Abstract
Systemic mastocytosis is a disease of mast cell proliferation that may be associated with hematologic disorders. There are no features on examination that allow the diagnosis of systemic disease, and mast cell–derived mediators, which may be elevated in urine or blood, may also be elevated in individuals with severe allergic disorders. Thus, the diagnosis usually depends on results of bone marrow biopsy. To facilitate evaluation, surrogate markers of the extent and severity of the disease are needed. Because of the association of mastocytosis with hematologic disease, plasma levels were measured for soluble KIT (sKIT) and soluble interleukin-2 receptor alpha chain (sCD25), which are known to be cleaved in part from the mast cell surface and are elevated in some hematologic malignancies. Results revealed that levels of both soluble receptors are increased in systemic mastocytosis. Median plasma sKIT concentrations as expressed by AU/mL (1 AU = 1.4 ng/mL) were as follows: controls, 176 (n = 60); urticaria pigmentosa without systemic involvement, 194 (n = 8); systemic indolent mastocytosis, 511 (n = 30); systemic mastocytosis with an associated hematologic disorder, 1320 (n = 7); aggressive mastocytosis, 3390 (n = 3). Plasma sCD25 levels were elevated in systemic mastocytosis; the highest levels were associated with extensive bone marrow involvement. Levels of sKIT correlated with total tryptase levels, sCD25 levels, and bone marrow pathology. These results demonstrate that sKIT and sCD25 are useful surrogate markers of disease severity in patients with mastocytosis and should aid in diagnosis, in the selection of those needing a bone marrow biopsy, and in the documentation of disease progression.
Introduction
Mastocytosis is characterized by an increase in tissue mast cells, particularly in the skin, bone marrow, lymph nodes, liver, and spleen. Activating mutations in c-kit, the receptor for stem cell factor, which is critical for mast cell proliferation, differentiation, and survival, have been found in both children and adults with this disease.1,2 The diagnosis and determination of the extent of disease and the assignment of prognosis in mastocytosis remain largely within the realm of clinical judgment influenced by the duration of the disease and tissue biopsy. Mast cell mediator levels may be elevated in plasma3,4(tryptases and histamine) and in urine5,6 (prostaglandin D2 and histamine metabolites). Mediator levels are generally believed to increase in parallel with an increase in tissue mast cells7 and thus are diagnostic aids that also may reflect the extent of disease. Confounding interpretation, levels of beta-tryptase, histamine, and prostaglandin D2 are also elevated in patients experiencing a severe systemic allergic reaction (anaphylaxis).8-10
In some patients, however, the increase in mast cell numbers occurs in the presence of an identifiable hematologic disorder, particularly dysmyelopoiesis.11-13 The identification in circulation of surrogate markers of hematologic disease would facilitate understanding the spectrum of pathologic findings in mastocytosis and also in identifying individuals exhibiting this process. Thus, 2 possible surrogate markers of bone marrow disease appeared promising. The first of these was soluble KIT (sKIT). KIT is expressed on primitive hematopoietic cells and on mast cells at all stages of maturation. The second candidate marker, soluble CD25 (sCD25), is the alpha chain of interleukin-2 (IL-2) receptor. CD25 is known to be expressed on bone marrow mast cells in mastocytosis. The soluble forms of both receptors are elevated in the plasma of patients with certain hematologic malignancies.14-19
As will be shown, both sKIT and sCD25 are increased in the plasma of patients with mastocytosis. Soluble KIT levels, in particular, seem to reflect the extent and severity of disease. Both sKIT and sCD25 correlate with plasma total tryptase levels and severity of bone marrow pathology. Thus, the ability to measure sKIT and sCD25 in the plasma of patients suspected of having mastocytosis adds insights into the pathophysiology of mastocytosis and should aid in assessing the severity of mastocytosis and assigning prognosis.
Patients and methods
Patients
In a study approved by the Institutional Review Board of the National Institute of Allergy and Infectious Diseases, 48 patients with mastocytosis were examined. All patients signed an informed consent document. The diagnosis of mastocytosis was based on the demonstration of characteristic bone marrow and/or skin biopsy findings. Each patient was assigned a category according to a consensus classification20 based on the pattern of the disease: Ia, indolent mastocytosis with skin involvement only (n = 8); Ib, indolent mastocytosis with systemic involvement as evidenced by characteristic mast cell aggregates in the bone marrow (n = 30); II, mastocytosis with an associated hematologic disorder (n = 7); III, aggressive mastocytosis with lymphadenopathy and peripheral eosinophilia (n = 3). The median age for the patients with mastocytosis was 44.5 years, and the range was 18 through 73 years. Approximately 40% were male. In addition, sKIT was assayed in 11 serum samples collected from adult patients (45% male), each with clinically suspected anaphylaxis showing an elevated serum beta-tryptase level (4 ng/mL or higher) and a total tryptase to beta-tryptase ratio of less than 6. Plasma samples from anonymous healthy adult donors were obtained from the Department of Transfusion Medicine at the National Institutes of Health Clinical Center. Peripheral blood was collected in EDTA-containing tubes. Plasma was separated following centrifugation of blood samples. Aliquots were kept at −70°C until analyzed.
Determination of sKIT
Soluble KIT was measured by enzyme-linked immunosorbent assay (ELISA) with the use of the monoclonal antibodies L6 and L15 as described (Nichirei Diagnostics, Nichirei, Japan).21 These antibodies do not block stem cell factor (SCF) binding to KIT, and the immunoassay is not affected by addition of up to 20 ng/mL free recombinant SCF. In this assay, 10 μL of each plasma sample was mixed with 100 μL of alkaline phosphatase-conjugated anti-KIT monoclonal antibody (mAb) L15 in a buffer containing 0.5% bovine serum albumin, 0.25 mol/L NaCl, 1 mmol/L MgCl2, 0.1 mmol/L ZnCl2, 0.1% NaN3, and 50 mmol/L Tris-HCl (pH 8.0). Then, 100 μL of this mixture was incubated for 3 hours at room temperature in separate wells of 96-well microtiter plates coated with purified anti-KIT mAb L6. The plates were then washed with 0.9% NaCl and 100 μL of a p-nitrophenyl phosphate solution added to each well followed by a 1-hour incubation. The reaction was stopped by adding 50 μL of 0.2 mol/L NaOH to each well. The optical density of each well was determined at 405 nm (Model HTS 7000 Bioassay Reader; Perkin Elmer, Foster City, CA). All samples were assayed in duplicate. Samples with optical density values higher than the confidence range of the assay (ie, 800 AU/mL) were diluted and reassayed. Preliminary experiments determined there was no difference in sKIT levels between serum and plasma samples.
Determinations of SCF, sCD25, and IL-2
Plasma levels of SCF, sCD25, and IL-2 were determined by commercially available ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. The SCF ELISA detects free SCF as well as SCF in complex with KIT. The optical density was read at 450 nm with correction at 570 nm. All samples were assayed in duplicate.
Determination of tryptase
Tryptase in serum or plasma was measured by means of sandwich immunoassays.3 Beta-tryptase levels were measured with the use of mAbs B12 for capture and biotin-G5 for detection. Total tryptase levels (alpha-tryptases plus beta-tryptases, primarily alpha-protryptase at baseline) were measured on the UniCAP system (Pharmacia-Upjohn, Peapack, NJ) with the use of mAbs B12 for capture and beta-galactosidase-G4 for detection.
Statistical analysis
Comparisons for statistical significance were performed with the use of the Wilcoxon rank sum (Mann-Whitney U) test. Correlations between observations were assessed by Spearman's rank correlation coefficient. Contingency table analyses were performed by Fisher's exact test. A P value of < .05 was considered statistically significant.
Results
Plasma sKIT levels in mastocytosis and systemic anaphylaxis
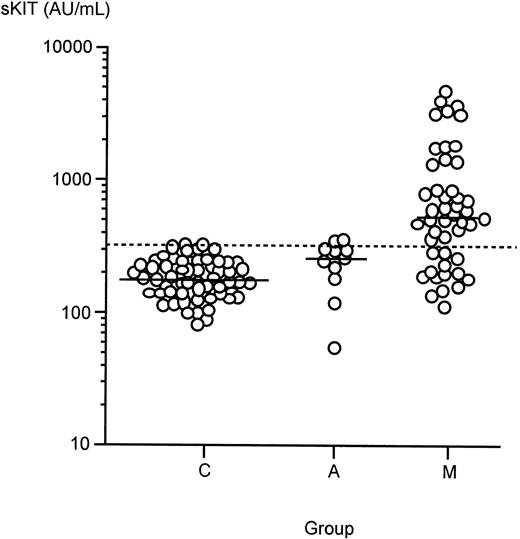
To determine if sKIT is elevated in mastocytosis and systemic anaphylaxis, we determined the plasma sKIT levels in 48 patients with mastocytosis and 11 patients with systemic anaphylaxis and compared the levels with those found in healthy controls. The control group had a median plasma sKIT level of 176 AU/mL (range, 81-322 AU/mL; n = 60) (Figure 1). These data are consistent with reported levels of sKIT in healthy adults.21 There was a statistically significant increase of sKIT in anaphylaxis serum when compared with healthy controls (median, 260 AU/mL; range, 55-356 AU/mL; P < .05). Plasma sKIT levels were also found to be significantly elevated in patients with mastocytosis, where the median value was considerably higher (median, 513 AU/mL; range, 112-5055 AU/mL; P < .00001 vs healthy controls andP < .005 vs anaphylaxis).
Plasma levels of sKIT in healthy controls (C) and in patients with mastocytosis (M) or systemic anaphylaxis (A).
Bars represent median values. Dashed line is the sKIT concentration (312 AU/mL), which corresponds to the mean + 2 SD of healthy control values.
Plasma levels of sKIT in healthy controls (C) and in patients with mastocytosis (M) or systemic anaphylaxis (A).
Bars represent median values. Dashed line is the sKIT concentration (312 AU/mL), which corresponds to the mean + 2 SD of healthy control values.
Correlation of plasma sKIT with disease category
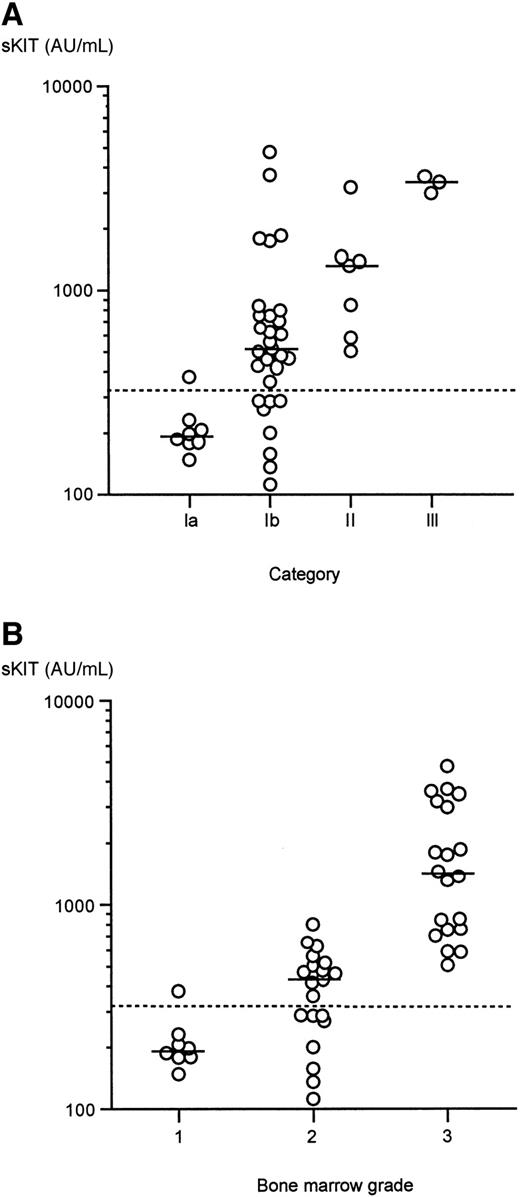
Because plasma sKIT levels were observed over a broad range in patients with mastocytosis, we determined whether this variation correlated with clinical patterns of disease. As can be seen in Figure2A, plasma sKIT levels were not significantly elevated in those with skin involvement only when compared with normal subjects (category Ia: median, 194 AU/mL; range, 148-377 AU/mL; statistically insignificant when compared with control group). However, levels were significantly elevated in systemic mastocytosis (ie, categories Ib, II, and III), and the level of elevation was greatest in those with more severe forms of disease. Thus, patients with systemic indolent disease (Ib) had a median level of 511 AU/mL (range, 112-4755 AU/mL; P < .00001 vs healthy controls and P < .002 vs category Ia). The median sKIT level for patients with an associated hematologic disorder was 1320 AU/mL (range, 506-3195 AU/mL; P < .00005 vs healthy controls, P < .002 vs category Ia, andP < .05 vs category Ib). Patients with aggressive mastocytosis who presented with lymphadenopathy and exhibited a rapidly progressive course (category III) had the highest median plasma level of sKIT (3390 AU/mL; range, 2910-3605 AU/mL; P < .005 vs healthy controls, P < .02 vs categories Ia and Ib,P < .05 vs category II).
Correlation of plasma sKIT levels with disease severity.
(A) Plasma sKIT levels correlate with disease severity based on clinical categorization. Category Ia, urticaria pigmentosa without systemic involvement; category Ib, systemic indolent mastocytosis; category II, mastocytosis with an associated hematologic disorder; category III, aggressive mastocytosis. (B) Plasma sKIT levels correlate with disease severity based on bone marrow pathology. Histopathological findings in bone marrow biopsies were graded as follows: grade 1: no evidence of increased mast cell numbers; grade 2: focal mast cell aggregates, normocellular marrow; grade 3: focal or diffuse mast cell aggregates and hypercellular marrow, or presence of a hematologic disorder. Bars represent median values.
Correlation of plasma sKIT levels with disease severity.
(A) Plasma sKIT levels correlate with disease severity based on clinical categorization. Category Ia, urticaria pigmentosa without systemic involvement; category Ib, systemic indolent mastocytosis; category II, mastocytosis with an associated hematologic disorder; category III, aggressive mastocytosis. (B) Plasma sKIT levels correlate with disease severity based on bone marrow pathology. Histopathological findings in bone marrow biopsies were graded as follows: grade 1: no evidence of increased mast cell numbers; grade 2: focal mast cell aggregates, normocellular marrow; grade 3: focal or diffuse mast cell aggregates and hypercellular marrow, or presence of a hematologic disorder. Bars represent median values.
Correlation of sKIT with bone marrow pathology
Mastocytosis may be associated with dysmyelopoiesis. Soluble KIT shed from mast cells and other KIT-bearing hematopoietic cells might be expected to increase as hematopoiesis becomes more disordered. We thus correlated the plasma sKIT concentrations with the extent of bone marrow pathology. Pathological findings in bone marrow biopsies from patients with mastocytosis were divided into 3 groups: (1) normocellular bone marrow with no evidence of increased mast cells (n = 8); (2) normocellular bone marrow with diagnostic focal increases in mast cell numbers (n = 20); and (3) focal or diffuse mast cell aggregates and hypercellular bone marrow or evidence of an associated hematologic disorder such as myelodysplasia or myeloproliferative state (n = 20). In the last patient group, 7 deaths occurred over a 2- to 42-month follow-up. No disease-related deaths were reported in groups 1 and 2 over a 1- to 11-year follow-up.
Elevations in sKIT were found to correlate with the extent of bone marrow involvement (Figure 2B), with the highest levels observed in group 3 (median levels: 193.5 AU/mL in group 1; 421.5 AU/mL in group 2; 1418 AU/mL in group 3). Soluble KIT levels in patients in groups 2 and 3 were significantly higher than in healthy controls and in group 1. Thus, the higher the levels of sKIT, the greater the likelihood that the patient will have an abnormal bone marrow at the time of the measurement.
Correlation of sKIT levels with detectability of c-kit mutation
The detectability of the somatic c-kit mutations at the amino acid position D816 (single-letter amino acid code) by restriction fragment length polymorphism analysis following reverse transcription polymerase chain reaction in the peripheral blood white cells has been shown to correlate with disease severity and a poorer prognosis.22 We therefore compared sKIT levels in patients who carried this molecular marker with those who did not. Results revealed a statistically significant increase in sKIT levels in patients who exhibited the D816 c-kit mutation in the peripheral blood (median levels of 421.5 AU/mL vs 1320 AU/mL,P < .005). This is consistent with the correlation found in categorizations based on clinical presentation and bone marrow pathology.
Correlation of plasma sKIT levels with plasma tryptase
Because plasma levels of tryptase have been shown to be elevated in mastocytosis, and proposed as an indicator of total body mast cell burden,3 we next determined tryptase levels in plasma samples from the same group of 48 patients with mastocytosis. Prior work suggested that total tryptase levels of 20 ng/mL or more in association with a total tryptase to beta-tryptase ratio of 20 or more identifies patients with systemic mastocytosis.3,7 The median total tryptase plasma level in healthy controls was 5.0 ng/mL. Statistically significant elevations in total tryptase were found in patients with all forms of the disease when compared with healthy controls: median tryptase levels (ng/mL) were 13 (range, 4-35) for category Ia; 61 (range, 16-400) for category Ib; 159 (range, 59-347) for category II; and 219 (range, 26-670) for category III. The beta form of tryptase was undetectable (ie, below 1 ng/mL) in 23 out of 48 patients and ranged from 1 through 53 ng/mL in the remaining patients. Such detectable levels of beta-tryptase are thought to reflect concurrent mast cell activation and cross-reactivity with alpha-protryptase in patients with biopsy-confirmed mastocytosis. There was no statistically significant difference in total tryptase levels or total tryptase/beta-tryptase ratios among categories Ib, II, and III. These data are consistent with previous reports.3
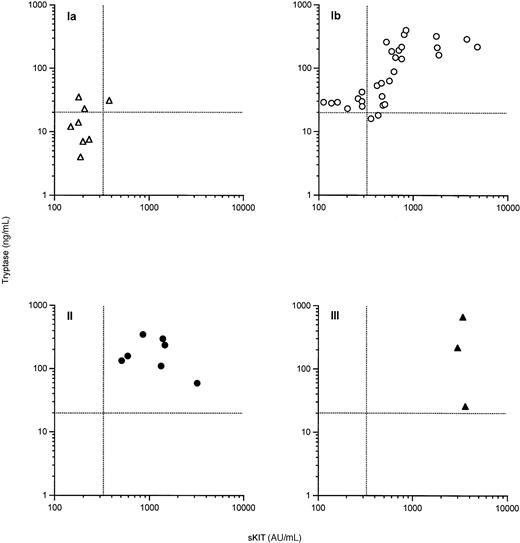
When plasma sKIT and total tryptase levels were compared within each disease category (Figure 3), a highly significant overall correlation was found between plasma sKIT and total tryptase levels (Spearman's r = 0.77P < .0001) and in patients with systemic indolent disease (category Ib) (r = 0.81, P < .0001). This correlation was not evident for patients with the mildest (Ia) or more extensive (II and III) categories of disease. Of 8 patients in category Ia, 3 had total tryptase levels exceeding 20 ng/mL, the cutoff value suggestive of a diagnosis of systemic mastocytosis, whereas only 1 patient in this category had an sKIT value greater than the highest normal control. Of the 30 patients classified as category Ib, 8 had an sKIT level lower than the highest normal control, whereas 2 had a total tryptase level below 20. This suggests that total tryptase levels are a more sensitive indicator than sKIT of an increased mast cell burden. On the other hand, sKIT elevations were not observed to be paralleled to the same extent by total tryptase values in advanced categories of disease (ie, II and III), although the levels of both proteins were increased. These data suggest that as the bone marrow pathology increases, it may not be accompanied by a parallel increase in total tryptase, but is better reflected by sKIT levels.
Correlation of plasma sKIT with plasma total tryptase in patients with mastocytosis.
▵ indicates category Ia; ○, category Ib; ●, category II; and ▴, category III. Dashed lines represent suggested cutoff values.
Correlation of plasma sKIT with plasma total tryptase in patients with mastocytosis.
▵ indicates category Ia; ○, category Ib; ●, category II; and ▴, category III. Dashed lines represent suggested cutoff values.
Lack of correlation between plasma sKIT and SCF
Soluble receptors may in some instances influence the circulating levels of their ligands by accelerating their metabolic clearance or by competing for binding to the cell-surface receptors.23 We therefore determined the plasma levels of SCF. The median SCF level in control samples was 983 pg/mL (range, 440-1838 pg/mL). Patients with mastocytosis had significantly higher plasma SCF levels than healthy controls (median, 1335; range, 272-2256, P < .00005). Analysis of SCF levels in different categories of disease revealed a significant increase only in category Ib (P < .00001). Patients with mastocytosis in categories Ia, Ib, II, and III had median SCF levels of 1211, 1615, 1312, and 553 pg/mL, respectively. There was no significant correlation between SCF and sKIT levels. All 10 patients in advanced categories (ie, II and III) had SCF levels below 1500 pg/mL, whereas only 16 of 38 patients (42%) with indolent disease had similar levels (Fisher's exact probabilityP < .001).
Correlation of hematologic parameters with plasma sKIT and SCF
Abnormalities in the numbers of circulating blood cells are encountered in systemic mastocytosis. In addition to the presence of a hematologic disorder,12 the presence of anemia, thrombocytopenia, and a hypercellular bone marrow implies more extensive disease and a poorer prognosis.24 However, we found no significant correlation between sKIT and hemoglobin levels, total white blood cell (WBC) count, and platelet count. Analysis of differential WBC counts for neutrophils, monocytes, and lymphocytes similarly failed to yield a significant correlation (data not shown). There was a marginal negative correlation between SCF levels and total WBC counts (Spearman's r = −0.30, P < .05) as well as lymphocyte counts (r = −0.33, P < .05). Because sKIT correlates with bone marrow pathology but not peripheral blood abnormalities, sKIT may indicate bone marrow disease prior to its reflection in peripheral blood counts.
Soluble CD25 in mastocytosis
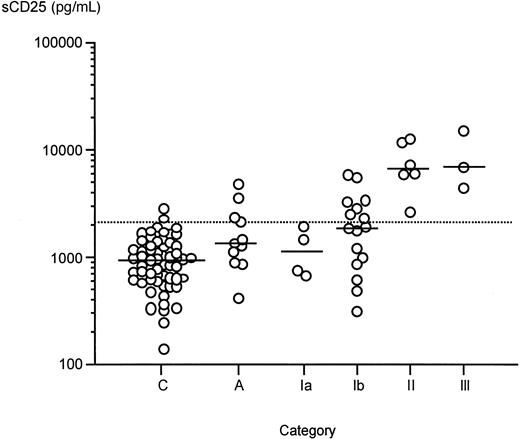
Demonstration of the elevation of sKIT in mastocytosis suggests that soluble forms of the membrane proteins highly or preferentially expressed by mast cells may be useful surrogate markers of disease activity and prognosis. This prompted an investigation of the plasma levels of sCD25. Because CD25 is reported to be expressed by the bone marrow mast cells of patients with systemic mastocytosis but not healthy individuals,25 we determined plasma sCD25 and IL-2 levels in a representative subset of 29 patients with mastocytosis and those with anaphylaxis. Results revealed an overall elevation of plasma sCD25 both in mastocytosis (median, 955 pg/mL; range, 138-2829 in controls, vs median, 2491; range, 310-15 020 in mastocytosis;P < .00001) and in anaphylaxis (median, 1361 pg/mL;P < .05 vs controls). Soluble CD25 was also significantly elevated in each category of systemic mastocytosis (categories Ib, II, and III) (Figure 4) when compared with controls (Ia median, 1092 pg/mL; range, 671-1933; Ib median, 1866; range, 310-5757; II median, 6589; range, 2677-12 566; III median, 6852; range, 4411-15 020). The elevations were more pronounced in categories II and III, both of which were significantly higher than categories Ia and Ib, although there was no significant difference between categories Ia vs Ib and II vs III. Analysis of sCD25 levels according to bone marrow pathology revealed that patients with hypercellular bone marrows had significantly higher levels than those with less or no significant pathology (P < .005 andP < .0001 when bone marrow pathology group 3 is compared with groups 1 and 2, respectively; data not shown). Patients who had detectable levels of D816V (single-letter amino acid code) messenger RNA in peripheral blood white cells similarly had significantly higher sCD25 levels than those who did not (P < .05). Plasma IL-2 levels in all patients were below the detection limit of the assay (ie, below 7 pg/mL) (data not shown).
Plasma sCD25 levels in mastocytosis, anaphylaxis (A) and healthy controls (C).
Bars represent median values. Dashed line represents mean + 2 SD value of sCD25 in healthy controls in this study (1150 pg/mL). Categories of mastocytosis are the same as described for Figure 2A.
Plasma sCD25 levels in mastocytosis, anaphylaxis (A) and healthy controls (C).
Bars represent median values. Dashed line represents mean + 2 SD value of sCD25 in healthy controls in this study (1150 pg/mL). Categories of mastocytosis are the same as described for Figure 2A.
Soluble CD25 plasma levels strongly correlated with those of sKIT and total tryptase in patients with mastocytosis (Spearman's r = 0.72,P < .0001, and r = 0.67, P < .0001, respectively) (Figure 5). A slight negative correlation was observed between sCD25 and SCF levels (r = −0.41, P < .05). There was no correlation between sCD25 levels and the total white cell, lymphocyte, neutrophil, monocyte, or platelet counts or hemoglobin levels (data not shown). These data thus suggest that sCD25, analogous to sKIT and tryptase, is a surrogate marker of disease severity in mastocytosis.
Correlation of plasma sCD25 with (A) sKIT and (B) total tryptase.
▵ indicates category Ia; ○, category Ib; ●, category II; and ▴, category III.
Correlation of plasma sCD25 with (A) sKIT and (B) total tryptase.
▵ indicates category Ia; ○, category Ib; ●, category II; and ▴, category III.
Discussion
This study documents that sKIT and sCD25 levels are significantly elevated in systemic forms of mastocytosis when compared with healthy controls and patients with suspected systemic anaphylaxis. There was overlap between normal values and those obtained from patients with milder forms of the disease and among systemic disease categories. The sKIT value corresponding to the mean + 2 SD in the normal population was 312 AU/mL. Soluble KIT levels in 9 of 11 patients with anaphylaxis were within healthy normal range (Figure 1). Out of 8 patients in category Ia, 7 (88%) had levels below this value. This overlap was less apparent (8 out of 30 or 27%) for patients with systemic indolent disease (category Ib) and did not exist for categories II and III. While half of the patients with an sKIT value below 300 AU/mL lacked systemic involvement, sKIT levels higher than 400 AU/mL were exclusively associated with systemic forms of the disease. All 3 patients in category III had sKIT levels exceeding 2900 AU/mL. Such high levels of sKIT have not been reported in other diseases.
Similarly, there was a significant difference in median sCD25 levels in systemic mastocytosis when compared with controls, although some values in healthy individuals overlapped with the values in indolent forms of the disease. Soluble CD25 levels in aggressive forms of the disease did not display this overlap. Thus, all the normal controls and patients without systemic involvement had sCD25 levels below 3000 pg/mL, while 8 of 9 patients (88%) with an associated hematologic disorder or aggressive disease had levels above this value.
Soluble KIT and sCD25 levels correlated not only with the classification based on clinical presentation but also with bone marrow pathology and detectability of the D816V mutation in blood. Because both sKIT and sCD25 are thought to be generated by the proteolytic cleavage of the membrane-bound receptors,26 it is possible that the increased levels in mastocytosis in part reflect increased mast cell numbers. This is supported by the finding that sKIT and sCD25 levels highly correlated with total tryptase levels, a proposed marker of mast cell burden.
Comparison of the diagnostic value of plasma sKIT measurements with that of total tryptase (primarily alpha-protryptase) shows that these 2 assays may be complementary in assessment of the disease status in mastocytosis and its differential diagnosis from idiopathic anaphylaxis. It appears that total tryptase is a more sensitive indicator of increased mast cell burden in early stages of the disease, because 5 out of 8 patients with category Ia and 28 of 30 patients with systemic disease had total tryptase levels greater than the suggested cutoff value of 20 ng/mL. On the other hand, there was considerable overlap of total tryptase measurements between patients with category Ia, Ib, and II forms of systemic disease. Therefore, it appears that total tryptase levels may best reflect the total body burden of mast cells, but not necessarily the bone marrow pathology of the underlying disease. In patients with aggressive disease, non–mast cell lineages that express KIT but not tryptase may be involved. Also, it is possible that mast cells in lesions of category III mastocytosis produce less tryptase and more KIT per cell than those associated with less aggressive disease.
Soluble KIT levels have been determined in a number of other clinical conditions. Among the hematopoietic neoplasms examined, acute myeloid leukemia subtypes M1 and M2 were associated with modest increases in sKIT levels.14 One study found increased levels of sKIT in chronic myeloid leukemia (CML) in acute myeloid blast crisis and advanced myelodysplastic syndromes,15 although another study showed no statistically significant difference in these disease groups.14 The highest level of sKIT in these studies was 1470 AU/mL, using the same ELISA system as ours, and was reported in a patient with CML in myeloid blast crisis. Similar to mastocytosis, elevated levels of sKIT in these malignancies are thought to be related to the higher surface expression of KIT in the immature neoplastic cells. Mild yet statistically significant increases have also been reported in chronic renal failure with a median value of 244 AU/mL.27 In contrast, low levels of sKIT were associated with graft-vs-host reaction,28 delayed engraftment after bone marrow transplantation,29 and systemic lupus erythematosus.21
In contrast to the relatively few clinical conditions in which sKIT is known to be elevated, sCD25 is elevated in a number of hematologic malignancies,16-19 solid tumors,30 rheumatic disorders,31-34 and infectious diseases.35-37These elevations may reflect the numbers of activated T cells or neoplastic cells displaying CD25 on their surface. Indeed, changes in sCD25 levels correspond to clinical response to therapy in hematologic neoplasms such as hairy cell leukemia and adult T-cell leukemia.18 38 Monitoring of sCD25 levels could similarly be used as a predictor of response to therapy in mastocytosis.
A modest increase in sCD25 similar to that of sKIT was found in patients with anaphylaxis in this study. This finding is consistent with an earlier report of elevated sCD25 in children with a history of anaphylactic reaction to food or atopic eczema.39 The cellular source of sCD25 in these patients is likely to be activated T lymphocytes, in line with the demonstration of activated T cells in hymenoptera hypersensitivity40 and down-regulation of their surface IL-2 receptors following rush hyposensitization.41
The levels of SCF were found to be elevated in patients with systemic indolent mastocytosis although they did not correlate with those of sKIT or the patterns of disease. There was a mild inverse correlation between SCF levels and WBC and lymphocyte numbers. A similar inverse correlation was observed in patients with renal failure, which was thought to reflect a physiological response to stimulate the bone marrow in cases of inefficient hematopoiesis.27 Other studies, however, failed to yield a similar negative correlation in other bone marrow failure states, such as aplastic anemias and myelodysplastic syndromes.42-44 Whether the high levels of sKIT in mastocytosis have a pathophysiological relevance is unknown. Soluble KIT circulates in approximately 30-fold molar excess of its ligand SCF under physiologic circumstances.45 According to simple dissociation kinetics, it is predicted that about 85% of SCF exists bound to sKIT. Since sKIT can compete with the membrane-bound KIT for binding of SCF, a negative regulatory role is proposed for this soluble receptor.26 In advanced mastocytosis, where plasma levels of sKIT are increased 10-fold or more, approximately 98% of SCF may be predicted to exist in complex with sKIT. Therefore, high levels of sKIT may contribute to the impaired hematopoiesis in patients with advanced categories of disease.
In conclusion, we have demonstrated that plasma sKIT and sCD25 levels are elevated in mastocytosis and that the level of elevation correlates with the severity of cellular and molecular parameters of pathology of the disease. Assessment of these markers together with tryptase levels provides valuable information relating to the probable severity of mastocytosis, may help determine which individuals should undergo a bone marrow biopsy, and will facilitate the differential diagnosis of patients evaluated after anaphylactic episodes. Finally, determination of the plasma levels of these receptors may help identify patient subpopulations that may be predicted to respond to biological therapies targeting these receptors on the neoplastic mast cells.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Cem Akin, Laboratory of Allergic Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Building 10, Room 11C205, 10 Center Drive, MSC 1881, Bethesda, MD 20892-1881; e-mail: cakin@niaid.nih.gov.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal