Abstract
The relapse rate in childhood acute lymphoblastic leukemia (ALL) is approximately 30% but few reinduction regimens have investigated the intensive use of polyethylene glycol Escherichia coliasparaginase (PEG-Asp). Therefore, we assessed the pharmocokinetics and efficacy of PEG-Asp in this setting. Children with B-precursor ALL, in first marrow and/or extramedullary relapse were eligible. Reinduction included doxorubicin on day 1, prednisone for 28 days, vincristine weekly for 4 weeks, and PEG-Asp either weekly or biweekly by randomization. Asparaginase levels and antibody to both E coli asparaginase and PEG-asp were measured weekly just before each PEG-asp dose. Overall, 129 of 144 patients (pts) (90%) achieved a complete remission (CR). There was a highly significant difference in CR rates between weekly (69 of 71; 97%) and biweekly (60 of 73; 82%) PEG-Asp dosing (P = .003). Grade 3 or 4 infectious toxicity was common (50%), but only 4 pts died of sepsis during induction. Other toxicities were infrequent and hypersensitivity was rare (6 of 144; 4%). Low asparaginase levels were associated with high antibody titers to either native (P = .024) or PEG asp (P = .0013). The CR rate was significantly associated with higher levels of asparaginase (P = .012). Patients with ALL in first relapse receiving weekly PEG-Asp had a higher rate of second remission compared with biweekly dosing. Low levels of asparaginase were associated with high antibody titers. Increased asparaginase levels may correlate with an improved CR rate. The use of intensive PEG-Asp should be explored further in the treatment of ALL.
Introduction
Despite recent progress in the treatment of acute lymphoblastic leukemia (ALL), 30% of affected children still develop marrow relapse.1-3 For patients who relapse early (defined as an initial remission duration of less than 24 to 36 months), the chance for long-term survival is poor.4,5 More successful reinduction schedules are a necessary prerequisite for prolonged second remissions. A 4-drug reinduction regimen utilizing Escherichia coli (native) asparaginase, doxorubicin or daunorubicin, vincristine, and prednisone achieves a second marrow remission in 80% to 85% of such patients.5-7 Similar results have been reported with alternative but more intensive chemotherapy regimens.8-10
E coli asparaginase can be covalently linked to the synthetic substrate polyethylene glycol (PEG) to produce PEG L-asparaginase (PEG-Asp).11,12 When compared with native asparaginase, this enzyme has a long half-life (almost 6 days)13 and reduced immunogenicity.14 15Because of this long half-life, it is preferable to administer the drug on an every other week interval. There are few data, however, investigating the utility and pharmocokinetics of PEG-Asp in children with relapsed ALL, with the aims of determining optimal dosing, interval of administration, and improving second remission rates. Therefore, we elected to study the efficacy and toxicity of 2 PEG-Asp dosing schedules (standard arm: every other week PEG-Asp; and experimental arm: weekly PEG-Asp) by means of a randomized trial of 4-drug reinduction in a large cohort of patients with ALL in first relapse.
Patients, materials, and methods
Patients
POG protocol 9310 was designed for patients with B-precursor ALL younger than 22 years of age at initial diagnosis and in first marrow relapse (more than 25% lymphoblasts; M3 marrow), with or without concomitant extramedullary relapse. Also included were patients with first isolated extramedullary relapse in sites other than the central nervous system (CNS) or with first CNS relapse combined with an M2 marrow (5% to 25% blasts). Patients with isolated CNS relapse, prior cumulative anthracycline dose more than 350 mg/m2, clinical evidence of cardiac dysfunction, or prior bone marrow transplantation (BMT) were not eligible. Signed informed consent according to the guidelines of each center's institutional review board was required.
Reinduction treatment
The induction treatment schedule is shown in Table1 and age-adjusted dosages for intrathecal therapy are shown in Table 2. On registration, but before initiation of therapy, patients were randomized using a permuted block design based on stratification by prior hypersensitivity toE coli asparaginase to receive either weekly (days 1, 8, 15, and 22) or standard biweekly (days 1 and 15) PEG-Asp at a dose of 2500 IU/m2 intramuscularly. Patients allergic to both E coli asparaginase and PEG-Asp were nonrandomly assigned to receive the Erwinia preparation. There were 73 patients who were randomly assigned to receive weekly PEG, 74 randomly assigned to receive every other week PEG, and one patient who was nonrandomly assigned to receive Erwina asparaginase before the initiation of treatment. This patient is not included in this report.
Induction treatment schedule
| Prednisone | 40 mg/m2 orally days 1-29 |
| Doxorubicin | 60 mg/m2 IV bolus over 15 minutes on day 1 |
| Vincristine | 1.5 mg/m2 (max 2 mg) IV days 1, 8, 15, 22 |
| Intrathecal therapy (IT)* | *(IT) days 1, 15, 29 |
| PEG-L-asparaginase | 2500 IU/m2 IM randomized to weekly (days 1, 8, 15, 22) or every other week (days 1, 15) |
| Prednisone | 40 mg/m2 orally days 1-29 |
| Doxorubicin | 60 mg/m2 IV bolus over 15 minutes on day 1 |
| Vincristine | 1.5 mg/m2 (max 2 mg) IV days 1, 8, 15, 22 |
| Intrathecal therapy (IT)* | *(IT) days 1, 15, 29 |
| PEG-L-asparaginase | 2500 IU/m2 IM randomized to weekly (days 1, 8, 15, 22) or every other week (days 1, 15) |
IV = intravenous(ly); IM = intramuscularly.
See Table 2 for age-adjusted dosages.
Age-adjusted dosages for intrathecal therapy
| . | <1 y . | 1 y . | 2 y . | 3-8 y . | >9 y . |
|---|---|---|---|---|---|
| HC (mg) | 7.5 | 8 | 10 | 12 | 15 |
| MTX (mg) | 7.5 | 8 | 10 | 12 | 15 |
| ARA-C (mg) | 15 | 16 | 20 | 24 | 30 |
| . | <1 y . | 1 y . | 2 y . | 3-8 y . | >9 y . |
|---|---|---|---|---|---|
| HC (mg) | 7.5 | 8 | 10 | 12 | 15 |
| MTX (mg) | 7.5 | 8 | 10 | 12 | 15 |
| ARA-C (mg) | 15 | 16 | 20 | 24 | 30 |
HC = hydrocortisone; MTX = methotrexate; ARA-C = cytosine arabinoside; CNS = central nervous system.
CNS disease at diagnosis: weekly IT therapy for a total of 5 doses.
Laboratory monitoring
Complete blood counts (CBCs) were performed twice weekly. Serum alanine aminotransferase (ALT), total and direct bilirubin, albumin, glucose, amylase and lipase, and plasma fibrinogen were measured on days 1, 15, and 29.
Toxicity/allergy recording
The National Cancer Institute's toxicity and complications grading scheme was utilized for all events, except for asparaginase allergy, which was scored as described below. Only grade 3 or 4 toxicity for ALT (more than 5 times normal), total/direct bilirubin (more than or equal to 1.5 normal), fibrinogen (less than 0.5 × normal), albumin (less than or equal to 2.5 g/dL), glucose (more than 250 mg/dL), and amylase and lipase (more than 2 × normal for both) were recorded and analyzed. There is no grade 4 toxicity for weight loss; therefore, only grade 2 or 3 weight change (10%-19.9%, greater than or equal to 20%, respectively) was assessed. Stomatitis/mucositis was analyzed if grade 3 (cannot eat or drink) or grade 4 (requires enteral/parenteral support) toxicity was evident. Grade 3 or 4 toxicity for neutrophil count, hemoglobin, and platelets was not analyzed because of coexisting marrow compromise and/or intensive chemotherapy. The allergic grading system utilized in this study follows that developed by Kurtzberg16 and is defined as follows:
Grade 0: no reaction; Grade 1: mild local reaction (less than 10 cm and/or less than 24 hours); Grade 2: urticaria; Grade 3: bronchospasm, serum sickness, severe local reaction (more than or equal to 10 cm and/or more than or equal to 24 hours); Grade 4: hypotension, anaphylaxis.
Dose modification
PEG-Asp was discontinued for clinical thrombosis or pancreatitis. Asparaginase dosing was not modified for hyperglycemia. Grade 1 or 2 hypersensitivity did not mandate switching asparaginase preparations, but any grade 3 or 4 allergy to PEG-Asp necessitated changing to the Erwinia preparation.
Remission status/off-study criteria
Bone marrow aspiration was performed on days 15 and 29 of reinduction therapy. Off-study criteria included an M3 marrow (more than 25% lymphoblasts) on day 15 or an M2 or M3 marrow on day 29. If the day 29 marrow was hypoplastic (defined by inability to discern trilineage bone marrow maturation), weekly CBCs and serial bone marrow aspirates were performed until remission or lack of response could be established. Remission status of those patients with isolated extramedullary relapse were clinically derived on day 29 of induction (ie, normal cerebrospinal fluid findings for isolated CNS disease and a normal testicular examination for isolated testicular relapse).
Assay for the measurement of serum asparaginase activity.
Serum asparaginase concentration was measured weekly on days 8, 15, 22, and 29 as trough levels. This assay is a coupled enzymatic reaction that indirectly measures the activity of L-asparaginase. During the reaction, L-asparagine is converted to L-aspartate in the presence of L-asparaginase. Subsequently, L-aspartate reacts with alpha-ketoglutarate to form oxaloacetate, which converts NADH to NAD+.17 This enzyme cascade reaction is monitored at 340 nm. L-asparaginase in the serum sample is calculated from an E coli asparaginase standard curve in the range of 0.02 to 0.3 U/mL. A low asparaginase level was defined as less than 0.03 U/mL, a value below which is indicative of the presence of asparagine and hence lack of enzyme activity.18 Patients were divided into 2 groups: those with asparaginase levels less than 0.03 U/mL and those with levels more than or equal to 0.03 U/mL to compare with asparaginase antibody levels (described below). Additionally, mean weekly asparaginase levels were calculated to ascertain the relationship between these trough levels and CR rate.
Asparaginase antibody assay.
Serum samples for E coli asparaginase and PEG-Asp antibodies were collected weekly on days 8, 15, 22, and 29 of reinduction and measured using an antibody capture enzyme-linked immunosorbent assay (ELISA). The mean ± SD was calculated on these weekly measurements and compared with corresponding levels of asparaginase as described previously. A reference standard pool with an approximate titer of 1 × 103 was prepared from the serum of patients allergic to E coli asparaginase. A standard curve ranging from 0% to 100% of the reference standard pool was generated in each ELISA run. The antibody titer for patient serum was expressed as percent of the reference standard and was calculated from the semilog standard curve fit. Positive and negative (normal human serum) controls were used when each set of specimens was analyzed.
Statistical methods
This trial was planned to detect a 15% increase in the initial response rate for the weekly PEG-Asp group (power = 0.80, α = 0.05, 2-sided) compared with the biweekly PEG-Asp group (standard treatment arm). The primary study endpoint was the complete response rate after induction therapy. Stratification was based on type of relapse (bone marrow versus extramedullary relapse) and asparaginase hypersensitivity as previously described. Patients were centrally randomized using a permuted block design and treatment arm assignment was balanced with respect to the stratification factors. The trial was closed when the projected accrual goal was achieved.
Contingency tables were constructed to determine the relationship between the induction response and the PEG-asparaginase schedules. Exact chi-square tests were performed and exact odds ratios (OR) along with 95% confidence intervals (CI) were generated using the StatXact 4 for Windows software (CYTEL Software Corporation, Cambridge, MA). Independent sample t tests and logistic regression analyses were performed using the Statistical Analysis System version 6.11 (SAS Institute Inc, Cary, NC). Two-sided statistical tests are reported.
Results
Patient characteristics
One hundred and forty-eight patients, 93 males and 55 females, were registered on this study. The mean age (± SD) was 9.4 ± 5.2 years, with a range of 0 to 20 years. Of the 148 patients enrolled, 10 had combined CNS and bone marrow involvement (M2 = 3; M3 = 7), 7 presented with both testicular and bone marrow relapse (all M3), 18 displayed isolated extramedullary relapse (16 testicular, 1 lymph node, and 1 subcutaneous), and 113 patients had isolated bone marrow involvement (M3).
Initial ALL treatment was quite varied, but the majority of patients (65%) received intensive parenteral methotrexate and 6-mercaptopurine during consolidation and nearly all (95%) received E coliasparaginase during their first induction therapy. Sixty-three of the 148 patients (43%) previously received an anthracycline. These were fairly equally divided between weekly (n = 34) and every other week PEG-Asp (n = 29) treatment schedules and one patient receivedErwinia asparaginase. Eighty-nine patients (60%) had an initial complete remission more than 24 months.
Patient characteristics (prior asparaginase hypersensitivity, sex, race, type of relapse, duration of first remission [CR1], age at diagnosis, age at relapse, and white blood cell count at relapse) by PEG-Asp treatment group is summarized in Table3. All patients (n = 147), except the patient receiving Erwinia asparaginase alone are included in this analysis. The duration of first remission was similar for patients assigned to both treatment arms (2.72 years for the weekly group and 2.74 years for the biweekly group). There were no statistically significant differences in the distribution of other patient characteristics between treatment arms.
Distribution of patient characteristics by treatment assignment
| Patient characteristic . | Weekly . | Every other week . | P . | ||
|---|---|---|---|---|---|
| N . | (%) . | N . | (%) . | ||
| Prior Asp hypersensitivity | |||||
| No | 64 | (87.7) | 62 | (83.8) | .503-150 |
| Yes | 9 | (12.3) | 12 | (16.2) | |
| Sex | |||||
| Male | 48 | (65.8) | 45 | (60.8) | .533-150 |
| Female | 25 | (34.2) | 29 | (39.2) | |
| Race | |||||
| White | 53 | (72.6) | 49 | (66.2) | .403-150 |
| Non-white | 20 | (27.4) | 25 | (33.8) | |
| Type of relapse | |||||
| Isolated BM | 47 | (83.6) | 52 | (86.5) | |
| BM + EMD | 19 | (6.8) | 12 | (0.0) | .313-150 |
| Isolated EMD | 7 | (9.6) | 10 | (13.5) | |
| Duration of CR1 (y) | |||||
| Mean ± SD | 2.72 ± 1.83 | 2.74 ± 1.83 | .953-151 | ||
| Age at diagnosis (y) | |||||
| Mean ± SD | 6.64 ± 4.95 | 6.61 ± 4.56 | .973-151 | ||
| Age at relapse (y) | |||||
| Mean ± SD | 9.36 ± 5.07 | 9.35 ± 5.30 | .993-151 | ||
| WBC count at relapse (per mm3) | |||||
| Median | 5100 | 4100 | .523-152 | ||
| [interquartile range] | [3000-7700] | [2500-8200] | |||
| Patient characteristic . | Weekly . | Every other week . | P . | ||
|---|---|---|---|---|---|
| N . | (%) . | N . | (%) . | ||
| Prior Asp hypersensitivity | |||||
| No | 64 | (87.7) | 62 | (83.8) | .503-150 |
| Yes | 9 | (12.3) | 12 | (16.2) | |
| Sex | |||||
| Male | 48 | (65.8) | 45 | (60.8) | .533-150 |
| Female | 25 | (34.2) | 29 | (39.2) | |
| Race | |||||
| White | 53 | (72.6) | 49 | (66.2) | .403-150 |
| Non-white | 20 | (27.4) | 25 | (33.8) | |
| Type of relapse | |||||
| Isolated BM | 47 | (83.6) | 52 | (86.5) | |
| BM + EMD | 19 | (6.8) | 12 | (0.0) | .313-150 |
| Isolated EMD | 7 | (9.6) | 10 | (13.5) | |
| Duration of CR1 (y) | |||||
| Mean ± SD | 2.72 ± 1.83 | 2.74 ± 1.83 | .953-151 | ||
| Age at diagnosis (y) | |||||
| Mean ± SD | 6.64 ± 4.95 | 6.61 ± 4.56 | .973-151 | ||
| Age at relapse (y) | |||||
| Mean ± SD | 9.36 ± 5.07 | 9.35 ± 5.30 | .993-151 | ||
| WBC count at relapse (per mm3) | |||||
| Median | 5100 | 4100 | .523-152 | ||
| [interquartile range] | [3000-7700] | [2500-8200] | |||
Asp = asparaginase; BM = bone marrow; EMD = extramedullary; WBC = white blood cell count.
χ2 test.
Independent samples t test.
Wilcoxon rank sum test.
Prior Escherichia coli asparaginase hypersensitivity
This information is summarized in Table 3. Twenty-one of the 144 patients (15%) evaluable for induction response had prior E coli asparaginase allergy as follows: grade 1 = 6 (biweekly = 5; weekly = 1); grade 2 = 6 (biweekly = 4; weekly = 2); grade 3 = 7 (biweekly = 3; weekly = 4); and grade 4 = 2 (all patients in weekly arm).
Weekly versus every other week PEG-Asp reinduction results
Efficacy and toxicity data were available on 144 of 148 patients. Of the 4 inevaluable patients, 3 were enrolled in the study but refused treatment before beginning induction. The other patient was allergic to both E coli asparaginase and PEG-Asp and was treated withErwinia asparaginase and removed from the analyses below. All 4 inevaluable patients had isolated bone marrow involvement (M3).
Overall, 129 of 144 patients (90%) achieved a second complete remission. The univariate reinduction results are shown in Table4. There was a highly significant association between PEG dosing schedule and response when the analysis was stratified by type of relapse; the exact summary OR was 0.26 (95% CI = 0.06 to 0.94). The association between dosing schedule and induction response was even stronger for patients with bone marrow involvement; those receiving weekly dosing had a much higher response rate (61 of 63; 97%) compared with those receiving biweekly PEG-Asp dosing (50 of 63; 79%) (OR = 0.126, 95% CI = 0.013 to 0.605). This corresponds to nearly an 8-fold decrease in the risk of induction failure for patients receiving weekly PEG-Asp dosing. All patients with isolated extramedullary relapse achieved a complete response.
Reinduction results comparing weekly and every other week PEG-Asp in patients with bone marrow and isolated extramedullary involvement
| Patient groups . | Complete remission . | Resistant disease4-150 . | Early death . | |||
|---|---|---|---|---|---|---|
| qw . | qow . | qw . | qow . | qw . | qow . | |
| All patients (n = 144)4-151 | 69 | 60 | 2 | 9 | 0 | 4 |
| Bone marrow involvement (n = 126)‡ | 61 | 50 | 2 | 9 | 0 | 4 |
| Isolated EM (n = 18) | 8 | 10 | 0 | 0 | 0 | 0 |
| Patient groups . | Complete remission . | Resistant disease4-150 . | Early death . | |||
|---|---|---|---|---|---|---|
| qw . | qow . | qw . | qow . | qw . | qow . | |
| All patients (n = 144)4-151 | 69 | 60 | 2 | 9 | 0 | 4 |
| Bone marrow involvement (n = 126)‡ | 61 | 50 | 2 | 9 | 0 | 4 |
| Isolated EM (n = 18) | 8 | 10 | 0 | 0 | 0 | 0 |
PEG-Asp = polyethylene glycol asparaginase; qw = weekly PEG-Asp; qow = every other week PEG-Asp; EM = extramedullary.
M3 marrow on day 15 or M2 or M3 marrow after reinduction.
P = .003.
P = .004.
The type of relapse (bone marrow vs isolated extramedullary [EMD]) and prevalent asparaginase hypersensitivity were used as stratification factors for allocation to weekly or every other week PEG-Asp dosing. We performed a multivariate logistic regression analysis to determine the independent relationship between the PEG-Asp dosing schedule and induction response, while simultaneously adjusting for the effects of CR1 duration and type of relapse (BM vs EMD). When adjusted for type of relapse and prior hypersensitivity, the weekly PEG-Asp schedule appeared to confer a 7-fold decrease in the induction failure rate (OR = 0.13, 95% CI = 0.028 to 0.599). Neither CR1 duration (P = .13) nor prior hypersensitivity (P = .41) were independently associated with response.
To determine whether the 4 early deaths from infection that occurred in the biweekly PEG-Asp group (described below under toxicity) unduly influenced the results, we reanalyzed the data omitting these patients. The conclusions were the same, ie, the complete response rate was significantly different between patients on the weekly and biweekly PEG-Asp dosing regimens.
Days 15 and 29 bone marrows
Details concerning days 15 and 29 bone marrow results for those 11 patients who failed induction are as follows: For the weekly PEG-Asp arm, the 2 induction failures both had marrow hypoplasia at day 15 and an M3 marrow at day 29. For the 9 patients on the biweekly PEG-Asp arm, 5 had an M3 marrow at day 15 and 4 had an M2 marrow at day 29 (2 of these 4 had an M2 marrow on both day 15 and day 29 and 2 had hypoplasia on day 15 and an M2 marrow at day 29). For those patients with an M2 marrow on day 15, 11 of 14 achieved remission by day 29. For those in CR at day 29, 7 of 11 were in the weekly PEG-Asp group and 4 of 11 were in the biweekly group. Three of 14 patients were removed from the study at day 29 and were all in the biweekly asparaginase group. One died of sepsis in the third week of induction and never had a day 29 bone marrow performed and the other 2 patients had M2 bone marrow examinations at day 29.
Toxicity
Table 5 lists the overall toxicity noted in this study. Infections were common but were responsible for only 4 deaths; 2 each due to Pseudomonas aeruginosa and fungal sepsis. Severe (grade 3 or 4) hepatic toxicity was infrequent as were moderate (grade 2 or 3) mucositis and weight loss. However, grade 3 or 4 hypoproteinemia, characterized by low fibrinogen and albumin concentrations, overall occurred in 25% and 20% of tested patients, respectively. Toxicity was also analyzed for the 2 treatment groups as well as several subgroups (bone marrow involvement vs isolated extramedullary; previous E coli asparaginase hypersensitivity vs the nonallergic group). Comparing the weekly versus biweekly PEG-Asp treatment arms, only low fibrinogen levels were more common (44% vs 25%) in patients on the weekly PEG-Asp treatment arm. One patient in the weekly PEG-Asp arm had excessive bleeding (intracranial hemorrhage described below) accompanied by a low fibrinogen level. Stomatitis was more common (37% vs 6%) for patients with bone marrow involvement compared with isolated extramedullary relapse. Additionally, stomatitis was more common in the E coli asparginase hypersensitivity group compared with the nonallergic group (57% vs 30%). There was no significant difference in toxicity for the other listed side effects between the 2 treatment arms or the subgroups described above.
Nonhematologic reinduction toxicity
| Toxicity . | Number of evaluable patients5-150 . | Number (percentage) with toxicity . |
|---|---|---|
| Infection5-151 | 143 | 72 (50%) |
| Low fibrinogen (≤ 50 mg/dL) | 127 | 32 (25%) |
| Hypoalbuminemia (≤ 2.5 g/L) | 142 | 29 (20%) |
| Mucositis (IV fluids needed) | 141 | 22 (16%) |
| Increased bilirubin (≥ 1.5 mg/dL) | 143 | 22 (15%) |
| Elevated ALT (≥ 150 U/L) | 143 | 13 (9%) |
| Weight loss (≥ 10%) | 143 | 9 (6%) |
| Toxicity . | Number of evaluable patients5-150 . | Number (percentage) with toxicity . |
|---|---|---|
| Infection5-151 | 143 | 72 (50%) |
| Low fibrinogen (≤ 50 mg/dL) | 127 | 32 (25%) |
| Hypoalbuminemia (≤ 2.5 g/L) | 142 | 29 (20%) |
| Mucositis (IV fluids needed) | 141 | 22 (16%) |
| Increased bilirubin (≥ 1.5 mg/dL) | 143 | 22 (15%) |
| Elevated ALT (≥ 150 U/L) | 143 | 13 (9%) |
| Weight loss (≥ 10%) | 143 | 9 (6%) |
IV = intravenous; ALT = alanine aminotransferase.
All patients were not fully evaluable for each toxicity.
Includes fever without identifiable source, documented bacterial or fungal sepsis, or localized serious infection (herpes stomatitis, fungal sinusitis, perirectal abscess). Four of these 72 patients died from sepsis (see text).
PEG-Asp toxicity
Nonallergic toxicities possibly attributable to PEG-Asp were infrequent. One patient had deep venous thrombosis located in the calf vein. Two other subjects had small clots at the tip of their central venous catheters. Three patients had CNS events possibly related to PEG-Asp. One had a CNS hemorrhage associated with a normal platelet count and a low fibrinogen level of 70 mg/dL. Another patient had a seizure without evidence of hemorrhage or thrombosis on magnetic resonance imaging. The third patient had transient (2 days) coma of unknown cause. This child had evidence of liver dysfunction and sepsis before initiation of chemotherapy. Two children had clinical pancreatitis. Five patients had hyperglycemia develop, requiring insulin therapy, but they continued receiving PEG-Asp and corticosteroids with resolution of the hyperglycemia by the end of induction. All 3 children with CNS events were receiving weekly PEG-Asp, but there were no other differences in toxicities between the 2 PEG-Asp treatment arms.
PEG-Asp–associated hypersensitivity was uncommon (n = 6 of 144; 4%) and usually mild. Interestingly, only 1 of the 6 patients with allergic reactions had a prior hypersensitivity reaction to the native E coli preparation. Four of these 6 patients had mild grade 1 allergic reactions (3 while receiving weekly PEG-Asp) and were able to continue treatment after premedication with diphenhydramine. The 2 patients who had a more severe, grade 3 hypersensitivity reaction were infants who had not received asparaginase during their initial leukemia treatment. Both of these patients were equally divided between each treatment arm and they were subsequently placed on Erwiniaasparaginase.
Pharmacokinetic studies
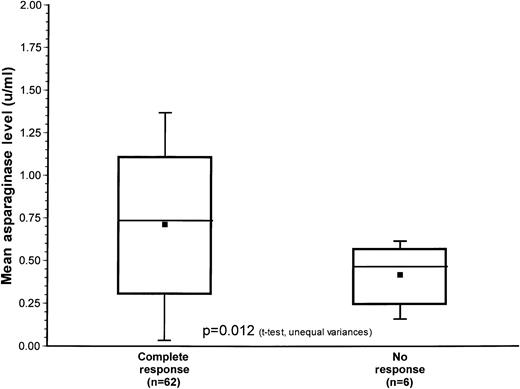
Table 6 summarizes the pharmacokinetic data correlating antibody production with asparaginase level. Low asparaginase levels (less than 0.03 u/mL) were associated with presence of a high titer of antibody against either native (P = .024) or PEG-Asp (P = .0013). Additionally, mean asparaginase levels were calculated in 62 patients with complete response (CR) and for 6 patients who showed no response (NR) to reinduction. Data from the remaining patients were unavailable. These data are represented in Figure 1. The mean asparaginase level for the CR group was 0.75 U/mL and 0.45 U/mL for the NR group. This difference was statistically significant (P = .012, t test, unequal variances).
Relationship between PEG-Asp antibodies and Escherichia coli asparaginase antibodies with asparaginase levels
| N . | Asparaginase level6-150 . | Asparaginase antibody . | |
|---|---|---|---|
| Asp Ab (mean % ± SD)6-151 . | PEG-Asp Ab (mean % ± SD)6-152 . | ||
| 60 | ≥ 0.03 U/mL | 5.4 ± 9.0 | 4.3 ± 14.1 |
| 16 | < 0.03 U/mL | 129 ± 196 | 48 ± 45 |
| N . | Asparaginase level6-150 . | Asparaginase antibody . | |
|---|---|---|---|
| Asp Ab (mean % ± SD)6-151 . | PEG-Asp Ab (mean % ± SD)6-152 . | ||
| 60 | ≥ 0.03 U/mL | 5.4 ± 9.0 | 4.3 ± 14.1 |
| 16 | < 0.03 U/mL | 129 ± 196 | 48 ± 45 |
Asp Ab = Escherichia coli asparaginase antibodies.
PEG-Asp Ab = polyethylene glycol asparaginase antibodies.
Asp level ≥ 0.03 U/mL corresponds to asparagine depletion.
P = .024.
P = .0013.
Box and wisker plot of the distribution of mean asparaginase levels for those with complete response (M1 marrow) versus no response (M2 or M3 marrow) at the end of induction.
The mean value of each group is represented by the closed square, the median value is the horizontal line within the box and the top and bottom lines delineate the 95% confidence interval.
Box and wisker plot of the distribution of mean asparaginase levels for those with complete response (M1 marrow) versus no response (M2 or M3 marrow) at the end of induction.
The mean value of each group is represented by the closed square, the median value is the horizontal line within the box and the top and bottom lines delineate the 95% confidence interval.
Discussion
This is the first large randomized study investigating the role of intensive PEG-Asp in patients with ALL in first marrow relapse. Almost all (95%) patients in this trial had received native asparaginase during initial therapy. The major aim of this study was to assess whether weekly PEG-Asp was superior to biweekly PEG-Asp dosing. On the basis of pharmocokinetic studies of PEG-Asp, we speculated that weekly PEG-Asp might be more effective and no more toxic than biweekly dosing. Our results suggest that weekly PEG-Asp may be superior to other forms and dosing schedules of asparaginase in inducing second remissions in children with ALL who are experiencing their first bone marrow relapse. The 97% reinduction rate with weekly dosing reported here was significantly higher than that noted using biweekly PEG-Asp. This observation is noteworthy because our study included many patients with first remissions less than 24 months in duration; 56 of 126 (44%) overall and 26 of 63 (41%) in the weekly PEG-Asp arm. Other studies have reported similar high reinduction rates (91%-100%) but have focused on patients with long initial remission durations (more than 36 months) or have included a smaller number of study subjects.4,10 19-23
A 4-drug reinduction schedule (prednisone, vincristine, asparaginase, and an anthracycline) has been utilized in relapsed ALL both in the United States and Europe for many years.5,6,24 Buchanan and colleagues7 used this approach in a large POG trial of patients in first marrow relapse during or within 6 months of cessation of initial therapy, and reported a reinduction rate of 82% in 247 patients. Italian investigators25 used a more intensive reinduction regimen, including idarubicin and cytarabine, but they encountered greater hematologic and infectious toxicity, while only achieving a second remission rate of 77%. Finally, Henze and coworkers10 used a 5-drug reinduction schedule (prednisone, vincristine, asparaginase, methotrexate, and higher dose cytarabine) and demonstrated an 88% reinduction rate with only modest toxicity.
The excellent results reported here utilizing PEG-Asp were achieved without marked toxicity. Pancreatitis, CNS events, and thrombosis were infrequent and no more common than the 2% to 3% incidence reported in the literature for native E coliasparaginase.26-28 Hypofibrinogenemia and hypoalbuminemia occurred at a frequency similar to that induced by native asparaginase (20%-25%) and were neither a management nor dose-limiting problem.29 The incidence of hepatic toxicity as measured by elevated ALT (9%) and total bilirubin level (15%) was more common with PEG-Asp compared with the low (1%-2%) incidence demonstrated with the use of E coli asparaginase,30 but these mild laboratory abnormalities were neither dose limiting nor clinically apparent.
Asparaginase is an effective reinduction agent, especially when given in larger doses than during initial therapy.24,31,32Asselin and colleagues33 have shown that lysis of lymphoblasts is proportional to the degree of asparagine depletion. As depicted in Figure 1, the superior reinduction rates noted with weekly PEG-Asp dosing on POG 9310 may have resulted from higher levels of asparaginase and more complete asparagine depletion. Unfortunately, there were not enough randomized patients with complete serial assays to compare weekly mean asparaginase levels between the 2 treatment arms. Recent information from a randomized trial in newly diagnosed ALL from the Children's Cancer Group, comparing one dose of PEG-Asp 2500 IU/m2 versus 6000 IU/m2 3 times per week ofE coli asparaginase, suggest that higher levels of asparaginase may correlate with a faster remission rate.34,35 Accordingly, it may be important to attempt to achieve asparaginase trough levels greater than 0.03 U/mL. Relatively low levels of asparaginase could be related either to an inadequate dosing schedule or short plasma half-life because of antibody production. Kurtzberg and colleagues36 have provided preliminary data to suggest that low asparaginase levels are significantly correlated with high asparaginase antibody and a lower response rate in patients in second or later relapse. Patients with prior clinical hypersensitivity to E coli asparaginase exhibit a decreased half-life of the enzyme compared with those who are asparaginase naive, whether the native or PEG asparaginase form is administered subsequently.37-39 The normal E coli asparaginase half-life (approximately 1 day in asparaginase naive patients) is markedly reduced in those with prior allergic symptoms.37 Similarly, patients who have not previously received PEG-Asp display a half-life of approximately 6 days, compared with 2 days if there is prior E coli asparaginase hypersensitivity.40 In our study, the weekly PEG-Asp treatment schedule produced higher asparaginase levels in patients with a prior history of clinical allergy, but there were insufficient data to perform a formal statistical comparison.
Many patients who have previously received asparaginase have elevated asparaginase antibody levels without clinical symptoms, so called “silent hypersensitivity.”39,41-43 In one study, the incidence of silent hypersensitivity to asparaginase was as high as 62%.36 Continued E coli asparaginase treatment in this group of patients is likely to result in inadequately low levels. Therefore, it would be ideal to periodically measure asparaginase antibody levels to optimize subsequent dosing.38
Effective use of intensive E coli asparaginase may be limited by clinical hypersensitivity reactions, which can occur in up to 75% of patients treated intensively with this agent during initial continuation therapy.44 A substantially lower hypersenstivitiy incidence of 20% to 35% occurs in patients treated with less intensive asparaginase dosing regimens.16,45,46PEG-Asp is thought to be less immunogenic than native asparaginase,11 but there are epitopes of the protein, which can still elicit an immunologic reaction, despite its conjugation to PEG. Allergic reactions to asparaginase during induction may in part be lessened to the concomitant administration of prednisone.47 Also, allergic reactions to PEG-Asp are more common if there has been previous clinical hypersensitivity to native asparaginase. For example, Ettinger and colleagues14 noted a 28% allergic reaction rate in patients receiving PEG-Asp who had a prior hypersensitivity reaction. These reactions to PEG-Asp, however, were less severe than the prior reactions to the native E coli protein. Our patient population had very few individuals with prior E coli asparaginase hypersensitivity, possibly contributing to the low incidence of clinical allergy in this study.
We draw several conclusions from our results. First, patients with ALL in first marrow relapse receiving weekly PEG-Asp had a greater chance of achieving a second remission than those receiving biweekly dosing. Second, toxicity due to either weekly or biweekly PEG-Asp in children with relapsed ALL is similar to that of previously reported studies utilizing native E coli asparaginase and lower than other more intensive reinduction schedules utilized in relapsed ALL. Third, higher asparaginase levels correlate with a better induction response rate. Finally, low serum levels of asparaginase correlate with the presence of antibody against native and PEG-Asp and may correlate with a lower CR. The use of intensive weekly PEG-Asp should be explored further in childhood ALL, ideally by means of a direct comparison with the less costly E colipreparation.
The following institutions participated in the pediatric oncology group study (National Cancer Institute grant numbers, if applicable, appear in parentheses): Alberta Children's Hospital; All Children's Hospital; Baylor (CA-03161); Boston Floating Hospital; Cancer Center of Hawaii; Children's Hospital (San Diego) (CA-28439); Children's Hospital Greenville System (CA-69177); Children's Hospital Michigan (CA-29691); Children's Hospital New Orleans/LSU CCOP; Christ Hospital (CA-07431); Cook-Ft Worth Children's Medical Center (CA-33625); Dartmouth Hitchcock (CA-29293); Duke University (CA-15525); East Carolina University (CA-69177); Emory University (CA-20549); Fairfax Hospital (CA-28476); Hackensack Medical Center; Hurley Medical Center (CA-29691); Joe DiMaggio Children's; Johns Hopkins University (CA-28476); Maine Children's (CA-41573); McGill University (CA-33587); Medical College Virginia; Miami Children's Hospital; Midwest Children's Cancer Center (CA-32053); Nemours/Jacksonville; Nemours/Orlando; Oklahoma University (CA-11233); Rhode Island Hospital (CA-29293); Roswell Park Cancer Institute (CA-28383); Sacred Heart Hospital; San Antonio MPC & BDC; Scott & White (CA-33625); Southwestern Medical School (CA-33625); SPOG Bern; SPOG Lausanne; St Christopher's Hospital; St Francis Regional; St Johns Hospital (CA-29691); Stanford University (CA-33603); Statistical Office (CA-29139); SUNY Syracuse; Sutter Community Hospital; Tampa Children's Hospital; UC/Davis; University of Alabama (CA-25408); University of Arizona (CA-33603); University of Arkansas; University of Florida; University of Kansas; University of Maryland (CA-69428); University of Miami; University of Missouri (CA-05587); University of Vermont (CA-29293); UT/San Antonio; Walter Reed Army Medical; Warren Clinics (CA-11233); Washington University (CA-05587); Yale University (CA-69428).
Reprint:Thomas C. Abshire, (#9310), c/o POG Operations Office, 645 N Michigan Ave, Suite 910, Chicago, IL 60611.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal