Abstract
We investigated the functions of adiponectin, an adipocyte-specific secretory protein and a new member of the family of soluble defense collagens, in hematopoiesis and immune responses. Adiponectin suppressed colony formation from colony-forming units (CFU)—granulocyte-macrophage, CFU-macrophage, and CFU-granulocyte, whereas it had no effect on that of burst-forming units—erythroid or mixed erythroid-myeloid CFU. In addition, adiponectin inhibited proliferation of 4 of 9 myeloid cell lines but did not suppress proliferation of erythroid or lymphoid cell lines except for one cell line. These results suggest that adiponectin predominantly inhibits proliferation of myelomonocytic lineage cells. At least one mechanism of the growth inhibition is induction of apoptosis because treatment of acute myelomonocytic leukemia lines with adiponectin induced the appearance of subdiploid peaks and oligonucleosomal DNA fragmentation. Aside from inhibiting growth of myelomonocytic progenitors, adiponectin suppressed mature macrophage functions. Treatment of cultured macrophages with adiponectin significantly inhibited their phagocytic activity and their lipopolysaccharide-induced production of tumor necrosis factor α. Suppression of phagocytosis by adiponectin is mediated by one of the complement C1q receptors, C1qRp, because this function was completely abrogated by the addition of an anti-C1qRp monoclonal antibody. These observations suggest that adiponectin is an important negative regulator in hematopoiesis and immune systems and raise the possibility that it may be involved in ending inflammatory responses through its inhibitory functions.
Introduction
Hematopoietic cell formation is regulated by features of the bone marrow (BM) microenvironment, including cytokines, extracellular matrix components, and stromal cells.1-3Several cytokines that regulate proliferation and differentiation of hematopoietic cells have been identified. Complex cell–cell or cell–matrix interactions are also important for development of hematopoietic stem/progenitor cells.4-7
There are several types of BM stromal cells, including mononuclear phagocytic cells, fibroblasts, endothelial cells, and fat-containing cells. Among these, adipocytes are one of the principal cellular elements in the BM microenvironment and produce a variety of proteins that act on hematopoiesis and metabolism.8 For example, leptin, which is the product of the obese gene and produced primarily by adipocytes, not only regulates nutrient intake and metabolism but augments the proliferation of myelocytic and primitive hematopoietic progenitor cells.9-12 In addition, adipogenesis alters the expression of the extracellular matrix, membrane proteins, and cytokines in BM stroma. For example, the expression of tenascin, a cytoadhesive extracellular component, decreases during adipocyte differentiation, whereas that of hyaluronan increases.13-16 In the production of collagens, a switch from fibrillar (type I and III) to basement membrane (type IV) collagens occurs along with adipocyte differentiation.17,18 The expression of macrophage colony-stimulating factor (M-CSF), one of the indispensable cytokines in hematopoiesis, is decreased in stromal adipocytes relative to preadipocytes.19 20 Thus, adipocytes are considered to influence hematopoiesis both directly and indirectly.
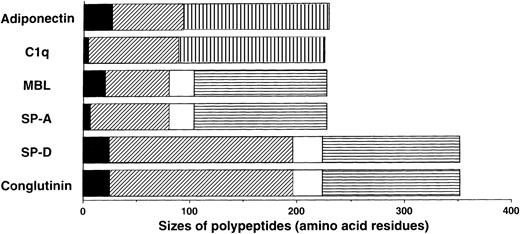
We previously isolated adiponectin as an adipocyte-specific secretory protein.21 The production of adiponectin increases in accordance with the differentiation of preadipocytes to adipocytes.22 Adiponectin is composed of 244 amino acid residues containing a short noncollagenous N-terminal segment followed by a collagen-like sequence (Figure 1). On the basis of structural analyses of its murine homolog Acrp30/AdipoQ,23 adiponectin is presumed to form a homotrimeric subunit with a collagen-like triple-helical structure and circulate through the body as a multimer of trimers. These structural features suggest that adiponectin belongs to a family of proteins identified as soluble defense collagens and including complement C1q and the collectins mannose-binding lectin (MBL), lung surfactant protein A (SP-A), lung surfactant protein D, and conglutinin (Figure1). The collectins play important roles in the innate humoral immune system.24-27 These proteins can identify foreign pathogens by detecting specific carbohydrate structures uniquely present on microorganisms, and they subsequently interact with phagocytic cells or the complement system to bring about killing and clearance of targets without involvement of antibodies. Lack or low levels of collectin expression cause increased susceptibility to infections, especially in infants, whose specific immune systems for various pathogens have not fully developed.28
Sequence features of adiponectin, complement C1q, and the collectins.
The monomer of adiponectin polypeptide (230 amino acids in its mature form) consists of 3 distinct regions: (1) a short N-terminal segment (solid black); (2) a collagen-like, glycine-X-Y repeating sequence (diagonal stripe); and (3) a C-terminal globular domain (hatched pattern). Adiponectin and C1q are similar in size, domain structure, and overall homology. They are different from the collectins in that they have no α-helix (solid white) regions and they contain C-terminal globular domains related to collagen VIII and X rather than the C-type lectin domains (horizontal stripe) in collectins.
Sequence features of adiponectin, complement C1q, and the collectins.
The monomer of adiponectin polypeptide (230 amino acids in its mature form) consists of 3 distinct regions: (1) a short N-terminal segment (solid black); (2) a collagen-like, glycine-X-Y repeating sequence (diagonal stripe); and (3) a C-terminal globular domain (hatched pattern). Adiponectin and C1q are similar in size, domain structure, and overall homology. They are different from the collectins in that they have no α-helix (solid white) regions and they contain C-terminal globular domains related to collagen VIII and X rather than the C-type lectin domains (horizontal stripe) in collectins.
We here describe unique functions of adiponectin in hematopoiesis and immune responses. Adiponectin serves as a negative regulator for myelomonocytic progenitor growth. In addition, adiponectin significantly inhibits functions of mature macrophages. Our data suggest that adiponectin may be involved in preventing excessive and prolonged inflammatory responses.
Materials and methods
Reagents and antibodies
We used highly purified recombinant human (rh) adiponectin and a murine monoclonal antibody (MoAb) reactive with adiponectin (9104; IgG1 isotype) that were described previously.29 Human serum albumin (HSA) and purified murine IgG1 fraction were purchased from Chemicon International (Temecula, CA) and Cappel (Durham, NC), respectively, and used as control proteins for adiponectin and MoAb 9104. Human C1q was purchased from Sigma (St Louis, MO). R3, a murine MoAb (IgM) reactive with human C1qRp, was generated as described previously.30 31 Purified murine IgM was purchased from Becton Dickinson (Mountain View, CA) and used as a control antibody for R3. In most experiments, the activities of adiponectin were examined in serum-free RPMI-1640 medium containing 10 μg/mL insulin-transferrin-sodium selenite supplement (Boehringer Mannheim, Indianapolis, IN), 1% chemically defined lipid concentrate (Gibco, Grand Island, NY), 0.2% bovine serum albumin (BSA), and 2 mmol/Ll-glutamine.
Source of cells
Human myeloid cell lines (KG1, HL60, THP1, KU812, and U937), erythroid cell lines (K562 and HEL), T-cell lines (MOLT4, Jurkat, and CCRF-CEM), and B-cell lines (Nalm6, BALL, ONHL1, and OPM2) were routinely maintained in RPMI-1640 medium supplemented with 10% fetal-calf serum (FCS). The human myeloid cell lines MO7E and F36p were cultured in RPMI-1640 medium supplemented with 10% FCS and 10 ng/mL rh granulocyte-macrophage colony-stimulating factor (GM-CSF). F36e, a human erythroid cell line, was cultured in RPMI-1640 medium supplemented with 10% FCS and 5 U/mL rh erythropoietin. The murine myeloid cell lines M1 and WEHI3 were maintained in RPMI-1640 medium supplemented with 10% FCS and Dulbecco modified Eagle medium supplemented with 10% FCS, respectively. A murine T-cell line (EL4) was maintained in RPMI-1640 medium supplemented with 10% FCS and 5 × 10−5 mol/L 2-mercaptoethanol. To make stable transformants of M1 cells expressing human Bcl-2, pcDNA3-hBcl-2 (a gift of Dr Itaru Matsumura, Osaka University, Osaka, Japan) was transfected into M1 cells by electroporation and the transformants were selected with 1 mg/mL G418 (Sigma).
Normal human BM was aspirated from the posterior iliac crest of healthy young volunteers after informed consent was obtained. Low-density BM mononuclear cells (BMMNC) were isolated by Ficoll-Hypaque density gradient centrifugation. To enrich the CD34+ cell population, BMMNC were negative-selected by using immunomagnetic beads conjugated with anti-CD3 and anti-CD11b MoAbs and then positive-selected by using immunomagnetic beads conjugated with an antihuman CD34 MoAb (Miltenyi Biotec, Berguisch, Gladbach, Germany) as described previously.5 In our series, more than 96% of the purified cells always expressed CD34 and more than 98% were viable.
Human monocyte-derived macrophages were isolated as described previously.32 Briefly, mononuclear cells were isolated from freshly collected buffy-coat preparations of whole human blood by Ficoll-Hypaque gradient centrifugation, and the cells were seeded in 24-well plastic plates (2 × 106 cells/well) or in 10-cm plastic tissue-culture dishes (2 × 107 cells/dish). The seeded cells were allowed to adhere for 1 hour in a humidified atmosphere at 37°C and 5% carbon dioxide (CO2), and nonadherent cells were removed by 3 washes with RPMI-1640 medium. The adherent cells, which were mainly monocytes, were grown in RPMI-1640 medium supplemented with 10% human type AB serum, with changing of the medium every 3 days. After incubation for 7 days, mature macrophages were obtained and used for subsequent experiments.
Assays of colony formation
To assess the colony formation of hematopoietic progenitor cells, serum-free methylcellulose progenitor assays were performed by using a modification of the technique described previously.33 Briefly, human BMMNC and CD34+cells were plated at a density of 5 × 104/mL and 5 × 102/mL, respectively, in serum-free methylcellulose medium (Veritas, Vancouver, Canada) consisting of Iscove modified Dulbecco medium, 0.9% methylcellulose, 10−4 mol/L 2-mercaptoethanol, 2 mmol/Ll-glutamine, 1% deionized crystallized BSA, human transferrin, bovine insulin, 3 U/mL rh erythropoietin, 50 ng/mL rh stem-cell factor, 10 ng/mL rh GM-CSF, 10 ng/mL rh granulocyte colony-stimulating factor (G-CSF), 10 ng/mL rh interleukin (IL) 3, and 10 ng/mL rh IL-6 with adiponectin or HSA. Cultures were set up in quadruplicate and incubated in a humidified atmosphere at 37°C and 5% CO2 for 16 days. Colony types and numbers were determined on days 12 to 16 of cultures by means of in situ observations using an inverted microscope according to established criteria34 and assessments of cytospin preparations of individual colonies of colony-forming units—granulocyte-macrophage (CFU-GM), colony-forming units—macrophage (CFU-M), colony-forming units—granulocyte (CFU-G), colony-forming units—mixed erythroid-myeloid (CFU-mixed), and burst-forming units—erythroid (BFU-E).
In some experiments, CD34+ cells were sorted singly into 96-well flat-bottomed microtiter plates with a fluorescence-activated cell-sorter scanner (FACScan) equipped with an automatic cell-deposition unit (Vantage; Becton Dickinson). In each well, the sorted cells were cultured in serum-free methylcellulose culture medium supplemented with 10 μg/mL adiponectin or HSA. Colony formation in each well was examined after 14 days of culture.
To evaluate precisely the effects of adiponectin on colony formation from myeloid progenitor cells, CFU-GM, CFU-M, and CFU-G colony formation from 1 × 105 BMMNC was assessed in methylcellulose culture containing 30% FCS with, respectively, rh GM-CSF alone (10 ng/mL), rh M-CSF alone (100 U/mL), and rh G-CSF alone (10 ng/mL). Colony types were verified by microscopical observation of cytospin preparations from individual colonies stained with May-Grünwald-Giemsa stain.
Assays of cell proliferation
To quantitate proliferation of cells, a tritium-thymidine incorporation assay was used as described previously.4Triplicate aliquots of cells (1 × 104/well) were cultured in 96-well flat-bottomed microtiter plates with 10 μg/mL HSA or adiponectin. Twenty-four hours after the initiation of culture, each well was pulsed for 4 hours with 0.0185 MBq tritium-thymidine. The cells were then harvested with a semiautomatic cell harvester (Pharmacia, Piscataway, NJ), and the incorporation was measured with a liquid scintillation counter.
Apoptosis assay
Nuclear DNA content was analyzed by flow cytometry as described previously.35 M1 cells were incubated with HSA or adiponectin for various times. Subsequently, the cells were fixed in cold ethanol and incubated at −20°C overnight. After centrifugation, the cells were resuspended in 300 μL propidium iodide (PI) solution containing 0.1% sodium citrate, 0.3% NP-40, 100 μg/mL RNase A, and 50 μg/mL PI and incubated in the dark at 37°C for 30 minutes. The fluorescence emitted from the PI-DNA complex was then quantitated with a FACScan (Becton Dickinson).
Fragmentation of DNA was analyzed as described previously.35 After incubation with HSA or adiponectin, cells were lysed in 0.4 mL lysis solution containing 200 mmol/L Tris-hydrochloric acid (HCl), 100 mmol/L EDTA, 1% sodium dodecyl sulfate, and 50 μg/mL proteinase K and incubated for 4 hours at 37°C. DNAs were extracted with phenol and then chloroform-isoamylalcohol. The aqueous phase was collected and precipitated with sodium chloride and ethanol. The ethanol-precipitated DNA was dried and resuspended in TE buffer (10 mmol/L Tris-HCl and 1 mmol/L EDTA) and treated with 50 μg/mL DNase-free RNase for 5 hours at 37°C and then with 300 μg/mL proteinase K for 5 hours at 37°C. DNAs were extracted twice and precipitated as described above. DNA pellets were resuspended in TE buffer, separated by electrophoresis in 1% agarose gel containing 0.5 μg/mL ethidium bromide (5 μg DNA per lane), and visualized under ultraviolet light.
Northern blot analysis
Total RNAs were isolated by using Trizol reagent (Gibco), electrophoresed through formaldehyde agarose gels (15 μg total RNA per lane), and transferred to nylon membranes (Amersham, Arlington Heights, IL). The cDNA fragments were labeled with phosphorus 32–deoxycytidine triphosphate using a random primed DNA labeling kit (Boehringer Mannheim) and hybridized to the membranes. Blots were then washed and autoradiographed. Fragments of the human Bcl-2, murine Bcl-xL, murine Bax, murine Bak, murine p53, murine β-actin, human tumor necrosis factor-α (TNF-α), human IL-1β, and human IL-6 genes and theB gene isolated from Chinese hamster ovary cells (a gift from Dr Toshio Hirano, Osaka University, Osaka, Japan) were used as materials for probes.35 36
Phagocytosis assay
Phagocytosis of human macrophages was evaluated with our previously described ingestion assays for adherent cells.32 Human macrophages were preincubated in 24-well flat-bottomed plates for 24 hours at 37°C in 500 μL serum-free conditioned RPMI-1640 medium with 10 μg/mL adiponectin, HSA, or medium alone. Fluorebrite fluorescent-microspheres (0.75YG; Polyscience, Warrington, PA) solution (50 μL 0.25% solids-latex in RPMI-1640 medium) was added to each well, and incubation allowed to proceed for 1 hour at 37°C. Nonadherent latex beads were removed by washing, and the cells were incubated for 30 minutes to obtain complete phagocytosis. Cells were then harvested by short-time treatment with EDTA and trypsin and washed vigorously 3 times with phosphate-buffered saline (PBS) to remove noningested beads. Subsequently, the amount of ingested beads was measured with a FACScan.
TNF-α secretion assay
Secretion of TNF-α from macrophages was determined by a heterologous 2-site sandwich enzyme-linked immunosorbent assay (ELISA) as described by Soell et al.37 First, 96-well microtiter plates were coated with 50 μL (10 μg/mL) rabbit anti-rh TNF-α IgG. After overnight incubation at 4°C, the plates were washed with PBS containing 0.05% Tween 20 and blocked with the same buffer containing 0.5% gelatin for 1 hour at 37°C. Macrophage supernatants (50 μL) were then added to the wells (2 hours at 37°C), and bound TNF-α was detected with biotinylated anti-rh TNF-α IgG (2 hours at 37°C). This was followed by incubation with alkaline phosphatase–labeled streptavidin (1 hour at 37°C) and the enzyme substrate. After 1 hour at 25°C, the plates were read at 405 nm. The readings were related to standard curves with rh TNF-α. The sensitivity of this ELISA was 20 pg/mL.
Results
Adiponectin inhibits proliferation of myelomonocytic progenitor cells
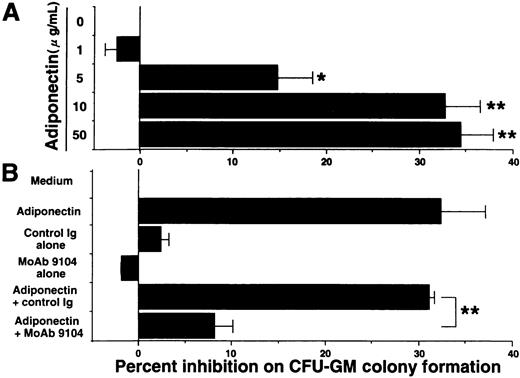
Our ELISA system previously revealed that adiponectin levels in plasma from healthy humans range from 1.9 to 17.0 μg/mL.29 Serum-free methylcellulose colony assays of human BMMNC were used to evaluate the influence of physiologic concentrations of adiponectin on hematopoiesis. First, we found that adiponectin reduced the number of CFU-GM colonies in a dose-dependent manner (Figure 2A). The number of CFU-GM colonies was decreased approximately 33% by the addition of 10 μg/mL rh adiponectin. The inhibitory effect was evident at 5 μg/mL and maximal at 10 to 50 μg/mL. In subsequent experiments, we used 10 μg/mL rh adiponectin. The antiadiponectin MoAb 9104, which can partly inhibit the binding of adiponectin to cells, markedly inhibited the reduction of CFU-GM formation by adiponectin, indicating that the reduction in colony formation of CFU-GM by rh adiponectin was specific (Figure 2B).
Effect of adiponectin on colony formation of colony-forming units—granulocyte-macrophage (CFU-GM) from human bone marrow mononuclear cells (BMMNC).
(A) Human BMMNC (5 × 104 cells) were cultured in serum-free methylcellulose medium containing a combination of growth factors with the indicated concentrations of adiponectin (0-50 μg/mL), and CFU-GM colony formation was estimated. (B) Purified antiadiponectin MoAb (MoAb 9104) or purified murine IgG1(30 μg/mL) was added to the methylcellulose culture containing a combination of growth factors with or without 10 μg/mL adiponectin, and CFU-GM colony formation from 5 × 104 human BMMNC was assessed. The data shown are the mean ± SD percentages of inhibition in 3 independent experiments in which each experiment was set up with quadruple cultures. Control (medium alone) colony numbers ranged from 54 to 77. Significant differences from control values are indicated by 1 (P < .05) or 2 (P < .01) asterisks.
Effect of adiponectin on colony formation of colony-forming units—granulocyte-macrophage (CFU-GM) from human bone marrow mononuclear cells (BMMNC).
(A) Human BMMNC (5 × 104 cells) were cultured in serum-free methylcellulose medium containing a combination of growth factors with the indicated concentrations of adiponectin (0-50 μg/mL), and CFU-GM colony formation was estimated. (B) Purified antiadiponectin MoAb (MoAb 9104) or purified murine IgG1(30 μg/mL) was added to the methylcellulose culture containing a combination of growth factors with or without 10 μg/mL adiponectin, and CFU-GM colony formation from 5 × 104 human BMMNC was assessed. The data shown are the mean ± SD percentages of inhibition in 3 independent experiments in which each experiment was set up with quadruple cultures. Control (medium alone) colony numbers ranged from 54 to 77. Significant differences from control values are indicated by 1 (P < .05) or 2 (P < .01) asterisks.
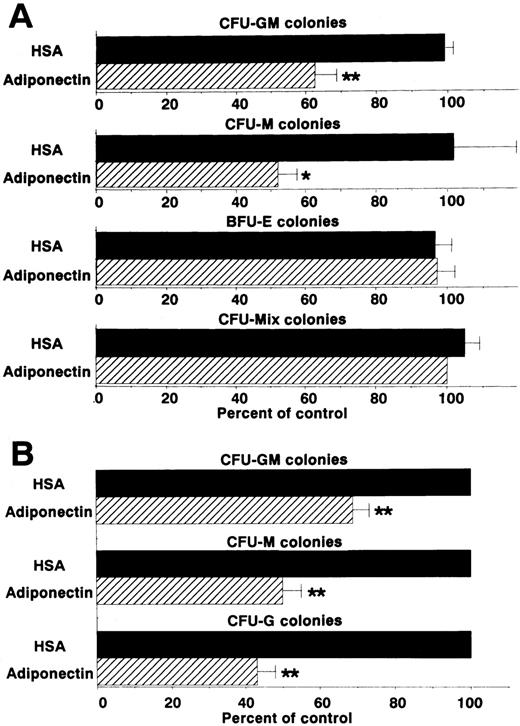
We then examined the effect of adiponectin on the colony formation of other progenitor cells by using the same assay system (Figure3A). CFU-M formation was also decreased approximately 50%. However, rh adiponectin had no influence on colony formation of BFU-E or CFU-mixed, indicating that the inhibitory effect was highly specific for myelomonocytic progenitor cells. To verify the inhibitory effects of adiponectin on colony formation from each type of myelomonocytic progenitor cell separately, colonies formed in the presence of rh GM-CSF alone, rh M-CSF alone, or rh G-CSF alone, with and without adiponectin, were examined. Colonies stimulated only with GM-CSF were mainly CFU-GM colonies (approximately 80%), with the remaining colonies containing only macrophages. Almost all colonies stimulated only with M-CSF were CFU-M colonies (> 95%). Colonies stimulated only with G-CSF were mainly CFU-G colonies (approximately 70%), with the remaining colonies containing both granulocytes and macrophages or only macrophages. No obvious shifts in these colony types were observed in the absence or presence of adiponectin (data not shown). As shown in Figure 3B, CFU-GM colonies formed with GM-CSF alone were decreased approximately 30% by 10 μg/mL adiponectin. CFU-M and CFU-G colonies were decreased approximately 50% and 60%, respectively, by adiponectin.
Effects of adiponectin on numbers of colonies.
(A) Human BMMNC (5 × 104 cells) were cultured in serum-free methylcellulose medium with a combination of growth factors containing 10 μg/mL adiponectin, human serum albumin (HSA), or medium alone, and colony formations from CFU-GM, colony-forming units—macrophage (CFU-M), burst-forming units—erythroid (BFU-E) and colony-forming units—mixed erythroid-myeloid (CFU-mixed) were evaluated. Control colony numbers (medium alone) ranged from 31 to 60 for CFU-GM, 4 to 13 for CFU-M, 62 to 99 for BFU-E, and 3 to 6 for CFU-mixed. (B) Colony formations by CFU-GM stimulated with recombinant human (rh) granulocyte-macrophage colony-stimulating factor (GM-CSF) alone, CFU-M stimulated with rh macrophage colony-stimulating factor (M-CSF) alone, and colony-forming units—granulocyte (CFU-G) stimulated with rh granulocyte colony-stimulation factor (G-CSF) alone from human BMMNC (1 × 105 cells) were evaluated in the absence or presence of 10 μg/mL adiponectin. Control (HSA) colony numbers ranged from 98 to 114 for CFU-GM, 73 to 86 for CFU-M, and 67 to 76 for CFU-G. The data shown are the mean ± SD percentages of control values from 3 independent experiments in which each experiment was set up with quadruple cultures. Significant differences from control values are indicated by 1 (P < .05) or 2 (P < .01) asterisks.
Effects of adiponectin on numbers of colonies.
(A) Human BMMNC (5 × 104 cells) were cultured in serum-free methylcellulose medium with a combination of growth factors containing 10 μg/mL adiponectin, human serum albumin (HSA), or medium alone, and colony formations from CFU-GM, colony-forming units—macrophage (CFU-M), burst-forming units—erythroid (BFU-E) and colony-forming units—mixed erythroid-myeloid (CFU-mixed) were evaluated. Control colony numbers (medium alone) ranged from 31 to 60 for CFU-GM, 4 to 13 for CFU-M, 62 to 99 for BFU-E, and 3 to 6 for CFU-mixed. (B) Colony formations by CFU-GM stimulated with recombinant human (rh) granulocyte-macrophage colony-stimulating factor (GM-CSF) alone, CFU-M stimulated with rh macrophage colony-stimulating factor (M-CSF) alone, and colony-forming units—granulocyte (CFU-G) stimulated with rh granulocyte colony-stimulation factor (G-CSF) alone from human BMMNC (1 × 105 cells) were evaluated in the absence or presence of 10 μg/mL adiponectin. Control (HSA) colony numbers ranged from 98 to 114 for CFU-GM, 73 to 86 for CFU-M, and 67 to 76 for CFU-G. The data shown are the mean ± SD percentages of control values from 3 independent experiments in which each experiment was set up with quadruple cultures. Significant differences from control values are indicated by 1 (P < .05) or 2 (P < .01) asterisks.
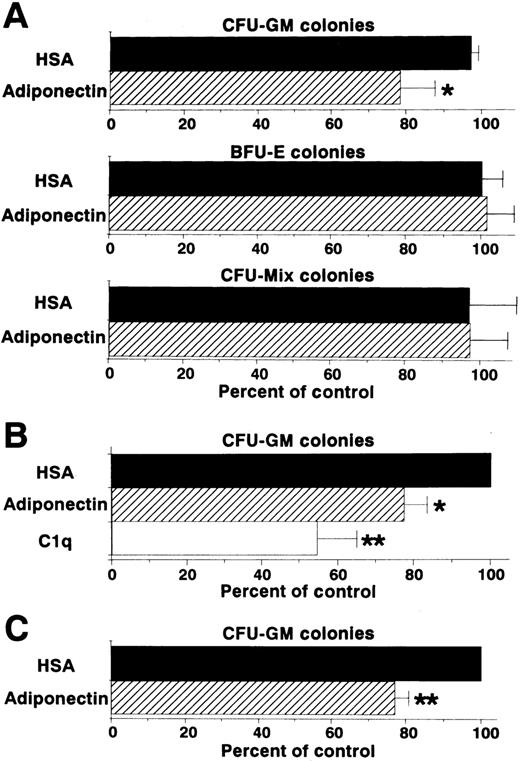
Colony formation from sorted CD34+ cells (enriched hematopoietic progenitor cells) was also analyzed in the presence of a combination of growth factors. Generation of CFU-GM colonies, but not that of BFU-E or CFU-mixed colonies, was inhibited by adiponectin (Figure 4A). This inhibitory effect on CFU-GM colony formation also occurred with complement C1q, which has a structure similar to that of adiponectin (Figure 4B). To eliminate the possibility that the inhibitory effect was mediated by accessory cells, the effect of adiponectin on CFU-GM colony formation from singly sorted CD34+ cells was examined. As shown in Figure 4C, CFU-GM colony formation was also significantly inhibited by adiponectin when only a single CD34+ cell was placed in each well.
Effect of adiponectin on colony formation from human CD34+ hematopoietic progenitors.
(A) Human CD34+ cells (500 cells) were cultured in serum-free methylcellulose medium with a combination of growth factors containing 10 μg/mL adiponectin, HSA, or medium alone, and colony formations from CFU-GM, BFU-E, and CFU-mixed were evaluated. Control (medium alone) colony numbers ranged from 28 to 47 for CFU-GM, 53 to 109 for BFU-E, and 14 to 22 for CFU-mixed. (B) Human CD34+cells (500 cells) were cultured in serum-free methylcellulose medium with a combination of growth factors containing 10 μg/mL C1q, adiponectin, or HSA, and CFU-GM colony formation was estimated. Control (HSA) colony numbers ranged from 49 to 70. (C) Single human CD34+ cells were placed in 96-well flat-bottomed plates (500 wells). The cells in each well were cultured with serum-free methylcellulose medium with a combination of growth factors containing 10 μg/mL adiponectin or HSA, and CFU-GM colony formation was estimated. Control (HSA) colony numbers ranged from 40 to 79. The data shown are the mean ± SD percentages of control values from 3 independent experiments.
Effect of adiponectin on colony formation from human CD34+ hematopoietic progenitors.
(A) Human CD34+ cells (500 cells) were cultured in serum-free methylcellulose medium with a combination of growth factors containing 10 μg/mL adiponectin, HSA, or medium alone, and colony formations from CFU-GM, BFU-E, and CFU-mixed were evaluated. Control (medium alone) colony numbers ranged from 28 to 47 for CFU-GM, 53 to 109 for BFU-E, and 14 to 22 for CFU-mixed. (B) Human CD34+cells (500 cells) were cultured in serum-free methylcellulose medium with a combination of growth factors containing 10 μg/mL C1q, adiponectin, or HSA, and CFU-GM colony formation was estimated. Control (HSA) colony numbers ranged from 49 to 70. (C) Single human CD34+ cells were placed in 96-well flat-bottomed plates (500 wells). The cells in each well were cultured with serum-free methylcellulose medium with a combination of growth factors containing 10 μg/mL adiponectin or HSA, and CFU-GM colony formation was estimated. Control (HSA) colony numbers ranged from 40 to 79. The data shown are the mean ± SD percentages of control values from 3 independent experiments.
Several lymphohematopoietic cell lines were cultured in serum-free conditions with rh adiponectin or HSA, and their proliferation was evaluated. In the tested myeloid cell lines, tritium-thymidine incorporation of THP1, KU812, M1, and WEHI3 cells was inhibited by incubation with adiponectin, whereas proliferation of KG1, HL60, MO7E, and F36p cells was not affected (Table1). Interestingly, the numbers of recovered viable cells were markedly decreased by adiponectin, especially in the THP1 and M1 cell lines. In these lines, treatment with adiponectin for 48 hours significantly increased the proportion of dead cells (by 45.3% ± 2.7% [THP1] and 30.9% ± 8.5% [M1]). Proliferation of the tested erythroid (K562, HEL, and F36e) and T- and B-cell lines (MOLT4, Jurkat, CCRF-CEM, EL4, Nalm6, ONHL1, and OPM2) was not affected by rh adiponectin, except in the case of BALL, a pre–B-cell line (Table 1). These results show that adiponectin serves as a negative regulator of the growth of limited cell lineages, especially myelomonocytic progenitors.
Effects of adiponectin on proliferation of hematopoietic cell lines
| Cell lineage/line . | Origin . | Proliferation (SI)A . | No. of viable cells (×104/well) . | |
|---|---|---|---|---|
| HSA . | Adiponectin . | |||
| Myeloid lineage | ||||
| KG1 | Myelogenous leukemia | 0.97 ± 0.02 | 1.71 ± 0.15 | 1.61 ± 0.02 |
| HL60 | Promyelocytic leukemia | 0.99 ± 0.06 | 3.60 ± 0.49 | 3.68 ± 0.22 |
| THP1 | Monocytic leukemia | 0.38 ± 0.03* | 1.79 ± 0.08 | 0.76 ± 0.08† |
| MO7E | Megakaryocytic leukemia | 1.04 ± 0.06 | 2.30 ± 0.24 | 2.15 ± 0.22 |
| KU812 | Myelogenous leukemia | 0.86 ± 0.04* | 1.44 ± 0.05 | 1.36 ± 0.04* |
| F36p | Myelodysplastic syndrome | 1.01 ± 0.05 | 2.65 ± 0.16 | 2.55 ± 0.20 |
| U937 | Histiocytic lymphoma | 1.02 ± 0.05 | 2.47 ± 0.10 | 2.65 ± 0.18 |
| M1 | Myelogenous leukemia | 0.73 ± 0.07† | 4.86 ± 0.25 | 2.13 ± 0.19† |
| WEHI3 | Myelomonocytic leukemia | 0.90 ± 0.06* | 2.42 ± 0.22 | 1.98 ± 0.10 |
| Erythroid lineage | ||||
| K562 | Erythroleukemia | 0.99 ± 0.04 | 4.57 ± 0.53 | 4.17 ± 0.25 |
| HEL | Erythroleukemia | 1.02 ± 0.02 | 3.43 ± 0.26 | 3.25 ± 0.20 |
| F36e | Myelodysplastic syndrome | 0.97 ± 0.07 | 0.41 ± 0.04 | 0.43 ± 0.03 |
| T-cell lineage | ||||
| MOLT4 | Lymphoblastic leukemia | 1.01 ± 0.03 | 5.40 ± 0.20 | 5.55 ± 0.41 |
| Jurkat | Lymphoblastic leukemia | 1.03 ± 0.03 | 2.38 ± 0.08 | 2.55 ± 0.19 |
| CCRF-CEM | Lymphoblastic leukemia | 0.94 ± 0.02 | 1.87 ± 0.19 | 1.73 ± 0.06 |
| EL4 | Malignant lymphoma | 0.99 ± 0.03 | 5.12 ± 0.06 | 4.80 ± 0.37 |
| B-cell lineage | ||||
| Nalm6 | Lymphoblastic leukemia | 1.04 ± 0.10 | 3.48 ± 0.06 | 3.22 ± 0.10 |
| BALL | Lymphoblastic leukemia | 0.62 ± 0.07† | 1.83 ± 0.05 | 0.81 ± 0.07† |
| ONHL1 | Malignant lymphoma | 0.99 ± 0.04 | 1.92 ± 0.27 | 2.13 ± 0.10 |
| OPM2 | Plasma cell leukemia | 0.98 ± 0.03 | 3.88 ± 0.18 | 4.00 ± 0.15 |
| Cell lineage/line . | Origin . | Proliferation (SI)A . | No. of viable cells (×104/well) . | |
|---|---|---|---|---|
| HSA . | Adiponectin . | |||
| Myeloid lineage | ||||
| KG1 | Myelogenous leukemia | 0.97 ± 0.02 | 1.71 ± 0.15 | 1.61 ± 0.02 |
| HL60 | Promyelocytic leukemia | 0.99 ± 0.06 | 3.60 ± 0.49 | 3.68 ± 0.22 |
| THP1 | Monocytic leukemia | 0.38 ± 0.03* | 1.79 ± 0.08 | 0.76 ± 0.08† |
| MO7E | Megakaryocytic leukemia | 1.04 ± 0.06 | 2.30 ± 0.24 | 2.15 ± 0.22 |
| KU812 | Myelogenous leukemia | 0.86 ± 0.04* | 1.44 ± 0.05 | 1.36 ± 0.04* |
| F36p | Myelodysplastic syndrome | 1.01 ± 0.05 | 2.65 ± 0.16 | 2.55 ± 0.20 |
| U937 | Histiocytic lymphoma | 1.02 ± 0.05 | 2.47 ± 0.10 | 2.65 ± 0.18 |
| M1 | Myelogenous leukemia | 0.73 ± 0.07† | 4.86 ± 0.25 | 2.13 ± 0.19† |
| WEHI3 | Myelomonocytic leukemia | 0.90 ± 0.06* | 2.42 ± 0.22 | 1.98 ± 0.10 |
| Erythroid lineage | ||||
| K562 | Erythroleukemia | 0.99 ± 0.04 | 4.57 ± 0.53 | 4.17 ± 0.25 |
| HEL | Erythroleukemia | 1.02 ± 0.02 | 3.43 ± 0.26 | 3.25 ± 0.20 |
| F36e | Myelodysplastic syndrome | 0.97 ± 0.07 | 0.41 ± 0.04 | 0.43 ± 0.03 |
| T-cell lineage | ||||
| MOLT4 | Lymphoblastic leukemia | 1.01 ± 0.03 | 5.40 ± 0.20 | 5.55 ± 0.41 |
| Jurkat | Lymphoblastic leukemia | 1.03 ± 0.03 | 2.38 ± 0.08 | 2.55 ± 0.19 |
| CCRF-CEM | Lymphoblastic leukemia | 0.94 ± 0.02 | 1.87 ± 0.19 | 1.73 ± 0.06 |
| EL4 | Malignant lymphoma | 0.99 ± 0.03 | 5.12 ± 0.06 | 4.80 ± 0.37 |
| B-cell lineage | ||||
| Nalm6 | Lymphoblastic leukemia | 1.04 ± 0.10 | 3.48 ± 0.06 | 3.22 ± 0.10 |
| BALL | Lymphoblastic leukemia | 0.62 ± 0.07† | 1.83 ± 0.05 | 0.81 ± 0.07† |
| ONHL1 | Malignant lymphoma | 0.99 ± 0.04 | 1.92 ± 0.27 | 2.13 ± 0.10 |
| OPM2 | Plasma cell leukemia | 0.98 ± 0.03 | 3.88 ± 0.18 | 4.00 ± 0.15 |
Triplicate aliquots of cells (1 × 104/well) were cultured in serum-free medium with 10 μg/mL adiponectin or human serum albumin (HSA), and their proliferation was examined by tritium-thymidine incorporation assay and by counting recovered viable cells. Tritium-thymidine incorporation was measured after 24 hours of culture, and the results were expressed as stimulation index (SI)A, which was calculated as SI = (tritium-thymidine incorporation with adiponectin)/(tritium-thymidine incorporation with HSA). Recovered viable cell numbers were evaluated after 48 hours of culture by using trypan blue dye exclusion.
P < .05 and
P < .01 for the difference from control (HSA) values. The data shown are the mean ± SD value from 3 separate experiments.
Adiponectin induces apoptosis in acute myelomonocytic leukemia cell lines
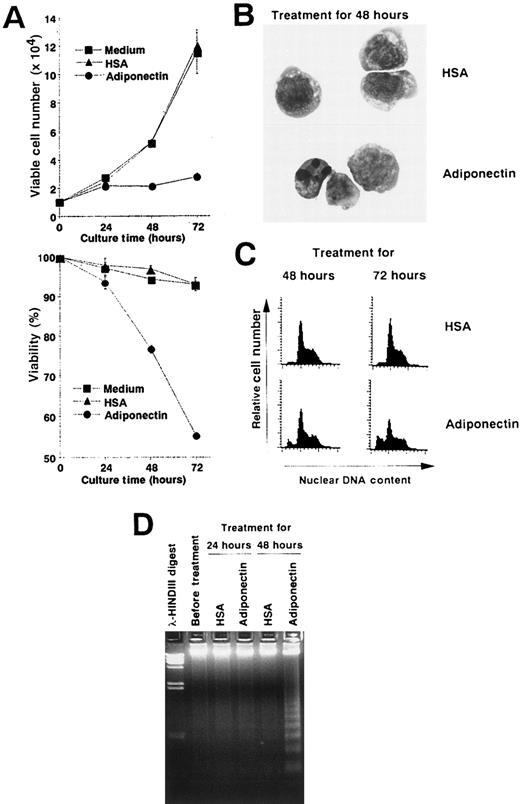
We performed experiments to analyze the mechanisms of the growth inhibition induced by adiponectin. Viable cell numbers and viability of M1 cells treated with rh adiponectin or HSA were evaluated every 24 hours (Figure 5A). Treatment with rh adiponectin strongly inhibited proliferation of M1 cells, and the cells lost their viability within 48 hours. Morphologic analysis after May-Grünwald-Giemsa staining indicated that treatment with adiponectin for 48 hours led to a distinctive condensation of chromatin and fragmented nuclei in M1 cells (Figure 5B). A subdiploid peak of DNAs appeared within 48 hours after initiation of rh adiponectin treatment and increased as treatment continued (Figure 5C). Thus, the proportions of the subdiploid population were 9.27% after 48 hours of rh adiponectin treatment and 25.35% after 72 hours, whereas the proportions with HSA were 2.64% at 48 hours and 3.55% at 72 hours. Moreover, DNA fragmentation into oligonucleosomal-sized pieces was readily visible in M1 cells after 48 hours of treatment with rh adiponectin but barely detected in M1 cells cultured with HSA (Figure5D). These results indicate that adiponectin induced apoptosis in an acute myelomonocytic leukemia cell line, M1. Essentially the same results were observed in an acute monocytic leukemia cell line, THP1 (data not shown).
Adiponectin inhibits the growth of an acute myelomonocytic cell line, M1, by inducing apoptosis.
(A) Triplicate aliquots of M1 cells (1 × 104 cells) were incubated in serum-free medium containing 10 μg/mL adiponectin, HSA, or medium alone for the indicated times. At various times after initiation of the culture, viable cell numbers (upper panel) and viability (lower panel) of M1 cells were determined by trypan blue dye exclusion. The results are shown as the mean ± SD values from triplicate cultures. (B-D) M1 cells were incubated in serum-free medium containing 10 μg/mL adiponectin or HSA for the indicated times and subjected to morphologic (B), nuclear DNA content (C), and DNA fragmentation (D) analyses. Each figure shows 1 of 3 similar experiments.
Adiponectin inhibits the growth of an acute myelomonocytic cell line, M1, by inducing apoptosis.
(A) Triplicate aliquots of M1 cells (1 × 104 cells) were incubated in serum-free medium containing 10 μg/mL adiponectin, HSA, or medium alone for the indicated times. At various times after initiation of the culture, viable cell numbers (upper panel) and viability (lower panel) of M1 cells were determined by trypan blue dye exclusion. The results are shown as the mean ± SD values from triplicate cultures. (B-D) M1 cells were incubated in serum-free medium containing 10 μg/mL adiponectin or HSA for the indicated times and subjected to morphologic (B), nuclear DNA content (C), and DNA fragmentation (D) analyses. Each figure shows 1 of 3 similar experiments.
Adiponectin modulates expression of apoptosis-related genes in M1 cells, and constitutive expression of Bcl-2suppresses adiponectin-induced apoptosis
To further investigate molecular mechanisms involved in the responses of M1 cells to adiponectin, we evaluated expression of some apoptosis-related genes by using Northern blot analysis. As shown in Figure 6A, M1 cells expressedBcl-2, Bcl-xL, and Bax genes. Expression of the Bcl-2 gene was down-regulated to an undetectable level by treatment with adiponectin for 48 hours. Expression of the Bcl-xL gene also appeared to be down-regulated by adiponectin treatment, although the initial expression level of this gene was weak. In contrast, expression ofBax was not affected by adiponectin. Neither Baknor the p53 gene was expressed in the cells at a level detectable with Northern blot analysis, and their expression was not induced by incubation with adiponectin (data not shown). Therefore, treatment with adiponectin down-regulated the expression of antiapoptotic genes, especially Bcl-2, in M1 cells, but had no effects on expression of the apoptosis-inducing genesBax, Bak, and p53.
Expression of apoptosis-related genes during cultures with adiponectin and effect of constitutive
Bcl-2 gene expression on adiponectin-induced apoptosis. (A) M1 cells were cultured in serum-free medium containing 10 μg/mL adiponectin or HSA for the indicated times. Total RNAs were isolated and subjected to Northern blot analyses using the complementary DNAs (cDNAs) of Bcl-2, Bcl-xL,Bax, and β-actin as probes. This figure shows 1 of 3 similar experiments. (B) Total RNAs from parent M1, M1 pcDNA3 (clone 1-3), and M1 hBcl-2 (clone 1-3) were isolated and subjected to Northern blot analyses using the cDNAs of Bcl-2 and β-actin as probes. (C) Triplicate aliquots of parent M1 cells, M1 pcDNA3 clones, or M1 hBcl-2 clones were incubated in serum-free medium containing 10 μg/mL adiponectin for the indicated times. The viability of each clone was determined by trypan blue dye exclusion every 24 hours after initiation of the culture. The results are shown as the mean ± SD values from triplicate cultures. Similar results were obtained in 3 independent experiments.
Expression of apoptosis-related genes during cultures with adiponectin and effect of constitutive
Bcl-2 gene expression on adiponectin-induced apoptosis. (A) M1 cells were cultured in serum-free medium containing 10 μg/mL adiponectin or HSA for the indicated times. Total RNAs were isolated and subjected to Northern blot analyses using the complementary DNAs (cDNAs) of Bcl-2, Bcl-xL,Bax, and β-actin as probes. This figure shows 1 of 3 similar experiments. (B) Total RNAs from parent M1, M1 pcDNA3 (clone 1-3), and M1 hBcl-2 (clone 1-3) were isolated and subjected to Northern blot analyses using the cDNAs of Bcl-2 and β-actin as probes. (C) Triplicate aliquots of parent M1 cells, M1 pcDNA3 clones, or M1 hBcl-2 clones were incubated in serum-free medium containing 10 μg/mL adiponectin for the indicated times. The viability of each clone was determined by trypan blue dye exclusion every 24 hours after initiation of the culture. The results are shown as the mean ± SD values from triplicate cultures. Similar results were obtained in 3 independent experiments.
Adiponectin down-regulated Bcl-2 gene expression. To evaluate the role of this change in adiponectin-induced responses, a human Bcl-2–expression plasmid, pcDNA3–hBcl-2, was stably transfected into M1 cells, and 3 individual clones that expressed humanBcl-2 under the control of a cytomegalovirus promoter (M1 hBcl-2 clone 1-3) were obtained. As controls, 3 individual transfectants with pcDNA3 alone were also established (M1 pcDNA3 clone 1-3). The amount of endogenous and exogenous Bcl-2 gene expression in each transfectant was confirmed by Northern blot analysis (Figure 6B). As shown in Figure 6C, M1 parent cells and pcDNA3 clones were severely deprived of viability by incubation with adiponectin, whereas M1 hBcl-2 clones evaded adiponectin-induced cell death. These findings indicate that Bcl-2 is sufficient to block adiponectin-induced apoptosis.
Adiponectin inhibits phagocytic activity of mature macrophages
We analyzed influences of adiponectin on the differentiation of monocytes to macrophages and the functions of mature macrophages. Human peripheral blood monocytes differentiate to mature macrophages when they are cultured in RPMI-1640 medium supplemented with 10% human type AB serum for 7 days. Addition of rh adiponectin to these cultures caused monocytes to have slightly rounded shapes and reduced proliferation (data not shown). However, their viability was not influenced and the cells grew to show macrophage-like morphologic features after 7 days of culture (data not shown). The viability of mature macrophages was also unaffected by treatment with adiponectin for at least 72 hours (data not shown).
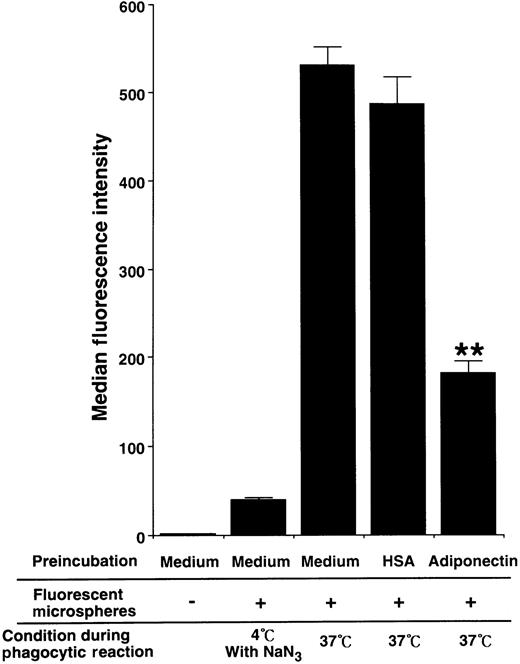
To evaluate the influence of adiponectin on phagocytic activity of mature macrophages, the cells were pretreated with rh adiponectin or HSA for 24 hours and then subjected to phagocytosis assays using Fluorebrite fluorescent microspheres. As shown in Figure7, treatment with adiponectin significantly suppressed the phagocytosis of macrophages (median fluorescence intensity, 181.67 ± 14.57 with rh adiponectin and 486.58 ± 32.11 with HSA).
Effect of adiponectin on the phagocytic activity of macrophages.
Human macrophages were incubated in serum-free medium containing 10 μg/mL adiponectin, HSA, or medium alone for 24 hours and subjected to phagocytosis assay. The fluorescence intensity level of nonspecifically binding microspheres was determined by using macrophages treated with 0.1% sodium azide on ice during incubation with fluorescent microspheres. The data represent the mean ± SD values from triplicate cultures. Significant differences from control (HSA) values are indicated by 2 (P < .01) asterisks. Similar results were obtained in 3 independent experiments.
Effect of adiponectin on the phagocytic activity of macrophages.
Human macrophages were incubated in serum-free medium containing 10 μg/mL adiponectin, HSA, or medium alone for 24 hours and subjected to phagocytosis assay. The fluorescence intensity level of nonspecifically binding microspheres was determined by using macrophages treated with 0.1% sodium azide on ice during incubation with fluorescent microspheres. The data represent the mean ± SD values from triplicate cultures. Significant differences from control (HSA) values are indicated by 2 (P < .01) asterisks. Similar results were obtained in 3 independent experiments.
Adiponectin inhibits lipopolysaccharide (LPS)-induced TNF-α production and TNF-α mRNA expression
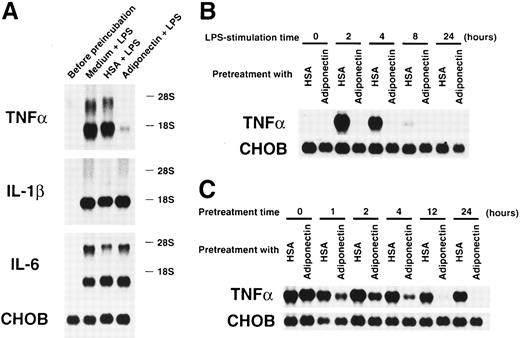
We also evaluated the effects of adiponectin on another macrophage function. Cultured macrophages were pretreated with rh adiponectin, HSA, or medium alone for 24 hours and stimulated with LPS for 4 hours, and gene expression of inflammatory cytokines was then analyzed. As shown in Figure 8A, LPS-induced expression of TNF-α mRNA was markedly suppressed by pretreatment with rh adiponectin, whereas induction of IL-1β and IL-6 mRNA expression was not affected. The inhibitory effect of adiponectin on LPS-induced TNF-α production was also confirmed by assessment of secreted protein levels in the supernatants. Adiponectin-treated macrophages failed to release TNF-α in response to LPS (Table2). When the kinetics of this inhibition was analyzed, we found that TNF-α mRNAs were strongly induced within 2 hours and detected for 8 hours when macrophages were stimulated by LPS (Figure 8B). However, no messages were detected during the observation periods when macrophages were pretreated with rh adiponectin for 24 hours. As shown in Figure 8C, adiponectin barely inhibited LPS-induced TNF-α expression of macrophages when it was added at the time of LPS stimulation (pretreatment time 0 hour). However, LPS-induced TNF-α gene expression was obviously reduced by pretreatment with rh adiponectin for 1 hour. In addition, longer pretreatment with rh adiponectin resulted in stronger suppression of TNF-α gene induction.
Effects of adiponectin on expression of proinflammatory cytokines in macrophages stimulated with lipopolysaccharide (LPS).
(A) After preincubation with 10 μg/mL adiponectin, HSA, or medium alone for 24 hours, human macrophages were stimulated with 10 μg/mL LPS for 4 hours. Total RNAs were then isolated and subjected to Northern blot analyses using the cDNAs of tumor necrosis factor α (TNF-α), interleukin-1 β (IL-1β), interleukin-6 (IL-6), and Chinese hamster ovary (CHO) cells as probes. This figure shows 1 of 3 similar experiments. (B) After preincubation with 10 μg/mL adiponectin or HSA for 24 hours, human macrophages were stimulated with 10 μg/mL LPS for the indicated periods. (C) After preincubation with 10 μg/mL adiponectin or HSA for the indicated periods, human macrophages were stimulated with 10 μg/mL LPS for 4 hours. The expression of TNF-α and CHO genes in the indicated cells was determined by Northern blot analyses. Each figure in B and C shows 1 of 2 independent experiments.
Effects of adiponectin on expression of proinflammatory cytokines in macrophages stimulated with lipopolysaccharide (LPS).
(A) After preincubation with 10 μg/mL adiponectin, HSA, or medium alone for 24 hours, human macrophages were stimulated with 10 μg/mL LPS for 4 hours. Total RNAs were then isolated and subjected to Northern blot analyses using the cDNAs of tumor necrosis factor α (TNF-α), interleukin-1 β (IL-1β), interleukin-6 (IL-6), and Chinese hamster ovary (CHO) cells as probes. This figure shows 1 of 3 similar experiments. (B) After preincubation with 10 μg/mL adiponectin or HSA for 24 hours, human macrophages were stimulated with 10 μg/mL LPS for the indicated periods. (C) After preincubation with 10 μg/mL adiponectin or HSA for the indicated periods, human macrophages were stimulated with 10 μg/mL LPS for 4 hours. The expression of TNF-α and CHO genes in the indicated cells was determined by Northern blot analyses. Each figure in B and C shows 1 of 2 independent experiments.
Effect of adiponectin on tumor necrosis factor α (TNF-α) secretion of macrophages stimulated with lipopolysaccharide (LPS)
| Treatment . | TNF-α level (pg/mL) . |
|---|---|
| Before LPS stimulation | < 20 |
| Medium + LPS | 878 ± 68 |
| Human serum albumin + LPS | 958 ± 177 |
| Adiponectin + LPS | < 20 |
| Treatment . | TNF-α level (pg/mL) . |
|---|---|
| Before LPS stimulation | < 20 |
| Medium + LPS | 878 ± 68 |
| Human serum albumin + LPS | 958 ± 177 |
| Adiponectin + LPS | < 20 |
Human macrophages were preincubated in serum-free medium containing 10 μg/mL adiponectin, HSA, or medium alone for 24 hours and subsequently stimulated with LPS (10 μg/mL) for 8 hours. Supernatants were harvested and their levels of TNF-α assayed. Plus-minus values are the mean ± SD results from triplicate cultures.
C1qRp is one of the cell-surface receptors for adiponectin
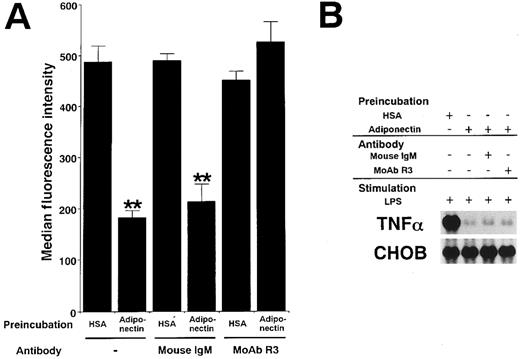
Adiponectin and complement C1q have a substantial sequence similarity. C1qRp is one of the receptors for C1q on phagocytic cells.30 31 To clarify whether the functions of adiponectin might be mediated by C1qRp, we examined the effect of R3, a murine MoAb blocking C1qRp, on those functions. As shown in Figure9A, MoAb R3 completely abrogated the suppression of phagocytosis of macrophages by adiponectin. However, MoAb R3 did not affect the suppression of TNF-α transcription by adiponectin (Figure 9B). In addition, neither the reduction of CFU-GM and CFU-M formation nor the inhibition of growth in myelomonocytic cell lines by adiponectin was canceled by MoAb R3 (data not shown). These results suggest that C1qRp is one of the receptors for adiponectin and mediates its inhibitory effect on macrophage phagocytosis.
Involvement of C1qRp in the suppressive effects of adiponectin on macrophage functions.
Human macrophages were incubated with 10 μg/mL adiponectin or HSA for 24 hours in the presence or absence of the indicated antibodies (30 μg/mL). (A) After incubation, the cells were subjected to phagocytosis assay. Significant differences from control (HSA) values are indicated by 2 (P < .01) asterisks. This figure shows 1 of 3 similar experiments. (B) After incubation, the cells were stimulated with 10 μg/mL LPS for 4 hours. Total RNAs were then isolated and subjected to Northern blot analyses. This figure shows 1 of 2 independent experiments.
Involvement of C1qRp in the suppressive effects of adiponectin on macrophage functions.
Human macrophages were incubated with 10 μg/mL adiponectin or HSA for 24 hours in the presence or absence of the indicated antibodies (30 μg/mL). (A) After incubation, the cells were subjected to phagocytosis assay. Significant differences from control (HSA) values are indicated by 2 (P < .01) asterisks. This figure shows 1 of 3 similar experiments. (B) After incubation, the cells were stimulated with 10 μg/mL LPS for 4 hours. Total RNAs were then isolated and subjected to Northern blot analyses. This figure shows 1 of 2 independent experiments.
Discussion
Adiponectin, an adipocyte-derived secretory protein, is a new member of the family of proteins that includes C1q and the collectins. The proteins in this family contain both collagen-like and noncollagen domains. In this study, we observed novel functions of adiponectin in hematopoiesis and immune responses. Adiponectin predominantly suppressed proliferation of myelomonocytic progenitors. It also inhibited mature macrophage functions, such as phagocytosis and cytokine production. These results suggest that adiponectin is an important regulator of inflammatory responses.
Adiponectin suppressed the colony formation of CFU-GM, CFU-M, and CFU-G, but had little or no effect on that of BFU-E and CFU-mixed. The suppression was unlikely to have been mediated by accessory cells because CFU-GM colony formation from single, isolated CD34+cells was also inhibited by adiponectin. In addition, cell growth in 4 of 9 myeloid cell lines but not in erythroid or lymphoid cell lines other than the BALL cell line was inhibited by adiponectin. Thus, the growth inhibitory effects of adiponectin were apparent mainly in cells of myelomonocytic lineage. The experiments using myeloid cell lines showed that growth inhibition by adiponectin was at least partly mediated by induction of apoptosis. Furthermore, down-regulation ofBcl-2 expression was shown to play a role in induction of apoptosis by adiponectin. However, induction of apoptosis was not obvious in CD34+ cells treated with adiponectin (data not shown). Several cytokines, such as TNFs, interferons, and transforming growth factors (TGFs), have the capacity to inhibit hematopoiesis,38 and these cytokines have been shown to suppress proliferation of a wide range of lymphohematopoietic progenitors. In contrast, target cells for adiponectin are restricted to myelomonocytic lineage committed progenitors.
Macrophages play a central role in immune responses by means of secretion of inflammatory cytokines, phagocytic activity, and antigen presentation.39 Our results show that adiponectin inhibits phagocytosis and LPS-induced TNF-α expression of mature macrophages and suggest that adiponectin may have anti-inflammatory effects. These inhibitory effects of adiponectin on macrophage functions are not due to killing of the cells because the viability of mature macrophages did not change. The mechanisms by which adiponectin cancels TNF-α production and TNF-α gene expression in macrophages stimulated with LPS remain unclear. Our kinetic studies indicate that it is unlikely that adiponectin directly neutralizes LPS or blocks LPS receptors. IL-1β and IL-6 gene expression induced by LPS was not affected by treatment with adiponectin, suggesting that signals to macrophages from adiponectin receptors attenuate the TNF-α gene transcription triggered by LPS stimulation. Several cytokines have been found to repress TNF-α synthesis in macrophages. IL-4 and IL-10 suppress inflammatory responses that can inhibit TNF-α synthesis in LPS-stimulated human macrophages. In contrast to adiponectin, IL-4 and IL-10 also inhibit synthesis of IL-1 and IL-6.40-42TGF-β is known to inhibit proinflammatory cytokine production in macrophages, but its inhibition of TNF-α secretion occurs after transcription.43 Thus, adiponectin is likely to be a unique suppressor of inflammatory responses because of its specific inhibition of TNF-α transcription.
Among the physiologic substances associated with inflammation, E-type prostaglandins (PGE) were shown to inhibit colony formation from CFU-GM and CFU-M but not that from BFU-E.44 Furthermore, PGE2 was reported to inhibit TNF-α production but not IL-1α or IL-2β.45-47 Target cells and functions of adiponectin are similar to those of PGE. However, we did not think that adiponectin was mediating its effects by inducing PGE because indomethacin, an inhibitor of PGE synthesis, could not abrogate adiponectin-induced suppression of colony formation and TNF-α expression (data not shown). Therefore, adiponectin seems to produce its effects on hematopoiesis and immune responses by means of mechanisms independent of PGE.
Members of the soluble defense collagen family, including C1q and the collectins, have similar structures and functions.24-27They can identify foreign pathogens and subsequently interact with phagocytic cells and complement to bring about killing and clearance of targets. Here, we found new functions of adiponectin. MBL and SP-A were shown previously to reduce TNF-α release from monocytes or macrophages activated by streptococcal rhamnose glucose polymers and LPS, respectively.37,48 Thus, attenuation of TNF-α expression may be a function of most members of the soluble defense collagen family. In contrast to adiponectin, C1q, MBL, and SP-A interact with macrophages and enhance their phagocytic activity.30,31,49,50 Although adiponectin appears to have functions similar to those of C1q and the collectins, it and other members of the soluble defense collagen family may also have unique functions.51 Of particular interest is that both adiponectin and C1q inhibit proliferation of myelomonocytic progenitors, which is a novel function for this family.
Adiponectin and complement C1q have a marked sequence similarity. C1q interacts with a variety of cell-surface receptors.30,52Although most C1q receptors have not yet been found to mediate cellular responses, C1qRp is known to enhance phagocytosis through binding to C1q.30 Interestingly, MBL and SP-A are also recognized by C1qRp.49,50 We conclude that adiponectin also modulates phagocytic activity of macrophages through C1qRp because we found that MoAb R3 completely blocked suppression of phagocytosis by adiponectin. However, the mechanism by which C1qRp mediates contrary functions on phagocytosis remains to be elucidated. It is possible that the functional receptor for regulating phagocytosis is a multisubunit complex including C1qRp, as previously speculated.30Adiponectin and C1q might use different associated molecules to transduce their signals into phagocytic cells. The lack of inhibition by MoAb R3 of the suppressive effects of adiponectin on the growth of myelomonocytic progenitors and the TNF-α transcription of LPS-stimulated macrophages suggests that adiponectin uses receptors other than C1qRp. Although adiponectin shares the C1qRp receptor with C1q, it is possible that adiponectin has unique and ligand-specific receptors. Identification of the adiponectin receptors responsible for its various functions would make it possible to selectively manipulate various adiponectin-mediated functions in a clinically advantageous manner. Experiments with this purpose are currently in progress.
Adiponectin is a plasma protein secreted exclusively from adipocytes. In plasma from healthy humans, it exists in concentrations ranging from 1.9 to 17.0 μg/mL. However, it remains unclear whether all in vivo adiponectin have the same activities. Although direct proof is lacking, some speculations about the in vivo status of adiponectin may be made on the basis of the fact that C1q is also a plasma protein and its structure is very similar to that of adiponectin. The cell-interaction site of C1q is located in a region that is normally masked in C1 component and becomes exposed as a result of dissociation of activated C1r2C1s2 components on binding to immune complexes or other ligands.53 A similar situation might exist in the mechanism of adiponectin activation. Adiponectin seems to be set up in vivo for immediate modification of immoderate inflammatory reactions occurring after invasion by foreign pathogens. On the other hand, C1q is thought to play a critical in vivo role in restraining autoimmunity by clearing apoptotic cells that provide the source of autoantigens.54,55 C1q-deficient mice have high titers of antinuclear antibodies and glomerulonephritis with multiple apoptotic cell bodies.55 In this case, part of adiponectin might constitutively function in vivo to maintain a homeostatic state in hematopoiesis and immune systems. In any case, adiponectin is quite different from other immune regulatory cytokines because they are barely produced until stimuli such as infections activate their secretory cells.
Adiponectin is likely to regulate inflammatory responses negatively through at least 2 mechanisms: suppression of mature macrophage functions and inhibition of growth of macrophage precursors. The former is considered to play an important role in the control of early responses of inflammation, and the latter may act in late events of inflammation to prevent immune responses from continuing chronically. All the data described here indicate that adiponectin is involved in the termination of inflammatory responses. Of course, the inflammatory responses to eliminate invasive foreign pathogens are important for host defense, but the systems to prevent those responses from spreading and persisting immoderately are also indispensable. TNF-α induces occlusion of blood vessels and plays an important role in containing local infection, but death can result when it is released systemically.56 In patients with septicemia, simultaneous release of TNF-α by macrophages in various organs causes septic shock and disseminated intravascular coagulation, frequently resulting in multiple organ failure and death. Therefore, our observation that treatment with adiponectin strongly inhibits LPS-induced TNF-α gene transcription in macrophages suggests that adiponectin may have therapeutic applications in diseases caused by excessive inflammatory responses. Clear and decisive information from additional studies should lead not only to a more precise understanding of mechanisms underlying regulation of inflammatory responses but should also suggest novel preventive and therapeutic procedures for inflammatory diseases.
Acknowledgment
We thank Dr Masahiro Muraguchi (Otuka Pharmaceutical Co Ltd, Tokushima, Japan) for excellent technical support.
Supported in part by grants from the Ministry of Education, Science and Culture of Japan; the Japan Society for the Promotion of Science; and the Yamanouchi Foundation for Research on Metabolic Disorders.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Takafumi Yokota, Department of Internal Medicine and Molecular Science, Graduate School of Medicine, Osaka University, 2-2 Yamada-oka, Suita, Osaka 565-0871, Japan; e-mail:yokota@imed2.med.osaka-u.ac.jp.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal