level of α-tubulin protein is included as a loading control.
No suppression on the tyrosine phosphorylation of STAT5 by the kinase inhibitors.
JYTF-1 cells were treated with PD98059 and LY294002 as described in Figure 6A. After stimulating with IL-5 or SCF, the tyrosine phosphorylation of the STAT5 protein was analyzed as described in Figure 3.
Discussion
In this study, we used 3 systems to demonstrate the importance of Mcl-1, a prosurvival member of the Bcl-2 gene family, in the antiapoptotic effects of SCF and IL-5. First, the ability of IL-5 to induce Mcl-1 expression is correlated with the ability of IL-5 to suppress apoptosis in TF-1 and JYTF-1 cell lines. IL-5 was unable to induce Mcl-1 expression in the IL-5Rα low-expressing cell line TF-1 in which IL-5 is unable to suppress apoptosis, although it is competent to activate mitogenesis (Figure 1A,B). Increase of the expression of IL-5Rα in JYTF-1 cells resulted in augmentation of the induction level of Mcl-1 and inhibition of apoptosis on the addition of IL-5 (Figure 1A,B). Second, the synergistic effect of SCF in hematopoietic progenitor cells depends on the ability of SCF to increase Mcl-1 expression. In TF-1 cells, in addition to IL-5, SCF costimulation was required for optimal growth effect (Figure 2A). The contribution of SCF in repressing apoptosis correlated well with the ability of SCF to increase the expression of Mcl-1 (Figure 2B). Third, blockage of cytokine-dependent growth by kinase inhibitors correlated with suppression of Mcl-1 induction. Incubation of cells with the inhibitors of either MEK or PI-3K abrogated the proliferation activity of IL-5 (Figure 5A), and both survival and mitogenic activities were severely impaired and the expression of Mcl-1 was suppressed to the basal levels. Our previous results indicated that overexpression of Mcl-1 in TF-1 cells greatly promotes survival in the absence of cytokine33 and that down-regulation of the Mcl-1 expression level by antisense transcripts resulted in impairment of cell viability in response to GM-CSF.33 Putting all these data together, our current results strongly suggest that Mcl-1 is one of the common targets of the survival signals of IL-5 and SCF in the human progenitor cell line TF-1.
mcl-1 was originally identified as an early gene induced by phorbol ester during differentiation of ML-1 myeloid leukemia cells.40 It was suggested that Mcl-1 may have a function in development and differentiation of hematopoiesis. Indeed, in terminally differentiated human neutrophils, the expression level of Mcl-1 was shown to be tightly correlated with cell viability promoted by certain agents including GM-CSF, IL-1β, sodium butyrate, and lipopolysaccharide.41 However, our present data suggest that Mcl-1 is also a target of SCF, which only acts on the early stage of hematopoiesis and not in mature differentiated cell lineages. In the primary IL-3–dependent progenitor cell culture derived from murine bone marrow, mcl-1 gene is highly inducible by murine SCF (our unpublished data). In vitro colony-forming assay42,43and in vivo phenotype of a mutation of SCF in mice44,45both suggest that SCF is required for the optimal production of clonogenic progeny from more primitive “stem cells” due to its ability to suppress spontaneous programmed cell death. Therefore, being the common target of the early-stage cytokine SCF and the late-stage cytokine IL-5, Mcl-1 may be partly responsible for survival in both the terminally differentiated mature and the stem/progenitor cell compartments. Mcl-1 expression also has strong correlation with viability in many other hematopoietic lineages including monocytic cells,46 granulocytic cells,46eosinophils,47 and lymphocytic B cells.48Furthermore, elevated expression of Mcl-1 at relapse of leukemia indicated that Mcl-1 overexpression has close correlation with death deregulation.49 It would be reasonable to suspect Mcl-1 of being a useful marker of cellular viability in the hematopoietic systems.
Our data also suggest that both the MEK/MAPK and PI-3K/Akt signaling pathways are essential in regulating Mcl-1 expression and the antiapoptotic activities of IL-5 and SCF. However, several lines of evidence suggest that these 2 signaling pathways do not cross-talk with each other. A Ras mutation (G12V/T35S), which effectively activates Raf/MAPK activity, does not stimulate PI-3K function.29Inversely, the G12V/V45E mutation of Ras causes the phosphorylation of S6 kinase via PI-3K without affecting the function of MAPK.29 In the present study, MEK kinase-specific inhibitor efficiently blocked the activation of MAPK by cytokines, but did not interfere with the function of Akt (Figure 6A,B). Overexpression of the constitutively active mutant of Akt (ie, Myr-Akt) also independently confers a survival ability on hematopoietic cells30 without activating MAPK (our unpublished data). Overexpression of the activated Ras mutants,29 which causes activation of either the Raf/MAPK or PI-3K/Akt pathway, independently prevents cytokine withdrawal-induced apoptosis suggesting that each signal is sufficient for suppressing apoptosis. These observations are consistent with the results from our Myr-Akt overexpression experiment, but not with the results from our kinase inhibitor experiments. For example, although SCF still fully activated the function of the PI-3K/Akt pathway in the presence of MEK inhibitor, SCF almost completely lost its antiapoptotic function (Figure 5B). In the presence of PI-3K inhibitor, SCF also completely lost its antiapoptotic activity (Figure 5B). These later observations suggested an essential, but not sufficient, role of each signaling pathway in achieving a survival effect from cytokine. We cannot currently account for these discrepancies among results reached by overexpression of constitutive active mutants29,30 and by chemical inhibitor treatments or from the expression of receptor binding site mutants.50 One possibility may be that the strength of the enzymatic activity of Akt in myr-Akt-expressing cells is much stronger than that of the endogenous Akt activated by cytokine receptors under the physiologic condition. Hypothetically, in this case the quantitative difference in the strength of the 2 identical Akt signals would result in the qualitative difference in physiologic responses; that is, the strong Myr-Akt signal protects cells from death, whereas the weak endogenous Akt signal does not.
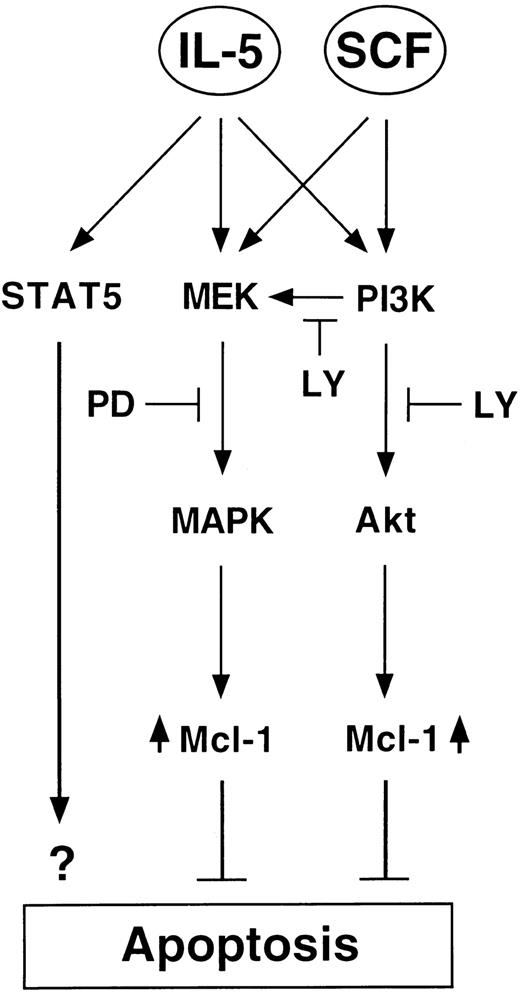
Intriguingly, although most of our results suggested that similar, if not identical, extents of activation in the MEK/MAPK and PI-3K/Akt pathways were achieved by IL-5 and SCF (Figure 3), JYTF-1 cells cultured in IL-5 were always slightly more viable than SCF-treated cells (Figure 2A). In addition, although the enzymatic activity of MAPK and Akt were suppressed to similar levels by kinase inhibitors (Figure6A,B), cells treated with IL-5 still possessed a better survival rate than cells cultured in SCF (Figure 5A,B). We demonstrated that STAT5 is activated only by IL-5, but not SCF, and that STAT5 was not affected by either PD98059 or LY294002 in our experiments (Figure 8). These differential responses of JYTF-1 cells toward IL-5 versus SCF may be due to the existence of an additional STAT5 signal of IL-5. Recently, expression of the dominant negative mutant of STAT5 was shown to be deleterious to the proliferation of factor-dependent cells.51 Furthermore, STAT5 was shown to be an essential antiapoptotic component of IL-3, IL-5, and GM-CSF in in vitro colony formation assays with STAT5a−/− and STAT5b−/− double knockout mice, but was not required for SCF function in the same assays.52 Therefore, it seems to be that in addition to MEK/MAPK and PI-3K/Akt pathways, the JAK/STAT5 pathway also contributes to the antiapoptotic function of IL-5, but not of SCF (Figure9). Together, our results suggest that cytokine may suppress cell death by activating multiple parallel antiapoptotic signaling pathways, wherein each individual signal is weak and essential, but not sufficient by itself, in achieving death suppression.
Schematic presentation of the hypothetical role of Mcl-1 in IL-5 and SCF antiapoptotic signaling pathways.
LY indicates LY294002 and is the PI-3K inhibitor. PD indicates PD98059 and is the MEK inhibitor. Both MEK/MAPK and PI-3K/Akt pathways were shown to be essential for the antiapoptotic activity of cytokines IL-5 and SCF, which results in the induction of the antiapoptotic protein Mcl-1. An additional JAK/STAT5 pathway, which is insensitive to both inhibitors, is involved only in IL-5 signaling and may also contribute to the survival effect of IL-5. A PI-3K–dependent MEK activation pathway, which is sensitive to LY treatment, is also indicated.
Schematic presentation of the hypothetical role of Mcl-1 in IL-5 and SCF antiapoptotic signaling pathways.
LY indicates LY294002 and is the PI-3K inhibitor. PD indicates PD98059 and is the MEK inhibitor. Both MEK/MAPK and PI-3K/Akt pathways were shown to be essential for the antiapoptotic activity of cytokines IL-5 and SCF, which results in the induction of the antiapoptotic protein Mcl-1. An additional JAK/STAT5 pathway, which is insensitive to both inhibitors, is involved only in IL-5 signaling and may also contribute to the survival effect of IL-5. A PI-3K–dependent MEK activation pathway, which is sensitive to LY treatment, is also indicated.
Acknowledgments
The authors thank Mr Yung-Luen Yu for preparation of the manuscript and Dr Hsin-Fang Yang-Yen for her careful reading of this manuscript and helpful suggestions.
Supported by grants from Academia Sinica and the National Science Council of Taiwan, NSC 86-2314-B-001-026 to J.J.-Y.Y.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jeffrey Jong-Young Yen, Institute of Biomedical Sciences, Academia Sinica, Taipei 11529, Taiwan; e-mail:bmjyen@novell.ibms.sinica.edu.tw.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal