Abstract
Recent knowledge gained regarding the relationship between erythropoietin, iron, and erythropoiesis in patients with blood loss anemia, with or without recombinant human erythropoietin therapy, has implications for patient management. Under conditions of significant blood loss, erythropoietin therapy, or both, iron-restricted erythropoiesis is evident, even in the presence of storage iron and iron oral supplementation. Intravenous iron therapy in renal dialysis patients undergoing erythropoietin therapy can produce hematologic responses with serum ferritin levels up to 400 μg/L, indicating that traditional biochemical markers of storage iron in patients with anemia caused by chronic disease are unhelpful in the assessment of iron status. Newer measurements of erythrocyte and reticulocyte indices using automated counters show promise in the evaluation of iron-restricted erythropoiesis. Assays for serum erythropoietin and the transferrin receptor are valuable tools for clinical research, but their roles in routine clinical practice remain undefined. The availability of safer intravenous iron preparations allows for carefully controlled studies of their value in patients undergoing erythropoietin therapy or experiencing blood loss, or both.
Several clinical settings have served as “natural experiments” that have furthered our understanding of the relationship between erythropoietin, iron, and the erythropoietic response to anemia in humans. In a review nearly 20 years ago, Finch1 summarized the knowledge gained primarily from studies of healthy persons, patients with hereditary hemolytic anemias, and patients with hemochromatosis. Under conditions of basal erythropoiesis in normal subjects, plasma iron turnover (as an index of marrow erythropoietic response) is little affected, whether transferrin saturation ranges from very low to very high levels. In contrast, the erythropoietic response in patients with congenital hemolytic anemia, in whom erythropoiesis is chronically raised as much as 6 times over basal levels,2 is affected (and limited) by serum iron levels and by transferrin saturation.3 Patients with hemochromatosis who underwent serial phlebotomy were observed to mount erythropoietic responses as much as 8 times over basal rates, attributed to the maintenance of very high serum iron and transferrin saturation levels in these patients,4 whereas healthy persons have been shown to have difficulty providing sufficient iron to support rates of erythropoiesis more than 3 times basal rates.5 These observations led Finch6 to identify a “relative iron deficiency” state that occurs when increased erythron iron requirements exceed the available supply of iron, even in the presence of storage iron. The recent practice of multiple phlebotomies through autologous blood donation in patients who are scheduled for elective surgery is also a natural experiment in blood loss anemia. This review summarizes insight gained in the past 20 years regarding the relationship between erythropoietin, iron, and erythropoiesis in patients with anemia, along with implications for patient management.
Blood loss anemia through autologous phlebotomy
Erythropoiesis mediated by endogenous erythropoietin
Patients undergoing autologous blood phlebotomy may donate 10.5 mL/kg (450 ± 45 mL) blood as often as twice a week until 72 hours before surgery.7 Under routine conditions, patients usually donate once a week.8 Oral iron supplements are routinely prescribed. This iatrogenic blood loss is accompanied by a response in endogenous erythropoietin levels that, though increased significantly over basal levels, remain within the range of normal (4-26 μm/mL).9 The erythropoietic response that occurs under these conditions is modest.8,10 A summary of selected prospective, controlled trials11-16 of patients undergoing phlebotomy is presented in Table 1. Calculated estimates of red blood cell (RBC) volume expansion (erythropoiesis in excess of basal rates) were determined17; 220 to 351 mL (11% to 19% RBC expansion,11,12 or the equivalent to 1 to 1.75 U blood18) are produced in excess of basal erythropoiesis, defining the efficacy of this blood conservation practice.
Endogenous erythropoietin-mediated erythropoiesis
| Patients (n) . | Blood removed (donated) . | Baseline RBC (mL) . | Blood produced . | Iron therapy . | Reference . | |||
|---|---|---|---|---|---|---|---|---|
| Requested/ donated (U) . | RBC (mL) . | RBC (mL) . | Expansion (%) . | |||||
| Standard phlebotomy | ||||||||
| 108 | 3 | 2.7 | 522 | 1884 | 351 | 19 | PO | 11 |
| 22 | 3 | 2.8 | 590 | 1936 | 220 | 11 | None | 12 |
| 45 | 3 | 2.9 | 621 | 1991 | 331 | 17 | PO | 12 |
| 41 | 3 | 2.9 | 603 | 1918 | 315 | 16 | PO + IV | 12 |
| Aggressive phlebotomy | ||||||||
| 30 | ≥3 | 3.0 | 540 | 2075 | 397 | 19 | None | 13 |
| 30 | ≥3 | 3.1 | 558 | 2024 | 473 | 23 | PO | 13 |
| 30 | ≥3 | 2.9 | 522 | 2057 | 436 | 21 | IV | 13 |
| 24 | 6 | 4.1 | 683 | 2157 | 568 | 26 | PO | 14, 15 |
| 23 | 6 | 4.6 | 757 | 2257 | 440 | 19 | PO | 16 |
| Patients (n) . | Blood removed (donated) . | Baseline RBC (mL) . | Blood produced . | Iron therapy . | Reference . | |||
|---|---|---|---|---|---|---|---|---|
| Requested/ donated (U) . | RBC (mL) . | RBC (mL) . | Expansion (%) . | |||||
| Standard phlebotomy | ||||||||
| 108 | 3 | 2.7 | 522 | 1884 | 351 | 19 | PO | 11 |
| 22 | 3 | 2.8 | 590 | 1936 | 220 | 11 | None | 12 |
| 45 | 3 | 2.9 | 621 | 1991 | 331 | 17 | PO | 12 |
| 41 | 3 | 2.9 | 603 | 1918 | 315 | 16 | PO + IV | 12 |
| Aggressive phlebotomy | ||||||||
| 30 | ≥3 | 3.0 | 540 | 2075 | 397 | 19 | None | 13 |
| 30 | ≥3 | 3.1 | 558 | 2024 | 473 | 23 | PO | 13 |
| 30 | ≥3 | 2.9 | 522 | 2057 | 436 | 21 | IV | 13 |
| 24 | 6 | 4.1 | 683 | 2157 | 568 | 26 | PO | 14, 15 |
| 23 | 6 | 4.6 | 757 | 2257 | 440 | 19 | PO | 16 |
Data are expressed as means.
PO, oral; IV, intravenous.
For patients subjected to more aggressive (up to 2 U a week) phlebotomy, the endogenous erythropoietin response is more substantial.13-16 In one clinical trial,14 a linear–logarithmic relationship was demonstrated between change in hemoglobin level and erythropoietin response,19 predicted previously by phlebotomy experiments in normal subjects.20Erythropoietin-mediated erythropoiesis in this setting is 397 to 568 mL (19% to 26% RBC expansion,13-16 or the equivalent of 2 to 3 U blood18).
Erythropoiesis mediated by erythropoietin therapy
Clinical trials have demonstrated a dose-response relationship between erythropoietin and red blood cell expansion.16 A study of “very low” dose erythropoietin therapy in autologous blood donors found that 400 U/kg administered over a 2-week interval resulted in clinically significant erythropoiesis.21 Table2 details red cell volume expansion in 134 patients treated with erythropoietin therapy during aggressive blood phlebotomy,14-16,22,23 ranging from 358 to 1764 mL (28% to 79% RBC expansion) over 25 to 35 days, or the equivalent of 2 to 9 U blood.18 The range in response (erythropoiesis) to dose (erythropoietin) is not related to patient gender or age,24 25 suggesting that patient-specific factors such as accompanying chronic disease, iron-restricted erythropoiesis, or other factors that normally cause the wide distribution of the hemoglobin level account for the variability in erythropoietic response to erythropoietin.
Erythropoiesis during blood loss and erythropoietin therapy
| Patients (n/sex) . | Total EPO dose (U/kg) . | Blood removed . | Baseline RBC (m) . | Blood produced . | Iron therapy . | Reference . | ||
|---|---|---|---|---|---|---|---|---|
| Units . | RBC (mL) . | RBC (mL) . | Expansion (%) . | |||||
| 10/F | 900 SQ | 3.4 | 435 | 1285 | 358 | 28 | IV | 22 |
| 24 | 900 IV | 5.2 | 864 | 1949 | 621 | 32 | PO | 16 |
| 10/F | 1800 SQ | 4.3 | 526 | 1293 | 474 | 37 | IV | 22 |
| 26 | 1800 IV | 5.5 | 917 | 2032 | 644 | 32 | PO | 16 |
| 11/F | 3600 IV | 4.9 | 809 | 1796 | 701 | 39 | PO | 14, 15 |
| 12/M | 3600 IV | 5.9 | 1097 | 2296 | 1102 | 48 | PO | 14, 15 |
| 23 | 3600 IV | 5.4 | 970 | 2049 | 911 | 45 | PO | 14, 15 |
| 18 | 3600 IV | 5.6 | 972 | 2019 | 856 | 42 | PO | 16 |
| 1/M | 4200 SQ | 8 | 1600 | 2241 | 1764 | 79 | Hemochromatosis | 23 |
| Patients (n/sex) . | Total EPO dose (U/kg) . | Blood removed . | Baseline RBC (m) . | Blood produced . | Iron therapy . | Reference . | ||
|---|---|---|---|---|---|---|---|---|
| Units . | RBC (mL) . | RBC (mL) . | Expansion (%) . | |||||
| 10/F | 900 SQ | 3.4 | 435 | 1285 | 358 | 28 | IV | 22 |
| 24 | 900 IV | 5.2 | 864 | 1949 | 621 | 32 | PO | 16 |
| 10/F | 1800 SQ | 4.3 | 526 | 1293 | 474 | 37 | IV | 22 |
| 26 | 1800 IV | 5.5 | 917 | 2032 | 644 | 32 | PO | 16 |
| 11/F | 3600 IV | 4.9 | 809 | 1796 | 701 | 39 | PO | 14, 15 |
| 12/M | 3600 IV | 5.9 | 1097 | 2296 | 1102 | 48 | PO | 14, 15 |
| 23 | 3600 IV | 5.4 | 970 | 2049 | 911 | 45 | PO | 14, 15 |
| 18 | 3600 IV | 5.6 | 972 | 2019 | 856 | 42 | PO | 16 |
| 1/M | 4200 SQ | 8 | 1600 | 2241 | 1764 | 79 | Hemochromatosis | 23 |
Data are expressed as means.
Studies in patients with the anemia of chronic disease (osteoarthritis26-28 or rheumatoid arthritis29,30) are summarized in Table3. Red cell volume expansion ranged from 157 to 353 mL (11% to 24%) for endogenous erythropoietin-mediated erythropoiesis and 268 to 673 mL (21% to 44%) with erythropoietin therapy. These erythropoietic responses are indistinguishable from those in patients with anemia from blood loss alone, noted in Tables 1and 2. A study of 17 patients with inflammatory bowel disease treated with erythropoietin and oral iron therapy demonstrated a similar response, with an estimated 20% increase in red cell volume over that in placebo-treated patients.31
Erythropoietin and erythropoiesis in patients with anemia of chronic disease
| . | Patients (n) . | RBC removed (U) . | RBC produced (mL) . | Expansion (%) . | Iron Rx . | Reference . |
|---|---|---|---|---|---|---|
| Osteoarthritis | ||||||
| 1. Placebo | 6 | 2.6 | 157 | 11 | PO | 26 |
| Placebo | 3 | 3.3 | 220 | 18 | PO + IV | |
| EPO (1800 IV)3-150 | 10 | 3.7 | 268 | 21 | PO | |
| EPO (1800 IV) | 9 | 5.2 | 560 | 43 | PO + IV | |
| EPO (3600 IV) | 8 | 4.0 | 289 | 22 | PO | |
| EPO (3600 IV) | 12 | 5.0 | 515 | 40 | PO + IV | |
| 2. Placebo | 77 | 3.0 | 353 | 24 | PO | 27 |
| EPO (3600 IV) | 75 | 4.5 | 673 | 44 | PO | |
| 3. Placebo | 26 | None | 4 | 0.3 | PO | |
| Placebo | 26 | None | 18 | 1 | IV | 283-151 |
| EPO (1200 SQ) | 26 | None | 219 | 14 | PO | |
| EPO (1200 SQ) | 26 | None | 220 | 15 | IV | |
| Rheumatoid arthritis | ||||||
| Placebo | 6 | 2.3 | 271 | 25 | PO | 29 |
| EPO (3600 IV) | 4 | 4.8 | 624 | 37 | PO | |
| EPO (1800 IV) | 11 | 2.6 | 291 | 27 | IV | 30 |
| EPO (800 SQ) | 11 | 2.5 | 337 | 27 | IV |
| . | Patients (n) . | RBC removed (U) . | RBC produced (mL) . | Expansion (%) . | Iron Rx . | Reference . |
|---|---|---|---|---|---|---|
| Osteoarthritis | ||||||
| 1. Placebo | 6 | 2.6 | 157 | 11 | PO | 26 |
| Placebo | 3 | 3.3 | 220 | 18 | PO + IV | |
| EPO (1800 IV)3-150 | 10 | 3.7 | 268 | 21 | PO | |
| EPO (1800 IV) | 9 | 5.2 | 560 | 43 | PO + IV | |
| EPO (3600 IV) | 8 | 4.0 | 289 | 22 | PO | |
| EPO (3600 IV) | 12 | 5.0 | 515 | 40 | PO + IV | |
| 2. Placebo | 77 | 3.0 | 353 | 24 | PO | 27 |
| EPO (3600 IV) | 75 | 4.5 | 673 | 44 | PO | |
| 3. Placebo | 26 | None | 4 | 0.3 | PO | |
| Placebo | 26 | None | 18 | 1 | IV | 283-151 |
| EPO (1200 SQ) | 26 | None | 219 | 14 | PO | |
| EPO (1200 SQ) | 26 | None | 220 | 15 | IV | |
| Rheumatoid arthritis | ||||||
| Placebo | 6 | 2.3 | 271 | 25 | PO | 29 |
| EPO (3600 IV) | 4 | 4.8 | 624 | 37 | PO | |
| EPO (1800 IV) | 11 | 2.6 | 291 | 27 | IV | 30 |
| EPO (800 SQ) | 11 | 2.5 | 337 | 27 | IV |
Patients had measurable storage iron. Data are expressed as means.
EPO is total dosage of erythropoietin administered, u/kg.
Perisurgical therapy without autologous phlebotomy.
Iron-restricted erythropoiesis and iron therapy
Blood loss and the endogenous erythropoietin response
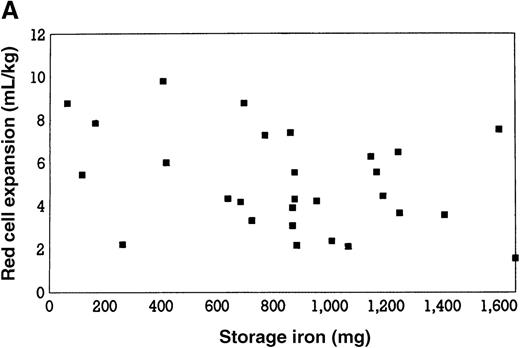
Erythropoiesis in response to aggressive autologous phlebotomy through endogenous erythropoietin has been estimated to increase by approximately 3 times.16,32 As illustrated in Figure 1, panel A, no relationship exists between basal iron stores and this magnitude of erythropoiesis, suggesting that serum iron and transferrin saturation for erythron requirements are adequately maintained by storage iron.13-16 Little or no benefit to oral iron supplementation was found in 2 studies,13,33 whereas a third study12 found some benefit (Table 1). Intravenous iron supplementation is of no value in enhancing erythropoiesis under these conditions.12 13
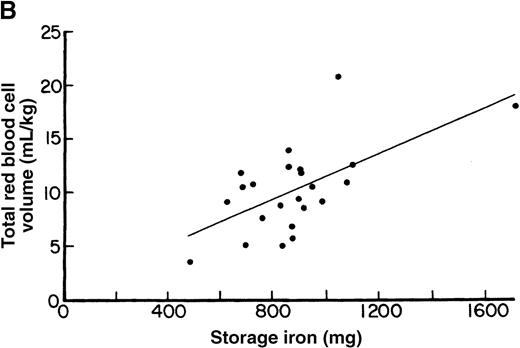
Initial storage iron and red blood cell volume expansion.
(A) Relationship between initial storage iron (mg) and red blood cell volume expansion (mL/kg) in patients undergoing aggressive phlebotomy, without erythropoietin therapy. Based on data from reference 32. Linear regression analysis (not shown) demonstrated no significant correlation (r = 0.06; P = .67). (B) Relationship between initial storage iron (mg) and red blood cell volume expansion (mL/kg) in patients undergoing aggressive phlebotomy, with erythropoietin therapy. Linear regression analysis demonstrated a significant correlation (r = 0.6; P = .02). Reprinted with permission.32
Initial storage iron and red blood cell volume expansion.
(A) Relationship between initial storage iron (mg) and red blood cell volume expansion (mL/kg) in patients undergoing aggressive phlebotomy, without erythropoietin therapy. Based on data from reference 32. Linear regression analysis (not shown) demonstrated no significant correlation (r = 0.06; P = .67). (B) Relationship between initial storage iron (mg) and red blood cell volume expansion (mL/kg) in patients undergoing aggressive phlebotomy, with erythropoietin therapy. Linear regression analysis demonstrated a significant correlation (r = 0.6; P = .02). Reprinted with permission.32
Blood loss and erythropoietin therapy
With enhanced erythropoiesis during erythropoietin therapy, iron-restricted erythropoiesis occurs even in patients with measurable storage iron (Figure 1B). Despite an 8-fold increase in gastrointestinal iron absorption,34 serum ferritin and transferrin saturation levels decline up to 50% with erythropoietin therapy.35 A 4-fold increase in erythropoietic activity is accompanied by declining reticulocyte counts and the appearance of hypochromic red cells by the second week of erythropoietin therapy.23,36 The superior erythropoietic response in a patient with hemochromatosis further suggests iron-restricted erythropoiesis in patients treated with erythropoietin (Table2).23
Basal red cell precursor mass is also a limiting factor. Half-maximal hemoglobin synthesis can be achieved by as few as 50 molecules of erythropoietin per target cell. This means that the high levels of serum erythropoietin present initially after parenteral injection are not entirely used for erythropoiesis.37-39 The biologic response is, therefore, maximal at lower levels than the erythropoietin concentration required to saturate all erythropoietin-binding sites. Consistent with this, reticulocyte responses in healthy subjects peak after a single erythropoietin dose of 1800 U/kg,40 and storage iron is mobilized more effectively after multiple-dose regimens than after single-dose regimens.41 A 72-hour interval between erythropoietin administrations is superior to a 24-hour interval.42 Erythropoietin therapy stimulates the gradual expansion of erythroblast mass,43 so that acute demands for erythropoiesis are met by an influx from pre-erythroid colony forming unit (CFU-E) pools, and chronic demands (eg, chronic hemolytic anemia) are met by an amplified pool of later erythroid precursors. Expansion and maturation of erythroid precursor cells are, therefore, limiting factors in the erythropoietic response to acute blood loss anemia and in treatment strategies using larger erythropoietin dosages. In a study of escalating (400%) erythropoietin dose administered to patients undergoing aggressive phlebotomy, the marrow erythropoietic index increased from 2.9 times (with endogenous erythropoietin stimulation) to 3.6 times over basal rates of erythropoiesis, representing only a 58% increase in erythropoiesis (Figure2). Emerging growth factors, such as novel erythropoiesis-stimulating protein,44 may have different pharmacokinetics regarding dose and response related to longer plasma residence time and erythron expansion.
Erythropoietic response, as reflected in the bone marrow erythropoietic index, in 4 cohorts of autologous donors treated with placebo or escalating doses of erythropoietin therapy.
(U/kg, given for 6 doses over 3 weeks.) Erythropoietic response (mL/kg per day) was estimated for each treatment group, according to the formula: bone marrow erythropoietic index = [RBC expansion] + [baseline RBC production] = [baseline RBC production]. Based on data from Goodnough et al.16
Erythropoietic response, as reflected in the bone marrow erythropoietic index, in 4 cohorts of autologous donors treated with placebo or escalating doses of erythropoietin therapy.
(U/kg, given for 6 doses over 3 weeks.) Erythropoietic response (mL/kg per day) was estimated for each treatment group, according to the formula: bone marrow erythropoietic index = [RBC expansion] + [baseline RBC production] = [baseline RBC production]. Based on data from Goodnough et al.16
Blood loss and iron therapy
Whether iron-restricted erythropoiesis is clinically important in patients with blood loss anemia is detailed in Table4.27,32 No differences in erythropoiesis stimulated by endogenous erythropoietin alone were seen between patients with or without measurable storage iron, in which the mean red blood cell expansion was 20% and 22%, respectively, in one study32 and 23% and 24%, respectively, in another study.27 When patients were administered erythropoietin therapy, those without measurable storage iron had reductions in erythropoiesis compared to patients with storage iron that reached statistical significance in one study (P < .05)27but not another (P = .07).32 These studies indicate that oral iron supplementation is sufficient for endogenous erythropoietin-mediated RBC expansion but may not be sufficient to prevent iron-restricted erythropoiesis during erythropoietin therapy.
Erythropoietin and erythropoiesis in patients with or without iron deficiency
| Cohorts . | Patients (n) . | RBC removed (U) . | Baseline RBC vol (mL) . | RBC produced (mL) . | Expansion (%) . | Iron Rx . | Reference . |
|---|---|---|---|---|---|---|---|
| Endogenous erythropoietin | |||||||
| Iron deficient | 6 | 3.8 | 1937 | 5.4 ± 2.8/kg | 22 | PO | 32 |
| Iron replete | 28 | 3.4 | 1942 | 4.8 ± 2.3/kg | 20 | PO | 32 |
| Iron deficient | 10 | 3.0 | 1503 | 367 ± 117 | 24 | PO | 27 |
| Iron replete | 77 | 3.0 | 1503 | 355 ± 157 | 23 | PO | 27 |
| Erythropoietin therapy (3600 U/kg)4-150 | |||||||
| Iron deficient | 13 | 4.9 | 1824 | 8.2 ± 3.0/kg | 34 | PO | 32 |
| Iron replete | 23 | 5.1 | 1894 | 10.2 ± 4.0/kg | 41 | PO | 32 |
| Iron deficient | 11 | 4.5 | 1535 | 540 ± 308 | 35 | PO | 27 |
| Iron replete | 75 | 4.5 | 1535 | 673 ± 190 | 44 | PO | 27 |
| Cohorts . | Patients (n) . | RBC removed (U) . | Baseline RBC vol (mL) . | RBC produced (mL) . | Expansion (%) . | Iron Rx . | Reference . |
|---|---|---|---|---|---|---|---|
| Endogenous erythropoietin | |||||||
| Iron deficient | 6 | 3.8 | 1937 | 5.4 ± 2.8/kg | 22 | PO | 32 |
| Iron replete | 28 | 3.4 | 1942 | 4.8 ± 2.3/kg | 20 | PO | 32 |
| Iron deficient | 10 | 3.0 | 1503 | 367 ± 117 | 24 | PO | 27 |
| Iron replete | 77 | 3.0 | 1503 | 355 ± 157 | 23 | PO | 27 |
| Erythropoietin therapy (3600 U/kg)4-150 | |||||||
| Iron deficient | 13 | 4.9 | 1824 | 8.2 ± 3.0/kg | 34 | PO | 32 |
| Iron replete | 23 | 5.1 | 1894 | 10.2 ± 4.0/kg | 41 | PO | 32 |
| Iron deficient | 11 | 4.5 | 1535 | 540 ± 308 | 35 | PO | 27 |
| Iron replete | 75 | 4.5 | 1535 | 673 ± 190 | 44 | PO | 27 |
Absent storage iron is defined as transferrin saturation <16% or ferritin <20 μg/L.
Total dose administered.
Intravenous iron can allow up to a 5-fold erythropoietic response to significant blood loss anemia in healthy persons.3,45 A greater rate of hemoglobin production is probably not possible unless red marrow expands into yellow marrow space, as is seen in hereditary anemias.2,45One limitation to intravenous iron therapy in patients not undergoing erythropoietin therapy may be that much of the administered iron is transported into the reticuloendothelial system as storage iron, where it is less readily available for erythropoiesis.46 For iron-deficient patients, 50% of intravenous iron is incorporated into hemoglobin within 3 to 4 weeks,47 whereas for patients with anemia of chronic disease or renal failure, intravenous iron is less rapidly mobilized from the reticuloendothelial system.48
The value of intravenous iron administration in patients undergoing erythropoietin therapy is not established. In one clinical trial,26 significantly greater erythropoietic responses were seen with intravenous iron therapy than with oral iron supplementation only (Table 3). However, a recent study28found no difference in red cell production between oral iron and intravenous iron therapy in patients before orthopedic surgery. Another study found that intravenous iron supplementation was not accompanied by a corresponding erythropoietic response to increasing doses of erythropoietin therapy; a 2-fold increase in erythropoietin dose was associated with only a 32% increase in red cell production,22 similar to the dose-response relationship using oral iron supplementation.16 Intravenous iron administered to normal subjects treated with erythropoietin abolished the marked reduction in serum ferritin and increased the reticulocyte hemoglobin content (a measure in g/L of the hemoglobin contained in all reticulocytes); however, the total number of reticulocytes generated in 8 days after therapy was not affected.49 Finally, perisurgical exposure to allogeneic blood is not different for autologous blood donors with or without measurable storage iron, regardless of oral27,32 or intravenous iron28administration. The current status of intravenous iron therapy in patients with blood loss anemia is summarized in Table5.50-55
Current status of intravenous iron therapy
| Beneficial (reference) . | No benefit (reference) . | Investigational (reference) . |
|---|---|---|
| Anemia of renal failure, with or without erythropoietin therapy52-54 Patients with ongoing blood loss35 51 Jehovah's Witness patients with iron deficiency,50 blood loss,51 or both | Autologous blood donation in patients with or without iron deficiency12 13 | Blood loss, iron deficiency, and erythropoietin therapy27,32 Anemia of chronic disease and erythropoietin therapy29,31,55 Perisurgical anemia, with or without erythropoietin therapy28 35 |
| Beneficial (reference) . | No benefit (reference) . | Investigational (reference) . |
|---|---|---|
| Anemia of renal failure, with or without erythropoietin therapy52-54 Patients with ongoing blood loss35 51 Jehovah's Witness patients with iron deficiency,50 blood loss,51 or both | Autologous blood donation in patients with or without iron deficiency12 13 | Blood loss, iron deficiency, and erythropoietin therapy27,32 Anemia of chronic disease and erythropoietin therapy29,31,55 Perisurgical anemia, with or without erythropoietin therapy28 35 |
Absolute iron deficiency is defined as ferritin <200 μg/L with or without iron saturation <20%, or relative iron deficiency (ferritin <400 μg/L in dialysis patients receiving erythropoietin therapy54 or the presence of >10% hypochromic erythrocytes, reticulocytes, or both.
Anemia of chronic renal failure or chronic disease
The success of erythropoietin therapy in correcting the anemia of chronic renal failure has led to substantial clinical experience and knowledge in erythropoietin, iron metabolism, and erythropoiesis in this setting.52,56 A distinguishing characteristic of the anemia in patients undergoing chronic renal dialysis is the presence of a normal mean corpuscular volume (MCV) in 85% of the patients and hypochromia in 96% of the patients.57 Hyporesponsiveness to erythropoietin therapy is a common phenomenon in these patients58 59 because of a variety of co-morbid conditions, particularly aluminum toxicity and iron deficiency.
Anemic patients undergoing dialysis may have suboptimal responses to oral iron therapy for several reasons. Under basal conditions, their absorption levels of food iron and therapeutic oral iron are similar to levels in normal subjects.60 During erythropoietin therapy, the absorption of iron increases as much as 5 times.61Nevertheless, external iron loss, including loss from hemodialysis and blood testing, exceed gastrointestinal iron absorption.52Poor compliance because of gastrointestinal symptoms is problematic, and significantly reduced iron absorption may occur with some newer iron formulations.62 Iron-restricted erythropoiesis is evident by clinical responses to ascorbate supplementation, thought to facilitate the release of iron from reticuloendothelial stores and increased iron use by erythrons,59 and by the success of intravenous iron therapy in reducing erythropoietin dosage.56
Because anemia is a determinant of life expectancy in patients on dialysis for chronic disease,57,63 intravenous iron administration has become standard therapy for many patients receiving erythropoietin therapy.64 Dialysis patients treated with intravenous iron (100 mg twice a week) achieved a 46% reduction in erythropoietin dosage, required to maintain hematocrit levels between 30% and 34%, compared with patients supplemented with oral iron.56 In a study of patients with chronic renal failure but not on dialysis,53 two thirds of patients who were unresponsive to oral iron responded to weekly intravenous iron therapy. Improved erythropoiesis occurred despite initial serum ferritin levels as high as 400 μg/L,65 indicating that biochemical markers of storage iron are not helpful in evaluating iron-restricted erythropoiesis.
The effect of intravenous iron therapy in patients with the anemia of chronic disease undergoing erythropoietin therapy is shown in Table 3. Patients with osteoarthritis and measurable storage iron doubled their red cell expansion, from a range of 21% to 22% (with oral iron) to 40% to 43% with intravenous iron.26 Intravenous iron therapy in iron-deficient patients with inflammatory bowel disease also resulted in improved responses to erythropoietin therapy55compared with a similar patient group who received oral iron supplementation.31 The clinical response to intravenous iron may be attributed to the salutary effect of erythropoietin on iron mobilization from the reticuloendothelial system into red cell precursors.66 The risk/benefit profile of intravenous iron is controversial in anemic patients on renal dialysis59,63,64,67 and in patients with anemia of chronic disease.68 Nevertheless, clinical settings in which the effect of intravenous iron therapy on erythropoiesis is beneficial, not beneficial, or undefined (investigational) are summarized in Table5.
Laboratory evaluation
Iron, transferrin, and transferrin saturation
The diagnosis of iron deficiency is traditionally based on a combination of parameters, including iron metabolism and hematologic indices.69-73 Technical and biologic issues limit the usefulness of these assays in the clinical setting,74-78and the value of iron, transferrin, and transferrin saturation is limited to uncomplicated iron deficiency. During repeated phlebotomy, there is little change in iron levels until iron stores are exhausted, after which iron declines to below 50 μg/dL.69 Once iron stores are depleted, transferrin increases linearly to approximately 400 μg/L. Transferrin saturation, therefore, falls below 16% only when iron stores are exhausted, in contrast to erythropoietin therapy-induced erythropoiesis, in which iron saturation falls even in the presence of storage iron.71 Hence, the detection of iron-restricted erythropoiesis during erythropoietin therapy6,35,41 71-73 poses additional challenges.
Ferritin
Ferritin is widely used as a marker of iron storage,79-81 with a log relationship between serum ferritin and liver iron (measured with magnetic spectrophotometry) and with a cutoff of 15 μg/L indicating absent iron stores in normal persons.82-84 However, 1 study found that 25% of women with no stainable bone marrow iron had serum ferritin levels above the 15 μg/L cutoff.85 Ferritin levels are elevated in conditions such as hyperthyroidism, inflammation/infection, hepatocellular disease, malignancies, alcohol consumption, and oral contraceptives.86 A cutoff level of 30 μg/L87to 40 μg/L88 for anemic patients is desirable to provide optimal diagnostic efficiency (positive predictive values of 92% to 98%, respectively), even without clinical evidence of infection or inflammation.
Subjects treated with erythropoietin exhibit a rapid decrease in ferritin to levels 50% to 75% below baseline.34,49,89Ferritin also decreased rapidly after intravenous iron administration in normal subjects treated with erythropoietin.49 Under these conditions, ferritin most likely reflects the iron content of a smaller, more labile pool in equilibrium with erythropoietic compartment and storage iron. Many patients have underlying disorders with “inappropriately high” serum ferritin levels. Two thirds of patients on renal dialysis respond to intravenous iron therapy; their mean ferritin levels of 94 μg/L and mean transferrin saturations of 22% are no different from those of patients not responsive to intravenous iron.65 This has led to suggested guidelines54 and algorithms90 for anemic patients with renal failure, in whom ferritin levels of less than 200 μg/L alone or less than 400 μg/L with a transferrin saturation less than 20% are used to determine the need for intravenous iron therapy90; only at transferrin saturations greater than 50% or ferritin levels in excess of 800 μg/L are these patients considered unlikely to benefit from iron therapy.54
Among patients with the anemia of cancer who are treated with erythropoietin, ferritin levels greater than 400 μg/L correctly predicted lack of response in 88%, whereas levels less than 400 μg/L correctly predicted response in 75%.91 However, several studies have failed to show a role for ferritin in predicting response to erythropoietin or in identifying functional iron deficiency in patients with cancer-related anemia.92-94 It is reasonable to assume that ferritin levels lower than 200 μg/L would predict response to intravenous iron in most patients receiving erythropoietin.
Erythrocyte ferritin and zinc protoporphyrin
Some studies have advocated a role for erythrocyte ferritin, rather than serum ferritin, in detecting iron deficiency in patients with anemia of chronic disorders.95-98 However, this difficult assay is insensitive to dynamic changes and becomes abnormally low only after most of the red cell population has been replaced by iron-deficient erythrocytes. A similar limitation of the zinc protoporphyrin measurement is that a significant proportion of the red cell pool must contain new red blood cells produced under iron-restricted conditions.99 Furthermore, the zinc protoporphyrin measurement is sensitive to interference by drug and plasma components.100 Zinc protoporphyrin, therefore, has little value in identifying iron-restricted erythropoiesis.101
Erythrocyte indices
Erythropoietin therapy in patients on dialysis is associated with the progressive appearance of hypochromic, microcytic erythrocytes.102 Values exceeding 10% (normally less than 2.5%) are compatible with iron-restricted erythropoiesis.54 Hypochromic erythrocytes are observed when erythropoietin is administered to normal subjects undergoing multiple phlebotomies,89 but not when it is administered to a patient with hereditary hemochromatosis treated similarly.23 Erythrocyte indices are helpful for monitoring iron status and the need for iron supplementation during erythropoietin therapy for the anemia of chronic renal failure.103-106Hypochromic erythrocytes also increase in patients with increased numbers of normal reticulocytes and young red cells. Therefore, the value of this assay has been questioned.107-109
Reticulocyte parameters
Because reticulocytes are normally released from the marrow 18 to 36 hours before their final maturation into erythrocytes, they provide a real-time assessment of the functional state of erythropoiesis. However, in the early phases of stimulated erythropoiesis, changes in absolute reticulocyte counts reflect the release from marrow of immature reticulocytes rather than the true expansion of erythropoiesis.45,48,110,111 It has been suggested that a response to erythropoietin can be assessed by measuring hemoglobin and reticulocyte counts after 4 weeks of therapy; a change in hemoglobin level by more than 1.0 g/dL or a change in absolute reticulocyte count by more than 40 × 109/L could indicate that the patient is a responder to erythropoietin therapy.93,112 113
Flow cytometric analysis of reticulocytes allows precise measurements of reticulocyte cell volume (MCVr), hemoglobin concentration (CHCMr), and hemoglobin content (CHr).114,115 In normal subjects, erythropoietin therapy induces an increase in MCVr and a decrease in CHCMr.43 Normal subjects treated with erythropoietin with baseline serum ferritin levels greater than 100 μg/L produce almost no hypochromic reticulocytes. Iron-restricted erythropoiesis is detected at an earlier stage if reticulocyte parameters rather than red cell indices are used.89,111,116 117
CHr has been studied in patients on dialysis. CHr demonstrated 100% sensitivity and 80% specificity and was a more accurate predictor of response to iron therapy than serum ferritin, transferrin saturation, or percentage hypochromic erythrocytes.107 Another study showed that a baseline CHr of less than 28 pg had 78% sensitivity and 71% specificity for detecting iron-restricted erythropoiesis, compared with 50% and 39% for traditional biochemical measures.118In dialysis patients treated with erythropoietin, CHr increases during intravenous iron therapy, indicating value as an early indicator of iron-restricted erythropoiesis,119 even with normal serum ferritin or transferrin saturation.120
Measurements of total reticulocyte hemoglobin, an integrated index derived from the absolute reticulocyte count and the CHr,121showed that reticulocyte–hemoglobin levels are much higher in subjects treated with intravenous iron.49 Moreover, in patients undergoing cardiac surgery, the administration of intravenous iron along with erythropoietin therapy abolishes the production of hypochromic reticulocytes, and CHr remains within the normal range.122 A recent study123 concluded that CHr was the strongest predictor of iron deficiency in children, and it should be considered an alternative to standard iron studies for the diagnosis of iron deficiency.
Another reticulocyte parameter now provided by automated analyzers is the immature reticulocyte fraction. Because it is sensitive to leukocyte interference124 and is difficult to standardize,125,126 its clinical use has been limited. The immature reticulocyte fraction reflects the degree of erythropoiesis, but it is not indicative of iron-restricted erythropoiesis.127 128
Transferrin receptor
The soluble transferrin receptor (TfR) is derived primarily from red cell precursor normoblasts129 and provides an estimate of the erythroid compartment mass. Both enhanced erythropoiesis and iron deficiency elevate TfR.129,130 Endogenous erythropoietin-mediated erythropoiesis through phlebotomy minimally influences TfR until iron-restricted erythropoiesis occurs, as illustrated in Figure 3.69Serum ferritin is the most sensitive and specific index of iron status when there are residual iron stores, whereas TfR is most sensitive in the presence of iron-restricted erythropoiesis.88
Endogenous erythropoietin-mediated erythropoiesis by phlebotomy minimally influences serum transferrin receptor (TfR) until iron-restricted erythropoiesis occurs.
Serial determinations of TfR during phlebotomy in 3 subjects with initial iron stores of 107 mg (▵), 335 (•), and 1,102 mg (○). Reprinted with permission.69
Endogenous erythropoietin-mediated erythropoiesis by phlebotomy minimally influences serum transferrin receptor (TfR) until iron-restricted erythropoiesis occurs.
Serial determinations of TfR during phlebotomy in 3 subjects with initial iron stores of 107 mg (▵), 335 (•), and 1,102 mg (○). Reprinted with permission.69
In a study of 43 healthy, nonanemic adult women, 17 (40%) had significant changes in TfR in response to oral iron therapy, indicating the presence of subclinical iron deficiency.131 In another study, 25% of patients undergoing routine ferritin tests, who were also studied for TfR measurements, were categorized as iron deficient by TfR (more than 2.8 mg/L) but not by ferritin (more than 12 μg/L).88 These values could represent iron-replete persons with increased erythropoiesis or iron-deficient patients with acute-phase increases of ferritin values. The clinical usefulness of the TfR may be limited to the subset of ill patients in whom iron deficiency is suspected but whose ferritin values are normal or raised,88 seen commonly in the anemia of chronic disease; numerous studies69,87,88 132-134 have shown TfR to be of value in differentiating iron-deficiency anemia (in which TfR is usually increased) from the anemia of chronic disease (in which TfR is usually normal). Meaningful comparisons of studies of TFR are difficult because of differences resulting from the reagents used.
The value of TfR in predicting the response to erythropoietin therapy and the adequacy of iron availability is modest. Although lower baseline or low-normal TfR levels predict the initial response to erythropoietin therapy in patients on dialysis,135 other studies have shown little predictive value for this assay in patients receiving erythropoietin because serum TfR values above normal are observed in iron deficiency and during erythropoietin-induced expansion of erythropoietic activity.136 The combination of TfR measurements, serum ferritin, and automated reticulocyte counts may be predictive of an erythropoietic response to increased erythropoietin dosage or of the need to ensure adequate iron replacement, such as by the intravenous administration of iron.137 138 Further studies are required to delineate the clinical usefulness of TfR measurements in these settings.
Erythropoietin assay
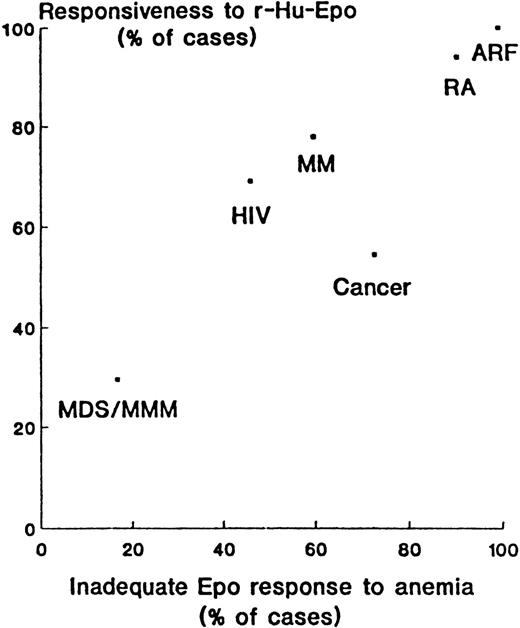
A classification of anemias has been proposed around the concept of adequate or inadequate erythropoietin response to degree of anemia139-141; patients with iron-deficiency or chronic hemolytic anemia would comprise the reference populations.142-144 The correlation between the percentage of patients showing an “inadequate” erythropoietin response to anemia and the percentage of patients responding to erythropoietin therapy (according to the author's criteria) can be illustrated (Figure 4) for several diseases, with a range in response to myelodysplastic syndromes,145 multiple myeloma,146 and rheumatoid arthritis.147
Correlation between the percentage of patients showing inadequate erythropoietin response to anemia and the percentage responding to erythropoietin therapy (according to the authors' criteria).
Numbers are derived directly or are calculated from reported data. ARF, anemia of renal failure; RA, anemia of rheumatoid arthritis; HIV, anemia in patients with human immunodeficiency virus; MM, anemia in multiple myeloma; Cancer, anemia of cancer; MDS/MMM, anemia in myelodysplastic syndromes and myelofibrosis with myeloid metaplasia. Reprinted with permission.141
Correlation between the percentage of patients showing inadequate erythropoietin response to anemia and the percentage responding to erythropoietin therapy (according to the authors' criteria).
Numbers are derived directly or are calculated from reported data. ARF, anemia of renal failure; RA, anemia of rheumatoid arthritis; HIV, anemia in patients with human immunodeficiency virus; MM, anemia in multiple myeloma; Cancer, anemia of cancer; MDS/MMM, anemia in myelodysplastic syndromes and myelofibrosis with myeloid metaplasia. Reprinted with permission.141
There are several problems with the use of erythropoietin levels in the management of patients. The interpretation of an erythropoietin level must take into account the degree of anemia at the time of measurement. Commercial assay results do not take this into consideration; hence, clinicians must have some familiarity with mathematical corrections, such as observed/predicted ratios.141 A retrospective analysis of erythropoietin therapy in anemic patients with cancer not undergoing chemotherapy148 found that pre-treatment erythropoietin levels of less than 200 mU/mL were correlated with red cell response to erythropoietin therapy. Subsequent analyses, however, have found that erythropoietin levels are not predictive for response in cancer patients undergoing chemotherapy.149,150 Because almost all anemic patients with cancer or on renal dialysis have erythropoietin levels that are inadequate for the degree of anemia,141 measuring erythropoietin levels is not useful in these settings. Furthermore, guidelines recommend that erythropoietin therapy be instituted before hemoglobin levels fall below 10 g/L, a level at which interpretation of erythropoietin level is not valid.141 The erythropoietin assay may be most useful as a determinant of response to therapy in certain patients, such as those with myelodysplasia.145
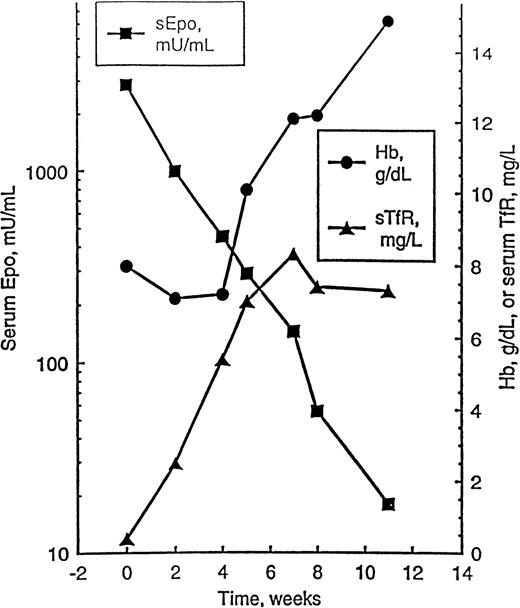
The proliferative state of bone marrow erythroid cells affects erythropoietin levels,151 as does iron status,152 hemolysis,153 and chemotherapy-induced endothelial damage.154 TfR has helped in the understanding of the relationship between hemoglobin level and serum erythropoietin. As illustrated in Figure5, a patient with pure red cell aplasia and a markedly elevated erythropoietin level was administered erythropoietin therapy for 4 weeks. Before hemoglobin level increased, the erythropoietin level decreased because erythroid activity measured by TfR reappeared. The increased plasma clearance of erythropoietin is probably related to an influx of early RBC precursors into CFU-E from primitive erythroid burst-forming units (BFU-E). CFU-E has a higher concentration of erythropoietin receptors than BFU-E,39 so late BFU-E through the proerythroblast stage defines the narrow window of erythroid cellular compartment that is erythropoietin responsive.151
Time course of hemoglobin (Hb) level, serum erythropoietin level (sEpo), and serum transferrin receptor (sTfR) in a patient with pure red cell aplasia (PRCA) responding to treatment.
The patient was treated with erythropoietin at a dose of 150 U/kg per day subcutaneously, 5 days a week; dosage was reduced to 3 weekly administrations when Hb level achieved 12 g/dL, and treatment was discontinued after 8 weeks. Reprinted with permission.151
Time course of hemoglobin (Hb) level, serum erythropoietin level (sEpo), and serum transferrin receptor (sTfR) in a patient with pure red cell aplasia (PRCA) responding to treatment.
The patient was treated with erythropoietin at a dose of 150 U/kg per day subcutaneously, 5 days a week; dosage was reduced to 3 weekly administrations when Hb level achieved 12 g/dL, and treatment was discontinued after 8 weeks. Reprinted with permission.151
A recent study of anemic children with systemic-onset juvenile chronic arthritis found that erythropoietin levels were appropriate for the degree of anemia. Variable iron status, measured by TfR levels, accounted for variations in hemoglobin levels, and intravenous iron therapy resulted in the normalization of TfR levels.155Another study in children with cancer found that TfR levels were inappropriately low for the degree of anemia, and a highly significant correlation between the logarithms of erythropoietin and hemoglobin levels was identified.156 The report concluded that anemia in children with cancer results from decreased erythropoietic activity, in contrast to anemia in adults with cancer.142 The anemia of chronic inflammatory disease is particularly multifactorial, caused not only by impaired erythropoietin response but also by defective iron supply to the erythron, along with inflammatory cytokine-mediated suppression of the erythropoietic response to erythropoietin.157,158 To effectively evaluate erythroid activity, an assay of TfR and mathematical correction of the erythropoietin level would be necessary,151 but this is not practical for clinical evaluation and patient management.
In summary, pre-therapy laboratory evaluation of erythropoietin, iron, or erythropoiesis in anemic patients may no longer be always appropriate. Rather, the epidemiology (ie, clinical setting) of the anemia may suggest a therapeutic trial of iron or erythropoietin therapy, with post-therapy laboratory evaluation determining the response. One example of this approach is the empiric use of oral iron therapy in otherwise well anemic, menstruating women, in which a follow-up blood count may serve as the sole laboratory assay. A second example is patients with chronic renal failure who remain anemic despite erythropoietin therapy and in whom an empiric trial of intravenous iron is recommended despite apparent laboratory evidence of storage iron. Follow-up transferrin saturations, serum ferritin, and blood counts then determine when intravenous iron therapy can be modified or withdrawn.54 Hematologic parameters are emerging as promising alternatives to biochemical markers in evaluating iron-restricted erythropoiesis.
Clinical management: iron therapy strategies
With significant on-going iron losses, oral iron supplementation is not enough to correct iron-deficient erythropoiesis. Patients on renal dialysis have such blood losses, and intravenous iron therapy allows the correction of anemia through the use of lower erythropoietin doses.54,159 Another role for intravenous iron therapy is in bloodless medical treatment and bloodless surgery for patients who decline blood transfusions because of religious beliefs.50These patients include pregnant women and patients with dysfunctional uterine bleeding who are scheduled for hysterectomy.51
Intravenous iron therapy has been closely scrutinized for risks and adverse events. Imferon (iron dextran BP, Merrill-Dow, Cincinnati, OH) was previously approved for parenteral use.160 This product was associated with a 0.6% risk of anaphylactoid reactions and a 1.7% risk of severe serum sickness-like reactions characterized by fever, arthralgias, and myalgias.161 Delayed reactions of up to 30% and severe reactions of 5.3% were subsequently described. They were attributed to changes in manufacturing processes,162 and this product was withdrawn from use.
InFed (iron dextran USP; Schein Pharm, Florham Park, NJ) has been approved for parenteral (intramuscular or intravenous) use in the United States. InFed (Schein Pharm) administered intravenously during dialysis is associated with significant adverse reactions in 4.7% of patients, of which 0.7% are serious or life threatening and another 1.7% are characterized as anaphylactoid.163 The prevalence of these reactions does not differ among patients receiving low-dose (100 mg) or higher-dose (250-500 mg) infusions.164 A recent review reported 196 incidences of allergy/anaphylaxis from iron dextran between 1976 and 1996, of which 31 (15.8%) were fatal.165
Safety aspects of parenteral iron dextran, ferric gluconate, and iron saccharate have been scrutinized.166-168 Iron saccharate is available in Europe but not in the United States. Ferric gluconate has been available in Europe for more than 20 years and was approved for intravenous use in the United States in 1999 (Ferrlecit; Schein Pharm) in patients on renal dialysis. Dosage is limited to 125 mg infused over 1 hour at each administration. The number of allergic reactions (3.3 episodes per million doses) is lower than that from iron dextran (8.7 episodes per million doses), and the safety profile is substantially better; among 74 severe adverse events reported from 1976 to 1996, there were no deaths.165
Adverse events associated with ferric gluconate include hypotension, rash, and chest or abdominal pain, with an incidence of 1.3% for serious reactions.170,171 Intravenous iron therapy can cause a clinical syndrome (nausea, facial reddening, and hypotension) that may be attributed to acute iron toxicity caused by oversaturation (more than 100%) of transferrin172 or nonspecific drug toxicity.173 The increased erythropoietic effect (4.5 to 5.5 times basal) of intravenous iron dextran (with an estimated half-life of 60 hours) is transient and lasts 7 to 10 days, after which the remaining iron is sequestered in the reticuloendothelial system and erythropoiesis returns to basal rates.3 Iron measurements and intravenous iron therapy are optimal at 2-week intervals.
A dose-response relationship between erythropoietin and erythropoiesis that is affected favorably by intravenous iron has important implications for erythropoietin dosage and cost.174 The current total recommended erythropoietin dose for patients scheduled for elective surgery175 ranges from 1800 U/kg176 to 4200 U/kg,177,178 which for a 70-kg patient would cost $1300 to $3000.179 However, an economic analysis of erythropoietin therapy in patients undergoing orthopedic surgery concluded that even the lower recommended dosage is not cost effective.180 Intravenous iron may potentiate the erythropoietic response in erythropoietin therapy by improving functional iron deficiency.
A multicenter trial in dialysis patients, designed to achieve normal (more than 42%) or low (more than 30%) hematocrits with a combination of erythropoietin therapy and intravenous iron (dextran USP) supplementation, was halted because of increased mortality in the high hematocrit cohort.63 These patients experienced a decline in dialysis adequacy and received intravenous iron in greater quantities than those in the low hematocrit group. Debated links between iron stores and morbidity or mortality rates include a predisposition to infection,181,182 increased death from infection183 in dialysis patients, and detrimental coronary outcomes in men.184-186 An accompanying editorial187 to a U.S. study of ferric gluconate170 concluded that its role in the management of anemia from renal disease was unclear until its relative efficacy, tolerability, and cost effectiveness is established. However, another editorial64 accompanying the target hematocrit study63 concluded that for patients with anemia of chronic renal disease, intravenous iron is recommended to reach target hematocrit goals of 33% to 36%. The current status of intravenous iron therapy for patients who are unresponsive to oral iron or in whom it is malabsorbed—along with opportunities for investigation—are presented in Table 5.
Conclusion
The development of new laboratory methods to assess iron-restricted erythropoiesis, along with clinical trials of blood phlebotomy and erythropoietin therapy, have furthered our understanding of the relationship between erythropoietin, iron, and erythropoiesis. Hematologic indices and reticulocyte parameters measured by new automated counters hold promise in the evaluation of iron-restricted erythropoiesis, but more studies are needed to define their role with iron and erythropoietin therapy. Erythropoietin and transferrin receptor assays are valuable tools for clinical research, but their roles in routine clinical practice remain undefined. The availability of a safer intravenous iron preparation allows an opportunity to study its value in patients with blood loss anemia, particularly those undergoing erythropoietin therapy. Given the low prevalence but potential side effects, the use of intravenous iron for patients other than those with chronic renal failure and those who decline blood because of religious beliefs must be defined by controlled clinical trials.
Reprints:Lawrence T. Goodnough, Division of Laboratory Medicine, Washington University School of Medicine, 660 South Euclid Avenue, Box 8118, St. Louis, MO 63110; e-mail:goodnough@labmed.wustl.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 2. Erythropoietic response, as reflected in the bone marrow erythropoietic index, in 4 cohorts of autologous donors treated with placebo or escalating doses of erythropoietin therapy. / (U/kg, given for 6 doses over 3 weeks.) Erythropoietic response (mL/kg per day) was estimated for each treatment group, according to the formula: bone marrow erythropoietic index = [RBC expansion] + [baseline RBC production] = [baseline RBC production]. Based on data from Goodnough et al.16](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/3/10.1182_blood.v96.3.823/5/m_bloo01549002x.jpeg?Expires=1766522947&Signature=Cxnmv8uRKrRu05PJpxJWDTjBs7JFc18Q5xDKS2c4z3XoEuBM3VOBhuGlBInFjIJQLSZatzXMmgupPreXxdP~ed8P2lBnn60WsN552txQmHWccGyeDNKKOa58AdDG-GWuzQ4ueR~9b34D4wIydIdG9-s4lJVjTxjJ8I4Z9DwxhlRbXWQMGsT2IKk-glAOurfaHE7~D2h~SHL2-j3N1UC6NAgWjq69FLd-kngWFCS~WBweWvEXWCnFbG~4~G2taXBKtx9ofjPEdOgEojRrQu7IwfahRjXNiw1X9DGHZKCkEsa-aL8ltirzlDeRG~9xNtTMP~zmtQJVLh0cof3QfbSxFQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal