Abstract
Erythrocyte maturation is accompanied by RNA degradation and release of mononucleotides. We have previously purified PN-I, a pyrimidine nucleotidase whose deficiency is associated with hemolytic anemia. Computer-aided analysis of PN-I tryptic and CNBr peptide sequences revealed substantial identity with tryptic peptide sequences reported for p36, an α-interferon-induced protein. PN-I partial sequences were matched through the expressed sequence tag database with different human complementary DNA (cDNA) clones, whose sequences were exploited to screen a human placenta cDNA library. PN-I cDNA, coding for a 286-residue protein, was expressed in Escherichia coli, yielding a fully active recombinant enzyme. The recombinant protein sequence comprised the peptide sequences determined for PN-I and p36. Rabbit antisera raised against two peptides deriving from p36 and PN-I tryptic digestions, respectively, recognized both wild-type and recombinant PN-I. Molecular properties of the two proteins were essentially the same. We conclude that p36 and PN-I are identical proteins.
Introduction
Erythrocyte pyrimidine 5′-nucleotidases are enzymes involved in the salvage pathway of nucleotides and in the catabolism of RNA, in that they catalyze the dephosphorylation of various pyrimidine nucleoside monophosphates to their respective nucleosides. Two human red cell soluble enzymes, termed PN-I and PN-II, were identified on the basis of their different molecular properties and substrate specificities.1-7 The deficiency of PN-I is associated to a hemolytic nonspherocytic anemia characterized by basophilic stippling at the Wright stain and accumulation of pyrimidine nucleotides,1 suggesting that in the red cell the fluctuation of pyrimidine levels is differently regulated with respect to its purine counterpart. Evidence has been reported on the accumulation of ribonucleic material as the cause of the basophilic stippling, indicating that PN-I deficiency is also closely related to incomplete RNA degradation. Recently it has been described that rat PN-I-like enzyme activity increased concomitantly with the maturation of new erythrocytes,8 suggesting a major role of this enzyme in the elimination, as diffusible nucleosides, of the pyrimidine nucleotides formed from internal RNA degradation. Likewise, in red blood cells of human fetuses from 17 to 23 weeks of gestation, the activity of pyrimidine 5′-nucleotidase was higher than in the adult cells.9 A transient increase of a similar activity has also been observed in chick embryonic erythrocytes in response to cyclic adenosine monophosphate stimulation of the enzyme synthesis.9 Furthermore, PN-I deficiency has been associated to the conversion of hemoglobin E disease into an unstable hemoglobinopathy-like disease.10
We have previously demonstrated that homogeneous pyrimidine nucleotidases from human erythrocytes possess phosphotransferase activities specific for pyrimidine nucleotides,11suggesting an additional role of these enzymes in nucleotide metabolism. To get insights into the physiological importance of PN-I, we decided to investigate on the molecular properties of the enzyme. In the present study the enzymatic protein was submitted to chemical and enzymatic digestions, and it has been shown that the sequences of the resulting peptides, when searched in protein data bank, matched with those of six peptides deriving from digestions of p36, an α-interferon-induced protein in cells forming lupus inclusions (LIs).12 LIs are abnormal cytoplasmic structures of unknown function, constituted by membranes, proteins, carbohydrates, and RNA.13 The PN-I complementary DNA (cDNA) was for the first time cloned and expressed. Its inspection reveals a sequence comprising, in addition to those stored in data bank, other sequences separately and independently determined both for p3612 and PN-I. Furthermore, rabbit antisera raised against synthetic peptides constructed on p36 and PN-I sequences cross-reacted with PN-I. These results indicate that p36 and PN-I are identical proteins.
Study design
A total of 10 μg homogeneous PN-I11 was both CNBr- and trypsin-digested.14 The fragments were separated on a Capillary Microblotter system and sequenced on an Applied Biosystems Procise sequencer.
Human placenta library was used as the source of PN-I cDNA, which was amplified for cloning by using polymerase chain reaction (PCR). The product, digested with BamH I and Pst I, was ligated into the expression vector pT7-7. Escherichia coliBL21, harboring the recombinant plasmid, was grown at 37°C, in luria-bertani (LB) medium. At different incubation times, cells were collected and disrupted by sonication. Samples were assayed for PN-I activity.11
Rabbit antisera raised against the peptides KSSVRIKNPTRVEEC and DNSNIILLGDSQGDC were obtained from Igtech (Salerno, Italy), according to standard procedure. The immunoreactive protein was detected as previously described.15
Results and discussion
Partial sequences obtained from PN-I digestions were used for similarity searches, using the NCBI BLAST server. In the “nr” database, the PN-I sequences matched with the 82-residue sequence obtained for p36,12 with the exception of only three residues (Figure 1a). In this regard, it should be pointed out that two of the residues are at the p36 peptide terminal positions, whose identification generally is more prone to ambiguity, and the third (Q) is just the deamidated form of the other (E). A search of the GenBank expressed sequence tag database (dbEST), using program “tblastn,” revealed a perfect match of all PN-I-derived peptides with several anonymous cDNA sequences. Each single cDNA sequence coded only for a part of the protein, and it was needed to overlap and combine the sequences to obtain the entire putative nucleotide cDNA sequence coding for PN-I, as well as the untranslated regions. Moreover, the translation of the combined cDNA into amino acid sequence made it possible to align the tryptic and CNBr peptide sequences, totalling 251 amino acids of the coded sequence. It should be pointed out that the PN-I-deduced amino acid sequence (Figure1b) also comprises p36 T42 (LQIITDFDM) and T50 (YM) residues, which are not present in the sequence determined for PN-I in our laboratory. This finding represents an additional evidence of the identity of p36 and PN-I proteins, and it is reinforced by the observation that the pI value of 5.4 calculated for the recombinant PN-I is superimposable to that of 5.6 reported for p36.12
Comparison of PN-I and p36 sequences.
(A) Alignment of p36 sequences, as reported,12 with those determined for PN-I. (B) Human PN-I cDNA sequence and its encoded amino acid sequence. The first base of the translation start codon is designated +1 and the stop codon is indicated by asterisks. The 251 amino acids obtained from PN-I peptide sequencing are underlined. Residues of fragments T42 and T50 of p36 that are not aligned with PN-I peptides (panel A) are boxed in the PN-I sequence.
Comparison of PN-I and p36 sequences.
(A) Alignment of p36 sequences, as reported,12 with those determined for PN-I. (B) Human PN-I cDNA sequence and its encoded amino acid sequence. The first base of the translation start codon is designated +1 and the stop codon is indicated by asterisks. The 251 amino acids obtained from PN-I peptide sequencing are underlined. Residues of fragments T42 and T50 of p36 that are not aligned with PN-I peptides (panel A) are boxed in the PN-I sequence.
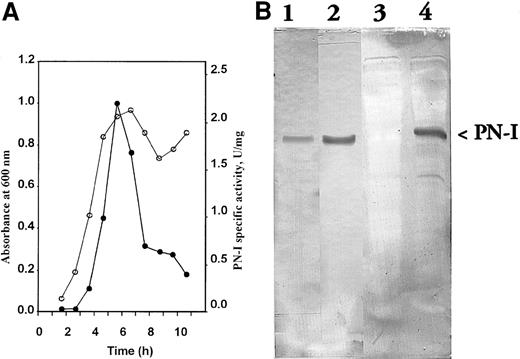
To clone and express PN-I cDNA, amplification experiments were carried out using a human placenta library. On the basis of the PN-I sequence, primers delimitating the cDNA portion of interest were chosen and added to specific restriction sites for cloning as described in the section “Study design.” PCR amplification yielded a product of 880 base pairs (bp), whose nucleotide sequence exactly corresponded to the data bank sequence. The expression resulted in a soluble and active PN-I. Such activity was not detectable in control extracts prepared from BL21 cells transformed with the nonrecombinant plasmid (not shown). In the cells harboring the recombinant plasmid, a spontaneous and transient expression of PN-I activity at the late logarithmic phase was found, followed by a rapid decrease at the stationary phase (Figure2a). Sodium dodecyl sulfate polyacrylamide gel electrophoresis of the same extracts revealed the appearance of a 36 000-Mr protein band, whose intensity paralleled the activity expression behavior (not shown).
Expression of PN-I activity in
E coli and PN-I immunoblot analysis. (A) The specific activity of the expressed PN-I (●) is plotted as a function of growth of BL21 cells transformed with the recombinant plasmid ○. (B) Antiserum raised against peptide KSSVRIKNPTRVEEC is incubated at 1:1500 dilution with blotted pure wild-type PN-I (lane 1); antiserum against peptide DNSNIILLGDSQGDC is incubated at 1:1500 dilution with blotted pure wild-type PN-I (lane 2), and blotted extracts of BL21 cells transformed with nonrecombinant (lane 3) and recombinant plasmid (lane 4).
Expression of PN-I activity in
E coli and PN-I immunoblot analysis. (A) The specific activity of the expressed PN-I (●) is plotted as a function of growth of BL21 cells transformed with the recombinant plasmid ○. (B) Antiserum raised against peptide KSSVRIKNPTRVEEC is incubated at 1:1500 dilution with blotted pure wild-type PN-I (lane 1); antiserum against peptide DNSNIILLGDSQGDC is incubated at 1:1500 dilution with blotted pure wild-type PN-I (lane 2), and blotted extracts of BL21 cells transformed with nonrecombinant (lane 3) and recombinant plasmid (lane 4).
To further ascertain whether PN-I and p36 indeed represent the same protein, rabbit antibodies were raised against two synthetic peptides constructed on the basis of two sequences: KSSVRIKNPTRVEEC, localized at the PN-I N-terminus, and DNSNIILLGDSQGDC, identical to the sequence of the p36 T29 fragment. The antibodies were able to recognize both wild-type and recombinant PN-I, when analyzed by Western blot (Figure2b), thus confirming both the expression of the recombinant protein and its identity with p36.
The significance of the identity of the two proteins is at the moment obscure. Computer-aided search of the 286-amino acid sequence revealed high similarity with hypothetical proteins of Caenorhabditis elegans and Arabidopsis thaliana, suggesting a conserved function of biological importance. We do not know whether p36 retains the ability to catalyze the hydrolysis of pyrimidine monophosphates. The occurrence of posttranslational modifications of p36, not affecting the apparently identical molecular and structural parameters but influencing the catalytic properties, cannot be discounted. However, our results might open a new possible scenario on the role played by p36 in immune diseases, in that LI form in the cytoplasm of reticuloendothelial cells of individuals with systemic lupus erythematosus 16 and acquired immunodeficiency syndrome.17 The availability of cDNA, together with full structural and catalytic characterization of the protein, now actively in progress in our laboratory, will be instrumental for more systematic experiments aiming at the elucidation of the function of PN-I in normal and pathological conditions.
Supported in part by CNR grant 98.00473.ct04 and CNR Target Project “Biotechnology.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Giulio Magni, Istituto di Biochimica, Facoltà di Medicina e Chirurgia, Università di Ancona, Via Ranieri 65, 60131 Ancona, Italy; e-mail: magnig@popcsi.unian.it.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal