Abstract
A-myb is a member of the myb family of transcription factors, which regulates proliferation, differentiation, and apoptosis of hematopoietic cells. A-Myb expression is normally restricted to the proliferating B-cell centroblasts and transgenic mice overexpressing A-myb displayed enhanced hyperplasia of the lymph nodes. Because A-Myb is highly expressed in several subtypes of human B-cell neoplasias, we sought to determine whether the A-myb gene promoted proliferation and survival of B lymphocytes, using the WEHI 231 and CH33 murine B-cell lymphomas as models. Here, we show that ectopic expression of A-mybrescues WEHI 231 and CH33 cells from growth arrest and apoptosis induced by anti-IgM treatment. Previously, we demonstrated an essential role of the c-myc gene in promoting cell survival of WEHI 231 cells in response to a variety of apoptotic stimuli. Furthermore, we and others have shown that the c-myc gene is potently transactivated by A-Myb in several cell types. Thus, we sought to determine whether c-Myc would mediate the A-Myb antiapoptotic effect in B cells. Here we show that ectopic expression of A-myb leads to maintenance of c-myc expression, and that expression of antisense c-myc RNA ablates A-Myb–mediated survival signals. Thus, these findings strongly implicate the A-myb gene in the regulation of B-cell survival and confirm the c-myc gene as one of the downstream targets of A-myb in these cells. Overall, our observation suggests that A-mybexpression may be relevant to the pathology of human B-cell neoplasias.
The A-myb gene belongs to the myb gene family, which includes the v-myb oncogene and the c-myband B-myb genes.1-4 Products of the mybgenes are structurally related and are able to activate transcription of artificial and natural promoters containing the myb DNA binding sequence (MBS) pYAACG/TG.5-9 Recently, we and others demonstrated that the A-myb gene potently transactivates MBS-driven constructs to an extent comparable to that of the v-myb oncogene,10-12 and identified the c-myc proto-oncogene as a target of A-myb–mediated transactivation in various cell types.13,14 Other targets of the A-myb gene include the c-myb,13mim-1,11 MD-1, and lysozyme genes.11,12Moreover, the A-Myb protein, like c-Myb, has been found to transactivate the human HSP70 promoter through a DNA-independent mechanism.11
The A-myb gene has a more limited pattern of expression than B-myb and c-myb. During mouse development, A-myb gene expression has been localized to the nervous system and urogenital ridge.12,15 In the adult mouse, A-myb expression was detected in the spleen, gut, testes, ovaries, and thymus. In the spleen, A-myb is expressed in the secondary follicles enriched with proliferating B cells.12Additionally, we have shown that A-myb is expressed in proliferating bovine vascular smooth muscle cells in culture.13 In normal human hematopoietic cells, A-myb expression is restricted to highly proliferating, centroblast germinal center B lymphocytes.16 A-mybexpression has also been detected in Burkitt's lymphomas (BLs), fresh B acute lymphoid leukemias (B-ALLs), and to a subset of chronic lymphocytic leukemias (B-CLLs).17 Ectopic expression of the A-myb gene under control of ubiquitously expressed promoters in transgenic mice display splenomegaly and lymphadenopathy due to aberrant proliferation of B lymphocytes.18 These findings strongly indicate a key role for A-myb both in regulation of normal B-cell proliferation and/or differentiation, as well as in the pathogenesis of BLs and lymphoid leukemias.
Evidence has also implicated the myb gene family in both positive and negative regulation of apoptosis in hematopoietic cells.19-23 With respect to the antiapoptotic roles played by these factors, the c-myb and v-myb genes have been found to promote survival via direct regulation of expression of the prosurvival bcl2 gene.24-26 Furthermore, mice lacking a functional A-myb gene displayed a block in spermatogenesis caused by massive testes tissue degeneration by apoptosis.27
WEHI 231 and CH33 murine B-cell lymphomas undergo cell death in response to treatment with an antiserum against their expressed surface IgM.28 Previously, we found that anti-IgM treatment of WEHI 231 cells down-regulated c-myc expression while inducing apoptosis.29,30 Ectopic c-myc expression rescued these cells from death induced by anti-IgM.31 Here, we report that A-myb and c-myc gene expression is coordinately down-regulated upon anti-IgM treatment of WEHI 231 cells. Thus, we sought to determine whether positive regulation of c-myc by A-myb is relevant to B-cell survival. A-Myb potently transactivated the c-myc promoter in WEHI 231 cells. Ectopic expression of A-Myb in WEHI 231 or CH33 cells significantly ablated apoptotic cell death induced by anti-IgM treatment, and in WEHI 231 cells, led to maintenance of c-myc gene expression that mediated survival signals. Thus, these findings indicate that the positive regulation of c-myc gene expression by A-myb is an important component in regulation of c-myc expression and apoptosis in murine B-cell lymphomas.
Materials and methods
Cell culture and treatment conditions
WEHI 231 and CH33 cells were maintained in Dulbecco's modified Eagle's medium (DMEM), supplemented as described previously.29,38 The A-myb WEHI 231 stable transfectants were prepared using 35 μg of pLL1 A-mybexpression vector10 and 5 μg pSV2Neo. Alternatively, WEHI 231 cells were transfected with 5 or 10 μg pSV2Neo. For transfection, WEHI 231 cells were resuspended in DMEM supplemented with 20% fetal bovine serum (FBS) at a concentration of 20 × 106 cells/mL. Cells (250 μL) were preincubated on ice for 10 minutes with DNA, and transfected by electroporation at 240 mV and 960 μF using a Gene Pulser (Bio-Rad, Hercules, CA). After incubation on ice for 5 minutes, the cell suspension was mixed with 1.75 mL of culture medium, and incubated for 10 minutes at room temperature, and then at 37°C. After 24 hours, 1 mg/mL G418 (GIBCO Laboratories, Gaithersburg, MD) was added to the culture medium and selective growth conditions maintained for approximately 2 weeks. Clones were isolated by limiting dilution. The pAW1-, 4-, 5-, and A-myb–expressing clones were obtained from a set of transfection independent from the pWA8 and 9 clones. For treatment, cells were incubated with 2 μg/mL anti-IgM antibody (Calbiochem, San Diego, CA) for the indicated periods.
Plasmids and transfection
The p1.6 Bgl chloramphenicol acetyl transferase (CAT) construct contains −1141 to +513 base pairs (bp) of the murine c-myc gene, containing promoter/upstream/exon 1 sequences.34 The pLL1 vector expresses a 3.1-kilobase (kb) human A-myb transcript, encoding full-length A-Myb protein.10 The pCAD1 vector directs expression of a mutant A-Myb protein that lacks the second and third tryptophan repeats within the DNA binding domain.10 This mutant retains the first tryptophan repeat but its DNA binding activity is inhibited by approximately 95% relative to the wild-type A-Myb.10 The pON407β-Gal expression vector is driven by the cytomegalovirus (CMV) promoter in which the 5 putative NF-κB elements have been removed.52 For transient transfection, cells were electroporated, as above, and transferred to petri dishes. Anti-IgM treatment was performed as above. After incubation at 37°C for 24 hours, cells were harvested, and the resulting extracts were normalized for total protein content by using the Bio-Rad protein quantitation kit. Equal amounts of lysates were assayed, in duplicate or triplicate, for β-Gal expression and CAT activity, as we have described previously.55 Standard deviation was obtained using the Student t test.
RNA isolation and analysis
Total cellular RNA was isolated by the guanidinium isothiocyanate method and samples (10-20 μg) subjected to Northern blot analysis, as described previously.54 The human A-myb pLL1 or the mouse c-myc cDNA clone pM-c-myc54 was used as probe. Quantitation by scanning densitometry was performed using a Molecular Dynamics #300A computing densitometer (Sunnyvale, CA).
Apoptosis and cell proliferation assays
For trypan blue exclusion assays, cells were incubated with 0.2% trypan blue (GIBCO Laboratories) for 10 minutes, and the percentage of cells, excluding dye (viable cells) or staining positive (dead cells) was determined. For the Non-Radioactive Cell Proliferation MTS assay (Promega, Madison, WI), cells were seeded at 20 × 104 in 100 μL volume in 96-well dishes. Cells were incubated, in triplicate, for 4 hours at 37°C in the presence of (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium inner salt (MTS) solution (333 μg/mL) and 25 μmol/L phenazine methosulfate (PMS), according to the manufacturer's directions. The A490 was measured in an enzyme-linked immunosorbent assay (ELISA) plate reader. For the β-Gal assay, cells were transfected with 5 to 10 μg pON407β-Gal expression vector in the presence or absence of the indicated constructs, as described above. After 6 hours, cells were treated in the presence or in the absence of 2 μg/mL anti-IgM and harvested at the 24-hour time point. On normalization for protein content, lysates (5-20 μg) were analyzed for β-Gal activity by using standard procedures. The A410 was measured in an ELISA plate reader and expressed in arbitrary units.
Results
A-myb expression is down-regulated after anti-IgM treatment of WEHI 231 cells
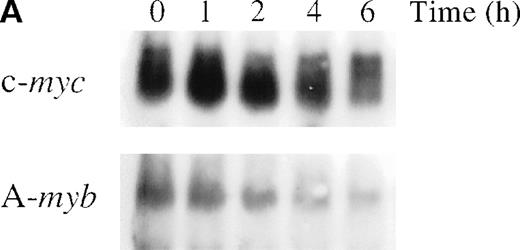
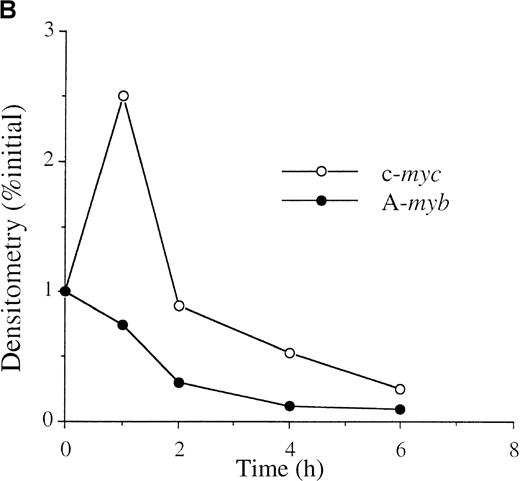
To determine whether anti-IgM treatment alters A-myb mRNA expression, WEHI 231 cells were treated with 2 μg/mL goat antimouse IgM antibody for 1, 2, 4, and 6 hours and mRNA isolated. As shown in Figure 1A (bottom panel), Northern blot analysis detected an approximately 5 kb A-myb messenger RNA (mRNA) in exponentially growing cells. A-myb mRNA level began to display a slight decrease after 1 hour of anti-IgM treatment and greatly decreased at the 2- and 4-hour time points. The amounts of A-myb mRNA at 2 and 4 hours were 30% and 18% relative to the initial value, respectively, as measured by densitometry (Figure 1B). Previously, we showed that anti-IgM treatment of WEHI 231 cells results in a transient increase in c-myc expression that is followed by a decline below basal levels, which precedes cell death.29Furthermore, we demonstrated that the nuclear factor-κB (NF-κB)/Rel transcription factors play a major role in the early up-regulation of c-Myc expression after anti-IgM treatment.30 As expected, Northern blot analysis showed up-regulation of c-myc mRNA levels by 1 hour of anti-IgM treatment of WEHI (Figure 1A, top panel). The level of c-myc mRNA then started to decline such that, at the 4- and 6-hour time points, they were at 53% and 25%, respectively, relative to the initial value (Figure 1B). Thus, the A-myb gene expression is down-regulated after anti-IgM treatment and this drop precedes cell death and the decline in c-myc mRNA levels.
Anti-IgM treatment down-regulates A-myb mRNA levels in WEHI 231 cells.
(A) WEHI 231 cells were treated with 2 μg/mL of anti-IgM antibody and total RNA was isolated from treated and untreated cells at the indicated time points. Samples (20 μg) were subjected to Northern blot analysis for RNA expression of c-myc (top panel) or A-myb (bottom panel). Equal loading was assessed by ethidium bromide staining of the gel (data not shown). (B) The resulting autoradiographs were subjected to densitometric scanning, and the values for signals of A-myb or c-myc are given as percentages relative to that observed in untreated cells (0 hour), arbitrarily set at 1. The exposure times were in the range of linearity as determined by multiple exposures.
Anti-IgM treatment down-regulates A-myb mRNA levels in WEHI 231 cells.
(A) WEHI 231 cells were treated with 2 μg/mL of anti-IgM antibody and total RNA was isolated from treated and untreated cells at the indicated time points. Samples (20 μg) were subjected to Northern blot analysis for RNA expression of c-myc (top panel) or A-myb (bottom panel). Equal loading was assessed by ethidium bromide staining of the gel (data not shown). (B) The resulting autoradiographs were subjected to densitometric scanning, and the values for signals of A-myb or c-myc are given as percentages relative to that observed in untreated cells (0 hour), arbitrarily set at 1. The exposure times were in the range of linearity as determined by multiple exposures.
Ectopic expression of A-Myb prevents anti-IgM–mediated apoptosis of WEHI 231 and CH33 cells
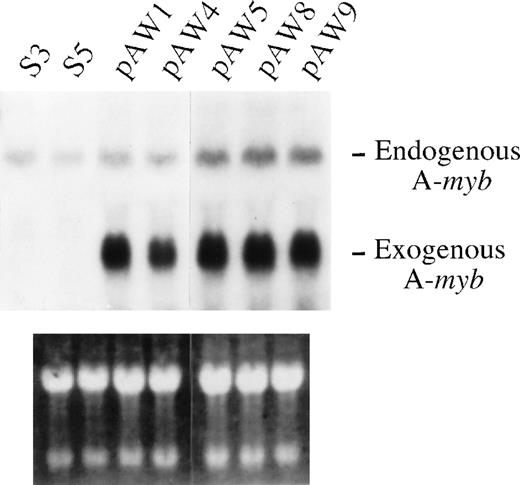
Recent findings have suggested that the A-myb gene might play a role in the control of B-cell survival.18 Thus, we sought to determine whether constitutive A-myb gene expression could prevent cell death of WEHI 231 cells on anti-IgM treatment. For this purpose, WEHI 231 cells were stably transfected with either the pSV2Neo neomycin resistance gene construct or pSV2Neo plus the vector pLL1 directing expression of the human A-myb gene,10 and clones isolated by limiting dilution. Total mRNA isolated from exponentially growing clonal transfectants were assayed by Northern blotting for A-mybexpression using the pLL1 A-myb DNA as a probe. Two of the pSV2Neo clones isolated, as exemplified by clones S3 and S5, and 5 A-myb clones, pAW1, 4, 5, 8, and 9, were found to express a 5-kb mRNA transcript corresponding to that of the endogenous A-myb gene (Figure 2, top panel). As expected, only the pLL1 transfected clones displayed the 3.1-kb long transcript from the human A-myb transgene. Equal loading of the samples was confirmed by ethidium bromide staining of the filters (Figure 2, bottom panel).
Ectopic A-Myb expression in WEHI 231 cell clones.
(A) WEHI 231 cells were transfected with 5 μg of the pSV2Neo and 35 μg of the human A-myb expression vector pLL1 (pAW1, -4, -5, -8, and -9) or the pSV2Neo vector alone (S3 and -5) and stable cell lines were selected by G418 resistance. Total mRNA was isolated from the cells in exponential growth, and samples (20 μg) subjected to Northern blot analysis using radiolabeled human A-mybpLL1 DNA, as probe (top panel). The positions of the transcripts from endogenous murine A-myb gene and the exogenous human A-myb gene are as indicated. As control for equal loading and integrity of the mRNA samples, the gel was stained with ethidium bromide (bottom panel).
Ectopic A-Myb expression in WEHI 231 cell clones.
(A) WEHI 231 cells were transfected with 5 μg of the pSV2Neo and 35 μg of the human A-myb expression vector pLL1 (pAW1, -4, -5, -8, and -9) or the pSV2Neo vector alone (S3 and -5) and stable cell lines were selected by G418 resistance. Total mRNA was isolated from the cells in exponential growth, and samples (20 μg) subjected to Northern blot analysis using radiolabeled human A-mybpLL1 DNA, as probe (top panel). The positions of the transcripts from endogenous murine A-myb gene and the exogenous human A-myb gene are as indicated. As control for equal loading and integrity of the mRNA samples, the gel was stained with ethidium bromide (bottom panel).
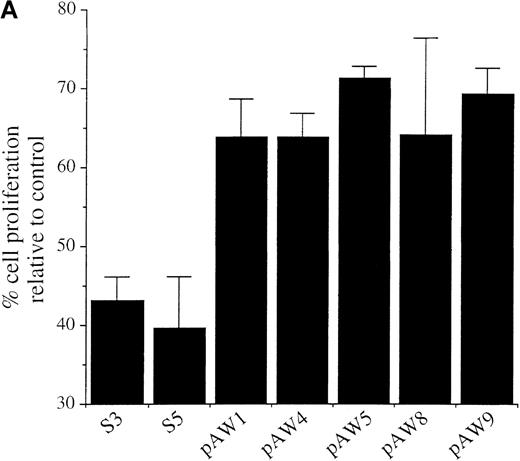
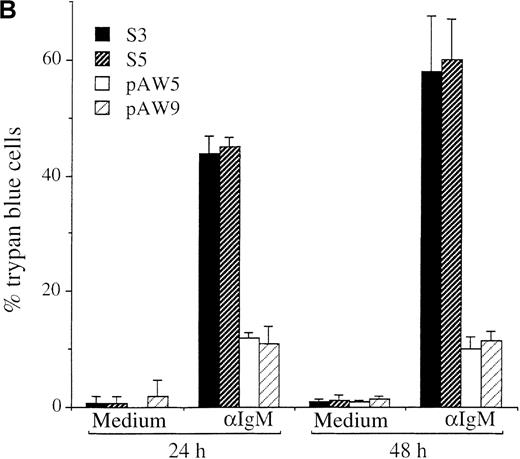
The WEHI 231 clones expressing ectopic A-myb gene and the pSV2Neo transfected clones were compared with respect to the effects of anti-IgM treatment on cell proliferation and apoptosis. Cultures were incubated for 48 hours in the presence of a goat antimouse IgM antibody, or medium alone as control, and proliferating cells assessed using the MTS assay. As observed previously,31 anti-IgM treatment of both S3 and S5 pSv2Neo transfected cells resulted in a significant block in cell proliferation of approximately 56% and 61%, respectively, relative to untreated control cells (Figure3A). The extent of this arrest in cell proliferation was comparable to that of anti-IgM–treated wild-type WEHI 231 cells, which was of about 66% (data not shown). In contrast, anti-IgM treatment had a much more modest effect on cell proliferation of the pAW A-myb expressing cells (Figure 3A). Loss of cell viability was further quantitated by trypan blue exclusion (Figure 3B). Cultures were incubated for 24 or 48 hours in the presence of the anti-IgM antibody or medium alone, as control. Anti-IgM treatment of the S3 and S5 clones resulted in a significant loss of viable cells. The levels of trypan blue positive cells at 24 hours were 43.8% ± 3% and 45% ± 1.7%, respectively, and increased at the 48-hour time point to 58.2% ± 9.5% and 60.2% ± 7.0%, respectively (Figure 3B). Ectopic A-Myb expression led to a very significant increase in cell survival. Only 12% ± 1.0% and 11% ± 3.0% of the pAW5 and pAW9 cells, respectively, were trypan blue positive after 24 hours of anti-IgM treatment. No significant increase in cell death was seen at the 48-hour time point, ie, 10% ± 2.1% and 11.5% ± 1.6%, respectively, of the trypan blue positive pAW5 and pAW9 cells (Figure3B). Thus, ectopic A-Myb expression prevents anti-IgM–induced growth arrest and apoptosis of WEHI 231 murine B-lymphoma cells.
Ectopic expression of A-Myb rescues WEHI 231 cells from anti-IgM–induced cell cycle arrest and apoptosis.
(A) Cultures of clones S3, S5, pAW1, pAW4, pAW5, pAW8, and pAW9 were incubated, in triplicate, for 48 hours in the presence of 2 μg/mL anti-IgM antibody or medium alone as control. Cell proliferation was quantitated by conversion of MTS dye to its formazan product. Data are plotted as the percentage of converted formazan by anti-IgM–treated cells relative to that of control cells. (B) S3, S5 PAW5 and pAW9 cells were incubated, in triplicate, for 24 or 48 hours in medium or with 2 μg anti-IgM (αIgM) and cell viability was determined by trypan blue exclusion assay. Trypan blue positive cell numbers are plotted as a percentage of the total cell number. The mean and SD are representative of 2 independent experiments carried out in triplicate.
Ectopic expression of A-Myb rescues WEHI 231 cells from anti-IgM–induced cell cycle arrest and apoptosis.
(A) Cultures of clones S3, S5, pAW1, pAW4, pAW5, pAW8, and pAW9 were incubated, in triplicate, for 48 hours in the presence of 2 μg/mL anti-IgM antibody or medium alone as control. Cell proliferation was quantitated by conversion of MTS dye to its formazan product. Data are plotted as the percentage of converted formazan by anti-IgM–treated cells relative to that of control cells. (B) S3, S5 PAW5 and pAW9 cells were incubated, in triplicate, for 24 or 48 hours in medium or with 2 μg anti-IgM (αIgM) and cell viability was determined by trypan blue exclusion assay. Trypan blue positive cell numbers are plotted as a percentage of the total cell number. The mean and SD are representative of 2 independent experiments carried out in triplicate.
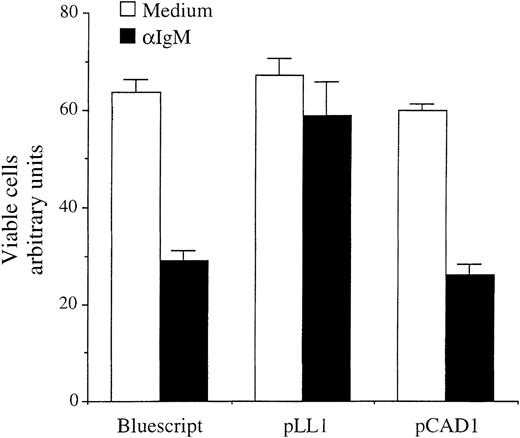
To extend this observation to another murine B-cell lymphoma, the immature B-cell line CH33, which similarly undergoes cell death after anti-IgM treatment, was tested using transient transfection analysis to evaluate cell death, described previously.32Exponentially growing CH33 cells were transiently transfected by electroporation with 5 μg of the β-galactosidase (β-Gal) expressing vector pON407, in which the 5 putative NF-κB sites within the CMV promoter have been removed (kindly provided by E. Mocarski), in the presence of 10 μg of vector, directing expression of either the complete human A-myb gene (pLL1) or a deletion mutant missing the second and third tryptophan repeats of DNA binding domain (pCAD1)10 or pBluescript DNA as control. After stimulation for 24 hours with 2 μg/mL anti-IgM, lysates of anti-IgM–treated or untreated cells were subjected to β-Gal staining, and the percentage of viable cells expressing β-Gal determined through absorbance reading. As expected, after 24 hours of anti-IgM treatment in cultures transfected with control Bluescript DNA, approximately 50% of the CH33 cells were no longer viable (Figure 4). In contrast, a significantly higher level of cell survival was seen when A-Myb was expressed in combination with anti-IgM treatment (approximately 90%). The deletion mutant A-Myb protein lacking a functional DNA binding domain, which is unable to transactivate heterologous promoters containing myb responsive elements,10 failed to rescue CH33 cells from anti-IgM–induced apoptosis (Figure 4). Thus, expression of A-myb reduces cell death of CH33 cells on anti-IgM treatment. Furthermore, an intact DNA binding domain is required for A-Myb–mediated cell survival.
A-Myb rescues CH33 lymphoma cells from anti-IgM–mediated apoptosis.
CH33 cells were transiently transfected by electroporation with 5 μg pON407 β-Gal expression vector in the presence or absence of 10 μg pLL1 or pCAD1 A-myb expression vectors, or Bluescript DNA, as control. The 2 μg/mL anti-IgM antibody was added to the medium 6 hours after transfection and cells were incubated for an additional 24 hours. Cellular lysates were subjected to β-Gal assay and absorbance quantitated in arbitrary units (vertical axis). The mean and SD are representative of 2 separate experiments, each carried out in triplicate.
A-Myb rescues CH33 lymphoma cells from anti-IgM–mediated apoptosis.
CH33 cells were transiently transfected by electroporation with 5 μg pON407 β-Gal expression vector in the presence or absence of 10 μg pLL1 or pCAD1 A-myb expression vectors, or Bluescript DNA, as control. The 2 μg/mL anti-IgM antibody was added to the medium 6 hours after transfection and cells were incubated for an additional 24 hours. Cellular lysates were subjected to β-Gal assay and absorbance quantitated in arbitrary units (vertical axis). The mean and SD are representative of 2 separate experiments, each carried out in triplicate.
A-myb potently transactivates the c-myc promoter in WEHI 231 cells
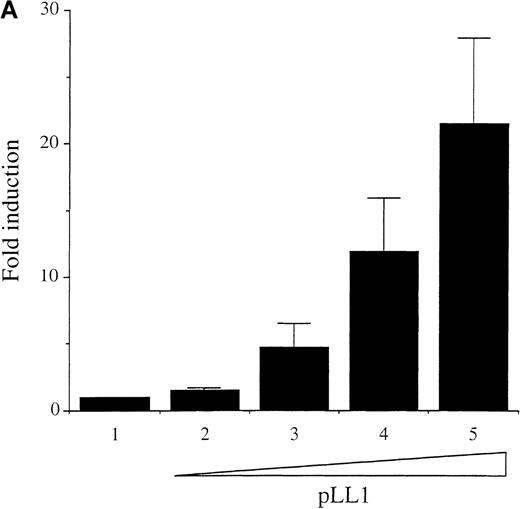
Previously, it has been noted that A-Myb is a potent transactivator of the c-myc promoter in several human B-cell lymphomas as well as other cell types.13,14 Because we noted a concomitant decrease in expression of A-myb and c-myc mRNA levels in WEHI 231 cells after anti-IgM treatment (Figure 1), we examined whether A-Myb could transactivate the c-myc promoter in these cells. Transfection analysis was performed with 10 μg of the c-myc promoter/upstream/exon1-CAT p1.6 Bgl construct, which contains both the proximal and distal Myb response regions (MRRs),33 linked to the CAT reporter gene.34Cotransfection of the expression vector pLL1, directing human A-myb expression, activated the p1.6 Bgl construct in a dose-dependent fashion up to 22-fold (Figure5A). Thus, A-myb potently transactivates the c-myc promoter in WEHI 231 cells to an extent similar to that observed in other B-cell lines.14
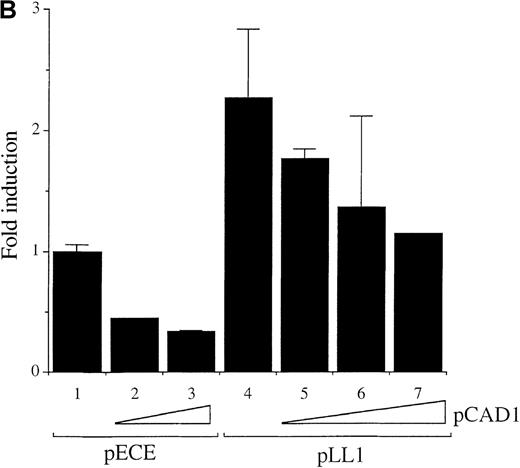
Wild-type A-Myb but not a DNA binding deletion mutant A-Myb transactivates the c-myc promoter in WEHI 231 cells.
(A) Exponentially growing WEHI 231 cells were electroporated, in duplicate, with 10 μg p1.6 Bgl in the absence (bar 1) or in the presence of 1, 5, 10, and 20 μg (bars 2-5, respectively) of the pLL1 A-myb vector, plus pECE parental vector DNA to have a final total DNA of 50 μg. Cell extracts were normalized for β-Gal expression and assayed for CAT activity. The data are presented as fold induction relative to the value of the p1.6 Bgl alone set at 1.0. Mean and SD were obtained from 2 independent experiments. (B) WEHI 231 cells were transiently transfected as described above, in the absence or in the presence of 2.5 μg of the empty pECE vector (bars 1-3) or 2.5 μg of the pLL1 A-myb construct (bars 4-7) with (bars 2, 3, 5, 6, and 7) or without (bars 1 and 4) increasing amounts of the pCAD1 vector: 5 μg (bars 2 and 5), 10 μg (bars 3 and 6), 20 μg (bar 7). CAT activity was determined as above and the data are representative of 2 experiments carried out in duplicate.
Wild-type A-Myb but not a DNA binding deletion mutant A-Myb transactivates the c-myc promoter in WEHI 231 cells.
(A) Exponentially growing WEHI 231 cells were electroporated, in duplicate, with 10 μg p1.6 Bgl in the absence (bar 1) or in the presence of 1, 5, 10, and 20 μg (bars 2-5, respectively) of the pLL1 A-myb vector, plus pECE parental vector DNA to have a final total DNA of 50 μg. Cell extracts were normalized for β-Gal expression and assayed for CAT activity. The data are presented as fold induction relative to the value of the p1.6 Bgl alone set at 1.0. Mean and SD were obtained from 2 independent experiments. (B) WEHI 231 cells were transiently transfected as described above, in the absence or in the presence of 2.5 μg of the empty pECE vector (bars 1-3) or 2.5 μg of the pLL1 A-myb construct (bars 4-7) with (bars 2, 3, 5, 6, and 7) or without (bars 1 and 4) increasing amounts of the pCAD1 vector: 5 μg (bars 2 and 5), 10 μg (bars 3 and 6), 20 μg (bar 7). CAT activity was determined as above and the data are representative of 2 experiments carried out in duplicate.
To establish whether the A-Myb DNA binding domain is required for c-myc transcriptional activation by A-Myb, cotransfection experiments were performed using the pCAD1 vector, which directs expression of the mutant A-Myb protein lacking part of the DNA binding domain.10 Transfection of 5 or 10 μg of the deletion mutant A-Myb vector pCAD1 failed to transactivate the c-mycpromoter, and instead a significant dose-dependent reduction in CAT activity of p1.6 Bgl to levels below baseline was observed (Figure 5B; compare lane 1 with lanes 2 and 3). Thus, intact 3 tryptophan repeats within the DNA binding domain of A-Myb are required to mediate c-myc promoter transactivation by A-Myb. This observation suggests that the A-Myb deletion mutant might compete with the endogenous A-Myb protein for unknown coactivator(s) required for the transactivation of the c-myc promoter by A-Myb. Thus, we next tested the effects of the mutant A-Myb on transactivation by wild-type A-Myb protein. Transfection of a suboptimal dose of the pLL1 A-myb expression vector (2.5 μg) resulted in a 2.5-fold transactivation of the p1.6 Bgl myc promoter (Figure 5B; compare lane 1 with lane 4). Cotransfection of increasing amounts of the pCAD1 vector down-regulated the pLL1 A-Myb vector-mediated transactivation of the c-myc promoter (Figure 5B; compare lane 4 with lanes 5, 6, and 7). These results suggest that the A-Myb deletion mutant can indeed compete with the wild-type A-Myb protein.
A-Myb rescues WEHI 231 cells from anti-IgM–mediated apoptosis through maintenance of c-myc gene expression
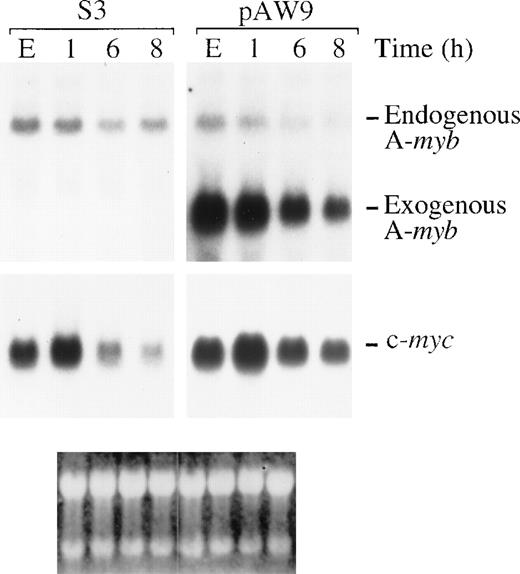
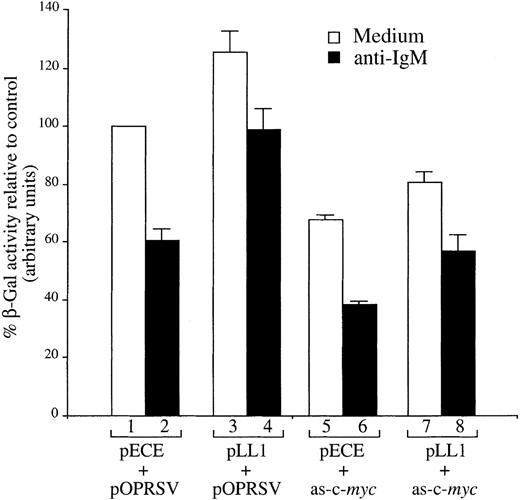
Previously, we showed that a drop in c-myc gene expression is necessary to promote anti-IgM–mediated apoptosis of WEHI 231 cells, and that ectopic expression of c-Myc significantly ablated apoptosis induced by anti-IgM treatment and cells continued to proliferate.31 Thus, we next determined whether positive regulation of c-myc gene expression by A-Myb could be responsible for protection from anti-IgM treatment of WEHI 231 cells. To this purpose, we tested c-myc mRNA levels in the stable WEHI 321 cell clones as a consequence of anti-IgM treatment. In exponential growth, before treatment, none of the WEHI 231 A-Myb–expressing clones showed significant variations in c-myc mRNA level (data not shown), probably due to the well-characterized autoregulation of c-myc gene expression.35 After anti-IgM treatment of the WEHI 231 A-Myb transfectant pAW9, or control pSV2Neo transfectant clone S3, the levels of A-myb and c-mycmRNA were characterized by Northern blotting (Figure6). Expression of the 5-kb endogenous A-myb gene transcription in the pAW9 cell line was down-regulated with essentially the same kinetics as the endogenous A-myb gene in the control S3 cells. Levels of the 3.1-kb transgene in the pAW9 cells decreased, but were maintained above endogenous control values (Figure 6, top panel). Intriguingly, the A-myb transgene expression was also partially affected by anti-IgM treatment (Figure 6, top right panel), possibly because of inhibition of NF-κB,36 which regulates the activity of SV40 early promoter driving A-myb expression in the pECE vector.10 When the same filters were analyzed for c-myc mRNA expression, both the S3 and pAW9 clones displayed c-myc mRNA induction at the 1 hour time point after anti-IgM treatment, as we observed previously,31 suggesting that these clones have retained their sensitivity to anti-IgM treatment. As expected, c-myc mRNA levels dropped subsequently. After 8 hours of anti-IgM treatment, c-myc mRNA levels in the S3 cells were 4.5-fold lower than those observed in exponentially growing cells. In contrast, c-myc mRNA levels were maintained (only 1.6-fold lower than basal level) after 8 hours of anti-IgM treatment of the pAW9 cells. These observations indicate that the c-myc gene expression indeed correlates with A-Myb–mediated survival. To confirm c-Myc expression actually mediates survival signals, we used an antisense strategy. If c-Myc mediates protection, we reasoned that inhibition of c-myc expression should block the enhanced WEHI 231 cell survival seen on ectopic A-Myb expression. To test this hypothesis, the β-Gal assay of cell survival, described above, was used. Exponentially growing WEHI 231 cells were transiently transfected by electroporation with the β-Gal expressing vector pON407 in the presence of (1) 10 μg pLL1 A-myb expression or pECE parental vector DNA, and (2) 10 μg pOPRSV-as-c-myc vector directing expression of antisense c-myc mRNA or pOPRSV empty vector DNA. After 6 hours, a portion of the cultures were treated with anti-IgM. After an additional 24 hours, β-Gal activity was measured, as above. Transfection of 10 μg as-c-myc and pECE parental DNA led to a decrease in cell viability of approximately 40% compared with control cells transfected with parental pOPRSV DNA (Figure7; compare lane 1 with lane 5). This observation is in good agreement with the extent of cell killing seen on induction of the same construct in mixed populations of stable WEHI 231 cell transfectants.37 As expected, treatment of cultures transfected with control pOPRSV vector with anti-IgM for 24 hours resulted in an approximate 40% loss of cell viability (compare lane 1 with lane 2). This cell killing was dramatically increased in the as-c-myc transfected WEHI 231 cells (approximately 60%; lane 6). In contrast, a significantly higher level of cell survival was seen when A-Myb was expressed in combination with the empty pOPRSV vector in anti-IgM–treated cells (approximately 95%; lane 4). However, when A-Myb was expressed in combination with as-c-myc, we observed much less protection from anti-IgM–induced cell death (compare lane 6 with lane 8). Taken altogether, these findings identify the c-myc gene as a downstream target of A-Myb–mediated rescue of WEHI 231 cells from apoptosis after anti-IgM treatment.
Ectopic expression of A-Myb in WEHI 231 cells leads to maintenance of c-myc mRNA levels on anti-IgM treatment.
Total mRNA was isolated from S3 or pAW9 cells in exponential growth (E) or after treatment with 2 μg/mL anti-IgM for 1, 6, or 8 hours. Samples (20 μg) were subjected to Northern blot analysis for mRNA expression of the A-myb and c-myc genes (bottom and center panels, respectively). Positions of the endogenous (end) and exogenous (ex) A-myb are as indicated. Ethidium bromide staining of the filter (bottom panel) was used to confirm integrity and equal loading of mRNA samples.
Ectopic expression of A-Myb in WEHI 231 cells leads to maintenance of c-myc mRNA levels on anti-IgM treatment.
Total mRNA was isolated from S3 or pAW9 cells in exponential growth (E) or after treatment with 2 μg/mL anti-IgM for 1, 6, or 8 hours. Samples (20 μg) were subjected to Northern blot analysis for mRNA expression of the A-myb and c-myc genes (bottom and center panels, respectively). Positions of the endogenous (end) and exogenous (ex) A-myb are as indicated. Ethidium bromide staining of the filter (bottom panel) was used to confirm integrity and equal loading of mRNA samples.
Cotransfection of a c-myc antisense expression vector ablates rescue of WEHI 231 cells from anti-IgM–mediated apoptosis by A-Myb.
WEHI 231 cells were transiently transfected by electroporation with 10 μg pON407 β-Gal expression vector in the presence of 10 μg either pLL1 or pECE, empty vector, in combination with 10 μg of either pOPRSV-as-c-myc or pOPRSV parental vector. Cultures were either left untreated (white bars) or treated 6 hours after transfection with 2 μg/mL anti-IgM antibody for an additional 24 hours (black bars). Cellular lysates were subjected to β-Gal assay and absorbance expressed as percentage of untreated cells transfected with control vectors alone (lane 1). The mean and SD are representative of 2 separate experiments, each carried out in duplicate.
Cotransfection of a c-myc antisense expression vector ablates rescue of WEHI 231 cells from anti-IgM–mediated apoptosis by A-Myb.
WEHI 231 cells were transiently transfected by electroporation with 10 μg pON407 β-Gal expression vector in the presence of 10 μg either pLL1 or pECE, empty vector, in combination with 10 μg of either pOPRSV-as-c-myc or pOPRSV parental vector. Cultures were either left untreated (white bars) or treated 6 hours after transfection with 2 μg/mL anti-IgM antibody for an additional 24 hours (black bars). Cellular lysates were subjected to β-Gal assay and absorbance expressed as percentage of untreated cells transfected with control vectors alone (lane 1). The mean and SD are representative of 2 separate experiments, each carried out in duplicate.
Discussion
In this study, we have shown that the A-myb gene acts as survival factor for murine B-cell lymphomas. Ectopic expression of A-Myb rescued WEHI 231 and CH33 cells from IgM-receptor–mediated cell death. We found that A-Myb potently transactivated the c-mycpromoter in WEHI 231 cells, similar to its effects in other cell types. Maintenance of c-myc expression in the A-Myb expressing WEHI 231 clones correlated with protection from anti-IgM–induced apoptosis. Furthermore, A-Myb–mediated protection from WEHI 231 apoptosis was blocked by the inhibition of c-myc expression by antisense mRNA. Previously, we demonstrated that maintenance of c-mycexpression is sufficient to ablate apoptosis of WEHI 231 cells resulting from anti-IgM31 or transforming growth factor (TGF)-β1 treatments.38 Here, we identify c-Myc as the target of A-Myb–mediated B-cell lymphoma survival. Thus, these findings implicate the A-myb gene in the development of self-tolerance, and suggest aberrant A-myb expression may play a role in the cause of autoimmune pathologies.
The myb factors have been extensively implicated in the regulation of cell proliferation and differentiation of hematopoietic tissues.39,40 On one hand, c-myb and B-mybgene expression was found to be cell cycle regulated in several hematopoietic cell types,41 and inhibition of c-Myb or B-Myb activity through antisense strategies ablated proliferation of myeloid, erythroid, and lymphoid cells.42-44 On the other hand, the c-myb viral counterpart v-myb was found to transform immature myeloid chicken bone marrow cells in vivo and in vitro.45 Accordingly, expression of c-myb and B-myb blocked the differentiation of several myeloid cell lines,46-48 and mice lacking a functional c-Myb protein showed impaired fetal hematopoiesis.49
Recently, the myb family member A-myb has also been implicated in the control of cell proliferation and differentiation, although its expression among hematopoietic cell types appears restricted to B-cell centroblasts, BLs, and to a subset of B-CLLs,17 and WEHI 231 and CH33 murine B-cell lymphomas (this study). Consistent with a role in differentiation of B cells, A-Myb expression was found to be down-regulated during CD40L, IL-2, and IL-10–mediated differentiation of germinal centers (GC) cell to centrocytes,16 and during progression of 1 case of CLL into Ritcher's syndrome.17 In agreement with a role of A-myb in the regulation of cell proliferation, ectopic expression of A-Myb in transgenic mice led to lymphoid hyperplasia in lymph nodes and spleen as a result of expansion of the follicular center B-cell population.18 In this study, we show that the down-regulation of A-myb mRNA levels parallels the decrease in WEHI 231 cell proliferation in response to anti-IgM treatment. Furthermore, ectopic A-Myb expression rescued WEHI 231 cells from anti-IgM–induced growth arrest. These findings suggest that the A-myb gene participates in control of murine lymphoma cell proliferation.
To date, mounting evidence has demonstrated a role for the mybgenes in the positive and/or negative regulation of apoptosis. Lipsick and coworkers22 found that ectopic expression of c-myb rendered chicken monoblasts more resistant to TPA-induced differentiation and cell death.22 Similarly, a Gag-myb-ETS fusion oncogene enhanced cell survival of the IL-3–dependent granulocytic FDC-P2 cells when these cells were transferred from IL-3 to hormone erythropoietin-containing media.23 Moreover, Frampton et al24 found that the v-myb oncogene contained in the E26 retrovirus acts as a survival factor in infected chicken myeloblasts by directly transactivating the anti-apoptotic gene bcl2. In agreement with this finding, ectopic c-Myb expression could rescue the IL-2–dependent cytotoxic T-cell line CTLL-2 from cell death induced by IL-2 deprivation or dexamethasone treatment via induction of bcl2 gene expression. In addition, a dominant negative c-Myb protein was found to induce programmed cell death when introduced in the thymoma cell line EL4 through selective down-regulation of bcl2 gene expression.25 In contrast, c-myb and B-mybgene expression accelerated TGF-β1–induced apoptosis of the murine M1 myeloid leukemia cell line.19,20 Also, the c-mybgene product stimulated apoptosis in both the murine promyelocytic 32D and the human osteosarcoma SOAS2 cell lines when coexpressed with p53.21 For the first time, our study shows that A-myb expression protects B-cell lymphomas from anti-IgM–mediated apoptosis. In agreement with a role for A-Myb in the regulation of apoptosis and proliferation of some cell types, the absence of a functional A-myb gene in breast ductal epithelium and spermatogonia, led to dramatic tissue degeneration by apoptosis.27
Previously, c-myb, B-myb, and A-myb genes have been shown to transactivate the c-myc promoter in a variety of cell types, including B-cell lymphomas.13,33,50-52 Here we found that A-Myb is a potent transactivator of the c-mycpromoter in murine B-cell lymphomas. Furthermore, we show that the DNA binding domain is required for A-myb–mediated c-myctransactivation. Previously, the DNA binding domain has been shown to be responsible for the specificity of the A-Myb activity in B versus T cells.14 Furthermore, it has been proposed that a 110-kd protein interacts with the A-myb DNA binding domain to confer B-cell specificity to the A-myb transcriptional activity.14 Our findings indicate an essential role of the A-myb DNA binding domain during A-Myb–mediated rescue from B-cell apoptosis. This evidence suggests that the expression of B-cell specific coactivators that interact with the A-Myb DNA binding domain might influence the anti-apoptotic function of the A-myb gene.
Overall, our findings extend to B-cell lymphomas the observation that A-Myb is an antiapoptotic factor. In addition, these observations indicate an important role of A-myb during B-cell clonal deletion with possible implications in autoimmune disease.
Acknowledgments
We are grateful to Dr E. Mocarski for the kind gift of the pON407 vector. We thank Dr M. FitzGerald for providing cellular lysates and Dr M. Wu for sharing the pOPRSV-as-c-myc construct and for helpful discussions. We thank Dr J. Foster for the use of the scanning densitometer. We also thank D. Sloneker for assistance in the preparation of this manuscript.
Supported by grants from the National Institutes of Health, CA 36355 (G.E.S.), the Cure For Lymphoma Foundation (CFL) (M.A.), from the Italian Association for Cancer Research (AIRC) (M.I.), and from the Istituto Superiore di Sanita' di Roma (ISS), AIOS 30A.29 (M.I.).
Reprints:Marcello Arsura, BUSM, Department of Biochemistry, 80 E Concord St, Boston, MA 02118; e-mail: marsura@bu.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal