Abstract
Using Cox models, we established a new prognostic system based on simple clinical parameters in a training series of 232 patients whose diagnoses were made before 1989. Adverse prognostic factors for survival (P < .01) were age 65 years or older, male gender, albumin level lower than 40 g/L, hemoglobin level lower than 12 g/dL, platelet count less than 150 × 109/L, white blood cell count less than 4 × 109/L, high number of cytopenias, and hepatomegaly. Taking age (age 65 years or older, 1 point; younger than 65 years, 0 points), albumin (less than 40 g/L, 1 point; 40 g/L or more, 0 points), and total number of cytopenias (no cytopenia, 0 points; 1 cytopenia, 1 point; 2 or 3 cytopenias, 2 points) into account, we separated the 232 patients into 3 groups with low (score 0 or 1), intermediate (score 2), or high (score 3 or 4) risk, associated with 5-year survival rates at 87%, 62%, and 25%, respectively (P < .0001). Only the presence of 2 or 3 cytopenias was an independent prognostic factor among patients younger than 65 years (P < .0001). Albumin level lower than 40 g/L and the presence of 1 or more cytopenia defined a prognostic system for patients 65 years and older. Patients at low risk, intermediate risk, and high risk had 5-year survival rates at 92%, 63%, and 27%, respectively (P < .0001). The 3 prognostic systems separated the 167 patients of a test series in groups with significantly different survival rates. The overall scoring system retained a significant prognostic value in 86 additional patients treated between 1990 and 1996. We conclude that the combination of age, albumin level, and blood cell counts might help to select patients with Waldenström macroglobulinemia for treatment and to evaluate therapeutic results.
Waldenström macroglobulinemia (WM) is a clonal proliferation of lymphocytes or plasma cells in bone marrow in which a large amount of monoclonal immunoglobulin M (mIgM) protein is produced.1 Clinical features include hyperviscosity, organomegaly, and cytopenia.2,3 Treatment usually consisted of prolonged low-dose chlorambucil therapy.1-4 More recently, long-term responses have been observed in 34% and 40% of patients with alkylating agent-resistant disease who were administered fludarabine5 and 2-chlorodeoxyadenosine,6respectively, and in 85% of patients with newly diagnosed disease.7 Although several analyses have indicated that age older than 60 years, male gender, and anemia were associated with a short survival times in patients with WM,1,3,8 only 1 prognostic system has been developed.9 The identification of patients at high or low risk should help to select patients who may benefit from new drugs administered early during the course of the disease. For this reason, we performed a prognostic analysis of survival in 232 patients with WM. Using multivariate analyses we developed a new, simple scoring system for clinical use, and we further validated it on 2 independent series of 1678 and 86 patients.
Patients and methods
Patients
Between March 1964 and October 1989, 232 patients were diagnosed with WM in the French university hospitals of Rouen, Rennes, and Saint Antoine (Paris). Two independent central reviews of the following inclusion criteria were performed (P.M., C.B.) to ensure the quality of the data collected by 2 other investigators (D.J., E.W.): serum mIgM level higher than 5 g/L and bone marrow (BM) infiltration by more than 25% normal lymphoid cells or lymphoplasmacytoid cells on BM smears or lymphoid infiltration on BM trephine biopsy.8
Serum mIgM was initially measured by radial immunodiffusion and later by a laser nephelometric technique. However, because both methods can give unreliable results with mIgM, all paraprotein levels were reevaluated from the densitometry tracing of the serum electrophoretic pattern. Bence–Jones protein was determined by immunoelectrophoresis or immunofixation. The M protein was measured on a warm sample in patients with cryoglobulinemia or cold agglutinin disease. Fifty-six patients had isotopic red cell mass and plasma volume determinations made. Although plasma volume increases in patients with anemia, we stated that plasma volumes higher than 50 mL/kg were probably not explained by anemia alone.10 Finally, the score described by Gobbi et al9 was assessed. This scoring system takes the following adverse prognostic factors into account: weight loss, cryoglobulinemia, hemoglobin less than 10 g/dL, and age older than 60 years. Patients at low risk have no or 1 adverse feature. The remaining patients are at high risk.
Treatment
Treatment criteria were the same in all patients. Chemotherapy was started only if a patient had hyperviscosity, other symptoms related to mIgM, cytopenias, or organomegaly. Sixty-five asymptomatic patients underwent no chemotherapy at diagnosis. Their median freedom-from-treatment interval was 28 months; the 95% confidence interval (CI) was 16 to 48 months. Only 8% of these patients remained untreated at the date of analysis. One hundred fifty-eight patients were treated at diagnosis with chlorambucil (0.1 mg/kg per day, administered until 6 to 12 months after a plateau mIgM had been obtained) alone (150 patients) or in association with prednisolone (8 patients). Nine patients underwent combination chemotherapy at diagnosis because of bulky disease (cyclophosphamide, vincristine, and prednisone [COP regimen], or cyclophosphamide, vincristine, doxorubicin, and prednisone [CHOP or VCAP regimens]). Fifteen patients required plasmapheresis. None received fludarabine or 2-chlorodeoxyadenosine therapy or underwent stem cell transplantation.
Statistical analysis
The following parameters were tested: age, sex, the presence of B symptoms, peripheral lymphadenopathy, splenomegaly, hepatomegaly, extranodal involvement, myelofibrosis, the percentage of lymphoid cells in bone marrow smears, blood cell counts, mIgM, albumin level, β2-microglobulin, plasma volume, antiglobulin test positivity, cryoglobulinemia, cold agglutinin, Bence–Jones proteinuria, type of light chain, Rai11 and Binet12 stages for chronic lymphocytic leukemia. β2-microglobulin and plasma volume were excluded from multivariate analyses because they were available in only few patients.
All analyses were performed with the statistical analysis software (6.12 release; SAS Institute, Cary, NC). Survival curves were drawn using the Kaplan–Meier product limit method.13 Survival was measured from diagnosis to death or to last follow-up. Statistical comparisons between actuarial survival curves were based on log rank tests. Continuous prognostic factors were categorized using the minimumP value approach with the correction proposed by Altman et al.14 After assessment of proportional hazards assumption by the Cox test,15 the parameters significant at the 0.05 level for survival were introduced in a proportional hazards model regression analysis of survival with the forward stepwise selection.16 Total number of cytopenias was a variable with 3 degrees of freedom (DF); therefore, it was coded by 3 binary covariates16 in all proportional hazards model regression analyses. These covariates corresponded to the presence of at least 1 cytopenia (versus no cytopenia), the presence of at least 2 cytopenias (versus no or 1 cytopenia), and the presence of 3 cytopenias (versus no, 1, or 2 cytopenias). The procedure was stopped when the Pvalue for entering an additional factor was greater than .05. Using regression coefficients for weighting each selected covariate, we obtained a categorical risk score for routine clinical use. In case of inclusion of age or gender in the scoring system, the same analyses were repeated in each subgroup of patients with similar demographic characteristics to establish an age-adjusted prognostic system. Each prognostic category (low, intermediate, or high) was retained in scoring systems if it had a significantly different survival (withP < .05 at log rank test) compared to each other category of the scoring system.
Test series
We first used 167 patients whose disease was diagnosed between January 1969 and December 1988 at Lille University Hospital as an independent test series (Lille test series) for validation of the prognostic model.8 Inclusion and treatment criteria were the same in this test series as in the training series. At the time of the current analysis, 85 patients had died. Albumin was estimated in these patients using the following equation: [serum albumin] = 12.6 + 0.4 × [protid] − 0.33 × [mIgM], obtained by linear regression analysis performed on the 232 patients of the training series (P = .0001; R2 = 0.3646).
To assess the effectiveness of the scoring system in patients treated during the 1990s who might have undergone more aggressive chemotherapy than chlorambucil, we applied the present scoring systems to an additional series of 86 patients whose disease was diagnosed at Lens, Paris, Rennes, and Rouen (LPRR test series) between January 1990 and December 1996. This group comprised 60 men and 26 women, 39 to 89 years of age (median age, 70 years). Inclusion and treatment criteria were the same as for other patients. However, most patients remained untreated (47) or received chlorambucil as first-line therapy (33) because of their advanced age. Only 7 patients received an anthracycline-containing chemotherapy regimen (at diagnosis in 5 patients, later in 2). Seven patients received fludarabine or 2-chlorodeoxyadenosine (at diagnosis in 1 patient, later in 6). None underwent autologous bone marrow transplantation or monoclonal antibody transplantation.
Results
Initial characteristics in the training series
Median patient age was 67 years (range, 30 to 100 years), and the male-to-female ratio was 2.46. Weight loss was recorded in 36 (15%) patients. One hundred twenty-four (53%) patients had organomegaly (lymphadenopathy, splenomegaly, or hepatomegaly). Clinical signs of hyperviscosity, including neurologic, ocular, or auditory impairment, were present in 23 (10%) patients, bleeding symptoms in 17 (7%), and peripheral neuropathy in 10 (4%). Patient characteristics are outlined in Tables 1 and2.
Initial characteristics of the 232 patients
| Characteristics . | No.patients . | (%) . | No. patients younger than 65* . | No. patients 65 or older* . |
|---|---|---|---|---|
| Total number of patients | 232 | 74 | 158 | |
| Sex | ||||
| Female | 67 | 29 | 21 (28) | 46 (29) |
| Male | 165 | 71 | 53 (72) | 112 (71) |
| Hepatomegaly | ||||
| Present | 49 | 21 | 17 (23) | 32 (20) |
| Absent | 183 | 79 | 57 (77) | 126 (80) |
| Splenomegaly† | ||||
| Present | 51 | 22 | 16 (22) | 35 (22) |
| Absent | 179 | 78 | 58 (78) | 121 (78) |
| Serum albumin (g/L)† | ||||
| Lower than 40 | 135 | 69 | 38 (60) | 97 (74) |
| At least 40 | 59 | 31 | 25 (40) | 34 (26) |
| Monoclonal IgM (g/L) | ||||
| Lower than 25 | 92 | 40 | 29 (39) | 63 (40) |
| At least 25 | 140 | 60 | 45 (61) | 95 (60) |
| Hemoglobin (g/dL)† | ||||
| Lower than 12 | 145 | 67 | 40 (57) | 105 (71) |
| At least 12 | 73 | 33 | 30 (43) | 43 (29) |
| Leukocytes (109/L)† | ||||
| Fewer than 4 | 16 | 8 | 5 (7) | 11 (7) |
| 4-10 | 176 | 77 | 56 (78) | 120 (79) |
| At least 10 | 32 | 15 | 11 (15) | 21 (14) |
| PMN count (109/L)† | ||||
| Fewer than 1.5 | 17 | 8 | 5 (7) | 12 (8) |
| 1.5 to 10 | 193 | 89 | 64 (89) | 129 (90) |
| More than 10 | 6 | 3 | 3 (4) | 3 (2) |
| Platelets (109/L)† | ||||
| Fewer than 150 | 46 | 20 | 11 (18) | 35 (25) |
| At least 150 | 167 | 80 | 61 (82) | 106 (75) |
| No. cytopenia† | ||||
| 0 | 57 | 28 | 28 (42) | 29 (21) |
| 1 | 105 | 52 | 30 (45) | 75 (55) |
| 2 | 33 | 16 | 6 (9) | 28 (20) |
| 3 | 8 | 4 | 3 (4) | 5 (4) |
| Characteristics . | No.patients . | (%) . | No. patients younger than 65* . | No. patients 65 or older* . |
|---|---|---|---|---|
| Total number of patients | 232 | 74 | 158 | |
| Sex | ||||
| Female | 67 | 29 | 21 (28) | 46 (29) |
| Male | 165 | 71 | 53 (72) | 112 (71) |
| Hepatomegaly | ||||
| Present | 49 | 21 | 17 (23) | 32 (20) |
| Absent | 183 | 79 | 57 (77) | 126 (80) |
| Splenomegaly† | ||||
| Present | 51 | 22 | 16 (22) | 35 (22) |
| Absent | 179 | 78 | 58 (78) | 121 (78) |
| Serum albumin (g/L)† | ||||
| Lower than 40 | 135 | 69 | 38 (60) | 97 (74) |
| At least 40 | 59 | 31 | 25 (40) | 34 (26) |
| Monoclonal IgM (g/L) | ||||
| Lower than 25 | 92 | 40 | 29 (39) | 63 (40) |
| At least 25 | 140 | 60 | 45 (61) | 95 (60) |
| Hemoglobin (g/dL)† | ||||
| Lower than 12 | 145 | 67 | 40 (57) | 105 (71) |
| At least 12 | 73 | 33 | 30 (43) | 43 (29) |
| Leukocytes (109/L)† | ||||
| Fewer than 4 | 16 | 8 | 5 (7) | 11 (7) |
| 4-10 | 176 | 77 | 56 (78) | 120 (79) |
| At least 10 | 32 | 15 | 11 (15) | 21 (14) |
| PMN count (109/L)† | ||||
| Fewer than 1.5 | 17 | 8 | 5 (7) | 12 (8) |
| 1.5 to 10 | 193 | 89 | 64 (89) | 129 (90) |
| More than 10 | 6 | 3 | 3 (4) | 3 (2) |
| Platelets (109/L)† | ||||
| Fewer than 150 | 46 | 20 | 11 (18) | 35 (25) |
| At least 150 | 167 | 80 | 61 (82) | 106 (75) |
| No. cytopenia† | ||||
| 0 | 57 | 28 | 28 (42) | 29 (21) |
| 1 | 105 | 52 | 30 (45) | 75 (55) |
| 2 | 33 | 16 | 6 (9) | 28 (20) |
| 3 | 8 | 4 | 3 (4) | 5 (4) |
PMN, polymorphonuclear.
Percentages in parentheses.
Data were unavailable for some patients.
Distribution of hemoglobin, white blood cell count, and platelet count according to the number of cytopenia in patients with Waldenström macroglobulinemia
| Blood cell counts . | Total no.cytopenias . | Total no.patients (%) . | ||
|---|---|---|---|---|
| Hemoglobin . | Leukocytes . | Platelets . | ||
| At least 12 g/dL | ≥4 × 103/mm3 | ≥150 × 103/mm3 | 0 | 57 (29) |
| <150 × 103/mm3 | 1 | 5 (2) | ||
| <4 × 103/mm3 | ≥150 × 103/mm3 | 1 | 1 (1) | |
| <150 × 103/mm3 | 2 | 2 (1) | ||
| Lower than 12 g/dL | ≥4 × 103/mm3 | ≥150 × 103/mm3 | 1 | 99 (48) |
| <150 × 103/mm3 | 2 | 26 (13) | ||
| <4 × 103/mm3 | ≥150 × 103/mm3 | 2 | 5 (2) | |
| <150 × 103/mm3 | 3 | 8 (4) | ||
| Blood cell counts . | Total no.cytopenias . | Total no.patients (%) . | ||
|---|---|---|---|---|
| Hemoglobin . | Leukocytes . | Platelets . | ||
| At least 12 g/dL | ≥4 × 103/mm3 | ≥150 × 103/mm3 | 0 | 57 (29) |
| <150 × 103/mm3 | 1 | 5 (2) | ||
| <4 × 103/mm3 | ≥150 × 103/mm3 | 1 | 1 (1) | |
| <150 × 103/mm3 | 2 | 2 (1) | ||
| Lower than 12 g/dL | ≥4 × 103/mm3 | ≥150 × 103/mm3 | 1 | 99 (48) |
| <150 × 103/mm3 | 2 | 26 (13) | ||
| <4 × 103/mm3 | ≥150 × 103/mm3 | 2 | 5 (2) | |
| <150 × 103/mm3 | 3 | 8 (4) | ||
Data were unavailable for some patients.
Hemoglobin less than 12 g/dL, thrombocytopenia (platelets less than 150 × 109/L), leukopenia (leukocytes less than 4 × 109/L) and lymphocytosis (circulating lymphocytes > 4 × 109/L) were present in 67%, 20%, 8%, and 7% of patients respectively. The antiglobulin test was positive at diagnosis in 22 patients (9%), and during the course of the disease in 5 additional patients. Symptomatic autoimmune hemolytic anemia was recorded in 15 of these 27 patients.
Median percentage of lymphoid cells in the bone marrow was 31%. Bone marrow trephine biopsy showed nodular lymphoid infiltration in 34 of the 136 patients and diffuse infiltration in the remaining patients. Serum mIgM concentration ranged from 5.1 to 84 g/L (median, 30 g/L). The light chain was the κ type in 83% of patients. Bence–Jones proteinuria was found in 36% of patients. It was probably underestimated because immunofixation had not been performed in the oldest patients. It was found in 27 of the 107 patients tested. Cryoglobulinemia was associated with arthralgia, neuropathy, or purpura in 13 patients.
Patients whose hemoglobin levels were lower than 12 g/dL had higher lymphoid cell percentages in bone marrow smears (mean, 58% vs 32%;P = .02), higher mIgM (mean, 32 g/L vs 25 g/L;P = .001), and higher plasma volumes (mean, 69 mL/kg vs 53 mL/kg; P = .002) than patients whose hemoglobin levels were 12 g/dL or higher. Plasma volume was less than 50 mL/kg in 10 of 56 tested patients. Mean serum mIgM and mean hemoglobin levels were, respectively, lower (23.3 g/L vs 37.7 g/L; P = .0032) and higher (12.8 g/L vs 9.5 g/L; P = .0002) than those of patients with high plasma volume. Plasma volume was not influenced by the presence of splenomegaly. Red cell mass and hemoglobin distributions were similar in both sexes.
Besides hemoglobin level, there was no difference in the distribution of other initial characteristics between patients with less than 31% or more than 31% lymphoid cells in the bone marrow. There was no difference either in the distribution of other initial characteristics, especially peripheral neuropathy and cold agglutinin hemolytic anemia, between patients with mIgM levels lower than 25 g/L or higher than 25 g/L.
Clinical course of patients of the training series
With a median follow-up of 51 months (range, 3 to 226 months), the median actuarial survival time was 61 months (95% CI, 53-68 months) (Figure 1). Survival time was not different in the patients who received no initial therapy than in the remaining patients (Table 3). One hundred seventy-five patients have died. Causes of death were known in 100 patients: 52 patients died from disease progression, and 32 died from unrelated disease; it was not possible to state accurately on the relationship between death and disease in 16 patients with progressive WM who died from cancer or from infection. Cancer was recorded in 9 patients before the diagnosis of WM (skin, 2; head and neck, 2; gastrointestinal tract, 1; lung, 1; breast, 1; uterus, 1; unknown, 1). It was diagnosed in 30 patients during the follow-up, 6 to 126 months after diagnosis (gastrointestinal tract, 10; skin, 9; lung, 4; kidney, 2; pleura, 1; head and neck, 1; chronic myelomonocytic leukemia, 1; liver, 1; eye, 1).
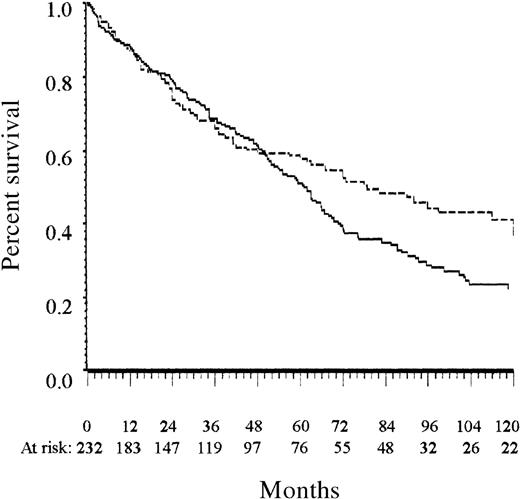
Overall survival of the training and the Lille test series.
The solid line indicates the training series; the dashed line, the Lille test series. All patients were diagnosed before 1990.
Overall survival of the training and the Lille test series.
The solid line indicates the training series; the dashed line, the Lille test series. All patients were diagnosed before 1990.
Results of univariate analysis for survival
| Characteristics . | No.patients . | Median survival (mos) . | 95% CI . | P3-150 . | Corrected P3-151 . |
|---|---|---|---|---|---|
| Age (years) | |||||
| <65 | 74 | 87.2 | (64.5-123.9) | ||
| ≥65 | 158 | 51.1 | (42.3-63.0) | .0009 | .022 |
| Sex | |||||
| Female | 67 | 107.9 | (55.1-150.9) | ||
| Male | 165 | 58.7 | (48.1-65.7) | .0001 | |
| Hepatomegaly | |||||
| Absent | 183 | 66.0 | (58.7-72.5) | ||
| Present | 49 | 34.5 | (27-52.7) | .0025 | |
| Splenomegaly3-152 | |||||
| Absent | 179 | 64.5 | (55.1-70.7) | ||
| Present | 51 | 51.1 | (36.5-69.9) | .15 | |
| Monoclonal IgM (g/L) | |||||
| Lower than 25 | 92 | 68.0 | (55.1-96.9) | ||
| At least 25 | 140 | 59.4 | (49.3-66.8) | .023 | .294 |
| Hemoglobin level (g/dL)3-152 | |||||
| Lower than 12 | 145 | 49.3 | (37.8-59.4) | ||
| At least 12 | 73 | 107.5 | (73.3-126.3) | .0001 | .003 |
| Platelets (109/L)3-152 | |||||
| Fewer than 150 | 46 | 40.2 | (33.3-51.1) | ||
| At least 150 | 167 | 67.2 | (59.4-76.6) | .0001 | .003 |
| Leukocytes (109/L)3-152 | |||||
| Fewer than 4 | 16 | 41.7 | (13.1-58.2) | ||
| At least 4 and fewer than 10 | 176 | 65.7 | (55.1-73.3) | ||
| At least 10 | 32 | 50.7 | (30.4-69.3) | .0057 | .250 |
| PMN count (109/L)3-152 | |||||
| Fewer than 1.5 | 17 | 28.1 | (4.7-49.3) | ||
| 1.5 to 10 | 193 | 64.6 | (58.7-72.3) | ||
| More than 10 | 6 | 30.4 | (7.5-71.3) | .0006 | .070 |
| No. cytopenia3-152 | |||||
| 0 | 57 | 123.9 | (73.4-150.9) | ||
| 1 | 105 | 60.7 | (42.9-67.7) | ||
| 2 or 3 | 41 | 34.5 | (23.5-48.1) | .0001 | |
| Serum albumin (g/L)3-152 | |||||
| Lower than 40 | 135 | 49.3 | (40.2-58.6) | ||
| At least 40 | 59 | 107.5 | (73.3-131.7) | .0001 | .003 |
| β2 microglobulin3-152 | |||||
| Up to 3 mg/L | 12 | 111.6 | (37.6-126.3) | ||
| More than 3 mg/L | 30 | 51.1 | (42.3-67.7) | .038 | |
| Plasma volume3-152 | |||||
| Less than 50 mL/kg | 10 | 150.9 | (15-NA) | ||
| More than 50 mL/kg | 46 | 38.9 | (28.1-51.5) | .0128 | |
| Binet staging3-152 | |||||
| A | 83 | 87.2 | (67.7-122.4) | ||
| B | 28 | 52.7 | (31.6-95.3) | ||
| C | 84 | 37.6 | (28-51.7) | .0001 | |
| Rai staging3-152 | |||||
| 0 or 1 | 53 | 111.6 | (72.3-142.5) | ||
| 2 | 28 | 67.2 | (38.9-122.4) | ||
| 3 or 4 | 142 | 51.7 | (37.6-61.8) | .0001 | |
| Gobbi staging3-152 | |||||
| Low risk | 109 | 72.3 | (64.6-90.4) | ||
| High risk | 53 | 48.3 | (33.2-58.8) | .004 | |
| Treatment | |||||
| No initial therapy | 65 | 66.8 | (48.3-118.4) | ||
| Initial therapy | 167 | 59.4 | (50.7-67.2) | .12 |
| Characteristics . | No.patients . | Median survival (mos) . | 95% CI . | P3-150 . | Corrected P3-151 . |
|---|---|---|---|---|---|
| Age (years) | |||||
| <65 | 74 | 87.2 | (64.5-123.9) | ||
| ≥65 | 158 | 51.1 | (42.3-63.0) | .0009 | .022 |
| Sex | |||||
| Female | 67 | 107.9 | (55.1-150.9) | ||
| Male | 165 | 58.7 | (48.1-65.7) | .0001 | |
| Hepatomegaly | |||||
| Absent | 183 | 66.0 | (58.7-72.5) | ||
| Present | 49 | 34.5 | (27-52.7) | .0025 | |
| Splenomegaly3-152 | |||||
| Absent | 179 | 64.5 | (55.1-70.7) | ||
| Present | 51 | 51.1 | (36.5-69.9) | .15 | |
| Monoclonal IgM (g/L) | |||||
| Lower than 25 | 92 | 68.0 | (55.1-96.9) | ||
| At least 25 | 140 | 59.4 | (49.3-66.8) | .023 | .294 |
| Hemoglobin level (g/dL)3-152 | |||||
| Lower than 12 | 145 | 49.3 | (37.8-59.4) | ||
| At least 12 | 73 | 107.5 | (73.3-126.3) | .0001 | .003 |
| Platelets (109/L)3-152 | |||||
| Fewer than 150 | 46 | 40.2 | (33.3-51.1) | ||
| At least 150 | 167 | 67.2 | (59.4-76.6) | .0001 | .003 |
| Leukocytes (109/L)3-152 | |||||
| Fewer than 4 | 16 | 41.7 | (13.1-58.2) | ||
| At least 4 and fewer than 10 | 176 | 65.7 | (55.1-73.3) | ||
| At least 10 | 32 | 50.7 | (30.4-69.3) | .0057 | .250 |
| PMN count (109/L)3-152 | |||||
| Fewer than 1.5 | 17 | 28.1 | (4.7-49.3) | ||
| 1.5 to 10 | 193 | 64.6 | (58.7-72.3) | ||
| More than 10 | 6 | 30.4 | (7.5-71.3) | .0006 | .070 |
| No. cytopenia3-152 | |||||
| 0 | 57 | 123.9 | (73.4-150.9) | ||
| 1 | 105 | 60.7 | (42.9-67.7) | ||
| 2 or 3 | 41 | 34.5 | (23.5-48.1) | .0001 | |
| Serum albumin (g/L)3-152 | |||||
| Lower than 40 | 135 | 49.3 | (40.2-58.6) | ||
| At least 40 | 59 | 107.5 | (73.3-131.7) | .0001 | .003 |
| β2 microglobulin3-152 | |||||
| Up to 3 mg/L | 12 | 111.6 | (37.6-126.3) | ||
| More than 3 mg/L | 30 | 51.1 | (42.3-67.7) | .038 | |
| Plasma volume3-152 | |||||
| Less than 50 mL/kg | 10 | 150.9 | (15-NA) | ||
| More than 50 mL/kg | 46 | 38.9 | (28.1-51.5) | .0128 | |
| Binet staging3-152 | |||||
| A | 83 | 87.2 | (67.7-122.4) | ||
| B | 28 | 52.7 | (31.6-95.3) | ||
| C | 84 | 37.6 | (28-51.7) | .0001 | |
| Rai staging3-152 | |||||
| 0 or 1 | 53 | 111.6 | (72.3-142.5) | ||
| 2 | 28 | 67.2 | (38.9-122.4) | ||
| 3 or 4 | 142 | 51.7 | (37.6-61.8) | .0001 | |
| Gobbi staging3-152 | |||||
| Low risk | 109 | 72.3 | (64.6-90.4) | ||
| High risk | 53 | 48.3 | (33.2-58.8) | .004 | |
| Treatment | |||||
| No initial therapy | 65 | 66.8 | (48.3-118.4) | ||
| Initial therapy | 167 | 59.4 | (50.7-67.2) | .12 |
PMN, polymorphonuclear; NA, not achieved.
P of the presented analysis.
Corrected P for the optimal cutpoint (see text).
Data were unavailable for some patients.
Univariate analysis of survival prognostic factors in the training series
Parameters associated with adverse prognostic significance at the 0.01 level were age 65 years or older, male gender, albumin level lower than 40 g/L, hemoglobin level lower than 12 g/dL, thrombocytopenia, neutropenia, leukopenia, and leukocytosis, total number of cytopenias, and hepatomegaly (Table 3). Patients with pancytopenia or 2 cytopenias had similar survival duration. Optimal cut-off values were evaluated for all tested covariates and then were used in the univariate analysis shown in Table 3. In addition to the scoring system of Gobbi (P = .004), Rai and Binet stages had a strong prognostic value for survival (P = .0001). Other adverse prognostic factors for survival at the 0.05 level were serum mIgM, β2 microglobulin level greater than 3 mg/L, and plasma volume greater than 50 mL/kg. In contrast, B symptoms, splenomegaly, Bence–Jones proteinuria, type of light chain, lymphocyte count, cryoglobulinemia, pattern of bone marrow involvement, myelofibrosis, cytologic features on bone marrow smears, and histologic features (in the 46 patients who had lymph node biopsy specimens taken at diagnosis) had no prognostic value at the 0.15 level in the present series.
Development of the scoring system in the training series
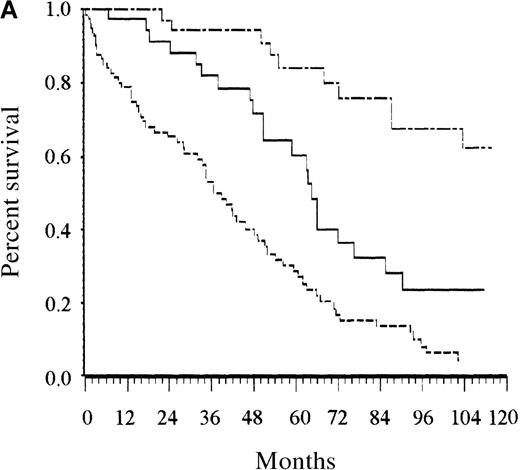
Among the parameters associated with adverse prognostic significance in the univariate analysis, the proportional hazards regression analysis selected 4 adverse parameters: at least 1 cytopenia, at least 2 cytopenias, low serum albumin level, and advanced age (Tables 4 and5). The same analysis was performed with categorized variables, and it selected the same covariates. They are listed in the order entered by the forward stepwise modeling procedure shown in Table 4. Taking the following adverse prognostic factors into account—age older than 65 years, albumin level less than 40 g/L, at least 1 cytopenia, and at least 2 cytopenias—and using estimated regression coefficients, risk for the 16 possible combinations of the 4 covariates fell into 3 groups. Finally, a score was defined as the total number of risk factors (Table 5). For routine clinical use, the 3 risk categories defined by the 2 binary covariates related to cytopenias are similar to the simpler categories defined by 0 for no cytopenia, 1 for 1 cytopenia, and 2 for 2 or more cytopenias. Patients with low risk (score, 0 or 1; 27% of patients), intermediate risk (score, 2; 27% of patients), and high risk (score, 3 or 4; 46% of patients) had estimated 5-year survival rates of 87%, 62%, and 25%, respectively (P < .0001; Figure 2A). The overall prognostic system remained able to separate patients in the training sample into 3 groups with significantly different survival rates when the analysis was restricted to patients whose disease was diagnosed before January 1980 and to those whose disease was diagnosed later (P = .0001 for both analyses).
Results of Cox multivariate analyses
| Codes and characteristics . | Relative risk . | 95% CI . |
|---|---|---|
| All patients | ||
| Step 1 | ||
| 0: 0 or 1 cytopenia | 1 | |
| 1: 2 or 3 cytopenias | 2.103 | (1.335-3.310) |
| Step 2 | ||
| 0: albumin at least 40 g/L | 1 | |
| 1: albumin lower than 40 g/L | 2.022 | (1.291-3.167) |
| Step 3 | ||
| 0: no cytopenia | 1 | |
| 1: 1 or more cytopenia | 2.046 | (1.240-3.377) |
| Step 4 | ||
| 0: age less than 65 years | 1 | |
| 1: age at least 65 years | 1.519 | (1.032-2.236) |
| Patients younger than 65 | ||
| Step 1 | ||
| 0: no or 1 cytopenia | 1 | |
| 1: 2 or 3 cytopenias | 8.14 | (3.323-19.943) |
| Patients 65 and older | ||
| Step 1 | ||
| 0: albumin at least 40 g/L | 1 | |
| 1: albumin lower than 40 g/L | 2.57 | (1.409-4.694) |
| Step 2 | ||
| 0: no cytopenia | 1 | |
| 1: 1 or more cytopenia | 2.19 | (1.131-4.266) |
| Codes and characteristics . | Relative risk . | 95% CI . |
|---|---|---|
| All patients | ||
| Step 1 | ||
| 0: 0 or 1 cytopenia | 1 | |
| 1: 2 or 3 cytopenias | 2.103 | (1.335-3.310) |
| Step 2 | ||
| 0: albumin at least 40 g/L | 1 | |
| 1: albumin lower than 40 g/L | 2.022 | (1.291-3.167) |
| Step 3 | ||
| 0: no cytopenia | 1 | |
| 1: 1 or more cytopenia | 2.046 | (1.240-3.377) |
| Step 4 | ||
| 0: age less than 65 years | 1 | |
| 1: age at least 65 years | 1.519 | (1.032-2.236) |
| Patients younger than 65 | ||
| Step 1 | ||
| 0: no or 1 cytopenia | 1 | |
| 1: 2 or 3 cytopenias | 8.14 | (3.323-19.943) |
| Patients 65 and older | ||
| Step 1 | ||
| 0: albumin at least 40 g/L | 1 | |
| 1: albumin lower than 40 g/L | 2.57 | (1.409-4.694) |
| Step 2 | ||
| 0: no cytopenia | 1 | |
| 1: 1 or more cytopenia | 2.19 | (1.131-4.266) |
Description of the outcome according to prognostic indexes
| . | No. risk factors . | Training series . | Lille test series . | LPRR test series . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients (%) . | Survival rate (%) . | Patients (%) . | Survival rate (%) . | % of patients . | Survival rate (%) . | |||||
| 2 y . | 5 y . | 2 y . | 5 y . | 2 y . | 5 y . | |||||
| Prognostic index, all patients5-150 | ||||||||||
| Low | 0 to 1 | 27 | 97 | 87 | 42 | 93 | 83 | 28 | 96 | 90 |
| Intermediate | 2 | 27 | 88 | 62 | 38 | 85 | 59 | 34 | 96 | 67 |
| High | 3 or 4 | 46 | 64 | 25 | 20 | 57 | 35 | 38 | 74 | 37 |
| Age-adjusted index, patients younger than 655-151 | ||||||||||
| Low | 0 | 86 | 94 | 75 | 80 | 91 | 74 | — | — | — |
| High | 1 | 14 | 53 | 13 | 20 | 50 | 33 | — | — | — |
| Age-adjusted index, patients 65 or older5-152 | ||||||||||
| Low | 0 | 12 | 100 | 92 | 5 | 100 | 80 | — | — | — |
| Intermediate | 1 | 23 | 78 | 63 | 38 | 81 | 52 | — | — | — |
| High | 2 | 65 | 64 | 27 | 56 | 59 | 35 | — | — | — |
| . | No. risk factors . | Training series . | Lille test series . | LPRR test series . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients (%) . | Survival rate (%) . | Patients (%) . | Survival rate (%) . | % of patients . | Survival rate (%) . | |||||
| 2 y . | 5 y . | 2 y . | 5 y . | 2 y . | 5 y . | |||||
| Prognostic index, all patients5-150 | ||||||||||
| Low | 0 to 1 | 27 | 97 | 87 | 42 | 93 | 83 | 28 | 96 | 90 |
| Intermediate | 2 | 27 | 88 | 62 | 38 | 85 | 59 | 34 | 96 | 67 |
| High | 3 or 4 | 46 | 64 | 25 | 20 | 57 | 35 | 38 | 74 | 37 |
| Age-adjusted index, patients younger than 655-151 | ||||||||||
| Low | 0 | 86 | 94 | 75 | 80 | 91 | 74 | — | — | — |
| High | 1 | 14 | 53 | 13 | 20 | 50 | 33 | — | — | — |
| Age-adjusted index, patients 65 or older5-152 | ||||||||||
| Low | 0 | 12 | 100 | 92 | 5 | 100 | 80 | — | — | — |
| Intermediate | 1 | 23 | 78 | 63 | 38 | 81 | 52 | — | — | — |
| High | 2 | 65 | 64 | 27 | 56 | 59 | 35 | — | — | — |
LPRR, Lens, Paris, Rennes, and Rouen.
Adverse prognostic factors: age 65 or older, at least 1 cytopenia, at least 2 cytopenias, and albumin level less than 40 g/L.
Adverse prognostic factor: at least 2 cytopenias.
Adverse prognostic factors: at least 1 cytopenia and albumin level less than 40 g/L.
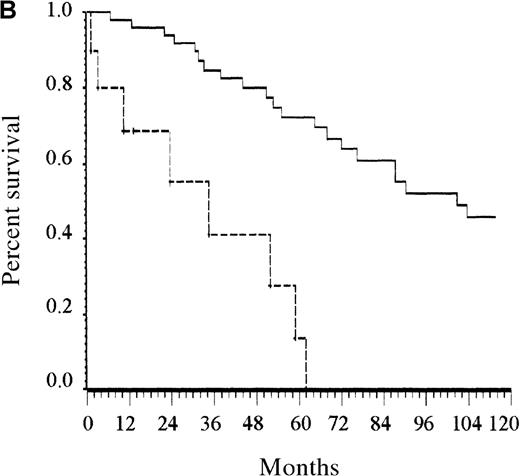
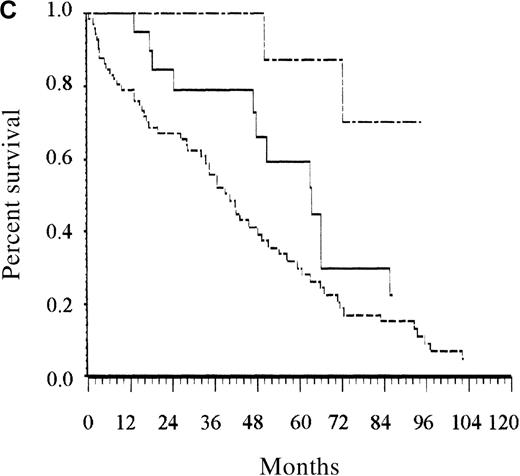
Survival of patients according to the scoring system in the training series.
(A) Overall scoring system (based on age, total number of cytopenias, and serum albumin; log rank = 60.72;P < .0001). The line of alternating dashes and dots indicates low risk (27% of patients; 54% died); the solid line, intermediate risk (27% of patients; 74% died), and the dashed line, high risk (46% of patients; 89% died). (B) Scoring system for patients younger than 65 years (based on total number of cytopenias; log rank = 27.07; P < .0001). The solid line indicates low risk (86% of patients; 69% died), and the dashed line, high risk (14% of patients; 89% died). (C) Scoring system for patients 65 years and older (based on total number of cytopenias and serum albumin; log rank = 26.34; P < .0001). The line of alternating dashes and dots indicates low risk (12% of patients; 43% died); the solid line, intermediate risk (23% of patients; 64% died); and the dashed line, high risk (65% of patients; 91% died).
Survival of patients according to the scoring system in the training series.
(A) Overall scoring system (based on age, total number of cytopenias, and serum albumin; log rank = 60.72;P < .0001). The line of alternating dashes and dots indicates low risk (27% of patients; 54% died); the solid line, intermediate risk (27% of patients; 74% died), and the dashed line, high risk (46% of patients; 89% died). (B) Scoring system for patients younger than 65 years (based on total number of cytopenias; log rank = 27.07; P < .0001). The solid line indicates low risk (86% of patients; 69% died), and the dashed line, high risk (14% of patients; 89% died). (C) Scoring system for patients 65 years and older (based on total number of cytopenias and serum albumin; log rank = 26.34; P < .0001). The line of alternating dashes and dots indicates low risk (12% of patients; 43% died); the solid line, intermediate risk (23% of patients; 64% died); and the dashed line, high risk (65% of patients; 91% died).
Comparison of the present scoring system with the Gobbi system
We assessed the prognostic value of this new scoring system within each risk group defined by the Gobbi system. Our scoring system was able to identify patients at low (25%), intermediate (12%), and high (63%) risk within the low-risk subgroup defined by the Gobbi scoring system (P = .0001) and patients at low (27%), intermediate (30%), and high (43%) risk within the high-risk subgroup defined by Gobbi score (P = .04). Conversely, the log-rank test for heterogeneity was not significant when the prognostic value of the Gobbi system was assessed in low, intermediate, and high-risk groups defined by the present score (P ≥ .88).
Survival prognostic factors in patients younger than 65 years in those 65 and older (training series)
Only the presence of at least 2 cytopenias remained an independent prognostic factor among patients younger than 65 years (Figure 2B). Albumin level lower than 40 g/L and the presence of at least 1 cytopenia were the 2 independent prognostic factors for patients older than 65 years. Counting the number of risk factors present at diagnosis allowed assignment of these patients to 1 of 3 risk groups. Accordingly, patients with low risk (no risk factor, 12% of the patients), intermediate risk (1 risk factor, 23% of patients), and high risk (2 risk factors, 65% of patients) had estimated 5-year survival rates of 92%, 63%, and 27%, respectively (P < .0001; Figure 2C).
Validation of the scoring systems in the test series
There was no difference in the initial characteristics and treatments between the Lille series and the training series, except the lower male-to-female ratio 1.26 in the Lille test series8(Table 5). Based on the overall scoring system, the estimated frequency of patients at high risk was lower in this test series than in the training series. Median survival time of patients in the Lille test series was 60 months (95% CI, 39-73; Figure 1). The overall prognostic system was able to separate patients of the Lille test series into 3 groups—low risk, intermediate risk, and high risk—with significantly different survival rates (P = .0001). Similarly, the presence of at least 2 cytopenias was a significant prognostic factor for survival of patients younger than 65 years in the Lille test series (P = .005). The scoring system developed for patients 65 years and older was able to separate these patients into groups with different survival rates (P = .05). Finally, there were no differences between the LPRR test series and the training series except a higher frequency of patients with low albumin levels (P = .004), a lower frequency of thrombocytopenia (P = .02), and patients with 2 or more cytopenias (P = .02) in the LPRR test series. Median survival time was not achieved in the LPRR series because of the shorter follow-up. The overall prognostic system was able to separate patients of the LPRR test series into low-, intermediate-, and high-risk groups with significantly different rates of survival (P = .001).
Discussion
The present analysis indicated that the combination of age, albumin level, and blood cell counts defined subgroups of patients with WM with significantly different survival rates. This simple scoring system was validated in 2 independent test series.
Although several studies have analyzed prognostic factors for survival in WM,1,3,8,9 only 1 multivariate analysis on 144 patients previously yielded a prognostic scoring system.9 The former reports identified the following adverse prognostic factors: age older than 60 years,1,3,8,9 male gender,8,17hemoglobin level lower than 10 g/dL,8,9,18thrombocytopenia,8,9 leukopenia,8 and granulocytopenia,8 whereas the latter selected 2 independent adverse prognostic factors besides age older than 60 years and hemoglobin level lower than 10 g/dL—namely, weight loss and cryoglobulinemia.9 The present study on 232 patients confirmed the strong prognostic value of age 65 years or older, male gender, low hemoglobin level, and thrombocytopenia for survival. In addition, the total number of cytopenias was shown to have an adverse prognostic value. For practical convenience, leukopenia was selected instead of granulocytopenia for the count of the total number of cytopenias because the prognostic values of both counts (evaluated by the χ2 of the log-rank test) were similar (data not shown). High β2 microglobulin, an adverse prognostic factor in many lymphoid malignancies,19 was associated with a short survival time in patients with WM. By contrast, we failed to confirm the prognostic value of 3 previously reported parameters—weight loss, cryoglobulinemia,9 and bone marrow histology.18,20 However, the latter characteristic has not been assessed in all patients and was not evaluated according to the Bartl18 criteria. It will be of interest to evaluate through large-scale, multivariate analyses the prognostic value of other parameters such as β2 microglobulin,3 lymph node histology, cytologic features,9,18,20 karyotypic abnormalities,21,22 p53 mutations, or proliferating cell nuclear antigen expression,20 which may better reflect the biologic heterogeneity of WM.
Stepwise regression analysis selected the number of cytopenias rather than hemoglobin level. However, the present results do not conflict with previous studies. Indeed, most patients with 1 or more cytopenias had low hemoglobin levels (Table 2). Although patients with hemoglobin levels lower than 12g/dL had higher percentages of lymphoid cells in bone marrow smears, isotopic studies and univariate survival analyses suggested that the adverse prognostic value of a low hemoglobin level might also be related, at least in part, to an increased plasma volume. Moreover, increased plasma volume was significantly associated with high level of mIgM but not with splenomegaly. Albumin level has not been studied in WM; however, the prognostic value of this parameter has been described in other low-grade lymphoid malignancies.23Because most of our patients underwent single low-dose chemotherapy, the prognostic values of age and albumin level are probably not related to a patient's impaired ability to tolerate treatment.
Differences in the findings of the present multivariate analysis and other reports might be attributed to the following reasons: large heterogeneity of features and outcomes in WM, large number of subjects required for the Cox proportional hazard model, statistical variability between analyses, selection of serum albumin (a variable not studied previously), and varying selection criteria. Indeed, Gobbi et al9 required that therapy start within 6 months of diagnosis because of the lack of defined distinction between asymptomatic WM and mIgM gammopathy of unknown significance. However, some patients with WM left untreated until symptoms of the hyperviscosity syndrome occur (usually when mIgM concentration exceeds 30 g/L) were excluded from this analysis, whereas other patients with lower mIgM concentrations, treated for symptoms related to cryoglobulinemia, peripheral neuropathy, or cold agglutinin hemolytic anemia, were eligible. Despite the varying inclusion criteria, the main initial clinical and hematologic characteristics of patients with WM in the present series agreed with previous analyses, which reported median age between 65 and 70 years, male predominance, thrombocytopenia,8,20 leukopenia, leukocytosis,8 and organomegaly,3,8respectively, in 22%, 10% to 25%, 18% to 30%, and 20% to 50% of patients. Fifty percent to 90% of patients had anemia.3,8,9 The median of mIgM ranged between 25 and 30 g/L.8,9 Bence–Jones proteinuria was found in only 36% of patients, probably because few patients had immunofixation. Cryoglobulinemia was present in 15% to 20% of patients,3,8 cold chronic hemolytic anemia and peripheral neuropathy in less than 10% of patients,3,8 and amyloidosis in less than 5% of patients.3,8 Similarly, the overall survival of the training series agreed with the 60-month median survival times previously reported.1,8 9
Cause-specific survival has been considered an appropriate endpoint in WM because it controls for the many unrelated deaths among older patients.3 However the frequency of unrelated deaths should probably be taken into account for treatment decision and evaluation; therefore, we analyzed overall survival, as previously performed for other prognostic scoring systems established for chronic malignancies such as chronic granulocytic leukemia,24 especially for malignancies occurring in the elderly, such as chronic lymphocytic leukemia,11,12 myelodysplastic syndromes,25 or agnogenic myeloid metaplasia.26 In addition, our data suggested that related and unrelated deaths may be very difficult to distinguish for many (16%) elderly patients because WM is a chronic disease with various complications during outcome.
The complete independence of the training and the 2 test series ensures that the prognostic system presented here received a valid evaluation. The slightly better survival of patients of the Lille test series (Figure 1) was probably attributable to the higher number of women8 and could be related to the lower estimated frequency of patients at high risk in this series than in the training series. The scoring system for patients 65 years or older had a lower prognostic value in the test series than in the training series. This may be related to the small number of patients at low risk who were 65 years or older in the Lille test series. The LPRR test series confirmed the prognostic value of the present score in patients treated during the 1990s.
We developed 2 separate models. One applied to all patients, and the other was age adjusted because the 2 prognostic systems may be applicable in different settings. For comparing therapeutic results in a series of patients of any age, the overall prognostic index would be more useful. In contrast, for the design of trials based on more experimental approaches, especially intensive approaches, which are restricted to younger patients, the age-adjusted index would be more useful. The present analysis indicated that only 13% to 20% of younger patients are at high risk at diagnosis. In contrast, new therapeutic approaches available for patients older than 65 might be considered early in the course of the disease in 56% to 65% of those patients. Indeed, WM treatment criteria have not yet been clearly codified to date. This is probably because few effective treatments were available for patients whose WM was resistant to an alkylating agent–glucocorticoid combination. However, for some patients with WM, as for those with other lymphoproliferative diseases, fludarabine,5 2-chlorodeoxyadenosine,6,7monoclonal antibody,27 or myeloablative therapy with autologous stem cell transplantation28 may be more effective when used early in the course of the disease.
Finally, the combination of age, albumin level, and blood cell counts provides a simple prognostic model for survival in WM, which may help to select patients for treatment and to evaluate therapeutic results.
Acknowledgments
The authors thank Alain Duhamel for helpful comments and Marie-Dominique Reynaud for assistance.
Reprints:Pierre Morel, Service d'Hématologie Clinique, Hôpital Schaffner, 62300 Lens, France; e-mail:pimorel@nordnet.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal