Abstract
Gap junctions are intercellular channels, formed by individual structural units known as connexins (Cx), that allow the intercellular exchange of various messenger molecules. The finding that numbers of Cx43-type gap junctions in bone marrow are elevated during establishment and regeneration of the hematopoietic system has led to the hypothesis that expression of Cx43 is critical during the initiation of blood cell formation. To test this hypothesis, lymphoid and myeloid development were examined in mice with a targeted disruption of the gene encoding Cx43. Because Cx43−/− mice die perinatally, initial analyses were performed on Cx43−/−, Cx43+/−, and Cx43+/+ embryos and newborns. The data indicate that lack of Cx43 expression during embryogenesis compromises the terminal stages of primary T and B lymphopoiesis. Cx43−/− embryos and neonates had a reduced frequency of CD4+ and T-cell receptor-expressing thymocytes and surface IgM+cells compared to their Cx43+/+ littermates. Surprisingly, Cx43+/− embryos/neonates also showed defects in B- and T-cell development similar to those observed in Cx43−/− littermates, but their hematopoietic system was normal at 4 weeks of age. However, the regeneration of lymphoid and myeloid cells was severely impaired in the Cx43+/− mice after cytoablative treatment. Taken together, these data indicate that loss of a single Cx43 allele can affect blood cell formation. Finally, the results of reciprocal bone marrow transplants between Cx43+/+ and Cx43+/− mice and examination of hematopoietic progenitors and stromal cells in vitro indicates that the primary effects of Cx43 are mediated through its expression in the hematopoietic microenvironment.
Gap junctions allow the direct exchange of ions, metabolites, and other messenger molecules between cells. These intercellular channels are formed by individual structural units known as connexins (Cx), which are encoded by a multigene family estimated to consist of approximately 20 members in vertebrates.1-7 Cx43 is among the most widely distributed and has been shown to be the predominant Cx expressed in the bone marrow, thymus, spleen, and other lymphoid tissues.8-14 That Cx43 protein in these tissues assembles into functional gap junctions has been demonstrated by the passage of low molecular weight dye or electronic current between stromal cells in the thymus9,10,15 and bone marrow.8,13,16 It has been suggested that gap junction-mediated intercellular communication between hematopoietic and stromal cells also occurs.10,12 However, the latter heterologous junctions are rare. Although most stromal cells in contact are coupled via gap junctions,8 only 0.1% of hematopoietic cells form gap junctions with the stroma.11
Considerable evidence suggests that Cx43 expression might be of particular importance during establishment or regeneration of the hematopoietic system. Rosendaal and colleagues have demonstrated that gap junction numbers are elevated at the sites of regeneration following cytoablative treatments and in the epiphyseal marrow of growing animals.11-13 For example, the number of gap junctions in the periosteal regions in growing mice was 80-fold greater than in adults in which steady-state hematopoiesis had established.12,13 Comparable increases in the number of gap junctions in the bone marrow of mice recovering from treatment with 5-fluorouracil (5-FU) or irradiation11 were also observed. Consistent with the interpretation that gap junctions are associated with active hematopoiesis are findings that during adipocyte differentiation of stromal cells, which is believed to result in the formation of yellow, inactive marrow, the number of gap junctions decreases.17 Taken together, these data have led to the hypothesis that expression of Cx43-type gap junctions in blood-forming tissues is critical during periods of intense hematopoietic growth and differentiation.
The present study tested this hypothesis by examining hematopoiesis in a recently described strain of mice with a targeted disruption of the gene encoding Cx43.18 Cx43−/− homozygotes die immediately after birth as a result of a ventricular malformation, indicating that Cx43 expression is required for normal cardiac development, even though multiple Cxs are expressed in the heart.18 By analogy, if Cx43 expression played an essential role during the most active periods of hematopoiesis, defects in blood cell formation should be apparent in Cx43−/− mice.
The results of this study clearly demonstrate a role for Cx43 during embryonic blood cell formation and during regeneration of the hematopoietic system and further indicate, based on findings of hematopoietic defects in Cx43+/− heterozygotes, a Cx43 gene dosage effect. Further characterization of the Cx43-deficient mice suggested that at least some hematopoietic effects of Cx43 are manifest via its expression in the hematopoietic microenvironment.
Materials and methods
Mice
Heterozygous male and female Gja mice, obtained from the Jackson Laboratory, Bar Harbor, ME, were bred in the vivaria of the Division of Laboratory Animal Medicine at UCLA or the University of Pennsylvania to generate Cx43+/+, Cx43+/−, and Cx43−/− mice. As a result of a cardiac malformation, mice die within hours of birth. Therefore, Cx43−/− animals were analyzed during late embryogenesis (days 17-19 of gestation) or immediately after birth. All heterozygote breeders and their progeny appeared healthy.
Genotyping of mice
The Cx43 mutation was generated by inserting a neomycin (neo) resistance gene into exon 2 of the Cx43gene.18 A polymerase chain reaction (PCR) protocol was developed to assess the Cx43 genotype of the mice being analyzed by incubating DNA aliquots with primers specific for Cx43 exon 2 and neo sequences. The primer sequences were as follows: Cx43: 5′-CTG TAC TTG GCT CAC GTG TTC TAT-3′ and 5′-CGT GGG AGT TGG AGA TGG TGC-3′13; neo: 5′-TGA CTG GGC ACA ACA GAC AAT CGG C-3′ and 5′-GTA GCC AAC GCT ATG TCC TGA TAG C-3′. PCR reactions were performed in 100 μL containing 100 mmol/L Tris-HCl (pH 8.3), 2.5 mmol/L MgCl2, 200 mmol/L each of dATP, dTTP, dGTP, dCTP, 2 mmol/L each of Cx43 and neo primers, 50 mmol/L KCl, and 2.5 U of Taq polymerase (Perkin-Elmer Cetus, Norwalk, CT). Thirty cycles (1 minute denaturation at 94°C, 1 minute annealing at 65°C, and 1 minute polymerization at 72°C) were performed. The final polymerization step was extended an additional 15 minutes. A Perkin-Elmer Thermal Cycler was used for all reactions. Products were run on a 1.2% agarose gel and the PCR fragments were detected by ethidium bromide staining. A single 800-bp fragment, the 800-bp product and a 600-bp fragment, or a single 600-bp product identifies Cx43+/+, Cx43+/−, and Cx43−/− mice, respectively (Figure1).
Representative blot showing identification of Cx43+/+, Cx43+/−, and Cx43−/−mice by PCR.
Liver DNA from mice was amplified using neomycin and Cx43 primers as described in “Materials and methods.”
Representative blot showing identification of Cx43+/+, Cx43+/−, and Cx43−/−mice by PCR.
Liver DNA from mice was amplified using neomycin and Cx43 primers as described in “Materials and methods.”
Preparation of cell suspensions
Single cell suspensions of thymus and spleen from embryos were prepared by gently pushing tissues through a 35-mm strainer fitted in the cap of 12 × 75-mm tubes (Falcon, Oxnard, CA) into α-Minimal Essential Medium (MEM; GIBCO, Grand Island, NY) supplemented with 5% fetal calf serum (FCS; Atlanta Biologicals, Atlanta, GA). Bone marrow cells were flushed from femurs and tibiae using a syringe fitted with a 30-gauge needle. Adult splenocyte suspensions were prepared by gently pressing the organs through a fine mesh screen. Cells were counted with a hemacytometer and cell viability was determined by eosin dye exclusion. In some experiments, testes were harvested for DNA preparation. All bone marrow data are expressed as number of white blood cells per 2 femurs and 2 tibiae.
Immunofluorescence analysis
Expression of specific cell surface determinants was detected by labeling cells with the following phycoerythrin (PE)-, fluorescein isothiocyanate (FITC)-, or biotin-conjugated antibodies: anti-CD11b (Mac-1, clone M1/70), FITC-conjugated antiheat-stable antigen (HSA, clone M1/69), biotin-conjugated anti-CD43 (clone S7), PE-conjugated anti-CD45R (B220, clone RA3-6B2), T-cell receptor (TCR) αβ (clone H57-597), CD4 (clone RM4-5), and CD8α (clone 53-6.7), all from Pharmingen, La Jolla, CA, or FITC-conjugated anti-IgM (Southern Biotechnology, Birmingham, AL). Prior to staining, cell suspensions were treated with NH4Cl to lyse erythrocytes and incubated with an antibody to Fcγ II and III receptors (CD16/32, clone 2.4G2, Pharmingen) to reduce nonspecific labeling of cells.
All staining protocols were conducted in calcium, magnesium-free phosphate-buffered saline (PBS) at 4°C. Dead cells were excluded based on their SSC versus FSC profile or their staining with propidium iodide, added to a final concentration of 0.5 μg/mL per sample. Cell analysis was performed on a Becton Dickinson FACScan (Becton Dickinson, San Jose, CA). Gates were set on the basis of staining with either an isotype control antibody conjugated to the same fluorochrome or the secondary reagent alone.
Treatment with 5-FU
The cytostatic drug, 5-FU (Sigma St Louis, MO) has been shown to induce an extensive phase of hematopoietic progenitor proliferation prior to cell replenishment of lymphoid organs.19 20Cx43+/− and Cx43+/+ mice received an intravenous injection of either 125 mg/kg of 5-FU in 0.9‰ saline or saline alone. Nine days after injection, mice were killed and the extent of recovery of their hematopoietic tissues assessed. This was done by calculating a ratio of reconstitution (ratio 5-FU/saline), obtained by dividing the organ cellularity (or population frequency) in mice treated with 5-FU by the corresponding organ cellularity (or population frequency) in the saline-treated mice for each lymphoid organ (or lymphoid cell population) considered.
Long-term bone marrow cultures
Long-term myeloid bone marrow cultures were established as described.21 Three weeks after recharge, cultures were switched to B lymphoid-permissive conditions.22 23 Cells were harvested from 8 cultures per group at weekly intervals and analyzed for the emergence of CD45R+, sIgM−, and CD45R+IgM+ cells by immunofluorescence.
In some experiments purified stromal cells from long-term bone marrow cultures were analyzed for Cx43 gene expression. Purified stromal cell cultures were generated as described by treating established myeloid long-term bone marrow cultures with mycophenolic acid.24 This procedure eliminates hematopoietic cells from the cultures but retains intact, functional stromal cells.
Bone marrow transplantation
Mice were preconditioned before transplantation with 900 R delivered from a 60Cobalt irradiator (41.28 R/min). Five hours later, recipient mice received an intravenous injection of 3 × 106 bone marrow cells resuspended in 0.9% saline. Hematopoietic tissue reconstitution was assessed 17 days later.
Purification of hematopoietic populations
In some experiments CD43+CD45R+ pro-B cells were purified on a Becton Dickinson FACStarplus cell sorter after labeling cells as described above and as previously described.25 In other experiments, populations containing hematopoietic stem cells were purified as described.26Briefly, lineage depleted (Lin−) bone marrow cells were isolated from femurs and tibiae of 5 to 7 BALB/c mice by incubation in PBS with a cocktail of optimal concentrations of rat antibodies against the lineage specific antigens CD2 (clone RM2-5), CD8 (clone 53-6.7), CD45R (clone RA3-6B2), Gr-1 (clone RB6-8C5), Mac-1 (clone M1/70), and TER-119 (all from Pharmingen). The washed cells were then exposed to antirat Ig -conjugated MicroBeads (Miltenyl Biotec, Auburn, CA) for 30 minutes at 4°C and subsequently passed through a magnetic field using a MACS separation column. Lineage-positive cells were retained on the column while the Lin− cells were recovered from the eluate. The Lin− fraction was then incubated with a tricolor (TC) conjugated antirat Ig for 20 minutes. After washing, TC antirat Ig-free binding sites were blocked by incubation with normal rat Ig (1 mg/106 cells) prior to the addition of PE-anti-c-kit (clone 2B8) and FITC- conjugated anti-Sca-1 (Ly6A/E, clone E13-161.7). Then, Lin− cells expressing Sca-1 and c-kit were purified using a FACSstar plus flow cytometer (Becton Dickinson). Reanalysis showed that the purity of the sorted Lin− cells obtained was more than 97%.
Reverse transcriptase-PCR (RT-PCR)
Messenger RNA (mRNA) was prepared from FACS purified populations according to the manufacturer's directions (Invitrogen, Carlsbad, CA) and reverse transcribed into complementary DNA (cDNA). The cDNA was amplified with primer sets to Cx43, described above, and the following reduced glyceraldehyde-phosphate dehydrogenase (GAPDH) primers: 5′ CCATGGAGAAGGCTGGGG and 3′ CAAAGTTGTCATGGATGACC. PCR reactions were performed in a final volume of 100 μL containing 10 mmol/L Tris-HCl (pH 8.3), 1.5 mmol/L MgCl2, 600 μmol/L concentrations each of the dATP, dGTP, dUTP, and dTTP, 1 μmol/L concentrations of the primers, 50 mmol/L KCl, and 2.5 U Taq polymerase (Perkin-Elmer Cetus). Cycles, repeated 30 times, consisted of 1 minute denaturation at 94°C, 1 minute annealing at 65°C, 1 minute polymerization at 72°C, and a final extension for 15 minutes at 72°C. A Perkin-Elmer Thermal Cycler was used for all reactions. PCR products were run on 1.5% agarose gels and stained with ethidium bromide.
Statistical analysis
Statistical analyses were performed using a Student ttest. Because all neonatal mice were analyzed immediately after birth and were thus the same developmental age, data from multiple litters were pooled. However, so as not to bias results, embryonic data were pooled only if animals were from the same litter or it could be confirmed through breeding records that embryos were the same gestational age. All data are expressed as mean ± SD.
Results
T-cell development in Cx43-deficient embryos and neonates
Whether or not failure to express Cx43 at normal levels affected embryonic T-cell development was assessed by examining thymopoiesis in Cx43−/−, Cx43+/−, and Cx43+/+ late gestation embryos (day 17-19), a time at which T-cell production is well established,27 and in neonates.
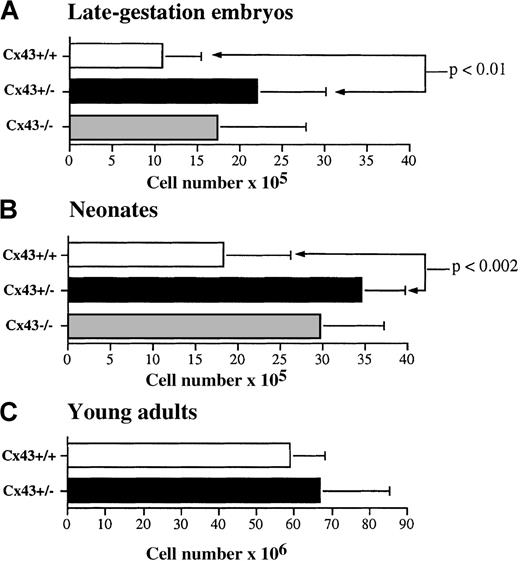
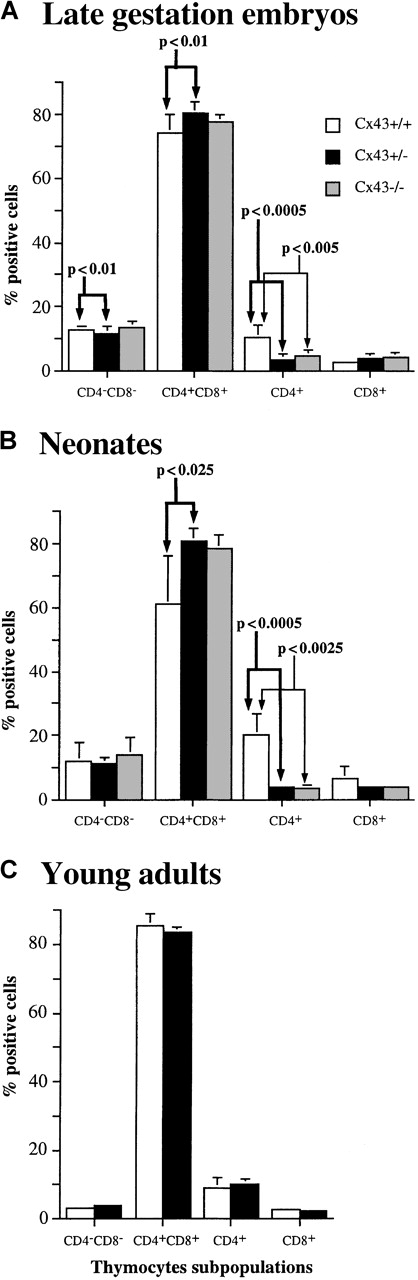
A consistent result was that thymic cellularity was on average higher in Cx43−/− and Cx43+/− embryos and neonates than in their Cx43+/+ littermates (Figure 2). Phenotypic analysis revealed additional developmental defects in the embryos and newborn mice. These were most demonstrable in the neonates in which the frequency of CD4+CD8+double-positive thymocytes, which generate CD4 and CD8 single-positive thymocytes, was higher and the frequency of CD4+single-positive cells significantly lower in the Cx43−/− and Cx43+/− mice as compared to their Cx43+/+ littermates. The decrease in the CD4+ cells correlated with a decrease in the frequency and absolute number of TCR-αβ T cells in the Cx43−/− and Cx43+/− neonates (Table1).
Thymus cellularity.
(A) Late gestation Cx43+/+ (n = 4), CX43+/− (n = 12), and Cx43−/− (n = 6) mice. (B) Neonatal Cx43+/+ (n = 6), Cx43+/− (n = 5), and Cx43−/− (n = 3) mice. (C) Young adult Cx43+/+ (n = 4) and Cx43+/− (n = 4) mice. The level of statistical significance between different groups of mice is indicated.
Thymus cellularity.
(A) Late gestation Cx43+/+ (n = 4), CX43+/− (n = 12), and Cx43−/− (n = 6) mice. (B) Neonatal Cx43+/+ (n = 6), Cx43+/− (n = 5), and Cx43−/− (n = 3) mice. (C) Young adult Cx43+/+ (n = 4) and Cx43+/− (n = 4) mice. The level of statistical significance between different groups of mice is indicated.
Absolute number and frequency of TCRβ+ T cells in the thymus of Cx43+/+, Cx43+/− and Cx43−/− neonates
| Genotype . | % TCRαβ+ cells* . | TCRαβ+cells × 105 . |
|---|---|---|
| Cx43+/+ (n = 6) | 22.6 ± 6.8 | 4.3 ± 1.4 |
| Cx43+/− (n = 5) | 11.8 ± 5.0 | 3.9 ± 1.2 |
| Cx43−/− (n = 3) | 7.9 ± 3.7 | 2.4 ± 1.4 |
| Genotype . | % TCRαβ+ cells* . | TCRαβ+cells × 105 . |
|---|---|---|
| Cx43+/+ (n = 6) | 22.6 ± 6.8 | 4.3 ± 1.4 |
| Cx43+/− (n = 5) | 11.8 ± 5.0 | 3.9 ± 1.2 |
| Cx43−/− (n = 3) | 7.9 ± 3.7 | 2.4 ± 1.4 |
Frequency of TCRαβ+ cells was significantly different at P < .01 for both Cx43+/+ and Cx43+/− mice and Cx43+/+ and Cx43−/− mice.
Because Cx43−/− mice die immediately after birth, further analysis of Cx43−/− adult animals was not possible. However, in view of the defects observed in Cx43+/− embryos and neonates, thymopoiesis was assessed in 12-week-old Cx43+/− mice to determine whether the thymic deficiencies persisted. As shown in Figures 2C and 3C, thymic cellularity and the frequency of CD4 and CD8 thymic subpopulations in Cx43+/− adult mice were normal.
Frequency of CD4−CD8−, CD4+CD8+, CD4+, and CD8+thymocyte subpopulations.
(A) Late gestation Cx43+/+ (n = 4), Cx43+/− (n = 12), and Cx43−/− (n = 6) embryos. (B) Neonatal Cx43+/+ (n = 6), Cx43+/− (n = 5), and Cx43−/− (n = 3) mice. (C) Young adult Cx43+/+ (n = 4) and Cx43+/− (n = 4) mice. The level of statistical significance for each thymocyte subpopulation between different groups of mice is indicated.
Frequency of CD4−CD8−, CD4+CD8+, CD4+, and CD8+thymocyte subpopulations.
(A) Late gestation Cx43+/+ (n = 4), Cx43+/− (n = 12), and Cx43−/− (n = 6) embryos. (B) Neonatal Cx43+/+ (n = 6), Cx43+/− (n = 5), and Cx43−/− (n = 3) mice. (C) Young adult Cx43+/+ (n = 4) and Cx43+/− (n = 4) mice. The level of statistical significance for each thymocyte subpopulation between different groups of mice is indicated.
B-cell development in Cx43-deficient embryos and neonates
The B lineage cell production peaks at approximately day 16 of gestation in the fetal liver; it initiates by the time of birth in the bone marrow and continues in that tissue throughout the life of the animal. In addition, B-cell progenitors can also be found in the spleen during embryogenesis and for up to 3 weeks after birth.28-30 Accordingly, B lymphopoiesis was analyzed by determining the frequency of B lineage cells in late-gestation fetal liver, the bone marrow, and spleen of Cx43−/−, Cx43+/−, and Cx43+/+ neonates. Because of the low number of hematopoietic cells that could be obtained from individual embryos and neonates, myelopoiesis in these mice was not analyzed.
The data in Table 2 demonstrate that the frequency of CD45R+, surface IgM− (sIgM) pro/pre-B cells in fetal liver of Cx43−/− and Cx43+/− mice was significantly higher than in their Cx43+/+ littermates. The sIgM+ B cells were either not detected or present at too low a frequency for reliable analysis in fetal liver at the time mice were examined (data not shown). Although there was no difference in the frequency of pro/pre-B cells in the bone marrow or spleen of Cx43−/− and Cx43+/− mice when compared to their Cx43+/+ littermates, the frequency of sIgM+ mature B cells was significantly lower in these organs (Table 2). This resulted in a significant reduction in absolute numbers of B cells in Cx43−/− and Cx43+/− mice as a result of the lower cellularity of their bone marrow and spleen (Table 2).
Effect of Cx43 deficiency on B lymphopoiesis
| Genotype . | Embryos . | Neonates . | Young adults . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fetal liver . | Bone marrow . | Spleen . | Bone marrow . | ||||||||
| Cells × 105 . | CD45R+ sIgM− . | Cells × 105 . | CD45R+ sIgM− . | sIgM+ . | Cells × 105 . | CD45R+ sIgM− . | sIgM+ . | Cells × 106 . | CD45R+ sIgM− . | sIgM+ . | |
| Cx43+/+ | 126.0 ± 26.9* | 6.7 ± 1.0#‡ | 7.9 ± 3.9* | 9.2 ± 0.6 | 2.1 ± 0.6 | 8.9 ± 4.6*‡ | 10.1 ± 0.8 | 12.8 ± 4.9‡ | 8.2 ± 2.3 | 12.6 ± 0.9* | 6.6 ± 1.5 |
| Cx43+/− | 105.9 ± 14.3* | 14.6 ± 7.0# | 3.6 ± 1.5* | 11.3 ± 2.7 | 1.2 ± 0.5 | 4.8 ± 3.8* | 12.9 ± 3.2 | 7.6 ± 5.6 | 9.5 ± 1.0 | 9.2 ± 2.6* | 6.9 ± 2.7 |
| Cx43−/− | 119.8 ± 44.5 | 18.2 ± 8.0‡ | ND | ND | ND | 3.5 ± 0.8‡ | 11.2 ± 7.4 | 3.6 ± 0.8‡ | ND | ND | ND |
| Genotype . | Embryos . | Neonates . | Young adults . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fetal liver . | Bone marrow . | Spleen . | Bone marrow . | ||||||||
| Cells × 105 . | CD45R+ sIgM− . | Cells × 105 . | CD45R+ sIgM− . | sIgM+ . | Cells × 105 . | CD45R+ sIgM− . | sIgM+ . | Cells × 106 . | CD45R+ sIgM− . | sIgM+ . | |
| Cx43+/+ | 126.0 ± 26.9* | 6.7 ± 1.0#‡ | 7.9 ± 3.9* | 9.2 ± 0.6 | 2.1 ± 0.6 | 8.9 ± 4.6*‡ | 10.1 ± 0.8 | 12.8 ± 4.9‡ | 8.2 ± 2.3 | 12.6 ± 0.9* | 6.6 ± 1.5 |
| Cx43+/− | 105.9 ± 14.3* | 14.6 ± 7.0# | 3.6 ± 1.5* | 11.3 ± 2.7 | 1.2 ± 0.5 | 4.8 ± 3.8* | 12.9 ± 3.2 | 7.6 ± 5.6 | 9.5 ± 1.0 | 9.2 ± 2.6* | 6.9 ± 2.7 |
| Cx43−/− | 119.8 ± 44.5 | 18.2 ± 8.0‡ | ND | ND | ND | 3.5 ± 0.8‡ | 11.2 ± 7.4 | 3.6 ± 0.8‡ | ND | ND | ND |
Cell numbers and frequency values significantly different at: (*)P < .05, (
) P < .01 and (#) P < .025. ND indicates no data.
The numbers of animals per experimental group were as follows: embryos: Cx43+/+ (n = 4), Cx43+/− (n = 12) and Cx43−/− (n = 6); neonates: Cx43+/+ (n = 6), Cx43+/− (n = 5) and Cx43−/− (n = 3); and young adults: Cx43+/+ (n = 4) and Cx43+/− (n = 4).
B lymphopoiesis was also assessed in 12-week-old Cx43+/− mice. The adult Cx43+/− mice exhibited a slight reduction in the frequency of bone marrow CD45R+sIgM−pro-B cells that was limited to the CD43+CD45+CD24 (HSA)+ pro-B cell fraction as defined by Hardy and colleagues31(Cx43+/− = 0.8 ± 0.3 and Cx43+/+ = 1.9 ± 0.5, difference significant at P < .005).
Hematopoietic recovery following cytoablative treatment is deficient in Cx43+/− mice
Although the above data suggest that Cx43 expression is critical during periods of active hematopoiesis, the small number of Cx43−/− animals that could be generated made it imperative to conduct additional experiments in a system where clear-cut effects would be apparent. If Cx43 expression is critical during periods of active blood cell formation, a prediction is that Cx43-deficient mice should exhibit defects during regeneration of the hematopoietic system. This hypothesis was tested by treating Cx43+/− mice with 5-FU. Treatment with 5-FU eliminates cycling hematopoietic cells and the surviving immature hematopoietic progenitors are driven into cell cycle to replenish the lymphoid and myeloid compartments.19 20
Defects were clearly evident in Cx43+/− mice treated with 5-FU. Recovery of bone marrow cellularity in Cx43+/− mice was not as efficient as in Cx43+/+ animals. As shown in Figure4A, bone marrow cellularity in Cx43+/+ mice treated with 5-FU had recovered to 70% of that in saline-treated Cx43+/+ mice. However, in Cx43+/− mice treated with 5-FU, recovery was only 20% of that in saline-treated Cx43+/− controls. Further analysis revealed a significant defect in the recovery of CD45R+ B lineage cells in the bone marrow (Figure 5A).
Cellularity in the bone marrow, thymus, and spleen.
(A) Bone marrow (BM) from 2 femurs and 2 tibiae); (B) thymus (THY); and (C) spleen (SPL) from Cx43+/+ and Cx43−/− mice 9 days after treatment with 5-FU or saline. Three to 4 mice of each genotype received the indicated treatment, and animals were processed individually. The ratio of recovery calculated as described in Materials and methods is indicated in parenthesis. The level of statistical significance between the different experimental groups is indicated. The results shown are from 1 of 2 identical experiments. Mice were 5 weeks of age when treated with 5-FU.
Cellularity in the bone marrow, thymus, and spleen.
(A) Bone marrow (BM) from 2 femurs and 2 tibiae); (B) thymus (THY); and (C) spleen (SPL) from Cx43+/+ and Cx43−/− mice 9 days after treatment with 5-FU or saline. Three to 4 mice of each genotype received the indicated treatment, and animals were processed individually. The ratio of recovery calculated as described in Materials and methods is indicated in parenthesis. The level of statistical significance between the different experimental groups is indicated. The results shown are from 1 of 2 identical experiments. Mice were 5 weeks of age when treated with 5-FU.
Phenotypes.
The phenotypes are shown for CD45R+ (A), TER-119+(B), and CD11b+ (C) cells in the bone marrow of Cx43+/+ and Cx43+/− mice 9 days after 5-FU or saline treatment. Mice are the same as those described in the legend to Figure 4. The level of statistical significance between the different experimental groups is indicated.
Phenotypes.
The phenotypes are shown for CD45R+ (A), TER-119+(B), and CD11b+ (C) cells in the bone marrow of Cx43+/+ and Cx43+/− mice 9 days after 5-FU or saline treatment. Mice are the same as those described in the legend to Figure 4. The level of statistical significance between the different experimental groups is indicated.
In addition, thymic cellularity in Cx43+/− mice was only 30% of that in saline-treated Cx43+/− mice 9 days following the 5-FU treatment, whereas it had returned to normal in Cx43+/+mice treated with 5-FU (Figure 4B). No difference in the frequency of thymocyte subpopulations defined by CD4 or CD8 (or both) expression was observed in the 5-FU- or saline-treated Cx43+/− and Cx43+/+ animals (data not shown).
Because sufficient cell numbers were recovered, it was also possible to assess myelopoiesis and erythropoiesis in these mice. Figure 5 further demonstrates that the recovery of these lineages in Cx43+/− mice treated with 5-FU was not as efficient as in Cx43+/+ mice treated with 5-FU (Figure 5B,C). These phenotypic data were corroborated by results of colony assays performed on bone marrow from the mice treated with 5-FU. In both saline-treated Cx43+/+ and Cx43+/− mice the frequency of day 11 colonies generated in response to interleukin (IL)-3, IL-6, and c-kit ligand was comparable (22 ± 6 and 30 ± 3 per 5 × 104 cells, respectively). However, although the number of colonies in Cx43+/+ mice 9 days after 5-FU treatment rose to 149 ± 53 per 5 × 104cells, the number of colonies generated in Cx43+/− mice treated with 5-FU was only 67 ± 21 per 5 × 104 cells.
Although recovery of splenic cellularity in Cx43+/− mice 9 days following 5-FU treatment was lower than in Cx43+/+ animals, the differences were not statistically significant (Figure 4C).
Cx43 bone marrow chimeras exhibit thymus defects
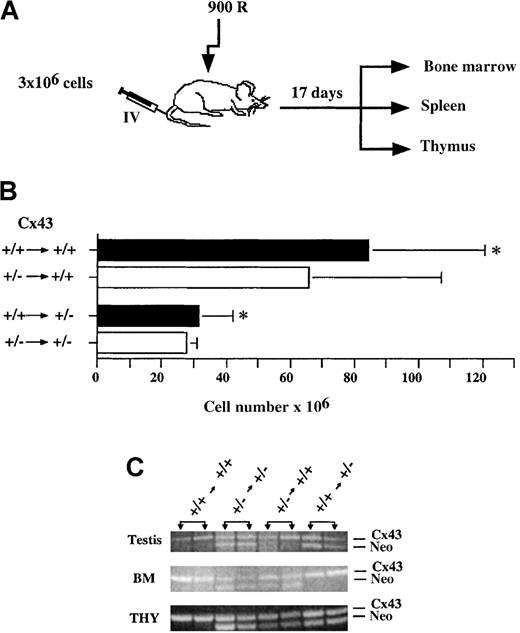
The above data demonstrate a clear requirement for Cx43 in the establishment/regeneration of the hematopoietic system but provide no information regarding in which cells expression is critical. In an attempt to distinguish between effects intrinsic to hematopoietic cells versus those in nonhematopoietic populations, bone marrow chimeras were generated by reciprocal bone marrow transplantation between Cx43+/− and Cx43+/+ mice. Irradiated Cx43+/+ and Cx43+/− mice received a graft of 3 × 106 bone marrow from either Cx43+/+ or Cx43+/− sex- and age-matched mice (Figure 6A). The frequency of c-kit+, Sca-1+, Lin− cells in the Cx43+/+ and Cx43+/− donor cells was determined and found to be identical (data not shown), therefore ensuring that mice received the same number of hematopoietic stem cells.
Thymus cellularity in Cx43+/+ or Cx43+/− mice after bone marrow transplantation.
(A) The transplantation protocol. (B) Thymus celllularity in Cx43+/+ or Cx43+/− recipients of Cx43+/+ or Cx43+/− bone marrow cells. (C) PCR analysis of testes, bone marrow (BM), and thymus (THY) from Cx43+/+ or Cx43+/− mice grafted with either Cx43+/+ or Cx43+/− bone marrow cells as indicated. Testis DNA was used to confirm the identity of the recipient mouse. Note that the genetic composition of the thymus of Cx43+/− mice that received Cx43+/+ bone marrow is not completely donor derived. Only 2 of 3 to 4 mice from each group are shown. The asterisk indicates values significantly different at P < .05.
Thymus cellularity in Cx43+/+ or Cx43+/− mice after bone marrow transplantation.
(A) The transplantation protocol. (B) Thymus celllularity in Cx43+/+ or Cx43+/− recipients of Cx43+/+ or Cx43+/− bone marrow cells. (C) PCR analysis of testes, bone marrow (BM), and thymus (THY) from Cx43+/+ or Cx43+/− mice grafted with either Cx43+/+ or Cx43+/− bone marrow cells as indicated. Testis DNA was used to confirm the identity of the recipient mouse. Note that the genetic composition of the thymus of Cx43+/− mice that received Cx43+/+ bone marrow is not completely donor derived. Only 2 of 3 to 4 mice from each group are shown. The asterisk indicates values significantly different at P < .05.
Mice were analyzed at 17 days after transplantation based on the rationale that progenitor cell proliferation is occurring at this time to replenish the various hematopoietic lineages. At this time, no differences in bone marrow cell recovery were observed between the 4 groups of mice (data not shown). However, as shown in Figure 6B, thymic cellularity in Cx43+/− recipients was significantly lower, regardless of the Cx43 genotype of the bone marrow donor. On the other hand, no significant difference in thymic cellularity was observed in Cx43+/+ mice that received either Cx43+/− or Cx43+/+ bone marrow cells. The frequency of thymocyte subpopulations defined by CD4 and CD8 expression was comparable in all of the different chimeras (data not shown).
Because the Gja/Cx43 knock-out strain has not been bred onto a genetic background that would allow phenotypic identification of donor cells in the recipients, the PCR protocol shown in Figure 1 was used to assess engraftment of donor cells in the chimeras. As shown in Figure 6C, the genotype of bone marrow cells in the chimeras was consistent with that of the donor cells, even though the extent of reconstitution by endogenous radioresistant populations could not be determined by this technique. Surprisingly, as opposed to what was observed when Cx43+/+ or Cx43+/− donor cells were grafted into Cx43+/+ hosts, cells of the Cx43+/− genotype were present in the thymus of Cx43+/− recipients of Cx43+/+ cells (Figure 7).
B lymphopoiesis in long-term bone marrow cultures from Cx43+/− and Cx43+/+ mice.
(A) After establishment of confluent adherent layers from Cx43+/+ or Cx43+/− bone marrow cells, cultures were recharged from the same pool of Cx43+/+ or Cx43+/− bone marrow, respectively, and maintained under myeloid long-term culture conditions for 3 weeks before transfer to B lymphoid culture conditions. Cells were harvested from cultures at weekly intervals thereafter and the frequency of CD45R+sIgM− and CD45R+sIgM+ cells determined. Groups different at ** = P < .0005; * = P < .005. There were no significant differences in cellularity between the cultures established with Cx43+/+ and Cx43+/− bone marrow (data not shown). Data are based on individual analysis of 8 cultures per experimental group. (B) Expression of Cx43 mRNA in sorted Lin−, Sca-1+, c-kit+ stem cells, sorted CD43+CD45R+ pro-B cells, and heterogeneous bone marrow stroma depleted of hematopoietic cells. RT-PCR was performed as described in “Materials and methods.”
B lymphopoiesis in long-term bone marrow cultures from Cx43+/− and Cx43+/+ mice.
(A) After establishment of confluent adherent layers from Cx43+/+ or Cx43+/− bone marrow cells, cultures were recharged from the same pool of Cx43+/+ or Cx43+/− bone marrow, respectively, and maintained under myeloid long-term culture conditions for 3 weeks before transfer to B lymphoid culture conditions. Cells were harvested from cultures at weekly intervals thereafter and the frequency of CD45R+sIgM− and CD45R+sIgM+ cells determined. Groups different at ** = P < .0005; * = P < .005. There were no significant differences in cellularity between the cultures established with Cx43+/+ and Cx43+/− bone marrow (data not shown). Data are based on individual analysis of 8 cultures per experimental group. (B) Expression of Cx43 mRNA in sorted Lin−, Sca-1+, c-kit+ stem cells, sorted CD43+CD45R+ pro-B cells, and heterogeneous bone marrow stroma depleted of hematopoietic cells. RT-PCR was performed as described in “Materials and methods.”
B lymphopoietic defects occur in the bone marrow of Cx43+/− mice
To determine whether any effects of Cx43 are directly mediated on the bone marrow, B lymphopoiesis was examined in the long-term bone marrow myeloid to lymphoid switch system using cultures established from Cx43+/− and Cx43+/+ mice. As described previously,22 on transfer of established myeloid long-term bone marrow cultures to B lymphoid permissive conditions, B-cell development initiates and predominates in the cultures by 4 weeks thereafter. Thus, these in vitro cultures provide a system to examine the “regeneration” B lymphopoiesis from immature hematopoietic precursors.
As shown in Figure 7A, by week 2 after the switch, a time by which myelopoiesis has declined and B lymphopoiesis is rapidly establishing, the frequency of CD45R+sIgM− and CD45R+sIgM+ B lineage cells was significantly higher in Cx43+/+ cultures. However, by week 4 after the switch, a time at which B lymphopoiesis has attained steady-state levels in the cultures, the frequency of B lineage cells in Cx43+/− and Cx43+/+ cultures was comparable.
Development of B cells in the myeloid to lymphoid switch cultures could initiate from pluripotential hematopoietic stem cells or more committed hematopoietic precursors or both. In view of reports suggesting that coupling occurs between immature hematopoieticcells and the stroma, albeit rarely, whether or not FACS purified Lin−, c-kit+, Sca-1+ and CD43+, CD45R+ cells expressed Cx43 was analyzed. The former population is enriched in pluripotential hematopoietic stem cells, whereas the latter phenotype includes the earliest B-cell committed progenitors. As shown in Figure 7B, Cx43 message was not detected in either population. However, consistent with previous results,8 Cx43 mRNA was present in bone marrow stromal cells.
Discussion
Based on findings that the number of Cx43 type gap junctions is up-regulated during establishment and regeneration of the hematopoietic system, it has been hypothesized that expression of Cx43 is critically important during periods of active blood cell formation (reviewed in reference 13). The aim of this study was to test this hypothesis by analyzing hematopoiesis in mice deficient in the expression of Cx43.
Analysis of lymphopoiesis in fetal and neonatal Cx43−/− mice demonstrated that Cx43 expression is required for embryonic hematopoiesis. Maturation of double-positive thymocytes into single-positive cells expressing CD4 and CD8 is clearly dependent on the expression of Cx43. For example, the frequency of CD4+CD8+ double-positive cells was higher and the frequency of mature cells that express CD4, CD8, or TCRαβ was lower in Cx43−/− mice than in their Cx43+/+ littermates. A similar effect on the terminal stages of B lymphopoiesis was observed as evidenced by a reduction in the frequency of mature sIgM+ cells in Cx43−/− and Cx43+/− embryos and neonates. This trend was particularly evident in the spleen, which functions as a hematopoietic organ during embryonic and early neonatal life.32 Cx43 expression has been detected in stromal-like cells in that organ.10
The Cx43+/− mice also exhibited lymphopoietic defects, further revealing that failure to express Cx43 at normal levels is critical to this process. There is precedent for proposing a Cx43 gene dosage effect. Reaume and coworkers18 noted that the frequency of lucifer yellow dye transfer between Cx43+/− fibroblasts was intermediate between values observed in Cx43+/+ and Cx43−/− cells. Additional studies have demonstrated that precise levels of Cx43-mediated gap-junction communication are necessary for processes such as neural crest cell migration and cardiac development,33,34 and other analyses have reported that ventricular contraction in Cx43+/− mice is 30% slower than in wild-type littermates.35 36
Even though lymphopoietic defects were observed in embryonic and neonatal Cx43+/− mice, hematopoietic defects in adult Cx43+/− animals were subtle to nonexistent. This result alone provided support for Rosendaal's hypothesis that expression of Cx43 at normal levels is most critical during the establishment of blood cell formation but not for steady-state hematopoiesis.11,13However, additional experiments were designed to test this possibility directly. Because recovery of hematopoiesis from cytoablative treatment may recapitulate aspects of embryonic blood cell formation, lymphoid and myeloid regeneration was measured in Cx43+/− adult mice treated with 5-FU. The results of these experiments showed a highly significant defect in the recovery of cellularity in the thymus and bone marrow of Cx43+/− animals when compared to Cx43+/+ mice, thus supporting the hypothesis that gap-junction expression is somehow involved in the regulation of hematopoietic progenitor cell proliferation.12 No difference in the frequency of thymocyte subpopulations was observed in the mice treated with 5-FU, but severe effects on recovery of most hematopoietic lineages were observed in the bone marrow. In addition to a delay in the recovery of marrow CD45R+ B lineage cells, the Cx43+/− mice also exhibited a severe retardation in the recovery of myelopoiesis and erythropoiesis. The major effects observed on the myeloid lineage are consistent with published observations showing that myelopoiesis recovers more slowly than B lymphopoiesis after 5-FU treatment.19 Interestingly, the number of progenitors that formed colonies in semisolid medium in response to IL-3, IL-6, and c-kit ligand was also significantly lower in Cx43+/− mice treated with 5-FU. Because this cytokine combination targets developmentally immature hematopoietic populations, these data provide additional evidence that failure to express normal Cx43 levels significantly affects the growth of stem and early progenitor cell populations.12
Although these data provide clear evidence that Cx43 expression at optimal levels is critical for normal hematopoiesis, it is much more difficult to establish precisely the particular cellular compartment in which expression is critical. It is conceivable that the ventricular defect somehow could impair circulation and affect the distribution of hematopoietic cells. This might explain the defects in the Cx43−/− embryos and neonates but not in Cx43+/− mice. Steady-state hematopoiesis in the heterozygotes is normal, and defects are only observed on hematopoietic challenge. In an attempt to distinguish between effects intrinsic to hematopoietic versus those in nonhematopoietic populations, chimeras were generated by reciprocal bone marrow transplantation between Cx43+/− and Cx43+/+ mice. No effects on bone marrow cellularity were observed. A thorough kinetic analysis would be needed to address whether differences might have been apparent at earlier or later time points during reconstitution. However, such experiments are difficult to perform due to the limited availability of the Cx43+/− mice. This strain breeds poorly, and the mothers often fail to nurse their litters after delivery (unpublished observations).
On the other hand, clear differences were apparent in the thymus 17 days after transplantation. Thymus cellularity in Cx43+/+ recipient mice was similar regardless of the Cx43 genotype of the bone marrow donor cells. This result suggested that Cx43+/− hematopoietic cells are able to develop in a normal environment. On the other hand, Cx43+/− recipient mice exhibited a reduced thymic cellularity whether they were transplanted with Cx43+/+ or Cx43+/− bone marrow cells, supporting the hypothesis that the effects of Cx43 expression are manifest at a nonhematopoietic level. An unexpected observation in these studies was that Cx43+/− message was detected in the thymus of Cx43+/− recipients of Cx43+/+ cells. This could be due to the fact that the ratio of stroma/thymocytes is higher in Cx43+/− recipients and the PCR reaction is detecting radioresistant host stromal cells. In any case, the results of the bone marrow transplantation studies strongly suggest that there is an environmental defect in Cx43+/− mice localized to the thymus stroma.
Because the transplantation experiments did not reveal effects on the bone marrow, the final set of experiments used the established myeloid to lymphoid switch culture system to assess whether or not effects of Cx43 occurred at that level. These “switch” cultures measure the initiation of B-cell development from immature hematopoietic precursors that do not yet express B lineage cell surface determinants.22 Thus, this system allows the “regeneration” of B-cell development to be assessed in vitro. The results obtained in vitro paralleled what was observed in Cx43+/− mice in vivo. During the exponential phase in which B lymphopoiesis is being “established,” between day 0 and week 2 following the transfer of cultures to B lymphoid permissive conditions, the frequency of both CD45R+sIgM− and CD45R+sIgM+ B lineage cells was significantly reduced in cultures from Cx43+/− mice. However, by 3 weeks after the switch, when B lymphopoiesis has established and the cultures are producing B lineage cells at steady-state levels, no differences in the frequency or number of CD45R-expressing cells in Cx43+/+ or Cx43+/− cultures were observed.
Stromal cells in situ and in long-term bone marrow cultures are well coupled, suggesting that they are the main site at which effects of Cx43 expression occurs. However, it has been reported that immature hematopoietic cells and stromal cells couple at a frequency of 0.1%.11 Because this is the approximate frequency at which stem cells are found in the bone marrow,37 the heterologous couplings may also be important in the regulation of stem cell growth or differentiation.38 To determine where in the bone marrow Cx43 expression is most critical, the expression of Cx43 in stromal and hematopoietic cells was analyzed. The data herein do not provide support for stromal cell-stem cell or for stromal cell-lymphocyte coupling in the regulation B-cell development. Although stromal cell expression of Cx43 was confirmed, no expression of Cx43 in stem cell purified populations or pro-B cells was detected. These data are consistent with previous results from this laboratory that consistently failed to detect dye transfer between stromal and B lineage cells.8 Thus, taken together with the thymus results, the data in this paper support a role for expression of Cx43 in the marrow and thymus microenvironments.
In addition to the need to investigate further how Cx43 might affect microenvironmental function, several other issues remain to be resolved. For example, the nature of the regulatory signals that are transmitted via gap junctions has not been identified, but a possibility is that they may ultimately regulate cytokines produced by the hematopoietic microenvironment. Proposing that failure to express gap junctions at normal levels compromises cytokine production by the hematopoietic microenvironment is not unprecedented, based on the finding that coupling of pancreatic secretory epithelia via gap junctions plays a critical role in their biosynthesis, storage, and release of specific secretory products.39 It is also not clear that the manner in which gap junctions regulate hematopoiesis during embryonic development is comparable to what occurs during regeneration of the hematopoietic system. In this regard, clear effects on the development of mature CD4+ and CD8+thymocytes were observed in Cx43-deficient embryos and neonates, and cellularity in the thymus of the mice was higher in Cx43−/− and Cx43+/− mice than in their Cx43+/+ littermates. However, opposite results were observed in adult Cx43+/− mice following bone marrow transplantation or recovery from 5-FU treatment. In these instances, defects in restoration of organ cellularity, rather than maturation effects, were observed. Efforts to delineate Cx43 effects on hematopoietic cell differentiation versus proliferation are needed in this regard.
These issues aside, there are additional implications of the present data that have demonstrated that a defect in the expression of Cx43 can compromise blood cell production. Cx expression and channel formation is sensitive to a variety of drugs.33 39 If one or more chemotherapeutic agents used in various clinical scenarios also interfere with gap-junction expression or function, this could have significant adverse effects on the recovery of blood cell production in treated individuals.
Acknowledgments
The authors thank A. Johnson and L. Collins for expert technical assistance, Dr Cecelia Lo for critical comments and provision of Cx43+/− mice, and Dr F. Zaera for statistical analysis.
Supported by grant HL60658 from the National Institutes of Health. The flow cytometry core at UCLA is supported in part by grant CA16042 from the National Institutes of Health.
Reprints:Kenneth Dorshkind, Department of Pathology 173216, UCLA School of Medicine,10833 Le Conte Avenue, Los Angeles, CA 90095-1732; e-mail: kdorshki@mednet.ucla.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal