Abstract
Hematopoietic stem cell gene therapy holds promise for the treatment of many hematologic disorders. One major variable that has limited the overall success of gene therapy to date is the lack of sustained gene expression from viral vectors in transduced stem cell populations. To understand the basis for reduced gene expression at a single-cell level, we have used a murine retroviral vector, MFG, that expresses the green fluorescent protein (GFP) to transduce purified populations of long-term self-renewing hematopoietic stem cells (LT-HSC) isolated using the fluorescence-activated cell sorter. Limiting dilution reconstitution of lethally irradiated recipient mice with 100% transduced, GFP+ LT-HSC showed that silencing of gene expression occurred rapidly in most integration events at the LT-HSC level, irrespective of the initial levels of GFP expression. When inactivation occurred at the LT-HSC level, there was no GFP expression in any hematopoietic lineage clonally derived from silenced LT-HSC. Inactivation downstream of LT-HSC that stably expressed GFPin long-term reconstituted animals was restricted primarily to lymphoid cells. These observations suggest at least 2 distinct mechanisms of silencing retrovirally expressed genes in hematopoietic cells.
Studies in mice over the last 15 years have shown that pluripotential hematopoietic stem cells can be transduced with retroviral vectors, thus establishing the experimental basis for current gene therapy approaches in humans.1-3 To date, the translation of experiments done in mice to larger animal models has largely been unsuccessful, which has highlighted the need for more basic research in the area of stem cell biology and in the development of better gene delivery systems.4 The poor success of gene therapy could be attributed to a number of factors, including the refractory nature of human stem cells to retroviral transduction,5-7 inefficient “seeding” of cells following adoptive transfer to nonmyeloablated recipients, or the lack of sustained gene expression from viral vectors.
The long-term maintenance of gene expression following bone marrow reconstitution has frequently been evaluated in a nonquantitative manner. In mice, a number of reports document both the maintenance8-11 and the lack of sustained gene expression12-14 from murine retroviral vectors. Complicating the interpretation of these results are differences in vector design and the cell population being assayed for expression (day-12 colony-forming units–spleen cells [CFU-S], in vitro colonies, peripheral blood of reconstituted animals), the time posttransduction when the assay was performed, and the means of determining expression (bulk-cell polymerase chain reaction [PCR], enzymatic assays, or fluorescence-activated cell sorter [FACS]-selectable markers). A perceived loss of expression may also have to do with the limited half-life of all hematopoietic progenitors and differentiated progeny that are derived from long-term self-renewing hematopoietic stem cells (LT-HSC). Transduction of more short-lived multipotential progenitors, typified by most day-12 CFU-S–forming cells or transiently reconstituting multipotent stem cells,15 would be evidenced by sustained expression for up to about 2 to 3 months, followed by a loss in retrovirus-positive, short-lived cells.
To address the issue of gene expression from retroviral vectors in LT-HSC, we have used a murine retrovirus expressing the green fluorescent protein (MFG-GFP) to transduce a purified population of LT-HSC isolated by FACS. The MFG retroviral backbone was chosen because previous studies showed that MFG-derived vectors generate high-titer virus and can result in significant long terminal repeat (LTR)-driven gene expression in reconstituted animals.11,16 17 Transduction of purified LT-HSC followed by transplantation of transduced cells at or near limiting dilution allowed us to quantitatively determine the percentage of donor stem cells that sustain retroviral gene expression in the reconstituted animal. This approach eliminates the “noise” associated with transplantation of transduced whole bone marrow cells and greatly facilitates analysis of factors that may influence gene expression from retroviral vectors.
Materials and methods
Generation of retrovirus
MFG-GFP retrovirus was produced by transient transfection of the retroviral packaging cell line, BOSC 23,18 by calcium phosphate coprecipitation. BOSC cells were seeded on 6-cm dishes at a density of 2 × 106 cells per dish the day before transfection. Ten minutes prior to transfection, the media was changed to Dulbecco's modified Eagle's medium (DMEM) supplemented with 50-μM chloroquine diphosphate. A total of 8 to 10 μg of DNA was used per transfection. Following the addition of DNA-calcium phosphate precipitate, plates were incubated for 8 hours in a carbon dioxide incubator at 37°C, the media were aspirated, and fresh DMEM was gently added to the dishes. After an additional 16 hours, the media were changed and the cells were then gently overlaid with a minimal amount of media (2 mL) to collect virus. Viral supernatant was collected after 24 hours and then centrifuged at 1200 rpm for 5 minutes at 4°C to pellet any packaging cells. Supernatants were then collected, aliquoted, and frozen. Titering was done using NIH 3T3 cells seeded on 6-cm dishes at 5 × 105 cells per dish the day before transduction. Typical titers ranged between 1 × 106 to 2 × 106IU/mL.
Isolation of hematopoietic stem cells
Bone marrow cells were obtained by flushing the tibias and femurs with Hank's balanced salt solution (Gibco BRL, Grand Island, NY) supplemented with 2% donor bovine serum (staining media). Collected cells were stained with a cocktail of rat monoclonal antibodies to antigens present on the surface of mature blood cell lineages: 6B2 (anti-B220), M1/70 (anti-Mac-1), 8C5 (anti-Gr-1), Ter-119 (anti-erythrocyte–specific antigen), KT31.1 (anti-CD3), 53-7.3 (anti-CD5), GK1.5 (anti-CD4), and 53-6.7 (anti-CD8). Stained cells were washed with staining media and then incubated with goat antirat phycoerythrin (PE). After washing, free sites on the antirat antibodies were blocked using normal rat serum or rat immunoglobulin G (1 μg/mL in phosphate-buffered saline). Stained cells were washed then incubated with biotinylated E13-161-7 (anti-Sca-1), fluorescein isothiocyanate–conjugated 19XE5 (anti-Thy-1.1), and allophycocyanin (APC)-conjugated 2B8 (anti-c-kit). Washed cells were then incubated with streptavidin microbeads (Miltenyi Biotec, Auburn, CA) for 10 minutes, followed by incubation with avidin-Texas Red (Caltag, Burlingame, CA) for an additional 10 minutes. Sca-1+ cells were enriched by running the sample through a mini-MACS column (Miltenyi Biotec) and eluting the magnetic fraction according to the manufacturer's instructions. All cell sorts were done using a dual laser flow cytometer in the shared FACS facility at Stanford University. Dead cells were excluded by gating out propidium-iodide positive cells. The LT-HSC phenotype was sorted as Sca-1+Lin−Thy-1.1loc-kit+.
Hematopoietic stem cell transduction and bone marrow reconstitution
Sorted stem cells were incubated in 200 μL of DMEM (Gibco BRL, high glucose) supplemented with 10% fetal calf serum (tested for growth of embryonic stem cells, Gibco BRL), sodium pyruvate, nonessential amino acids, and penicillin-streptomycin in a 96-well plate. The media also contained interleukin-6 (5 ng/mL), Steel factor (50 ng/mL), and 1 × leukemia inhibitory factor (ESGRO, Gibco BRL). Incubation with cytokines was carried out for 24 to 28 hours to induce stem cell cycling, after which time 150 μL of the media was removed and replaced with 125 μL of viral supernatant, 4 μg/mL of polybrene, fresh cytokines, and media to 150 μL. Infections proceeded for 20 to 24 hours before the cells were washed and resorted for GFP expression using the FACS. C57BL/J (Ly-5.1, Thy-1.2) mice were used as recipients for long-term reconstitution assays. Mice were irradiated with 920 rad given in a split dose separated by 3 hours. Cells were injected retroorbitally into anesthetized (Metofane) mice that were maintained on antibiotics (neomycin sulfate and polymyxin B sulfate) at least 1 month following reconstitution.
PCR of GFP provirus in single cells
Single cells from gated populations were sorted directly into PCR buffer (supplied by Boehringer Mannheim; final concentration: 1.5-mM MgCl2, 50-mM Tris [pH 8.3], 50-mM KCl) supplemented with 0.5% Triton X-100. Two rounds of nested PCR (30 cycles each) were done using the outside and inside primer sets listed below. Cycle conditions were 94°C for 1 minute, 55°C for 1 minute, and 72°C for 30 seconds. Five percent of the first-round reaction was used in second-round PCR as template. Amplified products were resolved on agarose gels and then blotted for Southern analysis using an internal, random-primed GFP probe. All primers are written in 5′ to 3′ orientation. Outside primer set: ATGAGTAAAGGAGAAGAACTTTTC; ATGGCGTTACTTAAGCTAGC; inside primer set: CTGTCAGTGGAG- AGGGTGAA; TTTGTATAGTTCATCCATGCC.
Day 8, CFU-S assay
Bone marrow was isolated from primary transplant recipients and then counted using Türk's solution. A total of 50 000 nucleated cells were retroorbitally injected into lethally irradiated congenic mice. After 8 days, spleens were isolated from reconstituted animals, and individual nodules were microdissected and placed in a proteinase K lysis solution for overnight digestion. Genomic DNA was purified, cut with BamHI, and then run on a gel for Southern analysis using a random-primed (Pharmacia kit, Piscataway, NJ) GFP probe that encompassed the entire GFP cDNA (a unique BamHI site is located just 3′ of the GFP coding sequences in MFG-GFP).
Results
Repopulating potential of LT-HSC transduced with MFG-GFP retroviral supernatants
In a recent study, we optimized conditions for the transduction of FACS-sorted, LT-HSC using retroviral supernatants (“Materials and methods”; Klug et al, submitted). Retroviral transduction of FACS-purified LT-HSC ensures a high multiplicity of infection (between 10 and 50) and minimizes potentially oncogenic integration events into more differentiated hematopoietic cells that have little utility in reconstitution assays. Transduced LT-HSC were marked by both an allelic marker, Ly-5.2, and the GFP reporter gene. Inclusion of the GFP gene in the vector allowed us to perform the repopulation assays using a pure population of transduced cells that were resorted for GFP expression 20 to 24 hours posttransduction (Figure 1).
Competitive repopulation assay using GFP+, Ly-5.2+ LT-HSC, and recipient whole bone marrow cells.
Increasing numbers of transduced (GFP+), donor LT-HSC (Ly-5.2+) were mixed with 2 × 105nucleated bone marrow cells of the recipient type (Ly-5.1+). The recipient cells would statistically contain 20 LT-HSC, only 2 of which would home to bone marrow and contribute to the host-derived hematopoiesis that is seen in each reconstituted animal. Numbers in the upper right quadrant of each FACS plot represent the percentage of donor cells in peripheral blood of the recipient animal.
Competitive repopulation assay using GFP+, Ly-5.2+ LT-HSC, and recipient whole bone marrow cells.
Increasing numbers of transduced (GFP+), donor LT-HSC (Ly-5.2+) were mixed with 2 × 105nucleated bone marrow cells of the recipient type (Ly-5.1+). The recipient cells would statistically contain 20 LT-HSC, only 2 of which would home to bone marrow and contribute to the host-derived hematopoiesis that is seen in each reconstituted animal. Numbers in the upper right quadrant of each FACS plot represent the percentage of donor cells in peripheral blood of the recipient animal.
To address whether the in vitro transduction conditions had altered the functional phenotype of transduced LT-HSC, a limit dilution dose of transduced (GFP+) cells in reconstitution of lethally irradiated (Ly-5.1+) recipient mice was established. Titration of input GFP+ stem cells showed that as few as 30 cells were sufficient to detect donor-derived peripheral blood cells of both lymphoid and myeloid origin for more than 5 months following reconstitution (Figure 1). The normal limit dilution dose for FACS-sorted LT-HSC freshly isolated from bone marrow is approximately 10 cells.15 Other studies have shown that actively dividing HSC reconstitute at lower efficiencies,19,20 which indicates that there was little loss of LT-HSC activity during the in vitro transduction procedure. Figure 1 shows data from all reconstituted animals used in the experiment for 30, 60, and 120 cells. Significantly, at all cell doses tested, there was an almost complete absence of donor-derived cells that remained GFP+. In an independent experiment, 2 of 3 animals were donor-reconstituted with 30 GFP+ cells at 26 weeks, although the percentage of donor hematopoiesis was only about 1% in the competitive assay (data not shown). A total of 3 of 3 mice were donor-reconstituted using 60 GFP+ cells, with a mean donor reconstitution of 27%. In all experiments, the percentage of donor-derived peripheral blood cells increased with the increase in number of injected donor stem cells (Figure 1), consistent with data that virtually all LT-HSC contribute over time to hematopoiesis.21 22 Strikingly, in almost every case, there was an almost complete absence of Ly-5.2+/GFP+ cells when low numbers of transduced cells were transplanted. The limit dilution dose for Ly-5.2+/GFP+ cells that persisted beyond 6 months post-reconstitution was approximately 300 input cells.
The lack of Ly-5.2+/GFP+ cells is not due to deletion of the provirus
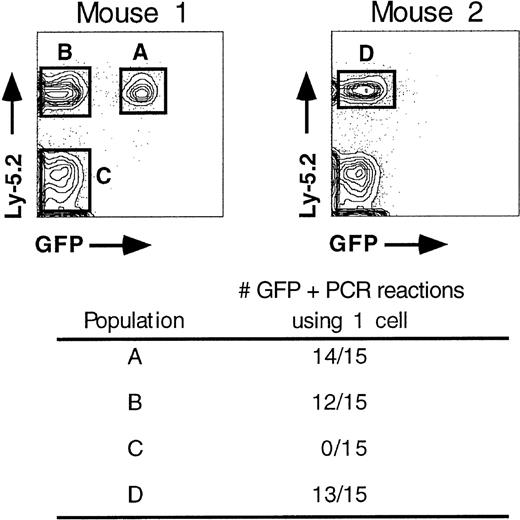
To test whether the lack of Ly-5.2+/GFP+peripheral blood cells was due to loss of the provirus or to silencing of retroviral gene expression, single-cell PCR was done using cells isolated from peripheral blood of animals reconstituted for 4 months. Single cells were sorted from Ly-5.2+/GFP+, Ly-5.2+/GFP−, and Ly-5.2−/GFP− populations (Figure2). Two rounds of PCR were done using nested primers that hybridize to GFP coding sequences. Products were resolved on a gel and then blotted for Southern analysis using an internal GFP probe. Virtually all donor-derived cells contained at least one copy of an integrated provirus (populations A, B, and D) whereas host-derived (Ly-5.1+) cells (population C) showed no evidence of a retrovirus even after prolonged exposures of the Southern blot. A repeat experiment with an additional reconstituted mouse showed 9 of 10 positive PCR reactions for both populations A and B, respectively, and 0 of 8 positive for population C (data not shown). In addition, 16 of 16 long-term reconstituted mice were positive for the presence of an intact provirus based on DNA Southern blotting of purified B, T, and myeloid cell populations, irrespective of the presence of Ly-5.2+/GFP+ cells (for example, see Figure 10 below). This indicates that the lack of GFP expression in donor-derived peripheral blood cells is due to transcriptional or translational silencing and that the silencing mechanism acts at early times post-reconstitution, usually within the first 6 weeks (Figures 1and 5). Proviral integrants that were not silenced early continued to express GFP in a significant fraction of Ly-5.2+(donor-derived) peripheral blood cell populations for at least 6 to 10 months post-reconstitution (Figure 3). This was seen in 13 of 13 animals. There was never evidence of complete inactivation of expression at later time points in any reconstituted animal.
Donor-derived cells in long-term reconstituted animals contain integrated provirus.
Single, Ly-5.2+ peripheral blood cells were isolated from animals reconstituted for 4 months and assayed for the presence of an integrated provirus using nested PCR. Single cells would predominantly be B, T, and myeloid cell types, which represent the major, circulating Ly-5.2+ cell populations.
Donor-derived cells in long-term reconstituted animals contain integrated provirus.
Single, Ly-5.2+ peripheral blood cells were isolated from animals reconstituted for 4 months and assayed for the presence of an integrated provirus using nested PCR. Single cells would predominantly be B, T, and myeloid cell types, which represent the major, circulating Ly-5.2+ cell populations.
Sustained expression of the GFP reporter gene in long-term reconstituted mice.
Peripheral blood of 4 long-term reconstituted animals from 2 independent experiments was assayed at the indicated time points for the persistence of GFP expression over 5 to 6 months. A total of 13 of 13 animals showed sustained long-term expression with little change in the percentage of GFP-expressing cells. The number of transplanted, GFP+ donor cells per animal are indicated. All donor cells were mixed with 2 × 105 recipient-type (Ly-5.1) marrow cells prior to transplantation.
Sustained expression of the GFP reporter gene in long-term reconstituted mice.
Peripheral blood of 4 long-term reconstituted animals from 2 independent experiments was assayed at the indicated time points for the persistence of GFP expression over 5 to 6 months. A total of 13 of 13 animals showed sustained long-term expression with little change in the percentage of GFP-expressing cells. The number of transplanted, GFP+ donor cells per animal are indicated. All donor cells were mixed with 2 × 105 recipient-type (Ly-5.1) marrow cells prior to transplantation.
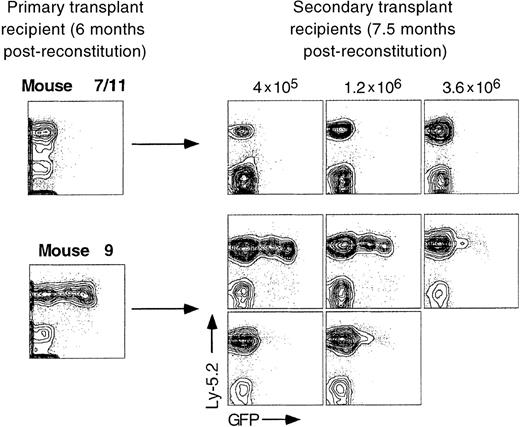
Secondary bone marrow transfer into irradiated recipient mice never resulted in reexpression of a silenced GFP reporter gene in a total of 15 animals examined although, in some cases, silencing of stably expressing proviruses may have occurred (Figure4, secondary recipients from mouse 9). It remains possible that GFP+ stem cell clones were not represented at a high enough frequency in the bone marrow inoculum used for secondary transplantation, so that apparent “silencing” in secondary recipients was really due to a lack of reconstitution by GFP+ stem cells. A limit dilution dose of whole bone marrow cells is approximately 1 × 105 to 2 × 105 cells, which indicates that a significant amount of self-renewal had to have occurred in stem cells that contributed to primary reconstitution of mouse 9.
Expression patterns seen in primary transplant recipients are generally maintained in long-term reconstituted secondary transplant recipients.
Increasing numbers of whole bone marrow cells from long-term reconstituted primary recipient mice were transplanted into lethally irradiated secondary recipients of the Ly-5.1 genotype. Peripheral blood was analyzed from 3 animals representing mouse 7/11 and 5 animals from mouse 9.
Expression patterns seen in primary transplant recipients are generally maintained in long-term reconstituted secondary transplant recipients.
Increasing numbers of whole bone marrow cells from long-term reconstituted primary recipient mice were transplanted into lethally irradiated secondary recipients of the Ly-5.1 genotype. Peripheral blood was analyzed from 3 animals representing mouse 7/11 and 5 animals from mouse 9.
Silencing is independent of initial levels of GFP expression
We next tested whether sites of retroviral integration that allowed for high-level GFP expression in LT-HSC might be predictive of integrants that would be buffered from inactivation. Immediately following in vitro transduction, cells that expressed low or extremely high levels of GFP were sorted for reconstitution into lethally irradiated recipient mice (Figure 5). A total of 350 GFP+ LT-HSC were injected into each reconstituted animal. Peripheral blood analysis showed that silencing occurred in both groups of animals regardless of the initial level of GFP expression in LT-HSC. Integrants that sustained expression could be found among animals that received low or high GFP-expressing cells.
Maintenance of gene expression is not predictable based on initial levels of GFP expression.
Recipient animals were reconstituted with 350 GFP+, LT-HSC that initially expressed either low (2 animals) or high levels (3 animals) of GFP 24 hours after stem cell transduction. Peripheral blood was analyzed at 3.5 weeks and 11.5 weeks post-reconstitution.
Maintenance of gene expression is not predictable based on initial levels of GFP expression.
Recipient animals were reconstituted with 350 GFP+, LT-HSC that initially expressed either low (2 animals) or high levels (3 animals) of GFP 24 hours after stem cell transduction. Peripheral blood was analyzed at 3.5 weeks and 11.5 weeks post-reconstitution.
GFP expression is silenced in lymphoid progeny that differentiate from GFP+ LT-HSC
To begin addressing the mechanisms for inactivation of GFP expression, stem cells were isolated from bone marrow of animals that had been reconstituted for more than 10 months. In one animal (L1, the first mouse in the GFP/low group from Figure 5), where the percentage of donor-derived GFP+ cells to donor-derived GFP− cells (about 50%) was similar in blood and unfractionated bone marrow, donor-derived stem cells (Ly-5.2+Sca-1+Lin−c-kit+) were almost entirely GFP+ (Figure6). The LT-HSC surface phenotype in long-term reconstituted primary recipients is similar to that found in normal (unreconstituted) bone marrow.23 In the absence of Thy-1.1 staining, this population is approximately 50% pure for LT-HSC activity. Thy-1.1 staining could not be done because of an inability to do 6-color analysis with the antibody combination used. In a second animal (L2, second animal in the low group from Figure 5), which had a very low percentage of GFP+ cells remaining in bone marrow 10 months after reconstitution, about 50% of the cells within stem cell gates were GFP+ (Figure 6). Given that approximately 50% of the cells within these gates are non-stem cells in the absence of Thy-1.1 staining, there could well be a similar majority of LT-HSC that remain GFP+ long after the primary reconstitution.
LT-HSC remain GFP+ in significantly higher proportion than developing bone marrow cells.
Two mice reconstituted for 10 months were analyzed for GFP expression in developing bone marrow cells and within the hematopoietic stem cell population using 5-color FACS analysis. Cells were stained for the donor marker (Ly-5.2, allophycocyanin), lineage markers (Cy5-PE), c-kit (PE), Sca-1 (Texas Red), and GFP (fluorescence in the fluorescein isothiocyanate channel). The numbers in parentheses indicate gating of lineage-marker negative-to-low cells according to the numeric scale given on the top of the lineage-marker histogram. Donor-derived, LT-HSC will be present at high purity in the Ly-5.2+, Sca-1+, c-kit+, Lin (0-80) gated population. The entire hematopoietic stem cell pool, which includes short-term reconstituting stem cells, will be present in the Lin (0-100) fraction.
LT-HSC remain GFP+ in significantly higher proportion than developing bone marrow cells.
Two mice reconstituted for 10 months were analyzed for GFP expression in developing bone marrow cells and within the hematopoietic stem cell population using 5-color FACS analysis. Cells were stained for the donor marker (Ly-5.2, allophycocyanin), lineage markers (Cy5-PE), c-kit (PE), Sca-1 (Texas Red), and GFP (fluorescence in the fluorescein isothiocyanate channel). The numbers in parentheses indicate gating of lineage-marker negative-to-low cells according to the numeric scale given on the top of the lineage-marker histogram. Donor-derived, LT-HSC will be present at high purity in the Ly-5.2+, Sca-1+, c-kit+, Lin (0-80) gated population. The entire hematopoietic stem cell pool, which includes short-term reconstituting stem cells, will be present in the Lin (0-100) fraction.
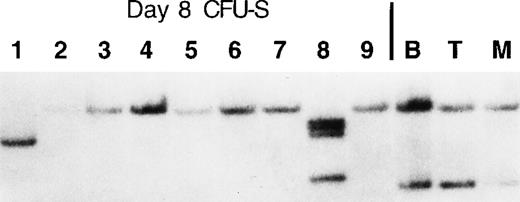
Southern blot analysis of purified B cells (B220+ cells from spleen), myeloid cells (Gr-1 and Mac-1+ cells from spleen), and developing T cells (whole thymus) from mouse L1 showed that there were 2 independent retroviral integrants that contributed most of the hematopoiesis seen in the bone marrow and peripheral lymphoid tissues (Figure 7). To determine whether 2 proviral integration events occurred in the same stem cell or in 2 independent stem cell clones, DNA from day 8 CFU-S was isolated following secondary transplantation of 50 000 nucleated bone marrow cells from long-term reconstituted mouse L1. Clonal analysis indicated that 2 independent stem cell clones (both of which remained GFP+, Figure 6) were responsible for most of the hematopoiesis seen in DNA samples isolated from mouse L1 and that other stem cells were also contributing to hematopoiesis at 10 months post-reconstitution at very low levels (samples 1 and 8). These rare clones can be seen in longer exposures of the Southern blot in the B, T, and myeloid cell lanes (data not shown).
Two independent LT-HSC clones contribute most of the hematopoiesis seen in mouse L1.
A portion of the bone marrow used for FACS analysis shown in Figure 6was used to inject secondary lethally irradiated mice for day-8 CFU-S. A total of 50 000 nucleated bone marrow cells were used per injection. Macroscopic spleen colonies were individually dissected 8 days post-reconstitution, and then genomic DNA isolated from each colony was used in Southern analysis to determine each proviral integration site. Whole B, T, and myeloid cell samples were obtained by positive magnetic bead selection (Miltenyi Biotech) using single cell suspensions of spleen (B and myeloid cells) and thymus (T cells).
Two independent LT-HSC clones contribute most of the hematopoiesis seen in mouse L1.
A portion of the bone marrow used for FACS analysis shown in Figure 6was used to inject secondary lethally irradiated mice for day-8 CFU-S. A total of 50 000 nucleated bone marrow cells were used per injection. Macroscopic spleen colonies were individually dissected 8 days post-reconstitution, and then genomic DNA isolated from each colony was used in Southern analysis to determine each proviral integration site. Whole B, T, and myeloid cell samples were obtained by positive magnetic bead selection (Miltenyi Biotech) using single cell suspensions of spleen (B and myeloid cells) and thymus (T cells).
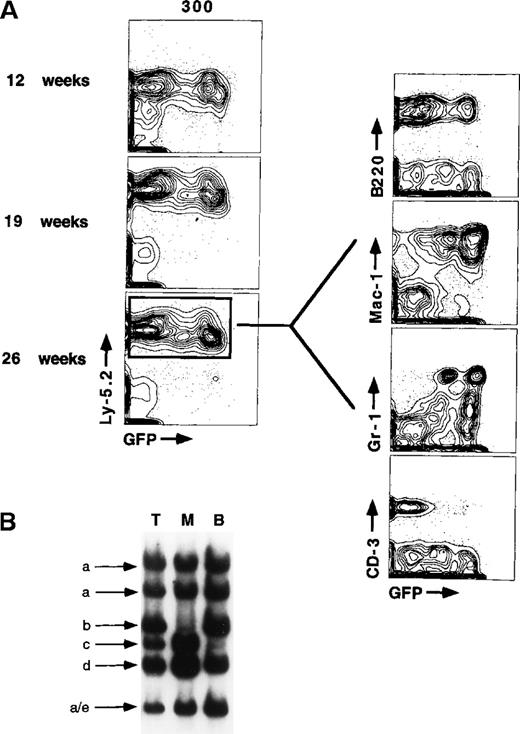
Even though the 2 major stem cell clones have remained GFP+(Figure 6), a significant percentage (31.6%) of donor-derived hematopoiesis is GFP− within the bone marrow of mouse L1. This suggests that silencing occurs early in the development of cell lineages that mature in bone marrow, which would primarily be erythrocytes, progenitor B cells, and myeloid cells. FACS analysis of donor-derived cells within bone marrow of mouse L1 (Figure8) shows that no inactivation occurred as LT-HSC differentiated into cells of the erythroid (Ter-119) or myeloid lineages (Mac-1 or Gr-1). Inactivation was only seen in progenitor B cells (B220+), T cells (CD3+), and natural killer cells (NK-1.1+). Notably, a small percentage of each of these populations remained GFP+. The data suggest that silencing can occur at a very early stage in lymphoid cell development, perhaps before the acquisition of B220 surface expression on progenitor B cells. Although we have no data for the number of stem cell clones contributing to hematopoiesis in mouse L2, there was clearly a large change in the ratio of GFP+ to GFP− cells within stem cell gates (Figure 6) versus whole bone marrow. This difference within bone marrow of mouse L2 indicates that inactivation can also occur as LT-HSC differentiate into myeloid cells, although it appears to be much less frequent than inactivation in lymphoid cells. Analysis of another independent animal reconstituted with 300 GFP+LT-HSC for 6.5 months showed a very similar GFP expression pattern in the peripheral blood cell lineages (compare GFP expression in myeloid and lymphoid cells at 26 weeks, Figure9). The molarity of the Southern blot bands indicates that at least 4 to 5 independent stem cell clones were contributing all of the hematopoiesis in the animal. This animal was nearly 100% GFP+ in the myeloid lineages, yet showed significant inactivation in peripheral B- and T-cell populations. This is in agreement with in vitro data showing that single GFP+LT-HSC sorted into 7-factor methylcellulose culture conditions favoring myeloid cell development do not inactivate GFP expression over the 10-day time of culture (Samuel Cheshier and Irving L. Weissman, data not shown).
Inactivation of GFP expression occurs primarily as stem cells differentiate into lymphoid cell populations.
Bone marrow cells were stained with antibodies directed against markers that define specific blood cell lineages, including B cells (B220), monocytes and neutrophils (Mac-1 and Gr-1), primitive erythrocytes (Ter-119), natural killer cells (NK-1.1), and T cells (CD3). Contour plots showing mature lineage marker expression are gated for all donor-derived (Ly-5.2) cells.
Inactivation of GFP expression occurs primarily as stem cells differentiate into lymphoid cell populations.
Bone marrow cells were stained with antibodies directed against markers that define specific blood cell lineages, including B cells (B220), monocytes and neutrophils (Mac-1 and Gr-1), primitive erythrocytes (Ter-119), natural killer cells (NK-1.1), and T cells (CD3). Contour plots showing mature lineage marker expression are gated for all donor-derived (Ly-5.2) cells.
GFP expression is largely maintained during myeloid differentiation from stem cells.
T, myeloid, and B cells were isolated from the bone marrow and spleen of a mouse reconstituted for 26 weeks using positive selection with magnetic beads. The molarity of the Southern bands indicates that at least 5 independent stem cell clones contribute most of the hematopoiesis in the animal (arrows a-e). The purity of column-enriched cells was generally 80% to 95% based on reanalysis of enriched cells. Nearly all donor-derived myeloid cells (monocytes and neutrophils) were GFP+ in the long-term reconstituted animal.
GFP expression is largely maintained during myeloid differentiation from stem cells.
T, myeloid, and B cells were isolated from the bone marrow and spleen of a mouse reconstituted for 26 weeks using positive selection with magnetic beads. The molarity of the Southern bands indicates that at least 5 independent stem cell clones contribute most of the hematopoiesis in the animal (arrows a-e). The purity of column-enriched cells was generally 80% to 95% based on reanalysis of enriched cells. Nearly all donor-derived myeloid cells (monocytes and neutrophils) were GFP+ in the long-term reconstituted animal.
Silencing can occur at the level of the LT-HSC
The previous observations show that inactivation of retroviral-mediated gene expression can occur as LT-HSC commit to differentiate along lymphoid cell pathways in vivo. However, a very significant number of all proviral integration events that initially express GFP (which permits resorting of cells following retroviral transduction) go on to inactivate expression from the integrated provirus (Figure 1). To assess whether LT-HSC remained GFP+in animals with no sustained GFP expression in peripheral blood, we analyzed stem cells in a GFP−, long-term reconstituted recipient that was highly donor-reconstituted (Figure10). Even though 46% of bone marrow was donor-derived, no GFP+ LT-HSC was detected in bone marrow. Based on Southern blot analysis, one major stem cell clone was contributing almost all of the donor blood cells in the primary transplant recipient (Figure 11). This indicates that inactivation occurring at the LT-HSC level is maintained in myeloid cell progeny that normally have the potential to express genes from the MFG vector (Figures 8 and 9).
Silencing occurs at the LT-HSC level.
LT-HSC were analyzed in an animal that showed no evidence of sustained GFP expression after long-term reconstitution even though the animal was highly donor-reconstituted. Donor hematopoietic stem cells were analyzed for being Ly-5.2+ (APC), Sca-1+ (Texas Red), lineage marker–negative (Cy5-PE), c-kit+ (PE), and GFP+ or GFP−.
Silencing occurs at the LT-HSC level.
LT-HSC were analyzed in an animal that showed no evidence of sustained GFP expression after long-term reconstitution even though the animal was highly donor-reconstituted. Donor hematopoietic stem cells were analyzed for being Ly-5.2+ (APC), Sca-1+ (Texas Red), lineage marker–negative (Cy5-PE), c-kit+ (PE), and GFP+ or GFP−.
Donor (Ly-5.2+) bone marrow cells contain integrated provirus based on Southern analysis.
Clonal assays to determine the number of proviral integrants per cell were done on isolated day 8 CFU-S colonies and from single stem cells FACS-sorted onto a stromal feeder layer (AC-6) that supports cell growth and expansion so that sufficient amounts of DNA could be isolated for analysis. B, T, and myeloid cell populations were purified by magnetic bead enrichment as described previously. The Southern blot was probed with a randomly primed GFP fragment.
Donor (Ly-5.2+) bone marrow cells contain integrated provirus based on Southern analysis.
Clonal assays to determine the number of proviral integrants per cell were done on isolated day 8 CFU-S colonies and from single stem cells FACS-sorted onto a stromal feeder layer (AC-6) that supports cell growth and expansion so that sufficient amounts of DNA could be isolated for analysis. B, T, and myeloid cell populations were purified by magnetic bead enrichment as described previously. The Southern blot was probed with a randomly primed GFP fragment.
Discussion
The studies reported here illustrate at least 2 levels of control that determine the active state of gene expression from the MFG retroviral vector in hematopoietic cells. Transplantation of limited numbers of transduced GFP+ stem cells shows that most retroviral integrants are silenced within the first 6 weeks following reconstitution (Figure 1). Silencing at the LT-HSC level may be much more rapid than 6 weeks due to the half-life of differentiated cells that are initially GFP+ following reconstitution. Proviral integrants that are completely silenced cease to express GFP at the LT-HSC level (Figure 10) and do not show evidence of GFP reexpression upon differentiation or upon transplantation into secondary irradiated recipient animals (Figure 4).
Others have demonstrated that a mechanism for retroviral inactivation could involve de novo methylation of viral sequences leading to stable propagation of the inactive state.12,24-27 Silencing of virally expressed genes has been observed in primary fibroblasts28,29 and in cultured cell lines.26,30 This points out that inactivation does not have to correlate with differentiation but, rather, may be a consequence of stochastic processes influenced by DNA replication. This could explain why some integrants, which were stably expressing GFP in long-term reconstituted primary recipients, were silenced upon secondary transfer (Figure 4). In the context of a reconstitution, presumably all stem cells would be stimulated to cycle rapidly in response to the hematopoietic needs of the animal. Understanding how chromatin conformation is reestablished following replication may shed light on why certain proviral integrants would remain active over successive rounds of replication while others are silenced. Silencing may occur more slowly in LT-HSC in primary transplant recipients because of the slower rate at which this population of cells is cycling in vivo.21 22
The second level of gene inactivation occurs downstream of LT-HSC and probably involves at least 2 mechanisms. In all primary transplant recipients that continued to express GFP 8 to 10 weeks after reconstitution (a total of 13 animals), expression remained stable for long periods. LT-HSC in these animals had a much higher ratio of GFP+ to GFP− cells than seen in whole bone marrow (Figure 6), with the most silencing occurring as LT-HSC differentiate into lymphoid cells (Figure 8). The early stage at which silencing was evident in progenitor B cells might suggest that silencing occurs as part of chromatin remodeling that accompanies lymphoid cell (or B cell) commitment. Assuming that retroviruses integrate into “open” chromatin present in hematopoietic stem cells,31-34 one consequence of differentiation along the lymphoid cell pathway might be the shutdown of open chromatin around stem cell genes and the emergence of new open chromatin domains that mark the unique set of genes defining a particular lymphoid cell type. Such a mechanism of inactivation would not be biased toward retroviruses but, rather, would affect all DNA that preferentially integrates into particular regions of open chromatin. The involvement of chromatin structure in silencing is strongly suggested by experiments that showed that completely silenced genes expressed from an adeno-associated viral backbone could be fully reactivated using chemical inhibitors of histone deacetylases.30Deacetylation of histones H3 and H4 is associated with the condensed, transcriptionally inactive state of chromatin.35
Another explanation for the lack of retroviral gene expression in most lymphoid cells could be the absence of positively acting transcription factors, or the presence of silencing factors, that control expression from the retroviral LTR. This is apparently the case for peripheral T lymphocytes, where only a few percent of GFP+ cells were also CD3+ in all animals tested, as has been in cases reported for human T cells.36-38 It is unlikely that this explanation is true for developing B lymphocytes in bone marrow or in the periphery in that some animals showed at least 50% positive B-lineage cells at early time points following reconstitution. This indicates that a high percentage of B cells have the potential for expressing a gene from the retroviral promoter. Another possibility is that lymphoid cell populations may express higher levels of de novo methylase activity than myeloid cells and are therefore more sensitive to silencing by a methylation-dependent mechanism.
Our observations are in agreement with a number of studies that document stable expression of retrovirally driven genes in myeloid cell populations such as day-12 CFU-S or peripheral blood monocytes and neutrophils.10,12,13 In some cases, we suspect that silencing would not be as readily observed in experiments done by coculturing stem cells with retroviral producer cells (which historically has been the most commonly used transduction approach) because of a higher number of integration events per stem cell. Multiple integrants would mask the actual frequency of silencing that we observe using a viral supernatant transduction protocol that, in general, leads to single integration events (13 of 15 single integrations based on clonal analyses). Although silencing at the LT-HSC level might be overcome through means that promote multiple integration events per cell, this has the disadvantage of increased potential for oncogenic integration events in long-lived cells that could accumulate additional mutations over time. Improvements in vector design, like murine stem cell virus (MSCV)39 and MND,40 may overcome the inactivation mechanism operating at the LT-HSC level that is independent of differentiation status. These vectors seem promising based on a number of recent studies,41-44 although the number of proviral integrants per cell were not evaluated at a clonal level. In one study,44 there were an estimated 2.5 proviral copies (using MSCV) per haploid genome based on Southern analysis of hematopoietic tissues from transplanted animals. If silencing did occur at frequencies of less than 50% of all integration events, this would be masked by the amount of provirus in each cell. Silencing at a very early stage in the development of lymphoid cells points out the need for further experiments to understand the nature of the inactivation process in this arm of the hematopoietic system. Vectors may need to have modifications designed to buffer integrated genes from chromatin effects or vectors that have modified LTR sequences that are permissive for expression in lymphoid cells. This will be critical for targeting a host of lymphoid deficiencies. Our preliminary experiments suggest that erythroid cell progenitors that express the Ter-119 antigen will behave similar to myeloid cells with respect to retrovirally mediated gene expression (Figure 8). Experiments addressing sustained gene expression in megakaryocytes and in dendritic cell populations have not yet been done.
Acknowledgments
We express our appreciation to Libuse Jerabek for laboratory management, Veronica Braunstein for antibody preparation, Tim Knaak for FACS operation, and Lucino Hidalgo for animal care. We also thank Michael Anderson and members of the Weissman laboratory for helpful and stimulating discussion.
Supported by grants from the National Cancer Institute (CA 42551), the National Institute of Diabetes and Digestive and Kidney Diseases (DK 54766-01), and from SyStemix/Sandoz.
Reprints:Christopher A. Klug, University of Alabama at Birmingham, Comprehensive Cancer Center, Room 387, Birmingham, AL 35294-3300; e-mail: chris.klug@ccc.uab.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.








This feature is available to Subscribers Only
Sign In or Create an Account Close Modal