Abstract
2′-Deoxycoformycin (dCF) as a single agent has been reported to be less effective against myeloid than against lymphoid malignancies in clinical trials. However, previous studies have shown that in the presence of 2′-deoxyadenosine (dAd), human monocytoid leukemia cell lines are much more sensitive to dCF with regard to the inhibition of cell proliferation. Thus, dCF might be useful for treating monocytoid leukemia with the aid of dAd analogs. The antiproliferative effects of dCF in combination with dAd or its derivatives were examined on normal and malignant blood and bone marrow cells. In the presence of 10 μmol/L dAd, the concentration of dCF required to inhibit the viability of primary monocytoid leukemia cells was much lower than that required to inhibit normal or non-monocytoid leukemic cells. Among the dAd analogs, 9-β-d-arabinofuranosyladenine (AraA) was also effective in combination with dCF. Athymic nude mice were inoculated with human monocytoid leukemia U937 cells and treated with dCF or a dAd analog or both. Although dCF alone slightly but significantly prolonged the survival of mice inoculated with U937 cells, combined treatment with dCF and AraA markedly prolonged their survival. These data suggest that the combination of dCF and AraA may be useful for the clinical treatment of acute monocytic leukemia.
Introduction
Although the frequency of complete remission of acute monocytic leukemia (AML) has increased, the median duration of remission is only about 6 months, even when remission is achieved by treatment with conventional cytotoxic antileukemic drugs.1These results clearly call for improved therapies.
There is a growing realization that cancer chemotherapeutic agents act primarily by inducing cancer cell death through the mechanism of apoptosis.2-7 Tumor cells that are intrinsically resistant to chemotherapy are unable to activate the apoptotic machinery and may therefore be fundamentally resistant to chemotherapeutic cell death. Several different mechanisms may be operating in the induction of apoptosis, depending on the stimulus such as cytokines, injury, stress, irradiation, starvation, and so forth. Understanding the specific mechanisms involved in tumor cell death may allow the identification of novel drug targets in various malignant cell types and the further development of more specific agents that are designed to specifically induce the apoptotic machinery of tumor cells.
In the presence of 2′-deoxyadenosine (dAd), human monocytoid leukemia cell lines are much more sensitive to 2′-deoxycoformycin (dCF), a specific and potent inhibitor of adenosine deaminase (ADA), with regard to the inhibition of cell proliferation.8 Deoxyadenosine triphosphate (dATP) effectively induced caspase-3 activation in the cytosol of monocytoid leukemia cells, but not in that from non-monocytoid cells, suggesting that dATP-dependent caspase-3 activation is at least partly involved in the preferential induction of apoptosis in monocytoid leukemia cells with this combination treatment.8 dCF has been effectively used to treat several lymphoid malignancies.9-13 Although dCF has been reported to be ineffective in myeloid leukemia, dCF might be useful for treating monocytoid leukemia with the aid of dAd or its derivatives. Therefore, in the present study, we examined the effects of dCF with or without dAd on normal monocytes and hematopoietic progenitor cells, and the therapeutic effect on monocytic leukemia in an experimental xenograft model.
Materials and methods
Materials
dCF was obtained from The Chemo-Sero-Therapeutic Research Institute (Kumamoto, Japan). Cladribine (CdA), fludarabine (Flu), dAd, daunorubicin (DNR), 9-β-d-arabinofuranosylcytosine (AraC), and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium (MTT) were purchased from Sigma Chemical Co (St Louis, MO). 9-β-d-Arabinofuranosyladenine (AraA) was obtained from Mochida Pharmaceutical Co (Tokyo, Japan).
Cell lines and cell culture
Human monocytoid (U937, THP-1, and HEL/S), myeloid (HL-60), erythroid (K562), and B-cell lymphoma (BALM3) cell lines were cultured in suspension in RPMI 1640 medium supplemented with 10% fetal bovine serum at 37°C in a humidified atmosphere of 5% CO2 in air.8
Drug-resistant U937 cells were isolated as described below. U937 cells were cultured in stepwise-increasing concentrations of DNR or AraC as follows: 5 transfers at 10−10 mol/L DNR or AraC, 5 transfers at 3 × 10−10 mol/L, 10 transfers at 10−9 mol/L, 10 transfers at 3 × 10−9mol/L, and 10 transfers at 10−8 mol/L. The variants remained resistant in the drug-free culture for more than 2 months.
Samples and cell processing
Peripheral blood leukocytes were obtained from 7 healthy volunteers. Normal bone marrow was obtained from 3 healthy volunteers. Bone marrow was obtained from 25 patients with acute leukemia and peripheral blood mononuclear cells were obtained from 9 patients with lymphoma. All samples were obtained after receiving informed consent. Mononuclear cells were isolated from the samples by Ficoll-Paque (Pharmacia, Uppsala, Sweden) density gradient centrifugation. After a 30-minute incubation of peripheral blood mononuclear cells on culture dishes, dish-attached cells and floating cells were used as monocytes and lymphocytes, respectively. The purity of monocytes was confirmed by an assay of nonspecific esterase activity, and more than 95% of the cells were positive.
Measurement of cell number and viability in cultures treated with dCF and dAd
Cells (105/mL) were cultured with various concentrations of dCF or dAd or both for 5 days. The cells were counted in a Model ZM Coulter Counter (Coulter Electronics, Luton, UK), and cell viability was examined by MTT assay, as described previously.14
Assay of hematopoietic colony-forming cells
Bone marrow cells were cultured in the semisolid medium Methocult GF H4434V, a complete pretested mixture of methylcellulose, fetal bovine serum, bovine serum albumin, erythropoietin, and recombinant human growth factors in Iscove medium (Stemcell Technologies Inc, Vancouver, British Columbia, Canada).
Bone marrow cells were plated in triplicate at 2 × 104/plate, and U937 and THP-1 cells were seeded at 1000/plate. Plates were kept in a humidified incubator at 37°C and 5% CO2 for 18 days. Colonies (> 40 cells) were counted at days 12 and 18 by light microscopy.
Assay for caspase-3 activity
Caspase-3 activity was assayed with the fluorogenic substrate acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin (DEVD-MCA) (Peptide Institute, Inc, Osaka, Japan), as described previously.8Enzyme activity was expressed as picomoles aminomethylcoumarin per minute per milligram protein.
Transplantation of U937 cells into nude mice
Seven-week-old female athymic nude mice with a BALB/c genetic background were supplied by CLEA Japan (Tokyo, Japan). They were injected intraperitoneally with cyclophosphamide (150 g/kg body weight) on each of 2 successive days. Twenty-four hours after the second injection, the mice were inoculated intraperitoneally with 3 × 106 U937 cells per animal. Three times per week, mice were given intraperitoneal injections of 0.2 mL of phosphate-buffered saline including dCF or AraA or both, with the first injection given 3 days after the inoculation of leukemia cells.
Results
Effects of dCF and dAd on the viability and growth of human peripheral blood and bone marrow cells
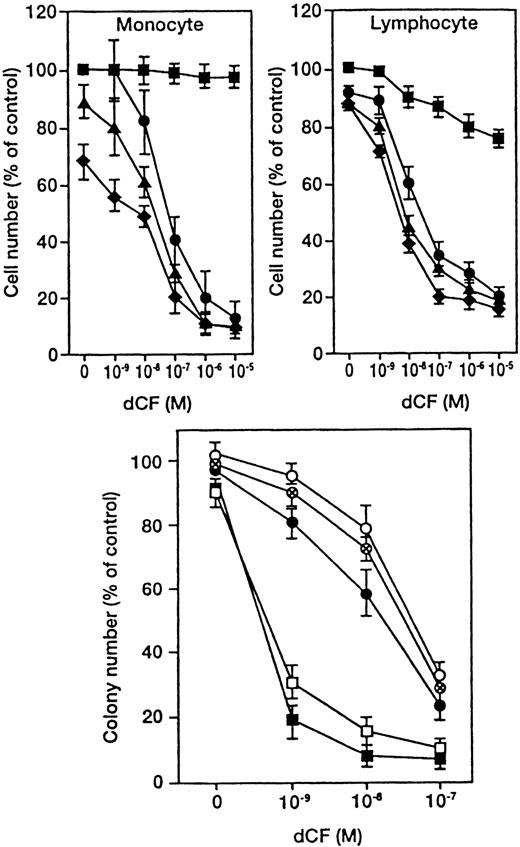
Peripheral lymphocytes and monocytes from 7 healthy volunteers were cultured with various concentrations of dCF in the presence of dAd (Figure 1, upper panel). dCF alone slightly inhibited the viability of lymphocytes, whereas it hardly affected the survival of monocytes. On the other hand, dAd alone slightly inhibited the viability of monocytes, whereas it hardly affected the survival of lymphocytes. Similar results were obtained in lymphoid and monocytoid leukemia cells (data not shown). The cells were cultured with various concentrations of dCF in the presence of 10, 30, and 50 μmol/L of dAd for 4 days, and concentrations of dCF that inhibited viable cell numbers by 50% (IC50) were calculated. The IC50 of normal monocytes was similar to that of lymphocytes, although the IC50 of monocytoid leukemia cells was 3 orders of magnitude lower than that of lymphoid leukemia cells.8 These results suggest that monocytoid leukemia cells, but not normal monocytes, are highly sensitive to the combined effects of dCF and dAd.
Combined effects of dCF and dAd on the viability of normal blood and hematopoietic cells.
(Upper panels) Human peripheral blood monocytes and lymphocytes were cultured with various concentrations of dCF in the presence of 0 (▪), 10 (●), 20 (▴), or 40 (♦) μmol/L dAd for 5 days. Values are the means ± SD of triplicate determinations for cells from 7 healthy volunteers. (Lower panel) Normal bone marrow cells (each circle indicates 1 of 3 cases) and monocytic leukemia cells (U937; ▪, THP-1; (■) were cultured in a semisolid medium with various concentrations of dCF in the presence of 10 μmol/L dAd. The values are the means ± SD for 4 determinations.
Combined effects of dCF and dAd on the viability of normal blood and hematopoietic cells.
(Upper panels) Human peripheral blood monocytes and lymphocytes were cultured with various concentrations of dCF in the presence of 0 (▪), 10 (●), 20 (▴), or 40 (♦) μmol/L dAd for 5 days. Values are the means ± SD of triplicate determinations for cells from 7 healthy volunteers. (Lower panel) Normal bone marrow cells (each circle indicates 1 of 3 cases) and monocytic leukemia cells (U937; ▪, THP-1; (■) were cultured in a semisolid medium with various concentrations of dCF in the presence of 10 μmol/L dAd. The values are the means ± SD for 4 determinations.
The effect of dCF plus dAd on the colony-forming capacity of cells from bone marrow of 3 healthy volunteers was measured by the assay of hematopoietic colonies in methylcellulose medium (Figure 1, lower panel). A total of 2 × 104 bone marrow mononuclear cells generated a mean of 236 (range, 201-264) colonies/clusters in methylcellulose cultures. Three samples of normal bone marrow progenitors were analyzed, and the results showed a very similar dCF/dAd-induced reduction in hematopoietic colony-forming capacity. Combined treatment with dCF and dAd did not affect the ratio of hemoglobinized colonies or colonies of monocyte/macrophages, suggesting that these drugs similarly inhibited different types of hematopoietic colonies including multilineage colonies (data not shown). The assays showed that monocytic leukemia U937 and THP-1 cells are more sensitive than normal bone marrow progenitor cells (Figure 1, lower panel).
Antiproliferative activities of several dAd analogs in the presence of dCF
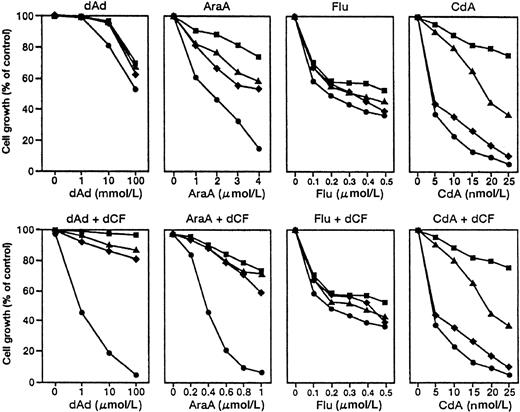
Although the combination of dCF and dAd preferentially induced apoptosis of monocytoid leukemia cells, the clinical use of dAd would be difficult because of the need for a high concentration of dAd. Therefore, we examined the effects of derivatives of dAd on the growth of myeloid and monocytoid leukemia cells in the presence or absence of dCF (Figure 2). AraA preferentially inhibited the growth of U937 cells, and the antiproliferative effect of AraA was greatly enhanced by dCF. However, Flu similarly inhibited the growth of U937 (monocytoid), HL-60 (myeloid), K562 (erythroid), and BALM3 (lymphoid) cells, and the growth-inhibitory effect of Flu was not enhanced by dCF. dCF did not augment the growth inhibition caused by CdA and Flu. The concentration of AraA that effectively inhibited the proliferation of U937 was 1/100 that of dAd (Figure 2). These results indicated that the combination of AraA and dCF was remarkably effective in inhibiting the growth of U937 cells. Similar results were obtained with other monocytic leukemia (THP-1 or HEL/S) cells (data not shown).
Growth-inhibitory effect of dAd analogs on human leukemia and lymphoma cell lines in the presence or absence of dCF.
U937 (monocytoid, ●), K562 (erythroid, ▪), HL-60 (myeloid, ▴), and BALM3 (lymphoma, ♦) cells were cultured with various concentrations of dAd, AraA, Flu, or CdA in the presence or absence of 10 nmol/L dCF for 5 days. Values are means of 4 separate experiments.
Growth-inhibitory effect of dAd analogs on human leukemia and lymphoma cell lines in the presence or absence of dCF.
U937 (monocytoid, ●), K562 (erythroid, ▪), HL-60 (myeloid, ▴), and BALM3 (lymphoma, ♦) cells were cultured with various concentrations of dAd, AraA, Flu, or CdA in the presence or absence of 10 nmol/L dCF for 5 days. Values are means of 4 separate experiments.
Induction of caspase-3 activity in U937 cells treated with AraA and dCF
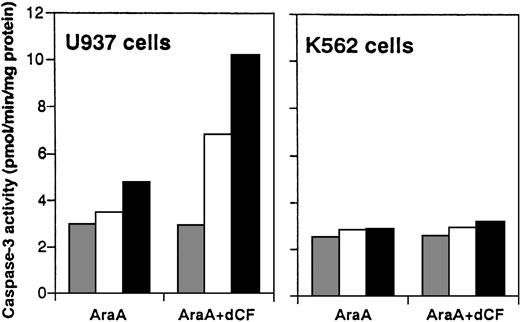
Activity of ADA in untreated U937 cells was similar to that in K562 cells, and there was no significant difference in the sensitivity of ADA activity in cell lysates to dCF between U937 and K562 cells, suggesting that ADA in U937 cells was quantitatively and qualitatively similar to that in K562 cells. Treatment with dCF for 24 hours inhibited ADA activity in leukemia cells in a concentration-dependent manner, and ADA activity in dCF-treated U937 and THP-1 cells was similar to that in non-monocytoid cells.8 There were no significant differences in the amount of dATP between monocytoid and non-monocytoid leukemia cells in the presence or absence of dCF.8 Induction of dATP-dependent caspase-3 activation accompanied the apoptosis of U937 cells induced by dCF plus dAd.8 To investigate whether AraA can activate caspase-3 in combination with dCF, we examined the activity of caspase-3 using DEVD-MCA as a substrate in extract of U937 or K562 cells treated with AraA plus dCF. Treatment with AraA in the presence of 10 nmol/L dCF induced proteolytic activity in a dose-dependent manner, whereas the activity in K562 cells was hardly affected (Figure3), indicating that AraA effectively induced caspase-3 activation in monocytoid leukemia cells but not in non-monocytoid leukemia cells.
Induction of caspase-3 activity by dCF and AraA.
Cell lysates from U937 and K562 cells treated with 0 ( ), 1 (□), or 3 (▪) μM AraA and/or 10 nmol/L dCF for 2 days were assayed for protease activity toward Ac-DEVD-MCA. Values are the means of 3 separate experiments.
), 1 (□), or 3 (▪) μM AraA and/or 10 nmol/L dCF for 2 days were assayed for protease activity toward Ac-DEVD-MCA. Values are the means of 3 separate experiments.
Induction of caspase-3 activity by dCF and AraA.
Cell lysates from U937 and K562 cells treated with 0 ( ), 1 (□), or 3 (▪) μM AraA and/or 10 nmol/L dCF for 2 days were assayed for protease activity toward Ac-DEVD-MCA. Values are the means of 3 separate experiments.
), 1 (□), or 3 (▪) μM AraA and/or 10 nmol/L dCF for 2 days were assayed for protease activity toward Ac-DEVD-MCA. Values are the means of 3 separate experiments.
Preferential inhibition of the survival of monocytoid leukemia cells by dCF and dAd or AraA in primary culture
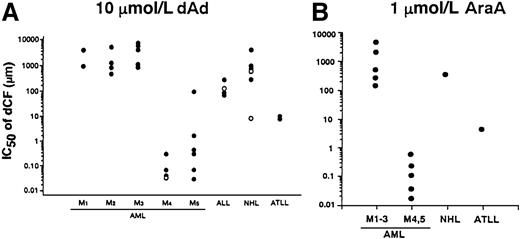
We examined the effect of dCF and dAd on the survival of bone marrow or peripheral blood mononuclear cells from patients with various hematologic malignancies. dCF alone hardly affected the number of viable cells in the culture of non-monocytoid AML cells, whereas it inhibited the survival of monocytic AML cells in a dose-dependent fashion. The inhibitory effects of dCF were augmented in combination with dAd. These findings that monocytic leukemia cells are more sensitive to treatment with dCF plus dAd were found in all of the hematologic malignancies we tested (Figure4A). Similar results were observed when the malignant cells were treated with dCF and AraA (Figure4B).
IC50 values of dCF in the inhibition of cell viability in primary culture of leukemia and lymphoma cells in the presence of dAd or AraA.
Cells were cultured with various concentrations of dCF in the presence of 10 μmol/L dAd (A) or 1 μmol/L AraA (B) for 5 days. The concentrations that inhibited the viable cell number by 50% (IC50) were calculated. Open circle in M4 indicates an eosinophilic variant. In the case of acute lymphocytic leukemia (ALL), open and closed circles indicate L1 and L2 subtypes, respectively. Follicular and diffuse type non-Hodgkin lymphoma (NHL) are open and closed circles, respectively.
IC50 values of dCF in the inhibition of cell viability in primary culture of leukemia and lymphoma cells in the presence of dAd or AraA.
Cells were cultured with various concentrations of dCF in the presence of 10 μmol/L dAd (A) or 1 μmol/L AraA (B) for 5 days. The concentrations that inhibited the viable cell number by 50% (IC50) were calculated. Open circle in M4 indicates an eosinophilic variant. In the case of acute lymphocytic leukemia (ALL), open and closed circles indicate L1 and L2 subtypes, respectively. Follicular and diffuse type non-Hodgkin lymphoma (NHL) are open and closed circles, respectively.
Antiproliferative effects of dCF plus dAd on U937 cells that are resistant to DNR or AraC
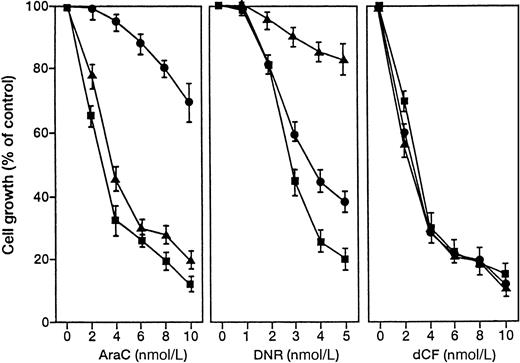
Daunorubicin and AraC are the most popular agents against AML including M4 and M5.1 We prepared U937 variants that were resistant to DNR or AraC and examined the effects of dCF and dAd (Figure 5). The drug-resistant U937 cells were similarly sensitive to treatment with dCF and dAd, suggesting that this treatment could be effective even when a monocytoid leukemia is refractory to conventional chemotherapy with DNR or AraC.
Effect of dCF on the growth of U937 cells resistant to AraC or DNR.
Parent (▪), AraC-resistant (●), and DNR-resistant (▴) U937 cells were cultured with various concentrations of AraC (left panel), DNR (middle panel), or dCF with 10 μmol/L dAd (right panel) for 5 days. Values are means ± SD of 3 separate experiments.
Effect of dCF on the growth of U937 cells resistant to AraC or DNR.
Parent (▪), AraC-resistant (●), and DNR-resistant (▴) U937 cells were cultured with various concentrations of AraC (left panel), DNR (middle panel), or dCF with 10 μmol/L dAd (right panel) for 5 days. Values are means ± SD of 3 separate experiments.
Effect of dCF on the survival of mice inoculated with U937 cells
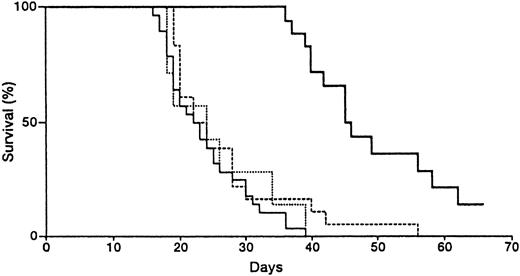
After the inoculation of 1, 10, and 100 × 105 U937 cells, all of the mice died of leukemia within 50.3 ± 12.4, 38.7 ± 8.6, and 16.7 ± 3.1 (mean ± SD, n = 7) days, respectively. To test the effect of dCF alone on the leukemogenicity of monocytic leukemia cells, we administered dCF intraperitoneally to nude mice that had been inoculated with 3 × 106 U937 cells. dCF significantly prolonged the mean survival time of mice inoculated with U937 cells (Table 1). Next, we examined the therapeutic effect of combined treatment with dCF and AraA. Administration of AraA alone did not prolong the mean survival time (Figure 6), and no appreciable adverse effects were observed in our experiment. Increasing the dose of AraA was not effective because of its low solubility. Mice inoculated with the monocytic leukemia cells were injected with various doses of dCF or a fixed dose (10 mg/kg) of AraA or both because of the insolubility of AraA. The mean survival time of mice inoculated with U937 cells was 24 days when treated with AraA only, but 46 days when treated with 10 mg/kg of AraA and 0.25 mg/kg of dCF (P = .0001, log-rank and generalized Wilcoxon tests) (Figure 6).
Effect of dCF on survival times of mice inoculated with U937 cells
| Experiment . | Dose (mg/kg) . | No. of mice . | Survival (d ± SD) . | T/C . |
|---|---|---|---|---|
| I | 0 | 10 | 22.0 ± 3.6 | |
| 0.025 | 10 | 23.7 ± 3.2 | 108 | |
| 0.25 | 10 | 25.2 ± 7.2 | 115 | |
| 2.5 | 10 | 29.2 ± 7.7* | 133 | |
| II | 0 | 8 | 21.5 ± 5.4 | |
| 0.25 | 8 | 28.6 ± 13.1* | 134 | |
| 2.5 | 8 | 31.2 ± 16.7* | 145 |
| Experiment . | Dose (mg/kg) . | No. of mice . | Survival (d ± SD) . | T/C . |
|---|---|---|---|---|
| I | 0 | 10 | 22.0 ± 3.6 | |
| 0.025 | 10 | 23.7 ± 3.2 | 108 | |
| 0.25 | 10 | 25.2 ± 7.2 | 115 | |
| 2.5 | 10 | 29.2 ± 7.7* | 133 | |
| II | 0 | 8 | 21.5 ± 5.4 | |
| 0.25 | 8 | 28.6 ± 13.1* | 134 | |
| 2.5 | 8 | 31.2 ± 16.7* | 145 |
Nude mice were inoculated with 3 × 106 U937 cells and treated with dCF 3 times per week. T/C indicates survival of treated mice as a percentage of that in the control.
P < .05, using Student t test.
Effects of dCF and AraA on the survival of nude mice inoculated with monocytic leukemia U937 cells.
Mice were inoculated intraperitoneally with 3 × 106 U937 cells. Three days after the inoculation, the mice were treated with saline alone (solid line), 10 mg/kg of AraA (dotted line), 0.25 mg/kg of dCF (broken line), or AraA plus dCF (bold line) 3 times per week. Twenty-eight mice were used in each of the “saline alone” and “AraA plus dCF” groups, and 15 mice were used in each of the “AraA” and “dCF” groups. The survival time of mice treated with dCF plus AraA was prolonged more than that of mice treated with dCF alone (P = .0001, log-rank and Wilcoxon tests).
Effects of dCF and AraA on the survival of nude mice inoculated with monocytic leukemia U937 cells.
Mice were inoculated intraperitoneally with 3 × 106 U937 cells. Three days after the inoculation, the mice were treated with saline alone (solid line), 10 mg/kg of AraA (dotted line), 0.25 mg/kg of dCF (broken line), or AraA plus dCF (bold line) 3 times per week. Twenty-eight mice were used in each of the “saline alone” and “AraA plus dCF” groups, and 15 mice were used in each of the “AraA” and “dCF” groups. The survival time of mice treated with dCF plus AraA was prolonged more than that of mice treated with dCF alone (P = .0001, log-rank and Wilcoxon tests).
A potential problem with combined therapy is increased toxicity, but the combined treatment in this experiment resulted in no gross difficulties, and no significant weight loss was observed in mice receiving AraA and dCF. The therapeutic effect was worse with 2.5 mg/kg of dCF and AraA (data not shown). The in vivo findings regarding the prolongation of survival times are compatible with those regarding the synergistic effect of dCF and AraA on cell growth.
Discussion
A key enzyme in the purine salvage pathway, ADA regulates intracellular adenosine and dAd levels through their deamination. ADA is widely distributed in mammalian tissues, with the highest activity found in lymphoid tissues.15 ADA activity is low in normal bone marrow cells, whereas myeloid leukemic blasts express a high level.16 dCF, a nucleoside analog produced byAspergillus nidulans, is a specific and potent inhibitor of ADA.17 Inhibition of ADA by dCF results in intracellular accumulation of dATP. Previously, we investigated the mechanism by which dCF plus dAd produces marked suppression of the growth of monocytic leukemia cell lines.8 First, we measured ADA activity in the cells. The ADA activity of U937 cells was not significantly different from that of non-monocytoid K562 cells, nor was there any remarkable difference in the suppression of ADA activity after treatment with dCF plus dAd. We also measured intracellular levels of dAd and dATP in the cells. The uptake of dAd and accumulation of dATP in dCF-treated K562 cells were comparable to those in U937 cells. These results are consistent with the hypothesis that the modulation of dAd metabolism is not involved in the preferential suppression of cell growth in monocytoid cells, although dATP formation is required for the action of dAd and dCF on monocytoid cells. On the other hand, dATP effectively induced caspase-3 activation in cytosol from monocytoid leukemia cells, but not in that from non-monocytoid cells, suggesting that dATP-dependent caspase-3 activation is involved in the preferential induction of apoptosis in monocytoid leukemia cells.8 Caspase inhibitors effectively suppressed the apoptosis caused by dCF plus dAd, and the activity of caspase-3 was increased in U937 cells 48 hours after treatment with dCF plus dAd or AraA. These results suggest that caspase activation is at least partly involved in the apoptosis of monocytoid cells induced by dCF plus dAd or AraA. With respect to the induction of apoptosis, the sensitivity of normal peripheral monocytes to dCF in the presence of dAd was similar to that of lymphocytes. Moreover, the growth of normal bone marrow progenitor cells was less sensitive to combined treatment with dAd and dCF than the growth of monocytic leukemia cells. These results suggest that the combined treatment is more effective against monocytic leukemia cells than against normal monocytes.
Administration of dCF alone significantly prolonged the survival of mice inoculated with U937 cells, but this effect was slight. The in vitro observation that both dCF and dAd are required for the remarkable antiproliferative effect suggests that combined treatment with dCF and dAd may be beneficial. However, it may be difficult to use dAd in such therapy, due to the high concentrations required and its rapid metabolism. In the present investigation, therefore, we examined whether clinically available adenosine analogs cooperated with dCF in inhibiting the growth of U937 cells. Flu and CdA are resistant to deamination by ADA, but such chemical modification also changes the activating enzyme from dAd kinase to deoxycytidine kinase.18 dCF did not cooperate with Flu or CdA in growth inhibition, and preferential inhibition of the growth of U937 cells was not observed in treatment with Flu or CdA (Figure 2). On the other hand, AraA preferentially inhibited the growth of U937 cells, and the antiproliferative effect of AraA was greatly enhanced by dCF. The growth inhibition caused by AraA was counteracted by 5′-amino-dAd, an inhibitor of dAd kinase,19 suggesting that AraA is phosphorylated by dAd kinase (data not shown). The antiproliferative effects of Flu and CdA were not counteracted by the inhibitor, suggesting that the mode of AraA action is different from that of Flu or CdA.
AraA is a functional analog of dAd with established antiviral activity.20 AraA is phosphorylated intracellularly to form AraATP, which inhibits DNA polymerase. AraA is a potent antiviral agent, but its antitumor activity is limited because of its low solubility and rapid deamination by ADA to 9-β-d-arabinofuranosyl hypoxanthine.21,22The limited pharmacologic inhibition of ADA by ADA inhibitors provides a potential mechanism for enhancing the antitumor activity of AraA. AraA and dCF were recently used to treat recurrent childhood acute lymphocytic leukemia, and this combined therapy was designed to potentiate the inhibitory effect of AraA against DNA polymerase and DNA synthesis by preventing its rapid destruction through the inhibition of ADA by dCF, because malignant lymphoid cells have high ADA activity.23 This therapy was effective, but produced severe adverse effects such as liver and renal dysfunction and neurologic toxicity. Consequently, the doses used in that study are clinically unsuitable.23 The intravenous administration of 250 mg/m2 dCF and 10 mg/m2 AraA for 3 days produced serum concentrations of 1.5 to 4.7 μmol/L dCF and 12 to 24 μmol/L AraA.24 25 In the present study, dCF effectively induced apoptosis of leukemia cells freshly prepared from patients with AML M4 and M5 at concentrations as low as 0.14 to 0.86 μmol/L in the presence of 1 μmol/L AraA (Figure 4B). With respect to growth inhibition, dCF was similarly effective against parent U937 and variant cells that were resistant to conventional chemotherapeutic agents. Moreover, the administration of dCF plus AraA markedly prolonged the survival of nude mice inoculated with U937 cells without any severe adverse effects. These results strongly suggest that this combined treatment may be a useful therapy against AML, although the nude mice model used has limitations and does not completely reflect the situation in patients with acute leukemia. dCF has been effectively used to treat lymphoid malignancies but is associated with profound immunosuppression. Further study will provide useful information for the development of a new therapeutic strategy against AML.
Supported in part by Grants for Cancer Research from the Ministry of Education, Science, Sports and Culture and the Ministry of Health and Welfare, Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yoshio Honma, Saitama Cancer Center Research Institute, 818 Komuro, Ina, Saitama 362-0806, Japan; e-mail: honma@cancer-c.pref.saitama.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal