Abstract
To analyze the transcriptional activity of the gene encoding the α subunit of the platelet integrin αIIbβ3during the hematopoietic differentiation, mice were produced in which the herpes virus thymidine kinase (tk) was introduced in this megakaryocytic specific locus using homologous recombination technology. This provided a convenient manner in which to induce the eradication of particular hematopoietic cells expressing the targeted gene. Results of progenitor cell cultures and long-term bone marrow (BM) assays showed that the growth of a subset of stem cells was reduced in the presence of the antiherpetic drug ganciclovir, demonstrating that the activation of the toxic gene occurs before the commitment to the megakaryocytic lineage. Furthermore theknock-in of the tk gene into the αIIb locus resulted in the knock-out of the αIIb gene in homozygous mice. Cultures of BM cells of these animals, combined with ultrastructural analysis, established that the αIIbglycoprotein is dispensable for lineage commitment and megakaryocytic maturation. Platelets collected from αIIb-deficient mice failed to bind fibrinogen, to aggregate, and to retract a fibrin clot. Moreover, platelet α-granules did not contain fibrinogen. Consistent with these characteristics, the mice displayed bleeding disorders similar to those in humans with Glanzmann thrombasthenia.
Introduction
Circulating blood cells arise from a limited number of pluripotent cells that have the capacity to self-renew and to differentiate into the various hematopoietic lineages. The proliferation and differentiation of pluripotent hematopoietic cells requires a highly complex series of cellular events that are not fully elucidated. Megakaryocytopoiesis starts with the commitment of pluripotent stem cells, continues with a series of mitotic divisions, endomitotic replication, and cytoplasmic maturation of cells, and culminates with the release of platelets into the circulation.1 Each of these phases is controlled by different hematopoietic growth factors and cytokines2 3; the molecular regulation of these events, however, is largely unknown.
Toxins encoded by genes expressed under the control of tissue-specific promoters represent a remarkable tool to analyze complex eukaryotic systems. In particular, the thymidine kinase (tk) gene of the herpes simplex virus, which encodes a protein that is not harmful for eukaryotic cells but could produce a toxic effect when ganciclovir is present, has been successfully used to eradicate selected cell populations.4-9 This has allowed the identification of cells in which specific genes are activated in a given developmental process.
Targeting the expression of an exogenous gene into the entire megakaryocytic pathway requires the use of cis-acting regulatory elements of a lineage-specific gene expressed at an early stage of the differentiation process. The gene encoding the α subunit of the platelet integrin αIIbβ3 fulfills this criterion.10 Although the β3 subunit is synthesized in tissues other than megakaryocytes and platelets and can be associated with the αv subunit, the distribution of the αIIb subunit appears to be restricted to the megakaryocytic lineage. After the stimulation of platelets with agonists such as adenosine diphosphate (ADP) or collagen, the αIIbβ3 complex becomes activated, binds to fibrinogen (resulting in platelet aggregation), and prevents blood loss at sites of vascular injury. Glanzmann thrombasthenia, an inherited bleeding disorder in humans, is caused by functional or quantitative abnormal exposure of the αIIbβ3 integrin at the surface of platelets caused, in molecular terms, by deletions or nucleotide transversions in either of the genes encoding the 2 subunits.11 Glanzmann thrombasthenia has been classified into type 1, type 2, and variants, depending on the amount of αIIbβ3, the amount of intracellular fibrinogen within platelets, and the residual capacity of platelets to retract clots.12 The ensuing disease is characterized by hemorrhagic episodes caused by the lack of platelet aggregation because of the inability of platelets to bind adhesive proteins when activated. Clinically, patients suffer from frequent mucocutaneous bleeding episodes. Their bleeding times are prolonged, but their blood coagulation times and their platelet counts are normal.
We have previously used fragments of different lengths of the human and the murine αIIb promoters to drive the expression of the tk gene in megakaryocytes.9 13 When these transgenic animals were exposed to ganciclovir, an acute cessation of megakaryocytopoiesis occurred that could be reversed by discontinuing the ganciclovir treatment. One of the interesting findings to come out of these studies was that the human and the murine promoters were found to be transcriptionally active in multipotent progenitor cells, suggesting that this megakaryocytic gene is expressed early in the differentiation pathway. The definition of the earliest hematopoietic cell capable of expressing the αIIb glycoprotein is of considerable importance because this could specify, at the genetic level, the mechanism(s) by which the megakaryocytic phenotype is established during the differentiation of hematopoietic cells.
Analysis with limited promoter fragments, however, may not account for all the cis-acting regulatory elements involved in the regulation of the expression of the αIIb gene. Therefore, in this study, we have used homologous recombination technology to insert the tk gene specifically into the αIIb locus to produce mice in which the expression of the knocked-in tk gene will mimic that of the targeted αIIb gene. Our results demonstrated that the tk gene is expressed in a pool of primitive multipotent stem cells. Furthermore, the insertion of the tk gene within the αIIblocus resulted in the disruption of the endogenous gene and, subsequently, in mice deficient in the αIIb glycoprotein. These animals exhibited a bleeding disease closely paralleling that seen in patients with type 1 Glanzmann thrombasthenia.
Materials and methods
Construction of the targeting vector
Three clones were isolated from a genomic library using the murine αIIb promoter probe as described.13,14 Partial sequencing indicated that the murine and human αIIb genes share extensive similarity. Consequently, a 6.3-kb HindIII fragment spanning exons 1 to 7 and 2.7 kb of 5′-flanking sequences of the αIIb gene were identified, cloned in pBluescript, and named pHIIIBS. The distal 5′-flanking region of the αIIb gene (positions −1914 to −114) was excised from pHIIIBS by BamHI/SalI. The fragment encompassing the tk gene, positioned under the control of the proximal 5′-flanking region of the αIIb gene, was excised from plasmid αIIbtk-pPNT13 byHindIII–SalI digestion and inserted into a Bluescript plasmid digested by the same enzymes. The distal 5′-flanking region (BamHI/SalI) was then added to assemble the entire promoter region. The neomycin-resistance (NeoR) cassette was introduced 3′ of the tk gene after HindIII–NotI digestion of plasmid pPGKNeobpA.15 The targeting vector was completed by the introduction, after the NeoR cassette, of a 3.4-kb PstI fragment encompassing the end of exon 1 to exon 7 (Figure 1A) and thus lacking a DNA fragment coding for the first 14 amino acids of the αIIbprotein. Linear vector-free insert DNA, prepared by digestion withBamH1, was electroporated into R1 embryonic stem (ES) cells16 17 provided by Dr A. Nagy (Mount Sinai Hospital, Toronto, Canada).
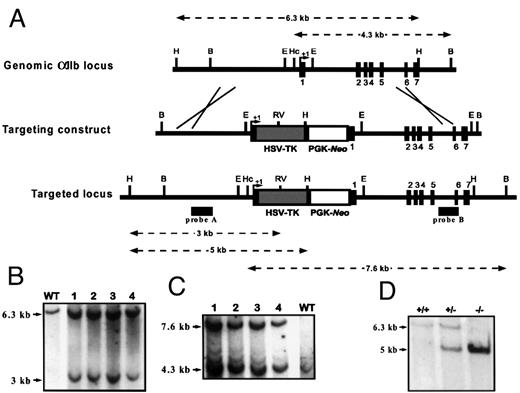
Generation of chimeric mice and progeny genotyping.
(A) Schematic representation of the wild-type αIIballele, the targeting vector, and the resultant recombinant αIIb locus. Filled rectangles indicate exons. Arrows indicate putative transcription initiation sites. Restriction endonuclease enzymes are abbreviated as follows: H,HindIII; B, BamHI; E, EcoRI; Hc, HincII; RV, EcoRV. Probe A was produced by PCR amplification using oligonucleotides 5′-GCCCATGTGTGTGTATGCC-3′ and 5′-CCTCAGGTCTCCATCTTGAACC-3′, located within the 5′-flanking region of the αIIb gene. Probe B was produced using 2 oligonucleotides, 5′-ACACAGAGAGACCCCCTGTC-3′ and 5′-CGGTAGCTGGAGATGATGTTC-3′, located within intron 6 and exon 7, respectively. Both probes were labeled by a random priming method using fluorescein-11-dUTP and hybridized according to the manufacturers' recommendations (Gene Image Random prime labeling and CDP star detection System, Amersham). (B) Southern blot analysis ofHindIII and EcoRV double-digested DNA of R1 ES cell line (WT) and recombinant clones (lanes 1-4) hybridized with probe A. Targeted clones have both the wild-type (6.3 kb) and the disrupted (3 kb) alleles. (C) Southern blot analysis of HincII andBamHI double-digested DNA of R1 ES cell line (WT) and recombinant clones (lanes 1-4) hybridized with probe B. Recombinant clones have wild-type (4.3 kb) and mutated (7.6 kb) alleles. (D) Southern blot analysis of progeny derived from heterozygous intercross. Tail DNA digested with HindIII and subsequently hybridized to probe A indicated the presence of all expected genotypes, including the predicted 6.3-kb band for the wild-type allele and the 5-kb fragment for the mutant allele. A wild-type (+/+), a heterozygous (+/−), and a homozygous mutant (−/−) offspring are represented.
Generation of chimeric mice and progeny genotyping.
(A) Schematic representation of the wild-type αIIballele, the targeting vector, and the resultant recombinant αIIb locus. Filled rectangles indicate exons. Arrows indicate putative transcription initiation sites. Restriction endonuclease enzymes are abbreviated as follows: H,HindIII; B, BamHI; E, EcoRI; Hc, HincII; RV, EcoRV. Probe A was produced by PCR amplification using oligonucleotides 5′-GCCCATGTGTGTGTATGCC-3′ and 5′-CCTCAGGTCTCCATCTTGAACC-3′, located within the 5′-flanking region of the αIIb gene. Probe B was produced using 2 oligonucleotides, 5′-ACACAGAGAGACCCCCTGTC-3′ and 5′-CGGTAGCTGGAGATGATGTTC-3′, located within intron 6 and exon 7, respectively. Both probes were labeled by a random priming method using fluorescein-11-dUTP and hybridized according to the manufacturers' recommendations (Gene Image Random prime labeling and CDP star detection System, Amersham). (B) Southern blot analysis ofHindIII and EcoRV double-digested DNA of R1 ES cell line (WT) and recombinant clones (lanes 1-4) hybridized with probe A. Targeted clones have both the wild-type (6.3 kb) and the disrupted (3 kb) alleles. (C) Southern blot analysis of HincII andBamHI double-digested DNA of R1 ES cell line (WT) and recombinant clones (lanes 1-4) hybridized with probe B. Recombinant clones have wild-type (4.3 kb) and mutated (7.6 kb) alleles. (D) Southern blot analysis of progeny derived from heterozygous intercross. Tail DNA digested with HindIII and subsequently hybridized to probe A indicated the presence of all expected genotypes, including the predicted 6.3-kb band for the wild-type allele and the 5-kb fragment for the mutant allele. A wild-type (+/+), a heterozygous (+/−), and a homozygous mutant (−/−) offspring are represented.
Generation of chimeras and progeny genotyping
Eight cell-stage embryos (2.5 days postcoitum) were isolated from superovulated OF1/Ico females (Iffa Credo, L'Arbresle, France) mated with males of the same strain. Chimeric mice were generated from these and recombinant ES cells by the aggregation method.18 The resultant mice were bred to derive heterozygous mice, and their genotypes were established by analyzing tail DNA either by Southern blot hybridization or polymerase chain reaction (PCR) amplification performed with αIIb(CCGGTGGTGGGAACTTCAGG, CTAGGACCCAAGAACAGTGGTG) and tk primers, as described.13
Assessment of gene expression
Gene expression analysis was performed by Northern blot and by reverse transcription (RT)-PCR of total RNA prepared from mouse tissues using a RNeasy kit (Qiagen, Hilden, Germany). RNA samples were transferred to Hybond N+ nylon membrane (Amersham Pharmacia Biotech, Piscataway, NJ) and hybridized with αIIb or tk probes. These probes were produced by PCR amplification of wild-type bone marrow (BM) RNA or from plasmid pPNT,19 respectively. RT-PCR was performed as described.13 For the amplification of blood mRNA, samples were diluted 5 times before reverse transcription into cDNA.
Real-time quantitative RT-PCR
A 2-μg sample of total RNA was treated with 1 U RQ1-DNAse. After phenol extraction and ethanol precipitation, cDNA was synthesized by MMLV-RT at 42°C for 30 minutes in a 100-μL cDNA synthesis reaction using random hexamers. A 5-μL cDNA aliquot was used to perform PCR reactions with a SYBR Green PCR kit (Perkin Elmer/Applied Biosystems, Foster City, CA), in the ABI PRISM 7700 detector.20 The conditions for each reaction were 2 minutes at 50°C and 10 minutes at 95°C, followed by 40 cycles (15 seconds at 95°C and 1 minute at 60°C). The primers were chosen with the assistance of the computer program oligo-4 (National Biosciences, Plymouth): αIIb, 5′-GTGGGGAAGACGACCTGTGTG-3′ and 5′-CAAGCCTCTCAAAGCCCTCAAT-3′; tk, 5′-CCCGGCCCTCACCCTCATC-3′ and 5′-AAGGTCGGCGGGATGAGG-3′. Statistics were obtained using Excel computer software (Microsoft, Redmond, WA).
Hematopoietic progenitor assays
BM cells flushed from the femoral cavity were cultured in methylcellulose media, as described.13 In some cases, G418 (1 mg/mL) or ganciclovir (from 1 to 10 μmol/L) were added to the media, as specified in the text.
For long-term cultures, marrow cells were flushed into M5300 liquid medium (Stem Cell Technologies) supplemented with hydrocortisone at a final concentration of 10−6 mol/L to establish adherent marrow feeder layers.21 After 3 weeks, BM of chimeric and control mice were seeded onto irradiated 20 Gy feeder layers. Cultures were maintained at 33°C with weekly half-medium changes and the concomitant removal of nonadherent cells. For the initial 4 weeks, ganciclovir was maintained in the culture medium at a dose of 1 μmol/L. This excluded the possibility that the production of granulocyte macrophage–colony-forming cell (GM-CFC)–derived colonies would arise from late progenitor cells that might persist through this period of culturing. Then ganciclovir was withdrawn from the medium, and the number of GM-CFC was evaluated on an aliquot of nonadherent and adherent cells after 2 additional weeks of culture. Controls without BM seeding were included in each experiment and were never found to have endogenous regeneration, as determined weekly by the absence of GM-CFC–derived colonies.
Immunoblotting of αIIb
The αIIb protein was assayed by Western blot analysis with a monoclonal antibody (D12A) directed against the human αIIb protein produced in our laboratory.
Hematologic parameters
Whole blood was drawn by cardiac puncture into anticoagulant (acid–citrate–dextrose; 10 vol blood:1 vol anticoagulant). Automated blood counts were performed with a Micros Blood Cell Analyzer (ABX).
Bleeding time
Mice were anesthetized with 60 μg avertine (Aldrich), and the bleeding time was measured by gently blotting emerging blood with Whatman 3MM paper every 5 to 10 seconds, as described.22
Fibrinogen binding
Fibrinogen from Diagnostica Stago was iodinated by the chloramine method, as described.23 The PRP was centrifuged at 800g, and the platelet pellet was washed twice in Tyrode buffer (NaHCO3, 12 mmol/L; NaCl, 130 mmol/L; KCl, 2.6 mmol/L; MgCl2, 2 mmol/L; bovine serum albumin, 2%; and dextrose, 5.5 mmol/L). Platelets were resuspended in the same buffer at 100 000 platelets/μL, and a 3-μL aliquot of sodium iodide I 125–fibrinogen (1.5 mg/mL) and 5 μL of CaCl2 (22.6 mmol/L) was added. One half of the platelet sample was activated with 5 μL of ADP (226 μmol/L); 5 μL of Tyrode buffer was added to the second half and used as the nonactivated platelet sample. After 25 minutes, a 50-μL aliquot of each sample was centrifuged on a 15% sucrose solution (in Tyrode buffer) to separate free from bound fibrinogen. Platelet-bound fibrinogen was measured by gamma counting of the cut-tip of the tube.
Standard electron microscopy and immunogold labeling on ultrathin cryosections
For standard electron microscopy, marrow samples were analyzed as described.13 24 For immunogold labeling, cryo-ultrathin sections placed on collodion-coated nickel grids were incubated successively for 1 hour at room temperature with the polyclonal antibodies and then with gold-labeled goat antirabbit IgG (1/50 dilution of AuroProbe EM G10; Amersham, Les Ulis, France).
The expression of the αIIbβ3 complex and GPIb in megakaryocytes was detected using polyclonal antibodies directed against the human αIIbβ3 complex and the human GPIb (a generous gift from Dr B. Steiner from Hoffman–La Roche, Basel, Switzerland). The presence of adhesive proteins, such as fibrinogen or von Willebrand factor (vWF), was detected with polyclonal antibodies (DAKO, Glostrup, Denmark).
Results
Generation of mice with the tk gene inserted into the αIIb locus
To monitor the transcriptional activity of the αIIbgene, homologous recombination technology was used to generate ES cells and mice harboring the tk gene located in the αIIb locus. Several clones having the predicted modification for a replacement event were obtained (Figure 1B,C).
Four of these recombinant ES clones were subsequently used in aggregation experiments. Of the 207 embryos transferred, 52 mice were born; 22 were chimeras, as judged by their pigmented coats, because the ES cells were derived from agouti embryos whereas the receivers were albinos. Thirteen chimeras, ranging from 60% to 100% coat color chimerism, were obtained. Most of these chimeras appeared to be sterile or did not transmit the mutation to their offspring. The sterility of females obtained after homologous recombination events in ES cells has been previously explained by the fact that the injection of ES cells derived from male 129Sv, thus XY, into female embryos results in inappropriate (XY→XX) combinations of sex genotypes. This might affect the pattern of sexual development.25,26Sterility of transgenic males harboring the tk gene had been related to the expression of tk from an endogenous cryptic promoter transcriptionally active in testes.27,28 In a few cases, fertile males have been produced9 29; nevertheless, the molecular mechanism has not been clearly identified. In the present study, one male exhibiting approximately 80% chimerism showed normal fertility and germline transmission after crossing with either Balb/c or C57Bl/6. The resultant heterozygous offspring were interbred to generate homozygous mutant mice. To verify whether the tk gene was precisely inserted in the αIIb locus, DNA of these mice was analyzed by Southern blot. All the restriction fragments obtained had the predicted size for a correct replacement event. TheHindIII restriction is represented in Figure 1D.
tk Expression analysis of mutant mice
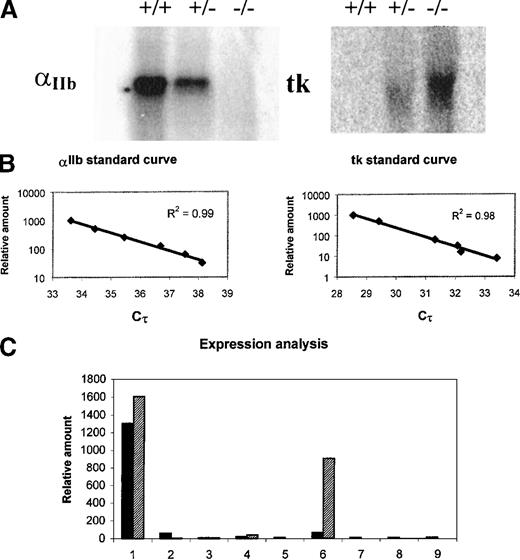
To determine whether the tk gene was active when inserted in the αIIb locus, BM RNA of wild type, heterozygous, and homozygous mice were analyzed by Northern blot. As illustrated in the upper part of Figure 2, the tk message was detected in heterozygous animals and at higher levels in homozygous mice. As expected, no tk transcripts were found in wild-type mice. To establish the consequence of tk insertion on the expression of the endogenous αIIb gene, the same analysis was performed using an αIIb probe. In this case, the levels of the αIIb mRNA were lower in heterozygous mice than in wild-type animals. No αIIb transcripts were found in homozygous mice. This clearly indicated that tk was expressed in these animals and that its integration into the αIIb locus impaired the transcription of the endogenous αIIb gene. Several other tissues besides BM were also tested. This was performed in heterozygous mice to compare the pattern of expression of the αIIb and the tk genes in the same animal. No αIIb or tk transcripts were detected in tissues other than BM by this method (not shown). A more sensitive quantification assay was developed to see whether extremely low levels of αIIb and tk transcripts were produced in tissues other than BM. The relative quantification was performed using a SYBR Green–based PCR method. A strong linear relationship between the Cτ (the cycle threshold value, predictive of the quantity of input target) and the log of the starting concentration of the cDNA (R2 ≥ 0.98) was always demonstrated (Table1, Figure 2B). The results confirmed those of Northern blot analysis, showing that the expression of αIIb and tk genes occurs mostly in BM. The tk transcripts, however, were slightly higher than αIIb. This might be attributed to the better efficiency and yield of the tk reactions (approximately 82.6%) relative to the αIIbreactions (approximately 81%) because of the inherent oligonucleotide sequence or message stability. It should be noted that significant tk transcripts were detected in testes. This was shown to be directed by a cryptic promoter within the tk coding region, which can drive its expression in this organ regardless of the upstream promoter used.27 28
Expression of the tk gene inserted into the αIIb locus.
(A) Results of Northern blot analysis of BM RNA prepared from wild-type mice (+/+), heterozygous (+/−), and homozygous tk-knocked mice (−/−) are represented. Each lane contains 30 μg total RNA. RNA integrity was determined by gel electrophoresis and visual examination of ethidium bromide-stained ribosomal bands. RNA samples were transferred to Hybond N+ nylon membrane (Amersham) and hybridized with αIIb (left) or tk (right) probes. The distortion of the tk band might result because tk transcripts comigrate with the 18S ribosomal RNA. (B) Standard curves for αIIb and tk transcripts. Serial dilutions of BM cDNA were used. 10 μL cDNA solution, corresponding arbitrarily to 2000 U, was used as the highest value. Cτ values are plotted against the relative concentration of serial-diluted cDNA. A strong linear relationship between the Cτ (the cycle threshold value predictive of the quantity of input target) and the log of the starting concentration of the cDNA is demonstrated (R2 ≥ 0.98). (C) Relative expression levels obtained after semiquantitative RT-PCR of RNA prepared from a variety of tissues of heterozygous mice. Duplicates for each sample were performed, but the data for only one is shown here. A negative PCR control without reverse transcription was performed to exclude PCR amplification of contaminating genomic DNA. Dark and dashed boxes correspond to αIIb and tk expression, respectively. Lane 1, BM; lane 2, spleen; lane 3, liver; lane 4, lung; lane 5, brain; lane 6, testes; lane 7, heart; lane 8, thymus; lane 9, kidney.
Expression of the tk gene inserted into the αIIb locus.
(A) Results of Northern blot analysis of BM RNA prepared from wild-type mice (+/+), heterozygous (+/−), and homozygous tk-knocked mice (−/−) are represented. Each lane contains 30 μg total RNA. RNA integrity was determined by gel electrophoresis and visual examination of ethidium bromide-stained ribosomal bands. RNA samples were transferred to Hybond N+ nylon membrane (Amersham) and hybridized with αIIb (left) or tk (right) probes. The distortion of the tk band might result because tk transcripts comigrate with the 18S ribosomal RNA. (B) Standard curves for αIIb and tk transcripts. Serial dilutions of BM cDNA were used. 10 μL cDNA solution, corresponding arbitrarily to 2000 U, was used as the highest value. Cτ values are plotted against the relative concentration of serial-diluted cDNA. A strong linear relationship between the Cτ (the cycle threshold value predictive of the quantity of input target) and the log of the starting concentration of the cDNA is demonstrated (R2 ≥ 0.98). (C) Relative expression levels obtained after semiquantitative RT-PCR of RNA prepared from a variety of tissues of heterozygous mice. Duplicates for each sample were performed, but the data for only one is shown here. A negative PCR control without reverse transcription was performed to exclude PCR amplification of contaminating genomic DNA. Dark and dashed boxes correspond to αIIb and tk expression, respectively. Lane 1, BM; lane 2, spleen; lane 3, liver; lane 4, lung; lane 5, brain; lane 6, testes; lane 7, heart; lane 8, thymus; lane 9, kidney.
Threshold cycle values obtained in semiquantitative RT-PCR
| Tissue . | Experiment . | αIIb Cτ . | tk Cτ . |
|---|---|---|---|
| Bone marrow | A | 33, 23 | 28, 18 |
| B | 32, 34 | 27, 31 | |
| Kidney | A | 37, 47 | 33, 46 |
| B | 38, 88 | 32, 77 | |
| Liver | A | 40 | 33, 49 |
| B | 40 | 33, 14 | |
| Lung | A | 38, 99 | 31, 69 |
| B | 37, 78 | 31, 31 | |
| Brain | A | 40 | 40 |
| B | 40 | 36, 86 | |
| Testes | A | 37, 4 | 28, 73 |
| B | 35, 99 | 28 | |
| Heart | A | 40 | 36, 78 |
| B | 39, 55 | 29, 33 | |
| Thymus | A | 40 | 37, 25 |
| B | 40 | 37, 09 | |
| Spleen | A | 40 | 36, 24 |
| B | 37, 32 | 37, 47 |
| Tissue . | Experiment . | αIIb Cτ . | tk Cτ . |
|---|---|---|---|
| Bone marrow | A | 33, 23 | 28, 18 |
| B | 32, 34 | 27, 31 | |
| Kidney | A | 37, 47 | 33, 46 |
| B | 38, 88 | 32, 77 | |
| Liver | A | 40 | 33, 49 |
| B | 40 | 33, 14 | |
| Lung | A | 38, 99 | 31, 69 |
| B | 37, 78 | 31, 31 | |
| Brain | A | 40 | 40 |
| B | 40 | 36, 86 | |
| Testes | A | 37, 4 | 28, 73 |
| B | 35, 99 | 28 | |
| Heart | A | 40 | 36, 78 |
| B | 39, 55 | 29, 33 | |
| Thymus | A | 40 | 37, 25 |
| B | 40 | 37, 09 | |
| Spleen | A | 40 | 36, 24 |
| B | 37, 32 | 37, 47 |
PCR amplifications for αIIb and tk were performed in the same sample.
Cτ values determine the cycle at which the fluorescence exceeds the threshold of the baseline.
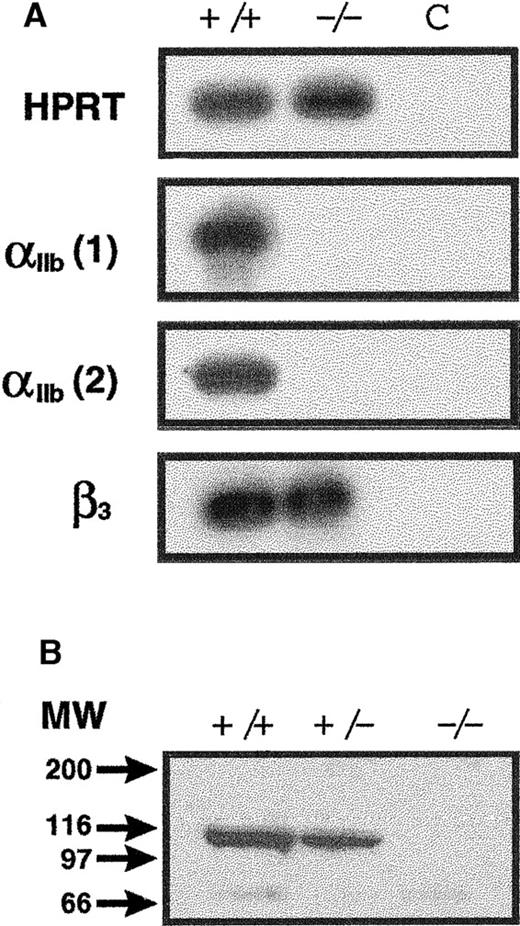
Considering that the αIIb and the β3 genes are closely linked in the same chromosome, the impaired expression of the αIIb gene might have altered the expression of the gene coding for the β subunit of the αIIbβ3complex. To test this possibility, the presence of the β3 mRNA was assessed by RT-PCR. The level of the β3-cDNA product was similar for homozygous mutant and control mice (Figure 3A), indicating that the transcription of the β3 subunit was unaffected in these animals. As a control for mRNA integrity, amplification of the hypoxanthine–phosphoribosyl transferase (HPRT) message was performed.
Molecular phenotype of the Glanzmann thrombasthenic mice.
(A) RT-PCR analysis of the expression of genes in wild-type (+/+) and αIIb-null mice (−/−). BM RNA was amplified using sense primers (A) and antisense primers (B) as follows. For the αIIb gene, 2 different 5′-oligonucleotides were used with the same 3′-primer: αIIb1 (A): CCTGTTTAGGACGTTTGGGAAG; αIIb2 (A): CACCACTGTTCTTGGGTCCTAG; αIib (B): GGGCGGTAGCTGGAGATGATG; tk (A): CCCCTGCCATCAACACGCG; tk (B): CGATGGGGATGGCGGTCGAAG; β3(A): ACTACACGCACTGACACCTGCATG; β3 (B): ACACTCCACACACTCCTTCTT; HPRT (A): GCTGGTGAAAAGGACCTCT; and HPRT (B): CACAGGACTAGAACACCTGC. The sizes of the amplified products are as follows: HPRT, 250 bp; tk, 411 bp; αIIb1, 777 bp; αIIb2, 677 bp; and β3, 123 bp. (C) Product from a control PCR with RT omitted. (B) Immunoblot analysis of platelet proteins from adult wild type (+/+), heterozygous (+/−), and homozygous (−/−) mice. Platelet pellets (50 × 106) were lysed in Tris buffer, 0.125 mol/L, pH 6.8, containing 2.5% SDS, 25% glycerin, 0.5 mg/mL leupeptin (Boehringer), and 2-mercaptoethanol 0.7 mol/L. The solubilized proteins were heated to 100°C for 5 minutes, electrophoresed on a 7.5% polyacrylamide gel, and transferred to a polyvinylidene fluoride membrane (Boehringer). Then αIIb was detected using the monoclonal antibody D12A raised against human αIIb. Bound primary antibody was developed with a goat antimouse alkaline phosphatase (AP) antibody (Sigma) and enhanced using the CDP-Star chemiluminescence system (Amersham). For the data shown, the film was exposed to the blot for 5 seconds. The only reactive species noted on the blot migrates as predicted for native αIIb protein.
Molecular phenotype of the Glanzmann thrombasthenic mice.
(A) RT-PCR analysis of the expression of genes in wild-type (+/+) and αIIb-null mice (−/−). BM RNA was amplified using sense primers (A) and antisense primers (B) as follows. For the αIIb gene, 2 different 5′-oligonucleotides were used with the same 3′-primer: αIIb1 (A): CCTGTTTAGGACGTTTGGGAAG; αIIb2 (A): CACCACTGTTCTTGGGTCCTAG; αIib (B): GGGCGGTAGCTGGAGATGATG; tk (A): CCCCTGCCATCAACACGCG; tk (B): CGATGGGGATGGCGGTCGAAG; β3(A): ACTACACGCACTGACACCTGCATG; β3 (B): ACACTCCACACACTCCTTCTT; HPRT (A): GCTGGTGAAAAGGACCTCT; and HPRT (B): CACAGGACTAGAACACCTGC. The sizes of the amplified products are as follows: HPRT, 250 bp; tk, 411 bp; αIIb1, 777 bp; αIIb2, 677 bp; and β3, 123 bp. (C) Product from a control PCR with RT omitted. (B) Immunoblot analysis of platelet proteins from adult wild type (+/+), heterozygous (+/−), and homozygous (−/−) mice. Platelet pellets (50 × 106) were lysed in Tris buffer, 0.125 mol/L, pH 6.8, containing 2.5% SDS, 25% glycerin, 0.5 mg/mL leupeptin (Boehringer), and 2-mercaptoethanol 0.7 mol/L. The solubilized proteins were heated to 100°C for 5 minutes, electrophoresed on a 7.5% polyacrylamide gel, and transferred to a polyvinylidene fluoride membrane (Boehringer). Then αIIb was detected using the monoclonal antibody D12A raised against human αIIb. Bound primary antibody was developed with a goat antimouse alkaline phosphatase (AP) antibody (Sigma) and enhanced using the CDP-Star chemiluminescence system (Amersham). For the data shown, the film was exposed to the blot for 5 seconds. The only reactive species noted on the blot migrates as predicted for native αIIb protein.
Homozygous mutant mice do not synthesize detectable levels of the αIIb protein
To examine whether the absence of the αIIb mRNA correlated with the absence of αIIb protein, immunoblot analysis of platelet proteins was performed using an antibody directed against human αIIb. This antibody cross-reacted with murine αIIb of wild-type mice. Results revealed a reduction in the level of αIIb for heterozygous mice and the absence of αIIb in the platelet extract of homozygous animals (Figure 3B). From these observations, we concluded that the integration of the targeting vector into the αIIb locus resulted in mice deficient in αIIb.
Primitive hematopoietic cells express the toxic gene
To characterize the precise stage of hematopoietic differentiation at which the expression of the tk gene was initiated, the toxic effect of ganciclovir was examined using progenitor cell assays. In the first set of experiments, BM was extracted from αIIb-deficient mice and cultured on methylcellulose. The level of tk in these mice proved to be insufficient to produce a toxic effect. This is consistent with the fertility of these male mice, as discussed above. Consequently, progenitor cell assays were performed in 2 other chimeric animals. The direct analysis of chimeric mice to evaluate the consequences of targeted mutations on hematopoiesis has been largely used.30-32 One prerequisite of this analysis, however, is to identify precisely those cells that are derived from the modified ES cells because in chimeras, mutant cells are mixed with wild-type cells. This implied a double selection of cells expressing both the tk and the NeoR genes. To this end, the 13 chimeric mice were tested for the expression of the knock-in tk gene in hematopoietic cells. This was achieved first by determining which of these animals expressed the tk gene in their blood by RT-PCR. Total RNA prepared from whole blood was amplified with tk-specific primers; tk expression represented by a 400-bp cDNA product was found in 6 chimeras. These animals were further analyzed to determine the level of chimerism in BM cells and to identify those hematopoietic progenitors that derive from modified ES cells. This is a critical issue to interpret unambiguously the subsequent results.
To this end, a strategy in which the growth of colonies was evaluated on semisolid assays in the presence of G418 was developed. It was rationalized that all the progenitor cells derived from modified ES cells, possessing the NeoR gene, should produce colonies on serum-free medium in the presence of this drug. In contrast, the growth of colonies derived from wild-type cells should be completely inhibited because these wild-type cells do not possess the NeoR gene. Consequently, BM cells of animals were plated on methylcellulose and cultured under optimal conditions for the growth of CFU-GEMMk and individual committed hematopoietic progenitor cells. First the BM of wild-type mice was cultured in the absence of G418 to determine the number and size of colonies derived from these early progenitor cells in normal conditions. Then it was cultured in the presence of G418 (1 mg/mL) to verify the activity of the drug. The results, summarized in Table 2, showed a total inhibition of the growth of all types of colonies in the presence of G418 for the control mice. When the BM of chimeric mice were plated on methylcellulose, colonies for all hematopoietic lineages were obtained in the presence of G418. This indicated that in these animals, hematopoietic cells of all lineages contained the NeoR and thus derived from a modified recombinant ES cell. Moreover, the number and size of colonies derived from chimeras in the presence or absence of the drug was similar to those derived from BM of a control mouse cultured in standard conditions, further indicating that the insertion of tk per se did not affect the development of hematopoietic cells.
Analysis of multipotent, mixed, and committed progenitor-derived colonies in semisolid assays
| Drug . | BFU-E . | GM-CFC . | CFU-Mk . | CFU-mix . | GEMMk . |
|---|---|---|---|---|---|
| Control cells | |||||
| None | 18 ± 2 | 130 ± 4 | 8 ± 1 | 10 ± 1 | 3 ± 2 |
| G 418 (1 mg/mL) | 0 | 0 | 0 | 0 | 0 |
| Ganciclovir (10 μM) | 17 | 129 ± 2 | 10 ± 1 | 8 ± 1 | 3 ± 1 |
| Chimeric cells | |||||
| None | 17 ± 1 | 140 ± 2 | 8 ± 1 | 7 ± 1 | 6 ± 2 |
| G 418 (1 mg/mL) | 26 ± 1 | 180 ± 1 | 17 ± 1 | 7 ± 1 | 4 ± 1 |
| Ganciclovir (10 μM) | 0 | 8 ± 3 | 0 | 0 | 0 |
| Drug . | BFU-E . | GM-CFC . | CFU-Mk . | CFU-mix . | GEMMk . |
|---|---|---|---|---|---|
| Control cells | |||||
| None | 18 ± 2 | 130 ± 4 | 8 ± 1 | 10 ± 1 | 3 ± 2 |
| G 418 (1 mg/mL) | 0 | 0 | 0 | 0 | 0 |
| Ganciclovir (10 μM) | 17 | 129 ± 2 | 10 ± 1 | 8 ± 1 | 3 ± 1 |
| Chimeric cells | |||||
| None | 17 ± 1 | 140 ± 2 | 8 ± 1 | 7 ± 1 | 6 ± 2 |
| G 418 (1 mg/mL) | 26 ± 1 | 180 ± 1 | 17 ± 1 | 7 ± 1 | 4 ± 1 |
| Ganciclovir (10 μM) | 0 | 8 ± 3 | 0 | 0 | 0 |
Marrow cells (5 × 104 per well) were obtained by femoral aspiration. Each number (± SD) is the mean of 3 wells in 3 different experiments, using 2 independent chimeric animals. CFU-mix consisted of colonies with erythroid and megakaryocytic component and colonies with granulocytic, macrophagic, and megakaryocytic without any erythroid component. Multipotent CFU-GEMMk were composed of granulocytic, erythrocytic, macrophagic and megakaryocytic components.
The fact that all the hematopoietic cells in chimeric animals originated from mutant ES cells enabled us to address the key question: at which stage during hematopoietic differentiation was the expression of the tk knock-in gene initiated? To this end, BM cells of chimeric and control animals were cultured on methylcellulose in the presence of ganciclovir. At a dose of 1 μmol/L, the number of all the progenitors derived from the mutant mice was drastically reduced, whereas the drug had no effect on control mice-derived cells, even at a dose of 10 μmol/L (Table 2). These results clearly indicated that the expression of tk was initiated in early multipotent progenitor cells.
Long-term BM cultures were then performed to investigate further whether the expression of tk was initiated in most primitive progenitors, usually referred to as long-term culture initiating cells (LTC-IC). In this set of experiments, control BM cells produced the same number of GM-CFC in the presence or absence of ganciclovir. In contrast, the number of GM-CFC derived from mutant cells was drastically reduced both in the supernatant and in the stromal layer (Table 3). This drastic failure of chimeric BM cells to produce GM-CFC colonies in long-term assays was consistent with the fact that the expression of tk exerted a cytotoxic effect in a pool ofuncommitted primitive hematopoietic cells. From these results we concluded that tk was transcriptionally active in primitive multipotent cells before the occurrence of a megakaryocytic commitment decision.
Ganciclovir-induced toxicity on LTC-IC after long-term culture assays
| . | Week 1 . | Week 6 . | |
|---|---|---|---|
| Supernatant . | Supernatant . | Adherent layer . | |
| Control (−) | 304 ± 40 | 64 ± 6 | 217 ± 10 |
| Control (+) | 400 ± 50 | 68 ± 7 | 213 ± 40 |
| Chimeric (−) | 184 ± 20 | 71 ± 9 | 300 ± 10 |
| Chimeric (+) | 20 ± 2 | 9 ± 5 | 7 ± 2 |
| . | Week 1 . | Week 6 . | |
|---|---|---|---|
| Supernatant . | Supernatant . | Adherent layer . | |
| Control (−) | 304 ± 40 | 64 ± 6 | 217 ± 10 |
| Control (+) | 400 ± 50 | 68 ± 7 | 213 ± 40 |
| Chimeric (−) | 184 ± 20 | 71 ± 9 | 300 ± 10 |
| Chimeric (+) | 20 ± 2 | 9 ± 5 | 7 ± 2 |
The presence of LTC-IC was determined weekly by the quantification of GM-CFC–derived colonies as described in the text. Values shown represent GM-CFC colonies/105 cells plated. Each number (± SD) is the mean of 3 wells in 2 separate experiments, using 2 independent chimeric animals. (−) Cultures performed without ganciclovir. (+) Cultures performed in the presence of ganciclovir.
Targeting the tk gene to the αIIb locus results in mice with a thrombasthenic-like syndrome
Analysis of αIIb-deficient mice revealed a hemostatic disorder that prevented them from controlling blood loss. Bleeding persisted for as long as it was monitored (more than 15 minutes). In 12 of 24 adult αIIb-null mice studied, severe bleeding diathesis were observed at autopsy. Five of these animals had subcutaneous bleeding, 2 had gastrointestinal hemorrhage, and 3 exhibited urogenital bleeding episodes. Three of 24 αIIb−/− mice developed visible intra-abdominal bleeding that did not prove to be fatal. Control and homozygous mutant mice did not show major differences in the number of white cells, red cells, or platelets (Table4), though in 3 of the αIIb-null mice, slight erythrocytopenia was associated with splenomegaly.
Hematologic analysis of control and mutant mice
| . | +/+ . | +/− . | −/− . |
|---|---|---|---|
| Platelets (× 109) | 6.8 ± 2 | 6.0 ± 1.8 | 7.8 ± 2.1 |
| Erythrocytes (× 1012) | 9.2 ± 1.2 | 9.7 ± 1.5 | 8.7 ± 0.6 |
| 5.4 ± 0.24-150 | |||
| Leukocytes (× 109) | 3.9 ± 1.4 | 3.970 ± 1.720 | 4.0 ± 1.6 |
| Spleen weight (g) | 0.071 ± 0.006 | 0.089 ± 0.015 | 0.090 ± 0.009 |
| 0.3 ± 0.064-150 | |||
| Age (d) | 100 ± 50 | 110 ± 40 | 104 ± 40 |
| . | +/+ . | +/− . | −/− . |
|---|---|---|---|
| Platelets (× 109) | 6.8 ± 2 | 6.0 ± 1.8 | 7.8 ± 2.1 |
| Erythrocytes (× 1012) | 9.2 ± 1.2 | 9.7 ± 1.5 | 8.7 ± 0.6 |
| 5.4 ± 0.24-150 | |||
| Leukocytes (× 109) | 3.9 ± 1.4 | 3.970 ± 1.720 | 4.0 ± 1.6 |
| Spleen weight (g) | 0.071 ± 0.006 | 0.089 ± 0.015 | 0.090 ± 0.009 |
| 0.3 ± 0.064-150 | |||
| Age (d) | 100 ± 50 | 110 ± 40 | 104 ± 40 |
Data are represented as mean ± SD of 10 independent measures.
The counts shown are per liter of blood volume.
3 of 24 animals examined as described in the text. +/+, wild-type animals; +/−, heterozygous mice; −/−, αIIb-deficient mice.
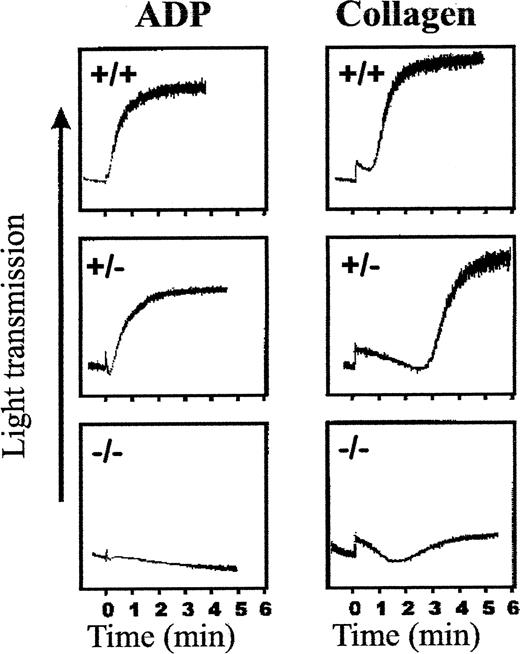
Platelets of these αIIb-deficient mice were next examined for their capacity to aggregate in response to stimulation. Platelet suspensions were prepared from αIIb+/+, αIIb+/−, and αIIb−/−mice and tested using standard aggregometer assays. Platelets of wild-type animals aggregated promptly, whereas platelets of homozygous mutant animals failed to aggregate in the presence of either ADP or collagen (Figure 4). Platelets from heterozygous animals supported substantial aggregation to both agonists, but the collagen response was significantly delayed, suggesting that an intermediate level of platelet fibrinogen receptors was sufficient to support aggregation. The observed delay in response to collagen, however, suggested that the kinetics of collagen stimulation, which requires ADP secretion, is more dependent on the density of αIIbβ3 receptors at the surface of the cell.
Aggregation response of platelets derived from αIIb+/+, αIIb+/−, and αIIb−/− mice.
Platelets diluted with PBS at a final concentration ranging from 0.5 to 1 × 108 in 336 μL were mixed with 14 μL CaCl2 (1 mmol/L final) and placed in an aggregometer (Chrono-log) at 37°C. Aggregation was initiated by the addition of 18.5 μL ADP (final concentration, 10 μmol/L) or 39 μL collagen (final concentration, 0.2 mg/mL) from Sigma (diagnostic kit for aggregation). Aggregation was monitored by recording the increase in light transmittance as a function of time.
Aggregation response of platelets derived from αIIb+/+, αIIb+/−, and αIIb−/− mice.
Platelets diluted with PBS at a final concentration ranging from 0.5 to 1 × 108 in 336 μL were mixed with 14 μL CaCl2 (1 mmol/L final) and placed in an aggregometer (Chrono-log) at 37°C. Aggregation was initiated by the addition of 18.5 μL ADP (final concentration, 10 μmol/L) or 39 μL collagen (final concentration, 0.2 mg/mL) from Sigma (diagnostic kit for aggregation). Aggregation was monitored by recording the increase in light transmittance as a function of time.
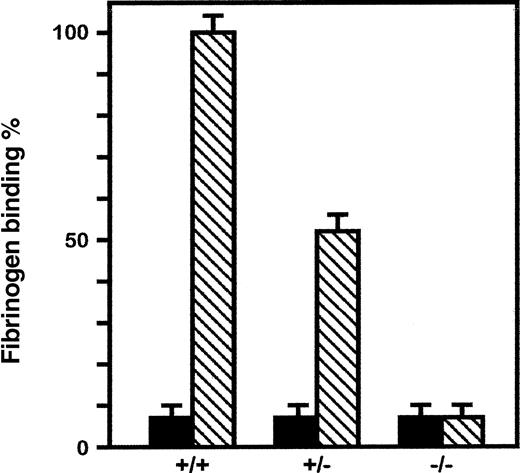
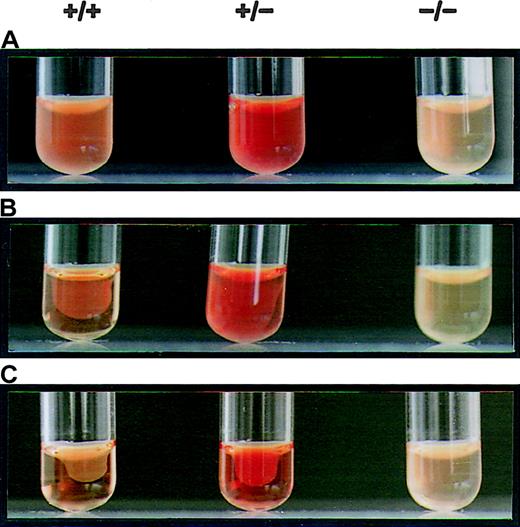
Binding experiments were performed to monitor the capacity of platelets from αIIb-deficient mice to interact with fibrinogen. Because of the scarcity of platelet samples derived from each mouse, test and control assays were performed using separate animals. Nonspecific binding was examined in the presence of a 100-fold excess unlabeled fibrinogen, and specific binding to wild-type platelets was taken as 100%. When compared to normal platelets, platelets from αIIb+/− exhibited a specific binding capacity of 57% (Figure 5). Platelets of αIIb-null mice maintained a specific and displaceable binding of approximately 6% ± 2% of normal. This residual binding might represent differences in genetic backgrounds, variation in fibrinogen samples, or additional fibrinogen binding to glycoproteins other than αIIbβ3, such as the αvβ3 integrin. The results, however, clearly indicated that platelets of αIIb−/−mice have a severely decreased capacity to bind fibrinogen. Finally, the clot reaction for wild-type mice was completed within a 2-hour period, whereas clot retraction with αIIb+/− PRP was delayed and incomplete during this period (Figure 6). PRP from null mice failed to undergo clot retraction as long as the reaction was monitored (15 hours). From these results we concluded that the impaired synthesis of the αIIb subunit in these mutant mice lead to a thrombasthenia-like phenotype. Otherwise, the αIIb-deficient mice did not reveal any gross anatomic abnormalities. The animals appeared normal at birth, grew to normal size, and were fertile.
Differences in fibrinogen binding of platelet suspensions prepared from wild-type (+/+), heterozygous (+/−), and homozygous (−/−) mice.
Filled boxes represent nonstimulated platelets, and striped boxes represent platelets stimulated with 10 μmol/L ADP. Platelets were incubated with 40 μg/mL labeled fibrinogen for 25 minutes at 22°C. Results represent specific fibrinogen binding. Nonspecific binding was evaluated on separate mice because of the scarcity of the platelet sample drawn from each animal.
Differences in fibrinogen binding of platelet suspensions prepared from wild-type (+/+), heterozygous (+/−), and homozygous (−/−) mice.
Filled boxes represent nonstimulated platelets, and striped boxes represent platelets stimulated with 10 μmol/L ADP. Platelets were incubated with 40 μg/mL labeled fibrinogen for 25 minutes at 22°C. Results represent specific fibrinogen binding. Nonspecific binding was evaluated on separate mice because of the scarcity of the platelet sample drawn from each animal.
Platelet-mediated clot retraction. PRP samples prepared from wild-type (+/+), αIIb+/−, and αIib−/− mice were clotted by the addition of thrombin, and the progress of clot retraction was followed for 15 hours at 37°C.
(A) t = 0 hours. (B) t = 2 hours. (C) t = 15 hours. The platelet-rich plasma (PRP) was obtained by centrifugation at 70g, and plasma was obtained by further centrifugation at 2500g in a F2402 rotor (Beckman). PRP at a concentration of 1 × 105 platelets/μL in 270-μL was incubated at 37°C in the presence of 30 μL CaCl2 (50 mmol/L). Clot formation was performed with 1 U prewarmed human thrombin (Sigma). PRP from wild-type animals had completed the retraction of the opaque clots in a 2-hour period, whereas the clot retraction for the αIIb+/− mice was incomplete. PRP of αIIb−/− failed to induce clot retraction for 15 hours.
Platelet-mediated clot retraction. PRP samples prepared from wild-type (+/+), αIIb+/−, and αIib−/− mice were clotted by the addition of thrombin, and the progress of clot retraction was followed for 15 hours at 37°C.
(A) t = 0 hours. (B) t = 2 hours. (C) t = 15 hours. The platelet-rich plasma (PRP) was obtained by centrifugation at 70g, and plasma was obtained by further centrifugation at 2500g in a F2402 rotor (Beckman). PRP at a concentration of 1 × 105 platelets/μL in 270-μL was incubated at 37°C in the presence of 30 μL CaCl2 (50 mmol/L). Clot formation was performed with 1 U prewarmed human thrombin (Sigma). PRP from wild-type animals had completed the retraction of the opaque clots in a 2-hour period, whereas the clot retraction for the αIIb+/− mice was incomplete. PRP of αIIb−/− failed to induce clot retraction for 15 hours.
Cellular morphology of BM in mice lacking the αIIb gene
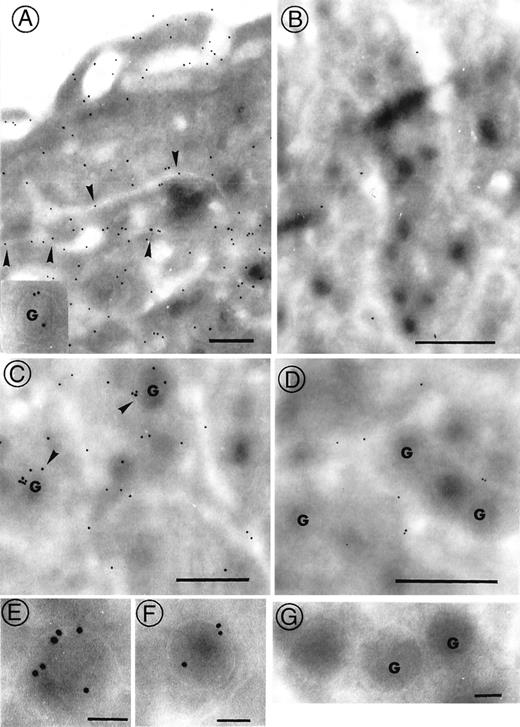
Evaluation of the BM ultrastructure of αIIbdeficient mice by electron microscopy revealed a normal morphology of cells of the megakaryocytic lineage. Megakaryocytes were observed near the vascular sinus in a normal bone microenvironment, where all myeloid lineages were represented. The demarcation membrane system (DMS) with well-defined platelet territories was observed in maturing megakaryocytes. Consequently, their fragmentation into proplatelets was normal. Immunogold staining using polyclonal antibodies revealed the αIIbβ3 complex in megakaryocyte membranes of wild-type mice. Labeling was seen on the surface, on the DMS, and in association with the α-granules membranes (Figure7A). In contrast, a total absence of αIIbβ3 complexes was found in all the membrane compartments of megakaryocytes from αIIb-deficient mice (Figure 7B). The occasional labeling observed was similar to that seen in control assays performed with nonimmune rabbit IgG and was considered to represent background staining. Labeling of the α-granules confirmed the presence of fibrinogen (Figure 7C) in wild-type mice, whereas no labeling was found associated with these organelles in the αIIb-deficient mice (Figure 7D). Occasional gold beads were present in the channels of the DMS. This labeling was significant and may represent αIIbβ3-independent fibrinogen endocytosis, consistent with the transport of fibrinogen from the plasma but without storage in α-granules. Labeling of the GP Ibα subunit and of vWF was similar for normal and αIIb-deficient mice (not shown).
Immunogold labeling of megakaryocytes.
Immunogold labeling of megakaryocytes with anti-αIIbβ3 antibody (A, B) and antifibrinogen antibody (C-G). In both cases, binding of antibodies was revealed with a goat anti-rabbit IgG adsorbed onto 10-nm gold particles. The cryosections embedded in a thin film of methylcellulose were observed with a JEOL JEM-1010 transmission electron microscope at 80 kV. In A, a normal megakaryocyte shows abundant labeling for αIIbβ3 on the plasma membrane and in the DMS (arrowheads). Occasional beads were seen on the membranes of α-granules (inset). In B, little or no staining for αIIbβ3 was seen on the membranes of the megakaryocytes from an αIIb-deficient mouse. In the megakaryocyte of a normal mouse (C), fibrinogen (arrowheads) is essentially present in the α-granules (g), as also illustrated at higher magnification (E, F). No labeling was found in the α-granules (g) of αIIb-deficient mice (D, G), though some labeling was present in externally accessible dilated channels. (A-D) Bars = 0.5 μm; (E-G) Bars = 0.1 μm.
Immunogold labeling of megakaryocytes.
Immunogold labeling of megakaryocytes with anti-αIIbβ3 antibody (A, B) and antifibrinogen antibody (C-G). In both cases, binding of antibodies was revealed with a goat anti-rabbit IgG adsorbed onto 10-nm gold particles. The cryosections embedded in a thin film of methylcellulose were observed with a JEOL JEM-1010 transmission electron microscope at 80 kV. In A, a normal megakaryocyte shows abundant labeling for αIIbβ3 on the plasma membrane and in the DMS (arrowheads). Occasional beads were seen on the membranes of α-granules (inset). In B, little or no staining for αIIbβ3 was seen on the membranes of the megakaryocytes from an αIIb-deficient mouse. In the megakaryocyte of a normal mouse (C), fibrinogen (arrowheads) is essentially present in the α-granules (g), as also illustrated at higher magnification (E, F). No labeling was found in the α-granules (g) of αIIb-deficient mice (D, G), though some labeling was present in externally accessible dilated channels. (A-D) Bars = 0.5 μm; (E-G) Bars = 0.1 μm.
Discussion
In this paper, we describe a strategy for analyzing the transcriptional activity of lineage-specific genes during hematopoiesis. A gene encoding a conditional toxin was targeted to a defined locus to induce a restricted cell knockout. The locus of the αIIb gene encoding a protein known to be active in platelets, which corresponds to the terminal phase of the differentiation process of the megakaryocyte, was selected. The results show that, although the αIIb gene is not critical for the development of the hematopoietic system, it is transcriptionally active in early hematopoietic progenitor cells. This supports the existence of a multilineage gene expression in the stem cell population. In addition, the knock-in of the tk gene into the αIIb locus impaired the synthesis of the α subunit of the platelet integrin αIIbβ3 and resulted in αIIb-null mice with a Glanzmann thrombasthenia-like syndrome. The strength of this model is that these mice possess a single molecular defect, and they provide unlimited material for experimental analysis. This is particularly useful given that it is difficult to obtain platelet or BM biopsies from patients with Glanzmann thrombasthenia because of the risk for hemorrhage.
The gene-targeting approach developed here led to the correct insertion of the tk gene in the αIIb locus. In heterozygous mice, αIIb and tk genes were coexpressed and were found mainly in BM. Using a sensitive quantification assay, trace amounts were detected in spleen and lungs, tissues where megakaryocytes could be found. The only difference seen was the presence of tk transcripts in testes. This testicular expression has been shown to be inherent to the tk gene, which has a cryptic promoter within its coding region that produces a shorter mRNA. Thus, even without a promoter, tk is expressed in this tissue.27,28 The possibility that this cryptic promoter could account for the slight difference in the tk expression and could limit the interpretation of the hematopoietic stem cell experiments cannot be excluded. Although both mRNA are indistinguishable by Northern blot or PCR analysis, such an effect would appear unlikely because the protein synthesized from the cryptic promoter is less efficient than the wild type for the eradication of cells,28 and, in some cases, this truncated mRNA does not result in translation into tk protein.29 Moreover, the same dose of ganciclovir did not eradicate testicular cells in previous αIIbtk transgenic mice expressing tk in testes (13 and our unpublished results).
Direct analysis of chimeric mice to evaluate the consequences of targeted lethal mutations on hematopoiesis has been previously used.30-32 A prerequisite of this analysis is to determine precisely whether the cells that are studied originate from the genetically modified cells. We found that mutated ES cells harboring the tk and NeoR genes contributed to the production of all hematopoietic lineages, with an apparent lack of compensation from the wild-type ES cells. This was deduced from results of BM cultures of chimeric mice showing that the growth of colonies derived from CFU-GEMM or all individual monopotent hematopoietic progenitors was not affected in the presence of G418.
The fact that all hematopoietic cells in chimeric mice were derived from mutant ES cells enabled us to undertake the analysis of the tk expression in BM of these animals. The toxic effect of ganciclovir was demonstrated in the multipotent CFU-GEMM progenitor, consistent with the tk expression in these cells. A possible explanation for the ganciclovir sensitivity of early progenitors could be that a nonspecific cell knock-out occurred as a result of the general release of toxic phosphorylated ganciclovir from dying cells specifically expressing tk. Although this bystander effect has been described in other systems,33,34 it would seem to be an unlikely event in this study because most cells are impermeable to phosphorylated nucleotides. This possibility was, in fact, tested in our previous studies in which BM cells of transgenic and control mice were cultured together.13 We showed that co-culturing BM cells of nontransgenic mice in the presence of transgenic BM cells did not reduce the number of expected colonies.
Long-term BM cultures performed on stromal feeders had been largely validated to characterize adherent and nonadherent early hematopoietic cells that have the capacity to express both myeloid and lymphoid differentiation potentials.35,36 These LTC-ICs maintain their capacity to repopulate the hematopoietic system of irradiated mice after long-term engraftment.37-39 Culturing BM cells of chimeric mice for 6 weeks indicated that the toxic gene was expressed in a subset of adherent and nonadherent LTC-ICs. This demonstrates that tk was expressed in a more primitive cell than the CFU-GEMM progenitor when located in the αIIb locus. This cell knock-out strategy provides a qualitative analysis, and the subsets of tk-expressing pluripotent hemopoietic stem cells (PHSC) remains to be identified.
The transcriptional activity of the toxic gene in a subset of PHSC indicates that the genetic programs of specific lineages are simultaneously activated in a primed totipotent stem cell. This establishes that the early, undifferentiated cells may express the full repertoire of lineage-specific genes. To date, increasing numbers of reports in the literature are consistent with this model. The coexistence of terminal erythroid and myeloid genetic programs in a permanent cell line exhibiting characteristics of PHSC and the presence of different lineage marker mRNA as c-kit, GATA-2, NF-E2, tal-1, and c-myb in the PHSC was demonstrated.40,41 Other transcription factors representing specific hematopoietic lineages were found in individual quiescent multipotent hematopoietic cells. Furthermore, recent studies show that alternative fates are preserved in stem and progenitor cells by the simultaneous low expression of genes for several lineages.42 43 All these observations challenge current ideas as to how immature cells become committed to producing specific types of progeny cells.
Early reports in the literature suggest the presence of the αIIbβ3 complex in a totipotent stem cell.44,45 More recent studies performed in the avian hematopoietic system providing evidence that αIIbβ3+ BM cells can differentiate into T cells46 are consistent with these results. Furthermore, the αIIb gene was found to be expressed as early as the mouse blastocysts.47 The protein encoded by the αIIb gene, however, is only known to be functionally important in the terminal phase of megakaryocytic differentiation. The functional or quantitative abnormal exposure of the αIIbβ3 at the surface of platelets results in the human inherited bleeding disorder, Glanzmann thrombasthenia. The mutant mice described in this study have a similar syndrome.
Mice lacking the αIIb subunit were born in the correct Mendelian ratio, demonstrating that the αIIb subunit is not essential for normal embryonic development. Females were fertile and did not have major delivery problems. In contrast, women with Glanzmann thrombasthenia have severe hemorrhagic episodes at this stage. Furthermore, secondary effects, such as splenomegaly, encountered in some αIIb-null animals but not commonly reported in patients with Glanzmann thrombasthenia, were also observed in β3-null mice.48 The propensity for intra-abdominal hemorrhage found in αIIb-null mice was also seen in mice deficient for αIIbβ3 ligands such as fibrinogen and vWF.22,49,50 However, mice lacking fibrinogen showed a more severe tendency to bleed. This is perhaps because fibrinogen is involved in other biologic processes51-53in addition to platelet aggregation and thrombus formation. Considering the similar roles of these ligands and the complexity of the systems in which they are involved, detailed comparative analyses of null mice might provide a means by which to define the precise role of each molecule.
Ultrastructural analysis and immunogold staining using electron microscopy further demonstrated the absence of αIIbβ3 complexes at the surface of αIIb-deficient mice megakaryocytes. These cells displayed normal morphology with normal α-granule organization. The development of platelet territories was indistinguishable from that of control mice as found for the human pathology.54
Platelet α-granules of αIIb-null mice did not contain fibrinogen. A correlation between the absence of the αIIbβ3 complex at the surface of platelets and the lack of granular fibrinogen within platelets has been reported in patients with Glanzmann thrombasthenia.12,55 56 The production of thrombasthenic mice, in which the only molecular defect is the absence of glycoprotein αIIb, provides a definite molecular basis for this correlation and further confirms that the presence of a functional integrin is a prerequisite for the uptake and storage of fibrinogen in α-granules. An interesting observation was that fibrinogen was localized within the DMS of mature megakaryocytes lacking the αIIbβ3 receptor. This suggests that endocytosis of circulating fibrinogen emerges as a complex event that may proceed, at least in part, through an αIIbβ3 independent mechanism occurring before the αIIbβ3 mediated storage in α-granules.
These results, combined with those obtained with BM cultures, indicated that the αIIb subunit does not appear to play a major role in stem cell commitment, hematopoietic progenitor cell development, or megakaryocyte differentiation. This raises the question of the possible role played by this glycoprotein in the establishment of the megakaryocytic phenotype or the differentiation of other hematopoietic lineages. One possibility is that in the absence of this integrin, a compensatory mechanism might develop by which other members of the integrin family would accomplish some of its biologic functions.
The platelet glycoprotein αIIbβ3 antagonist c7E3 has been approved for human use to reduce the risk for ischemic complications after percutaneous coronary intervention, angioplasty, or atheroctomy.57 However, it still has to be proven whether the beneficial effects of this antibody derive from the αVβ3 or the αIIbβ3 blockade. This is a critical issue for the successful design of effective protocols. Animals lacking αIIb described in this study, and those deficient in β3,48 might constitute valuable tools for developing comparative assays to define unambiguously the involvement of each integrin in physiological and pathologic processes.
Platelets are involved in a number of vascular diseases, including thrombosis, restenosis, and atherosclerosis. Different transgenic mouse models with an atherosclerotic phenotype have been developed.58,59 Thus, it is now feasible to create a second generation of atherosclerotic animals in which the precise roles of the αIIbβ3 integrin and platelet aggregation might be assigned. Furthermore, a model for arterial injury has been reported.60 Its use in animals such as αIIb-deficient mice could help determine whether this integrin influences the outcome of vascular insult.
In conclusion, αIIb-deficient mice constitute a reliable model for type 1 Glanzmann thrombasthenia. Furthermore, the present data strongly support the notion that the expression of lineage-specific genes occurs at the stem cell level. An interesting question that arises from these findings is whether a gene encoding an integrin subunit, which is functionally important in the terminal phase of the megakaryocytic differentiation, is transcriptionally active in a primitive totipotent cell. Clearly, the potential role of the αIIb subunit in early hematopoietic development must be further examined.
Acknowledgments
We thank Dr Nagy for providing the R1 ES cells, Muriel Vernet for assistance with ES cells culture and the production of mice, and Philippe Tropel for assistance with the experimental work. We thank Dr Roland Berthier for help with BM cultures, Dr Jean Jacques Ryckwaert for advice concerning platelet biochemistry, and HervéPointu for excellent technical assistance. Finally, we thank Yotis Senis for many valuable comments on the manuscript.
Supported by the Commissariat á l'Energie Atomique and by a grant from l'Association pour la Recherche sur le Cancer.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Diana Tronik-Le Roux, Commissariat àl'Energie Atomique, Département de Radiobiologie et Radiopathologie, DRR/LRGH, CEA-Evry, 2 rue Gaston Crémieux, 91057 Evry Cedex, France; e-mail: leroux@dsvidf.cea.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal