Abstract
β2-Glycoprotein I (β2GPI) is a major antigen for antiphospholipid antibodies, and its multiple in vitro functions have been reported. This glycoprotein not only down-regulates thrombin formation by inhibiting contact activation or prothrombinase activity, but also up-regulates coagulation by reducing protein C anticoagulant activity. However, the in vivo roles of β2GPI remain obscure. Coagulation and fibrinolytic characteristics were investigated in individuals with β2GPI deficiency. An apparently healthy woman and her brother are homozygotes for β2GPI deficiency. In these patients, Russell viper venom time was shortened (40.4 seconds; normal range, 47.8 ± 4.95 seconds), but all markers of thrombin generation and fibrin turnover were within normal ranges. Exogenous activated protein C adequately prolonged the clotting time of the β2GPI-deficient plasma, and euglobulin lysis time was also normal. Thus, elevated thrombin generation, enhancement of activated protein C response, and an altered fibrinolytic system were not found in congenitally β2GPI-deficient plasma.
Introduction
β2-Glycoprotein I (β2GPI), formed by 5 short consensus repeat domains, is a 50-kd phospholipid-binding protein with a plasma concentration of about 200 μg/mL.1 It bears the epitope(s) for anticardiolipin antibodies (anti-β2GPI antibodies) that are associated with the antiphospholipid syndrome.2 Despite studies on the function of β2GPI in vitro, the physiologic role of β2GPI has remained uncertain. β2GPI inhibits adenosine diphosphate (ADP)-induced platelet aggregation,3 factor XII activation,4prothrombinase activity,5 factor Va degradation by activated protein C,6 and factor Xa generation.7 Despite these multiple regulatory functions, Bancsi et al8 reported that a β2GPI-deficient family was apparently not at risk for thrombosis.
Recently we encountered 2 Japanese individuals (siblings) with homozygous β2GPI deficiency. To determine the significance of the physiologic roles of β2GPI, we examined the profile and behavior of coagulation and fibrinolysis indices in these 2 individuals.
Study design
Two individuals with congenital homozygous β2GPI deficiency were identified. Person 1 was a 36-year-old woman and person 2 was her sibling, a 34-year-old man. Genomic analysis of these persons showed that a thymine corresponding to position 379 of the β2GPI cDNA9 in exon 4 was deleted; hence, a frame shift would occur and this would make the gene code for an amino acid sequence unrelated to β2GPI beyond this position.10 These individuals had no history of thrombosis or abnormal bleeding.
Serum levels of mutated β2GPI were investigated by sandwich enzyme-linked immunosorbent assay, Western blotting, immunoelectrophoresis with rabbit polyclonal anti-human serum antibodies, and Ouchterlony double immunodiffusion with rabbit polyclonal anti-human β2GPI antibody (Diagnostica Stago, Asnieres, France).
In vitro platelet aggregability was investigated with the plasma from the homozygous β2GPI-deficient patients in the presence of thrombin or ADP.
Prothrombin time (PT), activated partial thromboplastin time (aPTT), plasma fibrinogen, antithrombin III (ATIII) activity and antigen, plasminogen, and α2-plasmin inhibitor (α2PI) were measured as hemostatic and fibrinolytic indices. Thrombin generation and fibrinolytic turnover in vivo were evaluated by measuring plasma levels of prothrombin fragment 1 + 2 (Enzygnost F1 + 2 micro; DADE Behring, USA), thrombin–antithrombin complex (TAT) (TAT test Kokusai F; Kokusai Shiyaku, Japan), plasminogen–plasmin inhibitor complex (PIC test Kokusai F; Kokusai Shiyaku), and D-dimer antigen (LPIA200 D-dimer; Dia-atron). Plasma protein C (Chromogenix, Sweden), protein S antigen (Asserachrom Total Protein S; Diagnostica Stago), and free protein S antigen (Asserachrom Free Protein S, Diagnostica Stago) were quantified. The effect of exogenous activated protein C (APC) in the plasma was tested with a clotting assay (Activated protein C resistance kit; Chromogenix).
To investigate the intensity of thrombin formation, we assessed the rate of thrombin generation by measuring the time taken to reach 50% maximal activity (T1/2).11 In addition, we evaluated PT using highly diluted tissue factor reagent (Dil-PT) to investigate the hypercoagulable state in a patient with β2GPI deficiency.
Fibrinolytic potential of the euglobulin fraction was also evaluated by fibrin-plate lysing assay in the presence or absence of 2.5 mg/mL of kaolin.
The effect of exogenous β2GPI on Russell viper venom time (RVVT) was tested using DVV-screen (American Diagnostica Inc, Greenwich, CT). β2GPI was purified from normal pooled plasma as described.12 To investigate the effect of exogenous β2GPI on RVVT, we added 25 μL of purified human β2GPI (0-1250 μg/mL final concentration) to 50 μL of the plasma sample from person 1 or to pooled healthy plasma. After incubation at 37°C for 120 seconds, RVVT was measured.
Results and discussion
Plasma mutated β2GPI was not detected by any of the methods described in “Study design.” Platelets were normally aggregated in β2GPI-deficient plasma by thrombin or ADP.
The results of hemostatic variables are shown in Table1. PT, aPTT (not done in person 2), fibrinogen, ATIII, plasminogen, and α2PI were normal in both individuals. No increased thrombin generation or fibrinolysis was indicated because levels of F1 + 2, TAT, PIC, and D-dimer were normal. Plasma levels of protein C activity were slightly increased in both individuals, but those of total protein S antigen and free protein S antigen were within normal values. The effects of exogenous APC in both β2GPI-deficient plasma samples did not differ from findings in plasma samples from healthy controls. There were no significant differences in T1/2 and Dil-PT between the β2GPI-deficient plasma and plasma samples of normal subjects. Therefore, no increased in vitro thrombin generation was suggested by these assays. Fibrinolytic activity in the euglobulin from the patients' plasma, as induced by the addition of kaolin, was similar to that in plasma from normal controls.
Hemostatic variables in individuals with β2-glycoprotein I deficiency
| Variable . | Normal range . | Person 1 . | Person 2 . |
|---|---|---|---|
| PT-INR, ratio | 0.85 -1.25 | 0.86 | 0.89 |
| APTT, s | 24.0 -32.6 | 32.3 | ND |
| Fibrinogen, mg/dL | 200 -400 | 388 | 259 |
| ATIII, % | 80 -130 | 106 | 95 |
| Protein C, % | 67 -127 | 137 | 142 |
| Free protein S, % | 65 -135 | 135 | 129 |
| Total protein S, μg/mL | 4.0 -13.8 | 13.8 | 10.9 |
| Plasminogen, % | 80 -130 | 125 | 97 |
| α2PI, % | 80 -130 | 115 | 88 |
| TAT, ng/mL | <3.5 | 1.0 | 1.2 |
| F1+2, nmol/L | 0.20 -1.04 | 0.73 | 0.71 |
| PIC, μg/mL | <0.8 | 0.6 | 0.3 |
| D-dimer, μg/mL | <1.0 | 0.32 | 0.27 |
| Variable . | Normal range . | Person 1 . | Person 2 . |
|---|---|---|---|
| PT-INR, ratio | 0.85 -1.25 | 0.86 | 0.89 |
| APTT, s | 24.0 -32.6 | 32.3 | ND |
| Fibrinogen, mg/dL | 200 -400 | 388 | 259 |
| ATIII, % | 80 -130 | 106 | 95 |
| Protein C, % | 67 -127 | 137 | 142 |
| Free protein S, % | 65 -135 | 135 | 129 |
| Total protein S, μg/mL | 4.0 -13.8 | 13.8 | 10.9 |
| Plasminogen, % | 80 -130 | 125 | 97 |
| α2PI, % | 80 -130 | 115 | 88 |
| TAT, ng/mL | <3.5 | 1.0 | 1.2 |
| F1+2, nmol/L | 0.20 -1.04 | 0.73 | 0.71 |
| PIC, μg/mL | <0.8 | 0.6 | 0.3 |
| D-dimer, μg/mL | <1.0 | 0.32 | 0.27 |
INR indicates international normalized ratio; ND, not done.
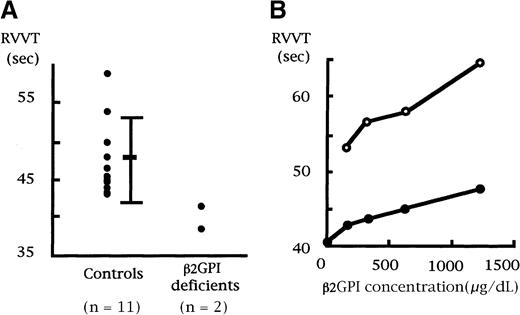
Shortened RVVT was found in both β2GPI-deficient persons as compared with controls (Figure1A). To investigate whether the shortened RVVT was due to the β2GPI deficiency, we added a series of concentrations of exogenous β2GPI to one of the β2GPI-deficient plasma samples and measured RVVT. The prolongation of the RVVT by exogenous β2GPI was dose dependent in the β2GPI-deficient plasma as well as a normal control plasma (Figure 1B). However, even an excess of exogenous β2GPI (1250 μg/mL) did not normalize the shortened RVVT. Moreover, Russell viper venom is a strong activator of factor X,13 but such potent substances have not been detected in the human coagulation system in vivo. Therefore, the relevance of the phenomenon remains obscure.
RVVT in 2 individuals with congenital β2GPI deficiency.
(A) Russell viper venom was added to plasma samples, and clotting time was recorded. Both samples with β2GPI deficiency displayed a shorter clotting time. (B) One plasma sample with β2GPI deficiency was preincubated with different concentrations of exogenous β2GPI for 120 seconds at 37°C, and RVVT was recorded. Prolongation of clotting time was dose dependent in both the β2GPI-deficient plasma (●●) and a control plasma sample (○○).
RVVT in 2 individuals with congenital β2GPI deficiency.
(A) Russell viper venom was added to plasma samples, and clotting time was recorded. Both samples with β2GPI deficiency displayed a shorter clotting time. (B) One plasma sample with β2GPI deficiency was preincubated with different concentrations of exogenous β2GPI for 120 seconds at 37°C, and RVVT was recorded. Prolongation of clotting time was dose dependent in both the β2GPI-deficient plasma (●●) and a control plasma sample (○○).
This is the first documentation that most hemostatic and fibrinolytic markers are normal and that there is no increased thrombin generation in individuals with congenital β2GPI deficiency. In physiologic circumstances, β2GPI is not a high-affinity phospholipid-binding protein14 and therefore may not interfere with the coagulation/fibrinolysis system. Our data partly support the observation of Bancsi et al8 that congenital β2GPI deficiency clinically may not be a risk for thrombosis and also show that congenital β2GPI deficiency is not associated with increased or decreased thrombin formation (ie, “subclinical” thrombotic or bleeding tendency) either in vivo or in vitro. Therefore, congenital β2GPI deficiency is not a risk factor for either thrombosis or a bleeding tendency.
Supported in part by a grant from the Japanese Ministry of Health and Welfare.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Tatsuya Atsumi, Department of Medicine II, Hokkaido University School of Medicine, N15 W7, Kita-ku, Sapporo 060-8648, Japan; e-mail: at3tat@med.hokudai.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal