Abstract
Thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS) are, in adults, clinically and pathologically indistinguishable except for the severity of renal failure. They are best described as a single disorder, TTP-HUS, because the diagnostic evaluation and initial management are the same. Treatment with plasma exchange, available for more than 20 years, has dramatically altered the course of disease in adults with TTP-HUS. Plasma exchange has improved survival rates from 10% to between 75% and 92%, creating urgency for the initiation of treatment. This has resulted in decreased stringency of diagnostic criteria, which in turn has resulted in a broader spectrum of disorders for which the diagnosis of TTP-HUS is considered. Long-term follow-up has revealed increasing frequencies of relapse and of chronic renal failure. Although the increased survival rate is dramatic and recent advances in understanding the pathogenesis of these syndromes are remarkable, clinical decisions remain empirical. Therefore, the management decisions for patients with suspected TTP-HUS rely on individual experience and opinion, resulting in many different practice patterns. Multipractice clinical trials are required to define optimal management.
Introduction
This article, “How I treat patients with thrombotic thrombocytopenic purpura–hemolytic uremic syndrome,” is no more than the title states. It is a concise review of the clinical syndromes, followed by a description of diagnosis and treatment. There are some observations from published case series that can guide us, but other than the randomized clinical trial demonstrating superiority of plasma exchange over plasma infusion,1 there is no firm evidence on which to base recommendations. Hence, there are no recommendations here, only opinions from my experience.
Defining the clinical syndromes
Thrombotic thrombocytopenic purpura (TTP) is a classic disease of hematology. The onset is often dramatic, as in the initially described patient,2 and the presenting features, when characteristic,3 are immediately recognizable. The defining clinical and pathologic features of TTP were described in a 1966 review3 of 271 patients: thrombocytopenia, microangiopathic hemolytic anemia, neurologic abnormalities, renal abnormalities, and fever were present in 88% to 98% of patients; the pathologic feature of thrombotic microangiopathy was demonstrated in 93% of patients. There was no effective treatment. Ninety percent of patients died.3 The course of TTP has changed dramatically since this initial definition.3 With plasma exchange treatment, it has become a curable illness. The availability of effective treatment has created an urgency for establishing the diagnosis, and this urgency has required decreased stringency of diagnostic criteria, from the classic pentad of clinical features3 to the current dyad1,4 (Table1). Inevitably, less stringent diagnostic criteria have resulted in a broader spectrum of disorders treated for possible TTP. Long-term follow-up of patients recovered from acute TTP has revealed an increasing frequency of recurrent episodes5-7 and chronic renal failure.5 7
Presenting signs and symptoms of patients with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome
| Primary diagnostic criteria: |
| Thrombocytopenia |
| Microangiopathic hemolytic anemia (defined by negative findings on direct antiglobulin test, red blood cell fragmentation, and evidence of accelerated red cell production and destruction) |
| No clinically apparent alternative explanation for thrombocytopenia and anemia |
| Other common clinical features: |
| Renal function abnormalities (proteinuria/hematuria common, acute renal failure and oliguria less common) |
| Neurologic abnormalities (mental status changes common, focal abnormalities less common) |
| Weakness |
| Abdominal symptoms (nausea, vomiting, diarrhea, pain) |
| Fever (high fever with chills is evidence against the diagnosis of TTP-HUS) |
| Primary diagnostic criteria: |
| Thrombocytopenia |
| Microangiopathic hemolytic anemia (defined by negative findings on direct antiglobulin test, red blood cell fragmentation, and evidence of accelerated red cell production and destruction) |
| No clinically apparent alternative explanation for thrombocytopenia and anemia |
| Other common clinical features: |
| Renal function abnormalities (proteinuria/hematuria common, acute renal failure and oliguria less common) |
| Neurologic abnormalities (mental status changes common, focal abnormalities less common) |
| Weakness |
| Abdominal symptoms (nausea, vomiting, diarrhea, pain) |
| Fever (high fever with chills is evidence against the diagnosis of TTP-HUS) |
The presence of the primary diagnostic criteria is sufficient to establish a presumptive diagnosis of TTP-HUS and to begin plasma exchange treatment.1,4 The additional clinical features support the diagnosis but may not be present. Profound weakness is a common symptom that appears to reflect more than just rapidly developing anemia. Abdominal symptoms are present in many more patients than those with infectious bloody diarrhea. Although fever may occur as part of the TTP-HUS syndrome,3 high fever with shaking chills more likely indicates sepsis as the primary diagnosis.
Hemolytic uremic syndrome (HUS) is a classic disease of nephrology, initially described as fatal renal cortical necrosis in children.8 It is defined by renal thrombotic microangiopathy, also the characteristic lesion of TTP,9and by thrombocytopenia and microangiopathic hemolytic anemia. Its course also has changed since its initial description, with the recognition in 1982 of Shiga toxin-producing Escherichia coli (typically 0157:H7) as a new human pathogen causing epidemic hemorrhagic colitis.10 These bacteria are now the predominant etiology of HUS in children10,11 and occasionally the etiology in adults.12 Children with epidemic diarrhea-associated HUS are not treated with plasma exchange, and nearly all recover from acute episodes.11 In contrast to children, adults with TTP-HUS after epidemic E coli0157:H7 infection have high mortality rates and appear to benefit from plasma exchange.12
Adults who have the defining diagnostic features of TTP (Table 1) but in whom acute renal failure is a prominent abnormality, with or without a diarrhea prodrome, have been described as having adult HUS, and some consider this as a distinct syndrome. However, the one prospective study of plasma exchange treatment of TTP-HUS in which patients were distinguished on the basis of renal failure demonstrated no differences in other clinical features. The severity of thrombocytopenia and anemia, and the frequency of focal neurologic signs or symptoms, were the same in patients with or without acute renal failure.1,13 This study also reported no difference in survival rates between patients without (78%)1 or with (83%)13 acute renal failure.
Yet it has been suggested that patients with HUS are usually unresponsive to plasma exchange.14 The origin for this impression is unclear. It may be related to observations that recovery of renal function is typically slow and often incomplete, in contrast to the recovery of neurologic and hematologic function, or to observations that continued plasma exchange does not seem to benefit persistent renal failure once hematologic recovery has occurred. Or it may be an extrapolation from experience with diarrhea-associated HUS in children, who recover without plasma exchange. However, the published observations12,13and our experience suggest that patients with acute renal failure respond to plasma exchange treatment as well as patients without renal failure, except for the unpredictable recovery of renal function. Certainly the survival rate of all patients with TTP-HUS, including patients with acute renal failure, is better than in the era before plasma exchange.3
This review, focused on evaluation and management in adults, will not distinguish between TTP and HUS but, rather, will use the term TTP-HUS to describe all patients. The presence or absence of acute renal failure may be important for prognosis, but the heterogeneity among TTP-HUS syndromes in adults is much greater than this single distinction. Table 2 presents a classification of TTP-HUS syndromes based on disease associations and probable etiologies. These adult syndromes are similar regarding diagnosis and management, despite their different etiologies, disease associations, and long-term outcomes.
Categories of clinical presentations of patients with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome
| Idiopathic |
| No apparent etiology or associated condition |
| Drug-induced |
| Allergic: quinine,39 ticlopidine32 |
| Dose-related toxicity: mitomycin C,40 cyclosporine,41pentostatin,42 gemcitabine43 |
| Pregnancy/postpartum |
| Bloody diarrhea |
| Infection, such asE coli 0157:H7 |
| Allogeneic bone marrow transplantation |
| Additional/alternative explanation for clinical signs and symptoms that may or may not be apparent at the time of initial diagnosis of TTP-HUS |
| Idiopathic |
| No apparent etiology or associated condition |
| Drug-induced |
| Allergic: quinine,39 ticlopidine32 |
| Dose-related toxicity: mitomycin C,40 cyclosporine,41pentostatin,42 gemcitabine43 |
| Pregnancy/postpartum |
| Bloody diarrhea |
| Infection, such asE coli 0157:H7 |
| Allogeneic bone marrow transplantation |
| Additional/alternative explanation for clinical signs and symptoms that may or may not be apparent at the time of initial diagnosis of TTP-HUS |
These categories describe the presentations of patients in The Oklahoma TTP-HUS Registry for whom plasma exchange treatment was begun for clinically suspected TTP-HUS.7 The approximate relative frequencies of these categories are: idiopathic, 40%; additional/alternative explanation, 20%; others, 10%. Not included among these categories are children, most of whom present with bloody diarrhea11 and who are not treated by plasma exchange; the youngest patient in our Registry is 14 years old. All patients fulfilled diagnostic criteria for TTP-HUS. The assignment of drug-induced categories as allergic or dose-related toxicity is based on clinical observations. Among patients with an additional or alternative explanation for the clinical features, some had an apparent additional diagnosis (eg, systemic lupus erythematosus44) when plasma exchange treatment was begun. In others an alternative explanation for the clinical features only became apparent later, and then treatment was stopped (see Table 3). Selected references are provided.
Pathogenesis
Systemic endothelial cell damage appears to be a central phenomenon in the pathogenesis of all TTP-HUS syndromes. Direct evidence for this are the demonstration of apoptosis of microvascular endothelial cells in spleens removed from patients with TTP15 and the demonstration that plasma from patients with TTP or adult HUS (distinguished from childhood epidemic diarrhea-associated HUS) can cause apoptosis of microvascular endothelial cells.16 Endothelial damage may cause the release of von Willebrand factor (vWF) multimers that are larger than vWF multimers in normal plasma.17 A plasma vWF-cleaving protease has been postulated to decrease the size of large vWF multimers to their normal size in plasma after secretion. Deficiency of this protease has been reported in patients with TTP, but not in patients with HUS (diagnostic criteria for distinguishing TTP from HUS were not reported in this study).18 vWF-cleaving protease deficiency may result in larger plasma vWF multimers that can cause platelet agglutination.19,20 These abnormalities may not be specific for TTP; patients with disseminated malignancies may have unusually large vWF multimers and deficient vWF-cleaving protease activity but no signs of TTP-HUS.21 Other studies have demonstrated enhanced proteolysis of vWF in patients with TTP-HUS, with an accumulation of abnormal low-molecular-weight multimers.22
The Oklahoma TTP-HUS Registry
Oklahoma has a unique opportunity to describe this clinical syndrome because all patients from our region with clinically suspected TTP-HUS are treated with plasma exchange by a single institution, the Oklahoma Blood Institute. The Registry began on January 1, 1989; 211 patients have been treated to the present (April 1, 2000). The number of patients treated per year has increased from 4 in 1989 to 28 in 1999, consistent with the 7-fold increase between 1981 and 1997 reported by the Canadian Apheresis Group.23 This remarkable increase may be attributed to the decreased diagnostic stringency described above, with the inclusion of a broader spectrum of disorders, and to the increased awareness of TTP-HUS. It is important to emphasize that The Oklahoma TTP-HUS Registry is not a case series of patients with TTP-HUS but of patients referred for plasma exchange treatment for clinically suspected TTP-HUS. This distinction is critical. Many patients had additional diagnoses when plasma exchange treatment for TTP-HUS was begun, or alternative diagnoses were subsequently discovered. Patients in whom alternative diagnoses became apparent (Table3) are probably excluded from most reported case series, yet on their initial evaluation TTP-HUS was the primary diagnosis. If these patients had died acutely with no autopsy, TTP-HUS would have remained the primary diagnosis. Since January 1, 1995, I have participated in the management decisions for 122 (85%) of 143 patients. Follow-up is complete to the current time for 115 of 116 living patients.
Additional/alternative diagnoses in patients presenting with clinically suspected TTP-HUS
| Additional diagnoses (apparent at the time of initiation of plasma exchange treatment): |
| Preeclampsia/eclampsia/HELLP syndrome |
| Autoimmune disorders (examples: systemic lupus erythematosus,44 scleroderma,9antiphospholipid antibody syndrome45) |
| Alternative diagnoses (only apparent or appreciated after plasma exchange is initiated) |
| Sepsis (examples: Rocky Mountain spotted fever, cytomegaloviral sepsis, disseminated aspergillosis, β-streptococcal sepsis) |
| Disseminated malignancy (examples: metastatic pancreatic or lung carcinoma,46 acute lymphocytic leukemia) |
| Malignant hypertension47 |
| Heparin-induced thrombocytopenia/thrombosis |
| Additional diagnoses (apparent at the time of initiation of plasma exchange treatment): |
| Preeclampsia/eclampsia/HELLP syndrome |
| Autoimmune disorders (examples: systemic lupus erythematosus,44 scleroderma,9antiphospholipid antibody syndrome45) |
| Alternative diagnoses (only apparent or appreciated after plasma exchange is initiated) |
| Sepsis (examples: Rocky Mountain spotted fever, cytomegaloviral sepsis, disseminated aspergillosis, β-streptococcal sepsis) |
| Disseminated malignancy (examples: metastatic pancreatic or lung carcinoma,46 acute lymphocytic leukemia) |
| Malignant hypertension47 |
| Heparin-induced thrombocytopenia/thrombosis |
In patients with established diagnoses of preeclampsia, TTP-HUS may be suspected because of the severity of neurologic and renal abnormalities and their persistence for longer than several days after delivery. Patients with an autoimmune disorder in whom hematologic, neurologic, and renal manifestations are severe and refractory to immunosuppressive treatment may be treated with plasma exchange for a possible additional diagnosis of TTP-HUS. In some patients, an alternative explanation for the signs and symptoms of TTP-HUS only becomes apparent or appreciated after plasma exchange is initiated; some examples from The Oklahoma TTP-HUS Registry are listed. Selected references are provided.
Diagnosis
In the absence of a gold standard-defining test, the diagnosis of TTP-HUS rests on the signs and symptoms presented in Table 1. The primary criteria are thrombocytopenia and microangiopathic hemolytic anemia. However, some patients may not initially be anemic, though their hematocrit levels may fall sharply after presentation, and in some patients red cell fragmentation may not be apparent.24 Serum lactate dehydrogenase (LDH) levels are increased, often to very high levels, reflecting not only hemolysis but also diffuse tissue ischemia.25 The absence of a clinically apparent alternative explanation for these abnormalities is the critical but difficult component of the diagnosis. For example, a patient with megaloblastic anemia may, on initial examination, have mental status changes, thrombocytopenia, and anemia characterized by many red cell fragments and elevated serum levels of bilirubin and LDH. If the initial evaluation suggests TTP-HUS but other etiologies are possible, can plasma exchange be withheld? Often it cannot. Table 3 shows the disorders that may accompany the diagnostic features of TTP-HUS. Many of these disorders—such as preeclampsia, autoimmune disorders, and malignant hypertension—may have the pathologic features of thrombotic microangiopathy that are characteristic of TTP-HUS.9 This diagnostic dilemma is not new; among the survivors in the defining case series were patients with systemic lupus erythematosus, and one had meningococcemia.3 Therefore, an initial diagnosis of TTP-HUS is often tentative, and the physician must remain vigilant for an alternative diagnosis.
Treatment
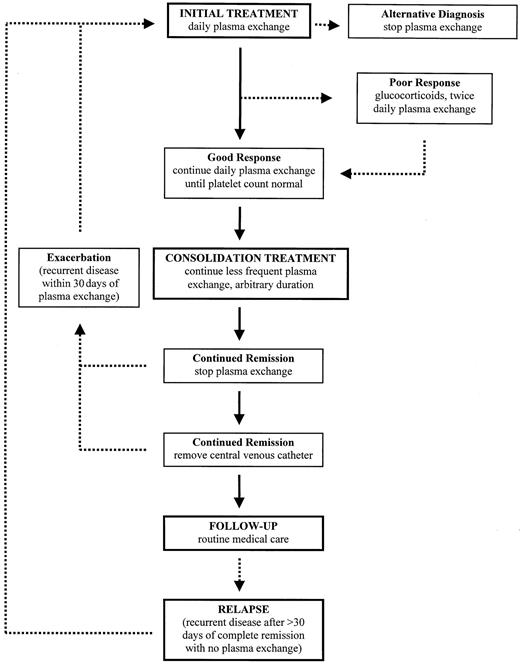
With the exception of the study demonstrating the superiority of plasma exchange over plasma infusion1 and a small study demonstrating the equivalence of fresh frozen plasma and cryosupernatant plasma for the initial treatment plasma,26there are no randomized clinical trials to provide data for management decisions. The following observations, then, are based on opinion, not on evidence. Because syndromes described as TTP or HUS may be indistinguishable (except for the presence of acute renal failure in HUS) and both respond to plasma exchange treatment (see above), vWF-cleaving protease assays cannot be expected to provide therapeutic guidance. The algorithm for our treatment is presented in Figure1.
Algorithm for the management of patients with TTP-HUS.
Solid arrows represent an ideal course; broken arrows represent common variations. The decision to initiate plasma exchange, often the most difficult step, is addressed in Tables 1 to 3. Once treatment is initiated, physicians must remain alert for alternative diagnoses. A “poor response” is not easily defined. It may indicate persistent, severe thrombocytopenia and hemolysis after several days of plasma exchange, or it may indicate recurrent, acute thrombocytopenia and hemolysis after an initial good response, or the appearance of new neurologic abnormalities, while the patient is continued on once daily plasma exchange. Consolidation treatment is empirical and is based only on observations that exacerbations of TTP-HUS are common when plasma exchange is stopped. Other considerations, such as complications with venous access, may override a decision to extend treatment. In many patients, repeated reinitiation of daily plasma exchange is required before durable complete remission is established. No maintenance treatment or specific diagnostic test appears to prevent or predict a relapse. The definitions of exacerbation and relapse, as occurring within or beyond 30 days of remission, are arbitrary.
Algorithm for the management of patients with TTP-HUS.
Solid arrows represent an ideal course; broken arrows represent common variations. The decision to initiate plasma exchange, often the most difficult step, is addressed in Tables 1 to 3. Once treatment is initiated, physicians must remain alert for alternative diagnoses. A “poor response” is not easily defined. It may indicate persistent, severe thrombocytopenia and hemolysis after several days of plasma exchange, or it may indicate recurrent, acute thrombocytopenia and hemolysis after an initial good response, or the appearance of new neurologic abnormalities, while the patient is continued on once daily plasma exchange. Consolidation treatment is empirical and is based only on observations that exacerbations of TTP-HUS are common when plasma exchange is stopped. Other considerations, such as complications with venous access, may override a decision to extend treatment. In many patients, repeated reinitiation of daily plasma exchange is required before durable complete remission is established. No maintenance treatment or specific diagnostic test appears to prevent or predict a relapse. The definitions of exacerbation and relapse, as occurring within or beyond 30 days of remission, are arbitrary.
Initial treatment
Plasma exchange is the most important treatment.1 The value of additional treatment modalities is unknown. In this report,1 all patients also received aspirin and dipyridamole; none received glucocorticoids. In another case series reported at the same time,27 all patients were treated with glucocorticoids, none with aspirin or dipyridamole. In both reports1,27 clinical outcomes were the same, suggesting that plasma exchange is the critical element of treatment and that neither aspirin, dipyridamole, nor glucocorticoids influenced recovery. Therefore, our practice has been to initiate treatment with only plasma exchange. Plasma exchange is performed once a day and replaces 1 plasma volume with either fresh frozen plasma or cryosupernatant plasma. A theoretical benefit for cryosupernatant plasma has been suggested because it is depleted of von Willebrand factor, and a retrospective survey suggested greater efficacy.28 However, findings from a small, randomized trial suggested equivalent outcomes with both products.26
Response to treatment
The response to plasma exchange treatment is variable, perhaps reflecting the heterogeneity of TTP-HUS syndromes. Nonfocal neurologic symptoms, such as mental status changes, may resolve immediately and dramatically. Thrombocytopenia typically requires several days for initial recovery to begin. Parameters of hemolysis, such as serum LDH levels, may improve promptly, yet anemia may continue to worsen and further red cell transfusion is often required. Renal failure may also worsen before improvement is noted; recovery from renal failure is unpredictable and often slow, and it may be incomplete.
The platelet count seems to be the most important parameter on which to base treatment decisions. Failure of the platelet count to respond requires intensifying treatment. Exacerbation of thrombocytopenia after initial recovery, while the patient continues to receive daily plasma exchanges, signals increased disease activity and necessitates more intense treatment. Recurrent or new neurologic abnormalities may occur during the course of treatment;1 these events are usually predicted by an exacerbation of thrombocytopenia. Often a prompt initial increase of the platelet count is followed by a period of several days with no change, suggesting a diminished response to plasma exchange; in these patients, simply continuing the daily plasma exchange is usually sufficient.
If the response to initial treatment is poor, with either no platelet count response or an exacerbation of disease activity during daily plasma exchange, glucocorticoids are added and the volume of plasma exchanged is increased. Glucocorticoid may be oral prednisone (1 mg/kg per day) or higher doses of intravenous methylprednisolone (125 mg twice daily). Plasma exchange may be intensified by increasing the volume of plasma replaced to 1.5 plasma volumes per exchange; our practice is to initiate twice daily exchanges of 1 plasma volume to minimize the recycling of infused plasma. This is a formidable procedure requiring nearly full-time personnel commitment, but refractory patients appear to respond to this plasma dose escalation. Many other modalities have been recommended (splenectomy, aspirin, dipyridamole, vincristine, other immunosuppressive agents, and IVIg), but their efficacy is unclear. The variable clinical course of TTP-HUS makes all anecdotal reports uninterpretable. Persistence and patience with plasma exchange may be the most effective practice.
Duration of treatment
No clinical parameters predict the required duration for plasma exchange. When remission is established (based on recovery of the platelet count to normal), nonfocal neurologic symptoms are resolved, the hematocrit level is stable but will not yet be normal, the serum LDH value is normal or nearly normal, and renal failure (it is hoped) is beginning to ameliorate. The decision to stop or to continue plasma exchange at this time is empirical. Prompt exacerbation of disease activity, principally manifested by a falling platelet count and requiring the resumption of daily plasma exchange, is common after the discontinuation of plasma exchange treatment, with reported frequencies of 29% to 82%.1,27 29-31 This seems to mandate additional treatment, analogous to “consolidation treatment” used in other disorders. Diminishing the frequency of plasma exchange to several times per week is practical for most patients. The feasibility of continued plasma exchange is often determined by practical, logistical issues such as the safe and efficient function of the central venous catheter or a patient's ability to return for outpatient treatment. Discontinuing plasma exchange treatment is the only way to know whether a durable remission has been achieved, and many cycles of stopping and resuming plasma exchange may be required. The additional modalities described above are often suggested for patients requiring prolonged treatment, but here also persistence with intermittent plasma exchange may be the best way to control disease activity until remission occurs.
The response to treatment may be related to the category of clinical presentation (Table 2). Prolonged courses of plasma exchange treatment, with frequent exacerbations when treatment is tapered or stopped, are characteristic of patients with idiopathic TTP-HUS. Patients with TTP-HUS caused by quinine or ticlopidine hypersensitivity, pregnancy, or an enteric infection with bloody diarrhea typically respond promptly and completely. Although the role of plasma exchange treatment in their recovery may be uncertain, in the acute urgency of initial management plasma exchange treatment cannot be withheld. A review of patients with ticlopidine-associated TTP-HUS has demonstrated decreased mortality rates in patients treated with plasma exchange.32 Patients with TTP-HUS caused by dose-related drug toxicity (eg, mitomycin C) or allogeneic marrow transplantation may not benefit from plasma exchange treatment. In contrast to other patients, these patients are often diagnosed and treated after several days of deliberation. Finally, the patient who has a clinically apparent additional diagnosis, such as systemic lupus erythematosus, has an unpredictable course. Management of plasma exchange treatment is often dictated by the activity of the associated condition. Prolonged courses of plasma exchange are uncommon; exacerbations and relapses of presumed TTP-HUS are rare.
Complications of treatment
Platelet transfusions may be dangerous in patients with TTP-HUS. Patients have been observed to have abrupt, striking deterioration after platelet transfusion, consistent with the exacerbation of thromboses.27 However, we have not observed adverse effects from platelet transfusions when they were required for an invasive procedure.
Plasma exchange treatment entails frequent and dangerous risks (Table4).33 In our prospective study of 71 consecutive patients treated for clinically suspected TTP-HUS, 21 (30%) patients had 27 major complications, including 12 systemic infections (1 fatal) and 2 episodes of hemorrhage after subclavian catheter insertion (1 fatal).33 Other major complications included pneumothorax, catheter thrombosis requiring removal of the central venous catheter, venous thrombosis requiring anticoagulant treatment, hypoxia and hypotension, and serum sickness.33 Consistent with previous observations,34 hemorrhage with catheter insertion was not related to the severity of thrombocytopenia; major bleeding did not occur in 17 patients whose platelet counts were lower than 20 000/μL. Because of the frequency of infections and thrombotic complications, central venous catheters should be removed as soon as possible, but this decision is balanced by the estimated risk for TTP-HUS exacerbation with the requirement for the resumption of plasma exchange.
Complications of plasma exchange treatment
| Complication . | Approximate frequency of major complications4-150 (% patients) . |
|---|---|
| Central venous catheter-related | |
| Insertion procedure (pneumothorax, hemorrhage) | 4 |
| Infection | 15 |
| Thrombosis (catheter obstruction, venous thrombosis) | 10 |
| Plasma-related | |
| Allergic (urticaria, serum sickness, hypoxemia, hypotension) | 4 |
| Alkalosis (paresthesias, tetany, nausea, vomiting) | — |
| Volume depletion | — |
| Transfusion-transmitted infection | 0 |
| Apheresis machine-related | |
| Persistent thrombocytopenia caused by unintentional plateletpheresis | — |
| Complication . | Approximate frequency of major complications4-150 (% patients) . |
|---|---|
| Central venous catheter-related | |
| Insertion procedure (pneumothorax, hemorrhage) | 4 |
| Infection | 15 |
| Thrombosis (catheter obstruction, venous thrombosis) | 10 |
| Plasma-related | |
| Allergic (urticaria, serum sickness, hypoxemia, hypotension) | 4 |
| Alkalosis (paresthesias, tetany, nausea, vomiting) | — |
| Volume depletion | — |
| Transfusion-transmitted infection | 0 |
| Apheresis machine-related | |
| Persistent thrombocytopenia caused by unintentional plateletpheresis | — |
Data are adapted from a study of 71 consecutive patients treated with plasma exchange for clinically suspected TTP-HUS.33Complications occurred in 43 (61%) of patients; complications were defined as major in 21 (30%) patients and caused 2 deaths.33 No transfusion-transmitted infections were detected. With some machines and settings, unintentional plateletpheresis may be an explanation for persistent thrombocytopenia when other features of TTP-HUS have resolved.
Major complications were defined by six criteria: systemic infections, prevention of treatment, requirement for an invasive procedure, red cell transfusion, systemic therapy other than Benadryl, hydrocortisone, or CaCl2, or transfer to an intensive-care unit. None of the patients with symptoms of alkalosis or volume depletion met criteria for a major complication. No frequency estimate is yet available for unintentional plateletpheresis. An additional 22 (31%) patients had minor complications, such as urticaria, hypotension or hypoxia, catheter obstruction that did not prevent completion of plasma exchange, and local infection at the catheter exit site.33
Follow-up
We have arbitrarily defined a recurrence of disease activity within 30 days of the discontinuation of plasma exchange treatment as an exacerbation of continuing active disease; when TTP-HUS recurs more than 30 days after the last plasma exchange, it is termed a relapse (Figure 1). Several patients have had relapses soon after 30 days, suggesting that this empiric duration may be too short. The variable frequency of reported relapses is dependent on the definitions used, from 2 weeks30 to 6 months6 after the completion of plasma exchange treatment, and on the duration of follow-up, given that relapse may occur after 8 to 10 years of complete remission.6 After the discontinuation of plasma exchange, frequent monitoring of blood counts and LDH values seems important; if these remain normal, the interval between evaluations is quickly extended. Then routine medical care becomes appropriate. One specific precaution we advise is to measure blood counts when symptoms of any systemic illness occur. No maintenance treatment, such as aspirin or intermittent plasma infusions,27 appears to be effective in preventing relapses. Among our patients, the frequency of relapse is different among different categories of TTP-HUS (Table2).7 Relapses are rare in patients who present during pregnancy or the postpartum period and among patients with an additional or an alternative explanation for their signs and symptoms; relapses are also rare among patients who present with bloody diarrhea; relapses have only occurred among patients with drug-induced TTP-HUS when the drug (quinine) was taken again. Among patients with idiopathic TTP-HUS, the relapse rate has been 20% and the mean follow-up 4.5 years. All patients with relapsed TTP-HUS have responded to plasma exchange treatment. We have rarely attempted to control relapses by splenectomy.35
A dilemma during follow-up is the observation of asymptomatic thrombocytopenia.5 Without knowledge of the patient's history, TTP-HUS would never be suspected, yet asymptomatic thrombocytopenia may be a prodrome for an acute relapse. In some patients retrospective evaluation suggests a long duration of signs and symptoms preceding the initial diagnosis.3,36 These observations challenge the common opinion that TTP-HUS, in contrast to idiopathic thrombocytopenic purpura, is either overtly active or in full remission. As the advent of plasma exchange treatment has created relapsing TTP-HUS, it may also reveal a new spectrum of chronic, smoldering TTP-HUS. The issue is when to intervene because asymptomatic thrombocytopenia itself does not require treatment. Glucocorticoids are simpler and safer than plasma exchange, but they may not be effective; however, the risks associated with central venous catheter insertion and plasma exchange are formidable.33
An issue specific for young women is the risk for future pregnancy. Limited experience suggests that pregnancy is associated with a risk for recurrence but that most subsequent pregnancies are uncomplicated.27
Long-term clinical outcomes
Relapse is not the only concern after successful treatment. Chronic renal failure, defined by a creatinine clearance less than 40 mL/min at 1 year after diagnosis, occurs in one fourth of our patients.7 This is comparable to the experience with children after diarrhea-associated HUS, in whom the frequency of renal function abnormalities and hypertension increases during long-term follow-up.37 38 Some patients report minor but persistent and troublesome problems with fatigue, memory, and concentration. Their significance is unknown. We are documenting and defining these symptoms by annual quality-of-life assessments.
Emotional support for patients is another important aspect of management. Coping with a disease unfamiliar to them, their families, and often their primary care physicians can be difficult, and they often have feelings of isolation and vulnerability. These problems led several of our patients to urge me to begin a support group. Although I initially had reservations, we began in 1996, and this has become a well-attended and appreciated occasion 3 times each year. We have also established a series of newsletters to patients and physicians that are distributed throughout our community and are posted on our website (http://moon.ouhsc.edu/jgeorge).
Priorities for future research
Lack of evidence on which to base recommendations for the care of patients with suspected TTP-HUS affects almost every decision a clinician commonly encounters. Rigorous clinical trial data are required to better define every aspect of diagnosis, treatment, and prognosis. The diagnosis of TTP-HUS is often uncertain, both at the initial episode and sometimes also at relapse, making the decision to initiate plasma exchange treatment difficult. There are no clinical features that help to predict how long plasma exchange treatment may be required. There are no data to support the efficacy of any treatment modality other than plasma exchange. Long-term clinical outcomes, such as chronic renal failure, may be more serious than we realize. Future clinical research must address the following issues:
Definition. There is a need to define accurately those patient groups in published case series, using explicit criteria to include and exclude patients, to define acute renal failure when patients are described as having HUS, to document the presence or absence of associated diseases and possible etiologies, and to describe clinical outcomes. Simply identifying patients as having TTP or having HUS is insufficient.
Diagnosis. There is a need to develop diagnostic tests and to define clinical features that can be helpful in determining which patients require treatment with plasma exchange and which patients may be safely observed without plasma exchange. Specifically, there is a need to determine the importance of the vWF-cleaving protease activity in diagnostic and management decisions.
Treatment. There is a need to develop diagnostic tests and to define clinical features that can predict the optimal duration of plasma exchange treatment. There is a need to determine whether early intervention with any treatment in addition to plasma exchange can improve the rate of initial recovery, decrease the frequency of exacerbations and relapses, and decrease the risk for chronic renal failure.
Prognosis. There is a need to describe long-term clinical outcomes of patients after recovery from an acute episode of TTP-HUS, stratified by associated conditions, and to document not only the risk for relapse but also the occurrence of renal failure and hypertension. There is a need to define the risks associated with future pregnancies.
It is hoped that with evidence from clinical trials, there will be no need for more opinion-based articles such as this on how to treat patients with TTP-HUS.
Acknowledgments
I thank my colleague, Sara Vesely, who has organized and analyzed the data of The Oklahoma TTP-HUS Registry and who provides continual critical assessment of clinical opinions. I thank the staff of the Oklahoma Blood Institute and the physicians of Oklahoma City for their support and our patients for their enthusiastic participation in our studies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
James N. George, Hematology-Oncology Section, The University of Oklahoma Health Sciences Center, PO Box 26901, Oklahoma City, OK 73190; e-mail: jim-george@ouhsc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal