Abstract
The administration of therapeutic doses of recombinant cytokines to patients with malignant disease can be complicated by systemic toxicities, which in their most severe form may present as a systemic inflammatory response. The combination of interleukin (IL)–18 and IL-12 has synergistic antitumor activity in vivo yet has been associated with significant toxicity. The effects of IL-18 plus IL-12 were examined in a murine model, and it was found that the daily, simultaneous administration of IL-18 and IL-12 resulted in systemic inflammation and 100% mortality within 4 to 8 days depending on the strain employed. Mice treated with IL-18 plus IL-12 exhibited unique pathologic findings as well as elevated serum levels of proinflammatory cytokines and acute-phase reactants. The actions of tumor necrosis factor–α did not contribute to the observed toxicity, nor did T or B cells. However, toxicity and death from treatment with IL-18 plus IL-12 could be completely abrogated by elimination of natural killer (NK) cells or macrophages. Subsequent studies in genetically altered mice revealed that NK-cell interferon–γ mediated the fatal toxicity via the signal transducer and activator of transcription pathway of signal transduction. These data may provide insights into methods of ameliorating cytokine-induced shock in humans.
Introduction
Natural killer (NK) cells are large granular lymphocytes that compose approximately 10% of peripheral blood mononuclear cells and, along with macrophages, granulocytes, eosinophils, and basophils, form the cellular arm of the innate immune system. NK cells can mediate the lysis of malignant and virally infected cells without prior sensitization and are also an important source of immunomodulatory cytokines such as interferon (IFN)–γ, tumor necrosis factor (TNF)–α, and granulocyte-macrophage colony–stimulating factor (GM-CSF).1-4 Macrophages infected with intracellular pathogens or stimulated by bacterial products are able to induce NK-cell production of IFN-γ via the secretion of stimulatory monokines such as interleukin (IL)–12, IL-15, and IL-18, factors for which NK cells constitutively express functional receptor complexes.5-7 IFN-γ is the prototypic macrophage-activating factor, and the early production of this cytokine by NK cells leads to marked enhancement of macrophage phagocytosis, cytocidal activity, and cytokine production, which in turn promote the effective clearance of infectious organisms.8,9 Thus, cooperative interactions between NK cells and macrophages are an important component of the innate immune response to infection in the time period that precedes the development of specific immunity.10
IL-12 is produced primarily by macrophages and appears to play a critical role in determining the outcome of infection through its ability to coordinate and activate the various compartments of cellular immunity.11,12 IL-12 induces the secretion of IFN-γ by both T cells and NK cells and promotes the maturation and activation of type-1 helper T (TH1) cells, thus favoring the development of cellular immunity and immunologic memory over humoral immunity. These properties of IL-12 suggested that it might be a particularly effective antitumor agent, and experiments in a number of murine models have confirmed the activity of IL-12 in this regard.13,14The ability of IL-12 to stimulate the production of IFN-γ has been confirmed in these models, and neutralization of this cytokine has been shown to seriously inhibit the antitumor activity of this cytokine treatment.15-17 In general, IL-12 has shown the greatest activity in murine models of melanoma and renal cell carcinoma. Although endogenous production of IFN-γ is clearly necessary for the antitumor effects of IL-12, there is evidence that other factors may contribute to the mechanism of action, since exogenous administration of IFN-γ is clearly less effective in the treatment of these malignancies.16 18
IFN-γ production can also be induced by IL-18, a cytokine recently cloned from the spleens of mice inoculated with Propionibacterium acnes and later challenged with lipopolysaccharide.19IL-18 is secreted in a precursor form, and processing by the enzyme caspase 1 is required for the active form of this cytokine to be released.20 IL-18 is produced by activated macrophages, dendritic cells, and Kupffer cells as well as by cells outside the immune system.21 The combination of IL-18 and IL-12 induces synergistic IFN-γ production in TH1 cells, presumably owing to the reciprocal up-regulation of IL-18 and IL-12 receptor components by each cytokine.21-23 IL-18 can activate NK cells independently of IL-12 effects; however, synergistic production of IFN-γ is obtained following costimulation with both factors.24-26 Combination therapy with IL-18 and IL-12 exhibited potent antitumor actions in a murine model of malignant melanoma, but at the cost of significant toxicities that included weight loss, diarrhea, and fatty degeneration of the liver.27 28 These toxicities, while unique to the combination of IL-18 and IL-12, underscore one of the major drawbacks to the use of cytokines in the treatment of cancer, namely the induction of systemic complications resembling the sequelae of septic shock.
In the present report, we have employed a murine toxicity model to characterize the lethal response to coadministration of IL-18 plus IL-12. While doses of the individual cytokines were well tolerated, the administration of IL-18 in combination with IL-12 induced a unique fatal systemic inflammatory reaction characterized by elevated serum levels of proinflammatory cytokines and acute-phase reactants as well as by multiorgan pathology. In the present report, we demonstrate that this toxicity is mediated by NK-cell production of IFN-γ and the signal transducer and activator of transcription (STAT) pathway of signal transduction.
Materials and methods
Reagents
Recombinant (r) murine (mu) IL-12 (specific activity = 3.3 × 106 U/mg) (Genetics Institute, Andover, MA) was administered intraperitoneally (IP) at a dose of 1 μg/d.29 Unless otherwise specified, rmuIL-18 (Vertex Pharmaceuticals, Cambridge, MA) was administered at 0.5 μg/d IP. IL-12, IL-18, or IL-18 plus IL-12 was administered daily until death of the animal. The rmuIL-10 was provided by Schering-Plough (Kenilworth, NJ) (specific activity = 11 × 106 U/mg). All cytokine reagents contained less than 0.015 experimental units (EU)/mL endotoxin as measured by the E-Toxate system (Sigma, St Louis, MO). Depletion of NK cells was accomplished via IP administration of an anti-asialo GM1 antibody (Wako BioProducts, Richmond, VA) every 3 days beginning 2 weeks prior to injection of cytokines (0.2 mg/injection).30 Mice were depleted of monocytes/macrophages via IP and intravenous injection of the F4/80 monoclonal antibody (mAb) (immunoglobulin G2b) 48 and 24 hours prior to cytokine therapy.31N-nitro-1-arginine methyl ester (L-NAME) was used at 0.2 mg per mouse daily IP to inhibit inducible nitric oxide synthase (iNOS) activity. N-nitro-d-arginine methyl ester (D-NAME) was used as a control reagent.32
Mice
C.B-17 scid/scid (SCID) mice (BALB/c background), inbred BALB/c control mice, and inbred C57BL/6 control mice were purchased from Taconic (Taconic Farms, Germantown, NY).33 CD3ε transgenic mice (B6 x CBA background), mice deficient in the IFN-γ gene (IFN-γ−/−, C57BL/6 background), and TNFR p55−/− mice (C57BL/6 background) were purchased from Jackson Laboratory (Bar Harbor, ME).34-36STAT1−/− mice (C57BL/6 background) were the gift of Dr Joan Durbin.37 STAT4−/− mice (B6 x 129 background) were the gift of Dr James Ihle.38 Mice deficient in the enzyme inducible nitric oxide synthase 2 (C57BL/6-Nos2tml/Lau, iNOS−/−) were purchased from Jackson Laboratory.39 All experimental groups contained at least 6 animals.
Analysis of cytokine-treated mice
Serum levels of IFN-γ, IL-1β, and IL-10 were measured by means of enzyme-linked immunosorbent assays (ELISAs) obtained from Endogen (Woburn, MA). TNF-α levels were measured by means of an ELISA obtained from Biosource International (Camarillo, CA). Macrophage inflammatory protein (MIP)–1α, IL-6, and GM-CSF levels were measured by means of ELISAs from R&D Systems (Minneapolis, MN). Serially diluted serum was analyzed for haptoglobin and α1-acid glycoprotein by immunoelectrophoresis, and the area under the precipitation peak was quantified in arbitrary units with the use of the National Institutes of Health Image program 1.61.40The data for each peak were converted into milligrams per milliliter by comparison with values obtained with calibrated mouse acute-phase plasma.
Detection of IFN-γ production by intracellular flow cytometry and propidium iodide staining
Spleens were harvested from C57BL/6 mice 24 hours after IP injection of IL-18 plus IL-12, minced with sterile scalpel blades to give a single-cell suspension, and cultured ex vivo for 4 hours in brefelden A to allow target protein to be sequestered in the Golgi apparatus.41 Cells were then surface-stained with DX5-PE (pan NK) mAb, washed in fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline [PBS] with 1% fetal calf serum), and fixed in 2% paraformaldehyde for 30 minutes at room temperature. After fixation, cells were permeabilized for 30 minutes in 25 μL PBS plus 0.5% saponin. Antimouse IFN-γ–fluorescein isothiocyanate (FITC) or isotype control-FITC mAbs were added at a final dilution of 1/100, and cells were incubated for 30 minutes at room temperature. Cells were washed and resuspended in 1 mL of FACS buffer for analysis on a Coulter XL (Coulter, Miami, FL) flow cytometer as described.26 NK cells were isolated from the spleens of cytokine-treated SCID mice and analyzed for endonucleolytic cleavage of cellular DNA via a flow-cytometric assay using propidium iodide, as described.42
Analysis of IFN-γ transcript by Real Time reverse transcriptase polymerase chain reaction
Real Time reverse transcriptase polymerase chain reaction (RT-PCR) was used to determine IFN-γ transcript levels in mouse spleens after treatment with cytokine.26,43 Spleens were harvested, snap-frozen in liquid nitrogen, and ground into a fine powder with a mortar and pestle on dry ice. Total cellular RNA was extracted by means of the RNAqueous total RNA isolation kit (Ambion, Austin, TX). Complementary DNA (cDNA) was generated by means of standard methods,26 with the use of 2 μg of cellular RNA and random hexamers (Perkin Elmer, Norwalk, CT) as primers for first-strand synthesis. The PCR mixture contained the following: cDNA template (2.5 μL); forward and reverse primers for muIFN-γ (900 nm each); 6-carboxy-fluorescein–labeled probe for muIFN-γ (125 nm); forward and reverse primers for 18S ribosomal RNA (rRNA, 50 nm each); 2,7-dimethoxy-4,5-dichloro-6-carboxyfluorescein–labeled probe (50 nm) for 18S rRNA (internal control); and 2X TaqMan Universal PCR Master Mix (PE Applied Biosystems, Foster City, CA). Reactions for analysis of muIFN-γ transcript and 18S rRNA (control) were performed in the same well of capped 96-well optical plates. The following amplification scheme was employed: 1 cycle at 50°C for 2 minutes (AmpErase uracil-N-glycosylase deactivation), 1 cycle at 95°C for 10 minutes (AmpliTaq Gold activation), followed by 40 cycles at 95°C for 15 seconds (denaturation), and 60°C for 1 minute (anneal/extension). Real-time PCR data were analyzed with the use of Sequence Detector software version 1.6 (PE Applied Biosystems). Final quantitation was derived by means of the comparative CT method26 and is reported as the n-fold difference in experimental cDNA (IL-12–, IL-18–, or IL-12– plus IL-18–treated mice) as compared with a calibrator cDNA (PBS-treated mice).
Statistical analysis
Statistical significance was analyzed by means of Student paired t test with P < .05 considered significant.
Results
Concomitant administration of IL-18 with IL-12 is lethal in mice
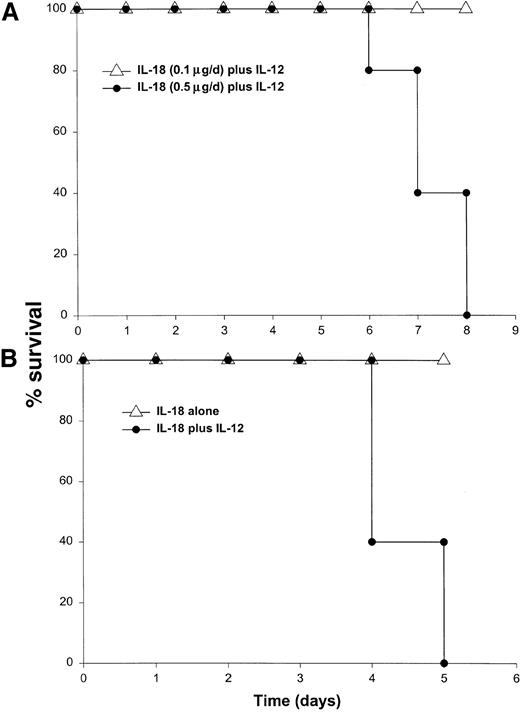
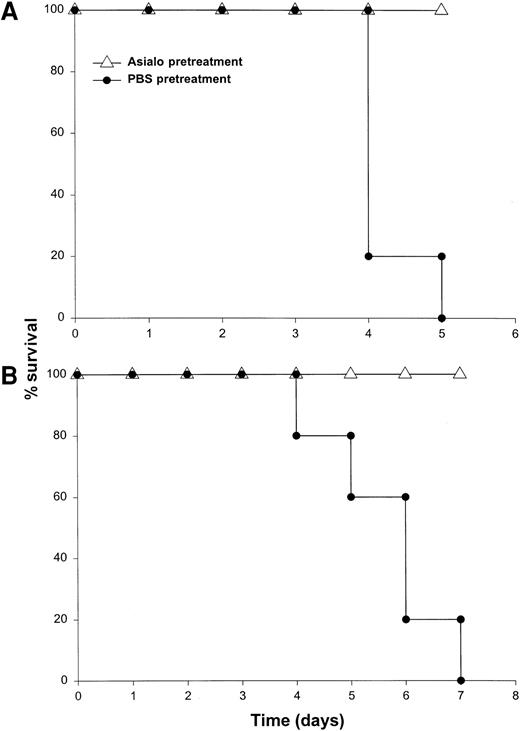
It has been previously demonstrated that IL-12 is well tolerated by inbred murine strains when administered at a dose of 1 μg/d.29 IL-18 exhibits antitumor actions in murine models of malignancy when administered daily at a dose of 0.5 through 1.0 μg/d.28 In order to establish the toxicity of this cytokine combination, rmuIL-18 (0.1 μg/d or 0.5 μg/d) was administered daily in combination with rmuIL-12 (1 μg/d) to C57BL/6 mice via the IP route (Figure 1A). Mice receiving IL-12 plus the lower dose of IL-18 exhibited only mild signs of systemic toxicity, and no deaths were observed even when injections were continued for a total of 14 days. In contrast, mice receiving IL-12 with the higher dose of IL-18 all died within 6 to 8 days. Similar results were obtained with IL-18 plus IL-12 in several different species of mice, including BALB/c mice, B6 x CBA mice, and C.B-17 mice bearing the scid/scid (SCID) mutation (Figure1B). Indeed, SCID mice, which lack T and B cells,33exhibited 100% mortality within 3 to 5 days of initiation of treatment. Of note, mice receiving daily injections of IL-18 or IL-12 alone exhibited minimal toxicity (Figure 1B and data not shown).
Administration of IL-18 in combination with IL-12 is lethal in C57BL/6 and SCID mice.
(A) Four- to 6-week-old female C57BL/6 mice were injected daily IP with rmuIL-18 (0.1 or 0.5 μg/d) plus rmuIL-12 (1 μg/d) and monitored for toxicity. No deaths were observed in mice receiving IL-18 or IL-12 alone (not shown). (B) Four- to 6-week-old female C.B-17 SCID mice were injected daily IP with IL-18 (0.5 μg/d) plus IL-12 (1 μg/d), or IL-18 alone and monitored for toxicity. These results are representative of 4 separate experiments.
Administration of IL-18 in combination with IL-12 is lethal in C57BL/6 and SCID mice.
(A) Four- to 6-week-old female C57BL/6 mice were injected daily IP with rmuIL-18 (0.1 or 0.5 μg/d) plus rmuIL-12 (1 μg/d) and monitored for toxicity. No deaths were observed in mice receiving IL-18 or IL-12 alone (not shown). (B) Four- to 6-week-old female C.B-17 SCID mice were injected daily IP with IL-18 (0.5 μg/d) plus IL-12 (1 μg/d), or IL-18 alone and monitored for toxicity. These results are representative of 4 separate experiments.
Histopathology
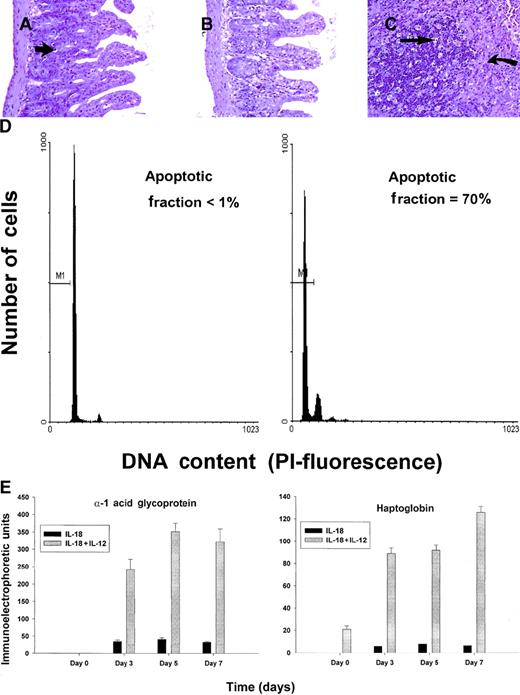
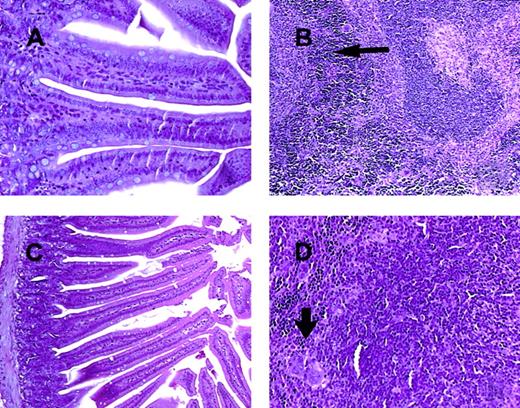
C57BL/6 mice receiving daily injections of IL-18 plus IL-12 were subjected to histopathologic evaluation. Acute changes within the gastrointestinal tract were prominent and consisted of a diffuse degenerative enteropathy within both the small and the large intestines (Figure 2A-B). Atrophy of the lymphoid tissues was also noted. This may have resulted from apoptotic events within these organs, as apoptotic bodies were frequently observed (Figure 2C). Analysis of nonadherent splenic NK cells from cytokine-treated SCID mice by propidium iodide staining confirmed this observation (Figure 2D). Examination of the liver revealed diffuse moderate histiocytosis (macrophage proliferation) as well as diffuse hepatocellular vacuolization. Pulmonary pathology consisted of moderate diffuse perivascular and septal inflammation associated with mononuclear cell infiltrates and alveolar edema (data not shown).
Histopathology and serum acute-phase proteins.
(A) (B) Photomicrographs of small intestine from C57BL/6 control mice (panel A) and SCID mice (panel B) treated with IL-18 plus IL-12 and killed at 72 hours. The small intestinal villi are severely blunted with crypt hyperplasia with abundant apoptotic cells in the crypt epithelium (short arrow). H & E stain at 200 ×. (C) The spleen of a C57BL/6 mouse treated with IL-12 plus IL-18 mice shows atrophy of the lymphoid tissue with abundant apoptotic lymphocytes (long arrow) and reticuloendothelial hyperplasia within the marginal zone (curved arrow). H & E stain, 200 × magnification. (D) Nonadherent splenocytes (85% to 90% NK cells by FACS) from PBS-treated (left panel) or IL-18 plus IL-12–treated SCID mice (right panel) were harvested at 48 hours and analyzed for the presence of apoptotic cells via a flow-cytometric assay using propidium iodide staining.42Apoptotic nuclei appear within the hypodiploid region of the DNA histogram (M1) owing to the endonucleolytic cleavage of DNA. (E) SCID mice received daily injections of IL-18 plus IL-12 or IL-18 alone for 72 hours. Serum was harvested at the indicated time points and analyzed for the presence of α1-acid glycoprotein (right panel) and haptoglobin (left panel) via immunoelectrophoresis. These results represent the mean of duplicate samples ± SEM.
Histopathology and serum acute-phase proteins.
(A) (B) Photomicrographs of small intestine from C57BL/6 control mice (panel A) and SCID mice (panel B) treated with IL-18 plus IL-12 and killed at 72 hours. The small intestinal villi are severely blunted with crypt hyperplasia with abundant apoptotic cells in the crypt epithelium (short arrow). H & E stain at 200 ×. (C) The spleen of a C57BL/6 mouse treated with IL-12 plus IL-18 mice shows atrophy of the lymphoid tissue with abundant apoptotic lymphocytes (long arrow) and reticuloendothelial hyperplasia within the marginal zone (curved arrow). H & E stain, 200 × magnification. (D) Nonadherent splenocytes (85% to 90% NK cells by FACS) from PBS-treated (left panel) or IL-18 plus IL-12–treated SCID mice (right panel) were harvested at 48 hours and analyzed for the presence of apoptotic cells via a flow-cytometric assay using propidium iodide staining.42Apoptotic nuclei appear within the hypodiploid region of the DNA histogram (M1) owing to the endonucleolytic cleavage of DNA. (E) SCID mice received daily injections of IL-18 plus IL-12 or IL-18 alone for 72 hours. Serum was harvested at the indicated time points and analyzed for the presence of α1-acid glycoprotein (right panel) and haptoglobin (left panel) via immunoelectrophoresis. These results represent the mean of duplicate samples ± SEM.
Taken together, these findings suggest that administration of IL-18 with IL-12 induced an acute systemic inflammatory response with marked injury to the liver, lung, and intestines. This interpretation is supported by our analysis of serum acute-phase reactants, which revealed the presence of high levels of haptoglobin and α1-acid glycoprotein in mice receiving the combination of IL-18 plus IL-12, but not in those receiving IL-18 alone (Figure 2E). We have previously demonstrated that IL-12 alone is a relatively weak stimulus for the induction of these acute-phase proteins.44 Taken together, these data suggest that IL-18 and IL-12 exert synergistic proinflammatory effects in the present model.
Serum cytokine levels
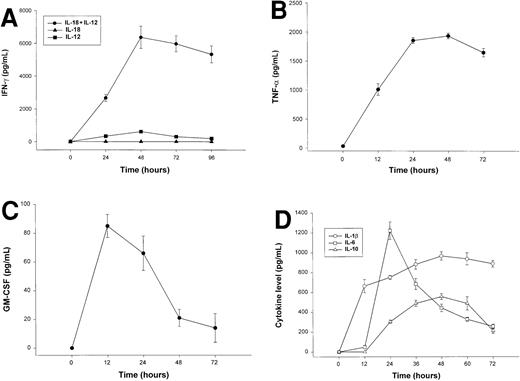
The combination of IL-18 plus IL-12 is a potent stimulus for NK-cell secretion of IFN-γ, TNF-α, GM-CSF, and MIP-1α/β in vitro,26,45 and we considered whether overproduction of these proinflammatory factors in response to coadministration of IL-18 plus IL-12 might be the origin of the observed toxicities. We therefore measured serum levels IFN-γ, TNF-α, GM-CSF, and MIP-1α in SCID mice receiving daily injections of IL-18 plus IL-12. IFN-γ and TNF-α levels rose rapidly in SCID mice treated with IL-18 plus IL-12, peaked at approximately 48 hours, and remained elevated until the death of the animal (Figure 3A-B). Serum levels of GM-CSF were only modestly elevated following treatment with IL-18 plus IL-12 (Figure 3C), and levels of MIP-1α were not significantly elevated at any time point (data not shown).45Administration of IL-18 alone did not stimulate significant endogenous production of IFN-γ, whereas modest serum levels of IFN-γ (below 500 pg/mL) were observed in mice receiving injections of IL-12 alone (Figure 3A).28 46 Likewise, mice receiving injections of IL-18 alone did not exhibit significant serum levels of TNF-α, GM-CSF, or MIP-1α (data not shown).
Serum cytokine levels in SCID mice receiving daily injections of IL-18 plus IL-12.
(A) Four- to 6-week-old female SCID mice received daily injections of IL-12 (1 μg), IL-18 (0.5 μg/d), or IL-18 plus IL-12. Serum was obtained from cytokine-treated mice at the indicated times and analyzed for the presence of muIFN-γ. (B) Serum levels of muTNF-α were measured by ELISA in SCID mice receiving daily injections of IL-18 plus IL-12. (C) Serum levels of muGM-CSF were measured by ELISA in SCID mice receiving daily injections of IL-18 plus IL-12. (D) SCID mice received daily IP injections of IL-18 plus IL-12. Serum was obtained from cytokine-treated mice at the indicated times and analyzed for the presence of IL-1β, IL-6, and IL-10 by ELISA. Mice receiving IL-18 plus IL-12 survived from 3 to 5 days. These results are representative of 3 separate experiments and represent the mean ± SEM of duplicate wells.
Serum cytokine levels in SCID mice receiving daily injections of IL-18 plus IL-12.
(A) Four- to 6-week-old female SCID mice received daily injections of IL-12 (1 μg), IL-18 (0.5 μg/d), or IL-18 plus IL-12. Serum was obtained from cytokine-treated mice at the indicated times and analyzed for the presence of muIFN-γ. (B) Serum levels of muTNF-α were measured by ELISA in SCID mice receiving daily injections of IL-18 plus IL-12. (C) Serum levels of muGM-CSF were measured by ELISA in SCID mice receiving daily injections of IL-18 plus IL-12. (D) SCID mice received daily IP injections of IL-18 plus IL-12. Serum was obtained from cytokine-treated mice at the indicated times and analyzed for the presence of IL-1β, IL-6, and IL-10 by ELISA. Mice receiving IL-18 plus IL-12 survived from 3 to 5 days. These results are representative of 3 separate experiments and represent the mean ± SEM of duplicate wells.
Elevated serum levels of IL-1β, IL-6, and IL-10 have been observed in animals and humans experiencing the toxic effects of high-dose cytokine therapy, and we therefore measured the levels of these factors in the serum of cytokine-treated mice (Figure 3C).47 IL-1β, IL-6, and IL-10 were markedly elevated in mice receiving IL-18 plus IL-12; peak serum levels, however, occurred at different points during the administration of cytokine treatments. In keeping with its role as an early proinflammatory factor, IL-1β levels peaked at 12 hours and remained elevated until the death of the animal.48 IL-6 is an important mediator of the acute-phase response, and serum levels of this cytokine appear to reflect the combined influence of systemic TNF-α and IL-1β.49 As expected, serum levels of this cytokine did not reach their peak until 24 hours, whereupon they steadily declined. In contrast, IL-10 has distinct anti-inflammatory properties and is normally released in quantity during the resolution phase of inflammatory processes.50 In keeping with this, we observed that IL-10 levels did not become significantly elevated until 36 to 48 hours following the initiation of cytokine treatments.
Role of IFN-γ and TNF-α in death induced by IL-18 plus IL-12
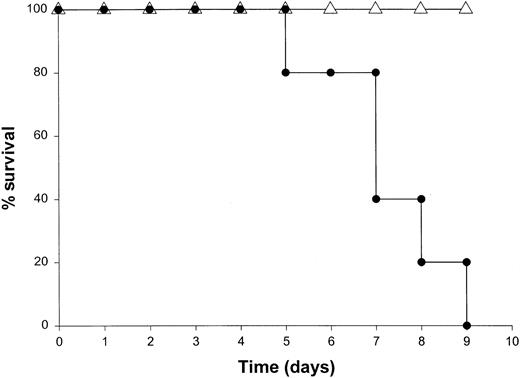
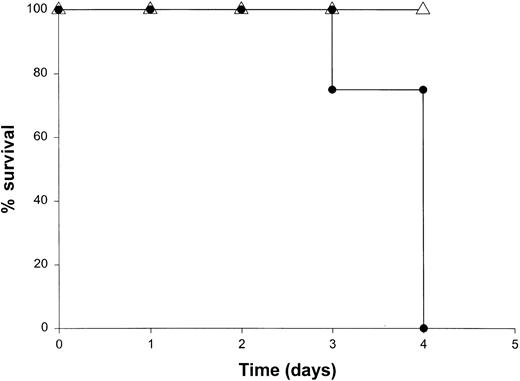
Administration of proinflammatory cytokines such as TNF-α or IFN-γ can induce a shocklike state in animals and humans, whereas neutralization of these factors can markedly attenuate septic shock in animal models.51 Given the presence of these factors in the serum of mice receiving IL-18 plus IL-12, we proceeded to investigate the toxicity of this cytokine treatment in mice that had been rendered genetically deficient in the p55 TNF receptor (R) or the IFN-γ ligand. TNFR p55−/− mice were not protected from the toxicity of IL-18 plus IL-12. In fact, mice lacking this receptor component died slightly earlier than similarly treated mice of the identical background (data not shown). In contrast, IFN-γ−/− mice were completely resistant to the toxic effects of IL-18 plus IL-12, whereas normal mice of the identical background all succumbed to this treatment within 5 to 9 days (Figure4). Gross examination of the IFN-γ−/− mice revealed minimal pathology outside of some minor changes within the spleen, which is in keeping with the complete lack of measurable IFN-γ in the serum of these mice (not shown). Importantly, serum levels of TNF-α and IL-1β were still significantly elevated at the 72-hour time point in IFN-γ−/− mice receiving IL-18 plus IL-12. These experiments indicate that the endogenous production of IFN-γ in response to IL-18 plus IL-12 is a critical component of the observed toxicity and also suggest that IL-18 plus IL-12 can induce the production of proinflammatory cytokines in an IFN-γ–independent fashion.
The role of IFN-γ in the lethal toxicity of IL-18 plus IL-12.
IFN-γ−/− (▵) mice and C57BL/6 control mice (●) received daily IP injections of IL-18 plus IL-12 and were monitored for toxicity. These experiments are representative of 2 separate determinations.
The role of IFN-γ in the lethal toxicity of IL-18 plus IL-12.
IFN-γ−/− (▵) mice and C57BL/6 control mice (●) received daily IP injections of IL-18 plus IL-12 and were monitored for toxicity. These experiments are representative of 2 separate determinations.
Intracellular staining of murine splenocytes and measurement of IFN-γ transcript via Real-Time PCR
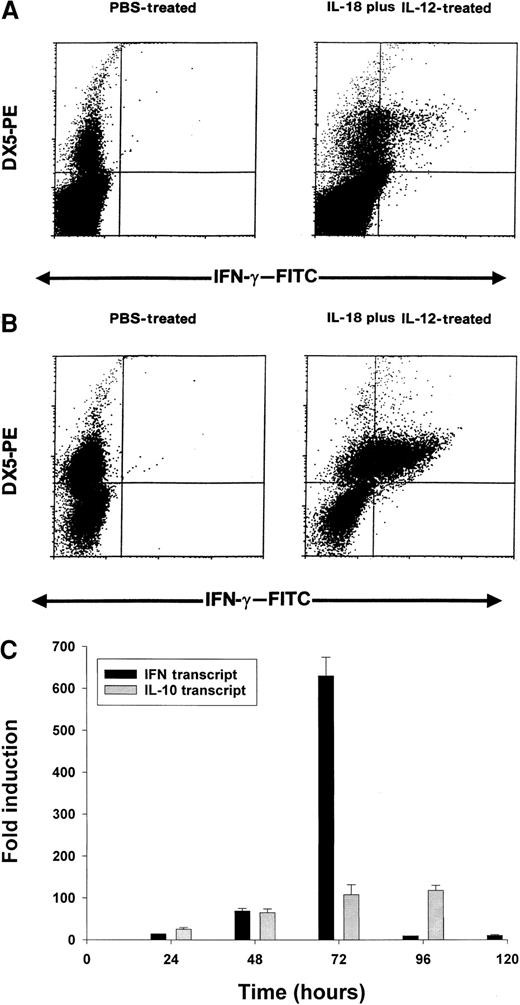
On the basis of the above results, we conducted a series of experiments to determine which cell population might be the primary source of IFN-γ in this toxicity model. Using an anti–muIFN-γ mAb conjugated to a fluorescent moiety, we performed intracellular staining of splenocytes obtained from cytokine-treated C57BL/6 mice at 24 hours. These results are presented in Figure 5A and reveal that NK cells are the major source of IFN-γ in mice receiving IL-18 plus IL-12. More dramatic results were obtained in SCID mice in which the percentage of NK cells are increased relative to normal mice (Figure 5B). In order to analyze the synergistic effects of IL-18 plus IL-12 at the transcript level, we administered PBS, IL-18, IL-12, or IL-18 plus IL-12 to SCID mice and analyzed splenocytes for IFN-γ transcript via Real-Time PCR at 24 hours. IL-12 alone stimulated significant transcription of the IFN-γ gene in comparison with PBS (50-fold induction), while IL-18 alone was completely ineffective in this regard (no induction). However, the combination of IL-18 plus IL-12 resulted in a significant 140-fold increase in transcript at the 24-hour time point as compared with PBS-treated cells (P < .05). Taken together, these experiments suggest that coadministration of IL-18 and IL-12 results in a marked and rapid increase in the transcription of the IFN-γ gene within the NK-cell compartment.
Induction of IFN-γ protein and transcript by IL-18 plus IL-12.
Four- to 6-week-old C57BL/6 mice (A) or C.B-17 SCID mice (B) received IP injections of IL-18 plus IL-12, and their spleens were harvested at 24 hours. Splenocytes were cultured for 4 hours in brefeldin A, permeabilized, stained with DX5-PE mAb (pan-NK) and anti–muIFN-γ–FITC mAb, and analyzed by flow cytometry with the use of a mononuclear cell gate. (C) Real Time RT-PCR analysis of SCID splenocytes for IFN-γ and IL-10 transcripts at various times after IP injection of IL-18 plus IL-12.
Induction of IFN-γ protein and transcript by IL-18 plus IL-12.
Four- to 6-week-old C57BL/6 mice (A) or C.B-17 SCID mice (B) received IP injections of IL-18 plus IL-12, and their spleens were harvested at 24 hours. Splenocytes were cultured for 4 hours in brefeldin A, permeabilized, stained with DX5-PE mAb (pan-NK) and anti–muIFN-γ–FITC mAb, and analyzed by flow cytometry with the use of a mononuclear cell gate. (C) Real Time RT-PCR analysis of SCID splenocytes for IFN-γ and IL-10 transcripts at various times after IP injection of IL-18 plus IL-12.
We next analyzed splenocytes for IFN-γ transcript over time using Real-Time PCR. We also examined levels of IL-10 transcript as we have previously determined that IL-10 is able to enhance NK-cell production of IFN-γ in vitro.52 This experiment is presented in Figure 5C and reveals that IFN-γ transcript levels are markedly induced between 24 and 72 hours, after which they drop rapidly. IL-10 transcript levels are also induced within this time frame, but these remain elevated until 96 hours. Thus, the drop in transcriptional activity of the IFN-γ gene is not easily ascribed to reduced production of IL-10.
Role of NK cells in the toxicity of IL-18 plus IL-12
Our results suggested that NK cells were the major source of IFN-γ in this model. To determine whether the toxicity of this model was critically dependent on the actions of NK cells, we administered IL-18 plus IL-12 to SCID mice depleted of NK cells by pretreatment with an anti-asialo GM1 antibody. We have previously determined that this regimen removes the majority of NK cells (approximately 98%) in most murine strains while leaving the macrophage compartment largely unaffected.30 This treatment completely abrogated the toxicity of IL-18 plus IL-12 in both SCID mice and normal C57BL/6 mice, whereas control mice all died within 5 days of the initiation of treatment (Figure 6A and data not shown). Importantly, antibody-treated mice exhibited very low serum levels of IFN-γ, TNF-α, and IL-1β (less than 10 pg/mL) at all time points (data not shown). To confirm the role of NK cells in the lethal reaction to IL-18 plus IL-12, we next administered IL-18 and IL-12 to CD3ε transgenic mice that completely lack mature NK cells and T cells owing to a developmental block.34 Control mice of the appropriate background died between 4 and 7 days, whereas CD3ε transgenic mice all survived this treatment (Figure 6B). In keeping with our hypothesis, CD3ε transgenic mice demonstrated very little reaction to IL-18 plus IL-12 at the histologic level (see Figure9A-B) and exhibited a complete lack of serum IFN-γ. Taken together. these data suggest that NK-cell–secreted IFN-γ is the major mediator of toxicity in mice receiving IL-18 plus IL-12.
Death induced by administration of IL-18 plus IL-12 is critically dependent on the NK-cell compartment.
(A) SCID mice were depleted of NK cells by pretreatment with an anti-asialo GM1 antibody (see “Materials and methods”). Control mice were pretreated with injections of PBS. Mice in both groups subsequently received daily IP injections of IL-18 plus IL-12 and were monitored for survival. This experiment was repeated 3 times with similar results. (B) CD3ε transgenic mice (▵) received daily injections of IL-18 plus IL-12 via the IP route. Normal mice of the identical background served as controls (●). This experiment was repeated twice with similar results.
Death induced by administration of IL-18 plus IL-12 is critically dependent on the NK-cell compartment.
(A) SCID mice were depleted of NK cells by pretreatment with an anti-asialo GM1 antibody (see “Materials and methods”). Control mice were pretreated with injections of PBS. Mice in both groups subsequently received daily IP injections of IL-18 plus IL-12 and were monitored for survival. This experiment was repeated 3 times with similar results. (B) CD3ε transgenic mice (▵) received daily injections of IL-18 plus IL-12 via the IP route. Normal mice of the identical background served as controls (●). This experiment was repeated twice with similar results.
Depletion/deactivation of monocytes/macrophages
Given the ability of NK-cell–derived cytokines to potentiate macrophage effector functions3,10 and the presence of activated macrophages in the liver of mice receiving IL-18 plus IL-12, the role of macrophages in the toxicity of this model was investigated. SCID mice can be depleted of monocytes and macrophages by 50% in bone marrow, spleen, and blood, and 100% in the peritoneal cavity by injection of the F4/80 mAb via the intravenous and IP routes 48 and 24 hours prior to the administration of IL-18 plus IL-12.44Alternatively, macrophages can be deactivated via administration of IL-10.44,53 Our preliminary experiments revealed that these maneuvers led to partial protection in the present model (approximately 50% survival), which is consistent with our experience in other models of cytokine-induced inflammation (not shown).44 Therefore, in order to deactivate any macrophages remaining after the administration of F4/80 mAb, mice also received IP injections of muIL-10 beginning 48 hours prior to the initiation of cytokine treatment. This regimen afforded significant protection from the toxicity of IL-18 plus IL-12, and mice in this group exhibited 100% survival (Figure7). Of note, NK cells obtained from the spleens of mice receiving F4/80 mAb and IL-10 were fully responsive to stimulation with IL-18 plus IL-12 in vitro, as measured by IFN-γ production (data not shown). Taken together, these data suggest that monocyte/macrophage effector functions play a significant role in mediating the lethal toxicity of IL-18 plus IL-12.
The role of the macrophage compartment in the toxicity of IL-18 plus IL-12.
SCID mice were partially depleted of macrophages by pretreatment with the F4/80 mAb (▵). Control mice were pretreated with injections of a control mAb (●). Mice that had been treated with F4/80 mAb also received daily IP injections of rmuIL-10 (5 μg) beginning 2 days prior to cytokine treatment.44 Mice in both groups subsequently received daily IP injections of IL-18 plus IL-12 and were monitored for survival. This experiment was repeated twice with similar results.
The role of the macrophage compartment in the toxicity of IL-18 plus IL-12.
SCID mice were partially depleted of macrophages by pretreatment with the F4/80 mAb (▵). Control mice were pretreated with injections of a control mAb (●). Mice that had been treated with F4/80 mAb also received daily IP injections of rmuIL-10 (5 μg) beginning 2 days prior to cytokine treatment.44 Mice in both groups subsequently received daily IP injections of IL-18 plus IL-12 and were monitored for survival. This experiment was repeated twice with similar results.
The toxicity of IL-18 plus IL-12 is not abrogated in mice exhibiting deficiencies in iNOS activity
Nitric oxide has been identified in the serum of mice receiving IL-18 plus IL-12 and has been implicated in the gastrointestinal toxicity observed in some murine models of infection.21 In addition, the fungicidal activity of murine peritoneal exudate cells is synergistically induced by treatment with IL-18 plus IL-12.54 This activity correlated with NK-cell production of IFN-γ, which in turn induced the production of nitric oxide. For these reasons, we investigated the role of nitric oxide in the toxicity of IL-18 plus IL-12 administration. Mice rendered deficient in the enzyme iNOS were treated with IL-18 plus IL-12 as were control mice of the identical background. Contrary to what we might have predicted, both groups of mice were equally susceptible to the toxic effects of this cytokine treatment. Similar results were obtained in SCID mice in which the activity of iNOS was inhibited via the administration of L-NAME (data not shown). Taken together, these results strongly suggest that nitric oxide is not a critical mediator of the lethal reaction to IL-18 plus IL-12.
Role of STAT signaling in the toxicity of IL-18 plus IL-12
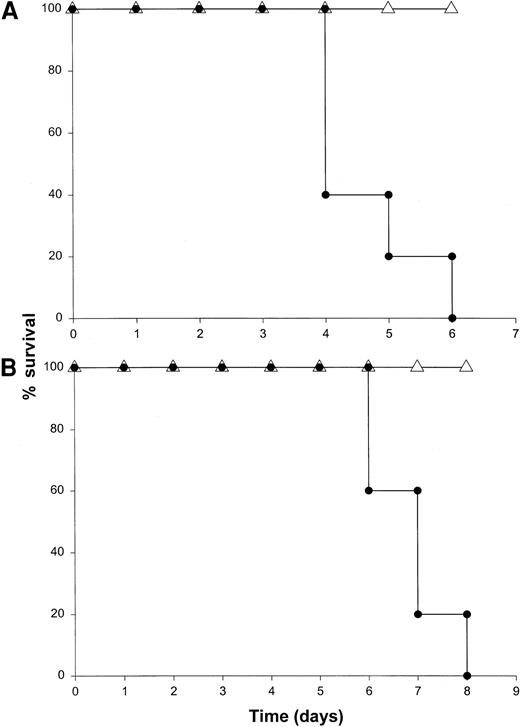
The binding of IL-12 to its specific receptor complex results in phosphorylation of STAT4 as well as STAT1, STAT3, and STAT5.55-57 Mice rendered genetically deficient in STAT4 do not produce IFN-γ following administration of IL-12 and exhibit distinct immunologic defects.38 In addition, costimulation of T cells with IL-18 and IL-12 results in nuclear translocation of phosphorylated STAT4 and AP-1, and these transcription factors must bind to specific regions of the IFN-γ promoter in order for transcription to be initiated.58 We were therefore interested in the effects of IL-18 plus IL-12 in mice exhibiting a genetic deficiency in the STAT4 transcription factor. STAT4−/− mice and mice of the appropriate background received IL-18 plus IL-12 daily via the IP route. Background mice all died within 6 days of the initiation of therapy, whereas the STAT4−/− mice all survived (Figure8A). In fact, these mice exhibited essentially no toxicity whatsoever even when injections were carried out for a period of 2 weeks. Histologic analysis of these mice confirmed the protective effects of the STAT4 mutation (not shown), and analysis of serum from these mice demonstrated only very low levels of IFN-γ (less than 50 pg/mL at all time points). These results suggest that the toxic effects of IL-18 plus IL-12 are dependent on the activation of STAT4 and confirm the importance of this transcription factor in the induction of IFN-γ gene expression.
The role of STAT proteins in mediating the toxicity of IL-18 plus IL-12.
(A) STAT4−/− (▵) mice and B6 x 129 background control mice (●) received daily injections of IL-18 and IL-12 via the IP route and were monitored for toxicity. (B) STAT1−/− (▵) mice and C57BL/6 control mice (●) received daily injections of IL-18 and IL-12 via the IP route and were monitored for toxicity. Both experiments were repeated once with similar results.
The role of STAT proteins in mediating the toxicity of IL-18 plus IL-12.
(A) STAT4−/− (▵) mice and B6 x 129 background control mice (●) received daily injections of IL-18 and IL-12 via the IP route and were monitored for toxicity. (B) STAT1−/− (▵) mice and C57BL/6 control mice (●) received daily injections of IL-18 and IL-12 via the IP route and were monitored for toxicity. Both experiments were repeated once with similar results.
Binding of IFN-γ to its receptor also results in activation of the Janus kinase (JAK)–STAT pathway of signal transduction in vivo. In fact, IFN-γ–mediated gene expression appears to require the translocation of STAT1 homodimers to the cell nucleus, where they bind to specific elements in the promoters of IFN-responsive genes.8 STAT1−/− mice exhibit marked deficiencies in antiviral immunity, and one might predict that high levels of IFN-γ might not induce significant toxicity in this murine strain.37 In order to examine the role of STAT1 in the toxicity of this cytokine treatment, we administered IL-18 plus IL-12 to STAT1−/− mice and normal mice of the identical background. STAT1−/− mice receiving this cytokine combination exhibited 100% survival whereas control mice all died within 6 to 8 days (Figure 8B). As was demonstrated with STAT4−/− mice, minimal systemic toxicity was observed following administration of IL-18 plus IL-12 to mice lacking the STAT1 protein. Serum IFN-γ levels in STAT1−/− mice were comparable to those observed in normal mice of the identical background treated with IL-18 plus IL-12, yet the pathology observed at the organ level was minimal (Figure 9C-D), which suggests that the toxic effects of IFN-γ are mediated largely via the JAK-STAT signaling pathway. Of note, serum levels of IL-1β and TNF-α were also maintained in the STAT1−/− mouse following administration of IL-18 plus IL-12, a finding that provides further support for the role of IFN-γ–induced gene regulation in the toxicity of this model.
Photomicrographs of intestine and spleen from CD3ε transgenic and STAT1-deficient mice treated for 72 hours with IL-18 plus IL-12.
In contrast to the lesions seen in C57BL/6 control mice and SCID mice (Figure 2A-C), the small intestinal villi of both CDεTG mice (A) and STAT1−/− mice (C) are normal. The spleen from both the CD3εTG mice (B) and STAT1−/− mice (D) show normal lymphoid tissue (white pulp and increased extramedullary hematopoiesis in the red pulp, arrows). H & E stain, 200 × (panels A and D) or 100 × (panels B and C) magnification.
Photomicrographs of intestine and spleen from CD3ε transgenic and STAT1-deficient mice treated for 72 hours with IL-18 plus IL-12.
In contrast to the lesions seen in C57BL/6 control mice and SCID mice (Figure 2A-C), the small intestinal villi of both CDεTG mice (A) and STAT1−/− mice (C) are normal. The spleen from both the CD3εTG mice (B) and STAT1−/− mice (D) show normal lymphoid tissue (white pulp and increased extramedullary hematopoiesis in the red pulp, arrows). H & E stain, 200 × (panels A and D) or 100 × (panels B and C) magnification.
Discussion
We have demonstrated that coadministration of IL-18 and IL-12 results in a systemic inflammatory response that is fatal within a matter of days. Analysis of mice treated with this cytokine combination revealed histopathologic evidence of systemic inflammation and a concomitant acute-phase response. The toxicity of this cytokine combination was abrogated in mice lacking NK cells, but not in T-cell– and B-cell–deficient SCID mice. Production of IFN-γ by activated NK cells appeared to be the mechanism of death as mice deficient in IFN-γ exhibited complete protection, while mice deficient in TNF-α signaling components remained susceptible to this treatment. Intracellular lymphocyte staining confirmed that NK cells were the potent producers of IFN-γ following coadministration of IL-18 and IL-12, not T or B cells. Interruption of IL-12 signaling in STAT4-deficient mice or abrogation of IFN-γ signaling in STAT1-deficient mice was also protective, thus confirming the importance of these signaling components and pathways in mediating the effects of this cytokine treatment. The complete protection afforded by the depletion of macrophages suggests that coadministration of IL-18 and IL-12 induces NK cells to secrete IFN-γ, which in turn activates macrophages via the STAT1 pathway to mediate the fatal inflammatory effect.
NK cells appear to be central to the toxicity of cytokine combinations utilizing IL-12. The combination of IL-2 plus IL-12 is similar to that of IL-18 plus IL-12 in that it exhibits synergistic antitumor activity in experimental systems, unfortunately at the cost of severe systemic toxicity.59 We recently examined the effects of IL-2 plus IL-12 in a murine model and found that this cytokine treatment induced a lethal inflammatory response that was similar in many respects to septic shock, as evidenced by the presence of increased pulmonary edema, multiple organ system toxicities, and elevated serum levels of proinflammatory cytokines and acute-phase reactants.44Cellular-depletion experiments revealed that this cytokine-induced toxicity reaction was dependent on the NK-cell compartment and its ability to mobilize the effector functions of monocytes/macrophages, as was the case in the present model. Interestingly, however, the lethal reaction to IL-2 plus IL-12 was not critically dependent on any of the known cytokine products or effector molecules of the NK-cell compartment (eg, IFN-γ, TNF-α, MIP-1α, STAT1, perforin, Fas) or on any of the factors currently thought to be involved the induction of septic shock (eg, IL-1β, IL-6, IL-1 converting enzyme (ICE), TNF-related apoptosis–inducing ligand (TRAIL), iNOS, or nuclear factor (NF)κB).44 In contrast to the results obtained in the current study, it is apparent that the toxicity of IL-2 plus IL-12 is not dependent on NK-cell production of IFN-γ or the actions of its downstream mediator, STAT1.44 We therefore predicted that STAT4−/− mice would be fully susceptible to the toxicity of IL-2 plus IL-12.38,60 Unexpectedly, we have recently determined that STAT4−/− mice are in fact completely protected from the toxicity of IL-2 plus IL-12 administration (W.E.C., unpublished data, January 2000). This finding implies that IL-2 plus IL-12 is able to induce systemic inflammation via a STAT4-dependent pathway that does not require the actions of IFN-γ. The existence of such a pathway is supported by the work of Simpson et al,61 who examined the role of IL-12 and STAT4 signal transduction in 2 separate models of experimental colitis. They determined that mice treated with anti–IL-12 antibodies or reconstituted with T cells from STAT4-deficient mice exhibited a significantly milder course of disease, whereas mice reconstituted with T cells from IFN-γ−/− mice proceeded to develop severe colitis with wasting. Thus, as with T cells, it is likely that the proinflammatory actions of STAT4 are not limited to the induction of IFN-γ gene expression in NK cells.
We have shown that the combination of IL-18 plus IL-12 induces a severe enteropathy characterized by villus atrophy, crypt hyperplasia, and abundant apoptotic cells in the crypt epithelium. Despite the severity of these gastrointestinal changes, we cannot say with certainty that they represent the primary cause of death. We observed only mild degenerative changes in the gastrointestinal tract of mice receiving IL-12 alone44 and believe that the addition of IL-18 to this regimen may somehow exacerbate this process. Work by other investigators suggests that overproduction of nitric oxide might play a role in mediating the pathologic tissue damage associated with dysregulated or inappropriate production of IFN-γ.28,62 Induction of iNOS has been reported following the administration of IL-12 to mice, and inhibition of this enzyme abrogates the gastrointestinal lesions associated withToxoplasma gondii infection in SCID mice.32 63However, this maneuver did not ameliorate the toxicity of IL-18 plus IL-12 despite the near complete lack of nitric oxide species in mice treated with an iNOS inhibitor. Similarly, no protection from gastrointestinal mucosal lesions was observed in iNOS-deficient mice treated with IL-18 plus IL-12. These findings suggest that nitric oxide is not the primary mediator of lethality in the present model, but they obviously do not exclude nitric oxide as a potential contributor to the observed gastrointestinal toxicity that results from treatment with IL-18 plus IL-12.
To the best of our knowledge, the toxicity of an anticancer cytokine treatment has never before been ascribed to a specific signaling pathway. This discovery suggests a distinct strategy for ameliorating the toxicity of this cytokine treatment as well as characterizing the factors that might mediate its antitumor effects. Further study of NK-cell activation following costimulation is also warranted given the role of this cell type in the toxicity of the present model. Current efforts in our laboratory are now directed at a closer analysis of the immunobiology associated with the administration of cytokine combinations that induce systemic inflammatory responses.
Supported by National Institutes of Health grants CA68326 and CA68458 and in part by National Institutes of Health grant P30 CA16058.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
William E. Carson III, Arthur G. James Comprehensive Cancer Center, The Ohio State University, N924 Doan Hall, 410 W 10th Ave, Columbus, OH 43210; e-mail:carson-1@medctr.osu.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal