Abstract
Surrogate light chains (λ5/VpreB) are selectively expressed in early precursors of B cells. B-cell defects in X-linked agammaglobulinemia (XLA) are caused by mutations in the gene for Bruton's tyrosine kinase. To elucidate the nature of early B-lineage cells in bone marrow (BM), samples from 13 XLA patients and 24 healthy controls of different ages were comparatively analyzed using an antihuman VpreB monoclonal antibody. Expression of surrogate light (SL) and μ-heavy chains were examined after cell membrane permeabilization because they are mainly expressed in the cytoplasm of early B-lineage cells. A flow cytometric analysis of normal BM identified 5 discrete cell types of B cells: μ−SL++ (pro-B [B-cell progenitor]), μlowSL++ (pre-B1a), μlowSL+ (pre-B1b), μlowSL− (pre-B2), and μhighSL− (B). The large cells, presumably in cycling states, were enriched in pre-B1a cells. The frequencies of B-lineage cells in BM were higher in young children, and declined with advancing age. In contrast, XLA showed a profound reduction in BM B-lineage cells. In XLA BM, an expansion of pro-B cells with some small pre-B1a cells was marked, but other cells were negligible. These observations illustrate a B-cell maturation defect in XLA as well as a normal human B-cell differentiation pathway. The results suggest that the genetic defect in XLA may impede the evolution of pro-B cells beyond the earlier pre-B stage into the later stage of pre-B cells in B-cell development.
In mammals, the generation of B lymphocytes from multipotent hematopoietic stem cells is found first in the fetal liver.1 After birth, the bone marrow (BM) becomes the major source of B-cell precursors and produces mature B cells throughout life.2-4 Many studies have demonstrated that the intracytoplasmic and surface expression of various developmentally regulated gene products is associated with progression along the B-cell differentiation pathway.3-10 Early B-cell progenitors (pro-B cells) express the enzyme terminal deoxynucleotidyl transferase (TdT), which functions in the completion of immunoglobulin (Ig) gene rearrangements.5 Pre-B cells were originally discriminated from mature B cells or other hematopoietic cells on the basis of an intracytoplasmic μ-heavy (μΗ) chain and a lack of cell surface IgM.3 One surface marker identified on early B-lineage cells is the Ig gene superfamily molecule, CD19, which is expressed until the latest stage of B cells.6,7 Other cell surface molecules defining early developmental stages of B cells include CD34, CD10, CD20, and CD227 and the surrogate light (SL) chains.8-10
Researchers have focused increasing interest on the SL chains and their biological significance in early B-cell development. The chains are composed of 2 polypeptides encoded by theλ511,12 and VpreB13 genes, which can associate with the μΗ chain to form the pre–B-cell receptor.14,15 A crucial role of the pre–B-cell receptor in early B-cell development has been verified by the findings that mutations in 1 of the pre–B-cell receptor genes cause development arrest at the pro–B-cell stage, thereby resulting in a severe impairment of B-cell generation.16-19 Production of the SL chains begins at the stage of pro-B cells, that is, before the formation of pre–B-cell receptors; maintains through the pre–B-cell stage; and halts at the immature B-cell stage.8-10 Thus, expression properties of the SL chains restricted to both pro–B-cell and pre–B-cell stages can serve as the potential marker for elucidation of distinct steps in early B-cell development.10 20-22
X-linked agammaglobulinemia (XLA) is a hereditary immunodeficiency caused by mutations in the gene coding for Bruton's tyrosine kinase (Btk) protein.23,24 XLA is characterized by the early onset of bacterial infection, very low serum Ig levels of all isotypes, and severely decreased numbers of peripheral B lymphocytes.25,26 The Btk protein consists of 5 functional domains: the pleckstrin homology (PH), the Tec homology (TH), the Src homology 3 (SH3), SH2, and the kinase domain (SH1).25,27Although the role of this molecule in B-cell development and activation remains poorly understood, an international registry for XLA shows that mutations in all 5 domains of the Btk gene cause the disease.28 The majority of Btk mutations have been shown to result in deficient expression of the Btk protein because of a reduction in Btk mRNA or an instability of the produced protein.23 29-31
We describe a rapid and simple method for detection of patients with XLA; the deficient status of the Btk protein expression in their monocytes is easily demonstrable by a flow cytometric assay using the anti-Btk monoclonal antibody (mAb).31 This method has identified atypical XLA patients who exhibited mild or even no clinical symptoms until adult life, despite the paucity of circulating B cells with hypogammaglobulinemia.32 Regarding the B-cell defect in XLA, confusing observations have been obtained from earlier studies using XLA BM samples.33-37 Some studies33,34have found that XLA patients show normal numbers of pre-B cells in BM, whereas other studies34,35 have found that pre-B cells are seen in only low or undetectable numbers. Furthermore, investigations using early B-cell markers, such as TdT, CD19, CD10, and CD34, have disclosed that normal or increased frequencies of pro-B cells are present in XLA BM.19 35-39
In the present study, we employed an mAb specific for the human VpreB, as a component of the SL chains, to examine the distribution of early B-lineage cells in BM samples from XLA patients. The patients were diagnosed by demonstration of both Btk protein deficiencies andBtk gene mutations compared with healthy controls of different ages. Flow cytometric analysis was used to understand the normal B-cell differentiation pathway in humans and the nature of the Btkmutation that caused the B-cell maturation defect in XLA.
Patients, materials, and methods
Subjects
After receiving informed consent, we obtained BM from 13 patients with XLA and from 24 healthy individuals of various ages as controls. Each XLA patient had received care in one of our clinics. Clinical features and Btk mutations of the XLA patients are summarized in Table 1. The present age of patients with XLA ranged from 4 months to 30 years. Patients P6, P7, and P8 were analyzed for Btk mutations in a previous study,29and patients P4 and P12 were diagnosed by a flow cytometric demonstration of the deficient Btk expression in monocytes in another study.31 Btk mutations in the latter 2 patients were detected according to the method described previously.29 The remaining 8 patients were diagnosed as XLA+ by a flow cytometric demonstration of a monocyteBtk deficiency and a genetic analysis of Btk mutations (Kanegane et al, unpublished data, April 1999).
Percentages of peripheral B cells and Btkmutations in XLA patients
| Patient no. . | Age at analysis, y (except where indicated) . | Age at diagnosis, mo or y . | Peripheral B cells, % . | Btkmutation . | |
|---|---|---|---|---|---|
| Nucleotide change35 . | Consequence . | ||||
| P1 | 4 mo | 4 mo | 0.10 | deletion of G787 | FS (stop) |
| P2 | 1 | 5 mo | 0.60 | deletion of G1089 and T1090 | FS (stop) |
| P3 | 2 | 1 y | 0.14 | 1 nt insertion between A612 and A613 | FS (stop) |
| P4 | 4 | 1 y | 0.10 | intron 5 −2A → G | exon 6 skip |
| P5 | 4 | 3 y | <0.1 | 5 nt deletion/13 nt insertion (exon 18) | exon 16-18 skip |
| P6 | 4 | 6 mo | <0.1 | 342 nt deletion between G1699 and G2040 | exon 16-18 skip |
| P7 | 5 | 3 y | 0.50 | deletion of G1089 and T1090 | FS (skip) |
| P8 | 6 | 10 mo | 0.10 | 60 nt insertion between G1764 and T1765 | Stop* |
| P9 | 10 | 3 y | 0.25 | 60 nt insertion between G1764 and T1765 | Stop* |
| P10 | 12 | 7 y | 0.30 | G324 → T | Val64 → Phe |
| P11 | 18 | 5 y | <0.1 | 1 nt insertion between G1176 and A1177 | FS (stop) |
| P12 | 21 | 1 y | <0.1 | deletion of G787 | FS (stop) |
| P13 | 30 | 18 y | <0.1 | A1924 → G | Tyr598 → Asp |
| Patient no. . | Age at analysis, y (except where indicated) . | Age at diagnosis, mo or y . | Peripheral B cells, % . | Btkmutation . | |
|---|---|---|---|---|---|
| Nucleotide change35 . | Consequence . | ||||
| P1 | 4 mo | 4 mo | 0.10 | deletion of G787 | FS (stop) |
| P2 | 1 | 5 mo | 0.60 | deletion of G1089 and T1090 | FS (stop) |
| P3 | 2 | 1 y | 0.14 | 1 nt insertion between A612 and A613 | FS (stop) |
| P4 | 4 | 1 y | 0.10 | intron 5 −2A → G | exon 6 skip |
| P5 | 4 | 3 y | <0.1 | 5 nt deletion/13 nt insertion (exon 18) | exon 16-18 skip |
| P6 | 4 | 6 mo | <0.1 | 342 nt deletion between G1699 and G2040 | exon 16-18 skip |
| P7 | 5 | 3 y | 0.50 | deletion of G1089 and T1090 | FS (skip) |
| P8 | 6 | 10 mo | 0.10 | 60 nt insertion between G1764 and T1765 | Stop* |
| P9 | 10 | 3 y | 0.25 | 60 nt insertion between G1764 and T1765 | Stop* |
| P10 | 12 | 7 y | 0.30 | G324 → T | Val64 → Phe |
| P11 | 18 | 5 y | <0.1 | 1 nt insertion between G1176 and A1177 | FS (stop) |
| P12 | 21 | 1 y | <0.1 | deletion of G787 | FS (stop) |
| P13 | 30 | 18 y | <0.1 | A1924 → G | Tyr598 → Asp |
Of the patients, P1 was a nephew of P12; P2 and P7 were brothers; and P8 and P9 were cousins. The percentages of peripheral B cells were evaluated by coexpression of CD19 and CD20. Nt indicates nucleotides; FS (stop), frame shift resulting in secondary termination.
Indicates that a stop codon appeared in the intron sequence.
The healthy controls comprised 10 infants and toddlers (5 months to 3 years), referred to in this report as infants; 9 children (5-10 years); and 5 adults (21-30 years). All of the children underwent diagnostic BM aspiration for medical indications, and all had morphologically normal BM. None of them had received cytotoxic drugs. During this study, we had the chance to see a 4-month-old girl with severe combined immune deficiency (SCID) who lacked both T and B cells and showed no mutations in the recombination activation genes,RAG-1 and RAG-2. We included this patient as 1 subject in our study.
Cell preparation
Approximately 0.5 mL BM was aspirated from the iliac crest into a heparinized syringe and mixed with 5 mL Roswell Park Memorial Institute medium (RPMI 1640) with 10% fetal calf serum (FCS) and antibiotics. Mononuclear cells were separated from diluted BM by centrifugation on a Ficoll-Hypaque gradient (Histopaque 1077; Sigma Chemical Co, St Louis, MO), followed by lysis of contaminated red blood cells (RBCs) using 0.83% ammonium chloride buffer, and washed 3 times in phosphate-buffered saline (PBS). BM cells were then resuspended in a staining buffer (PBS with 1% FCS and 0.1% sodium azide). Cell viability, assessed by trypan blue dye exclusion, was more than 95% in all the experiments.
Antibodies
The hybridoma producing the mouse mAb specific for human VpreB (HSL96) was generated as described previously.22 Purified anti-VpreB mAb was labeled with phycoerythrin (PE) (PharMingen, San Diego, CA). Other mAbs and antibodies (Abs) employed for 3-color immunofluorescence analysis of BM were: PE-cyanin 5.1–labeled (PC5-labeled) anti-CD19 (Immunotech, Marseilles, France) and fluorescence isothiocyanate–labeled (FITC-labeled) TdT (Dako Japan, Kyoto, Japan) mouse mAbs; FITC- and PE-labeled goat F(ab′)2 antihuman μH chain Abs (Southern Biotechnology Associates, Inc, Birmingham, AL); and the corresponding fluorochrome-labeled irrelevant mAbs or Abs (Dako Japan and Immunotech) as controls.
Flow cytometric analysis
We used 3-color immunofluorescence analysis to identify various B-lineage cells in the BM. In most experiments, the BM cells were fixed and then permeabilized to analyze the cell surface antigens and intracellular molecules simultaneously. In brief, the BM cells were fixed with 4% paraformaldehyde (Wako Pure Chemical Industries, Ltd, Osaka, Japan) in PBS for 15 minutes at room temperature and then treated with 0.5% saponin (Sigma) in a staining buffer for 15 minutes on ice. These fixed permeabilized cells were incubated with a combination of PC5-labeled anti-CD19 mAbs and other FITC-labeled or PE-labeled mAbs or Abs for 20 minutes on ice and then washed twice in a staining buffer. In some experiments, viable BM cells were just stained for cell surface antigens. In other instances, the BM cells were stained for certain cell surface antigens prior to cell membrane permeabilization and then subjected to intracellular staining. The stained cells were analyzed by flow cytometry (EPICS XL-MCL; Beckman Coulter KK, Tokyo, Japan).
Statistic analysis
The unpaired Student t test was used to analyze data.
Results
Flow cytometric analysis of B-lineage cells in BM defined by VpreB expression
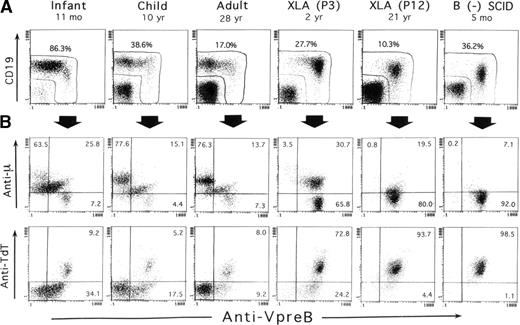
SL chains are mainly expressed in the cytoplasm of early B-lineage cells, but at very low levels in their cell surface.8,9,21,22 In this study, the VpreB expression in BM was evaluated after fixation and permeabilization of BM cells in the 3-color immunofluorescence assay. In addition to surface CD19 staining, this treatment conveniently resulted in the simultaneous staining of TdT, as well as of the μH chain, intracellularly expressed in early B-precursor cells. Figure 1 shows representative staining profiles of BM samples from 3 controls of different ages, 2 XLA patients, and 1 B-cell− SCID patient. Although CD19 is conventionally used as an earliest marker for B-lineage cells, evidence has suggested that the commitment to B-lineage cells appears to precede CD19 expression.40 41Apparently confirming this evidence, analysis of CD19 and VpreB expression by BM lymphoid cells indicated that some cells expressing VpreB intensely tended to decrease or lack CD19 expression, which was marked in XLA or SCID BM samples (Figure 1A).
Three-color immunofluorescence analysis of early B-lineage cells in BM based on intracellular VpreB expression.
BM cells from 3 controls of different ages, 2 XLA patients (P3 and P12), and 1 B-cell− SCID patient were fixed, permeabilized, and then stained with PE-labeled anti-VpreB and PC5-labeled anti-CD19 mAbs in combination with FITC-labeled anti-TdT mAb or antihuman μH chain Ab. The corresponding fluorochrome-labeled irrelevant mAbs or Abs were used as controls. The cells were gated for lymphoid cells by light scatter characteristics upon a flow cytometer and analyzed in the 3-color manner. (A) As demarcated in the figure, the combined use of anti-VpreB and anti-CD19 mAbs appeared to detect the presumably whole populations of B-lineage cells in BM. (B) The relation between VpreB and the μH chain or TdT expressed by BM-lineage cells, which were gated by a combined use of CD19 and VpreB expression. The number indicates the percentage of cells in each quadrant.
Three-color immunofluorescence analysis of early B-lineage cells in BM based on intracellular VpreB expression.
BM cells from 3 controls of different ages, 2 XLA patients (P3 and P12), and 1 B-cell− SCID patient were fixed, permeabilized, and then stained with PE-labeled anti-VpreB and PC5-labeled anti-CD19 mAbs in combination with FITC-labeled anti-TdT mAb or antihuman μH chain Ab. The corresponding fluorochrome-labeled irrelevant mAbs or Abs were used as controls. The cells were gated for lymphoid cells by light scatter characteristics upon a flow cytometer and analyzed in the 3-color manner. (A) As demarcated in the figure, the combined use of anti-VpreB and anti-CD19 mAbs appeared to detect the presumably whole populations of B-lineage cells in BM. (B) The relation between VpreB and the μH chain or TdT expressed by BM-lineage cells, which were gated by a combined use of CD19 and VpreB expression. The number indicates the percentage of cells in each quadrant.
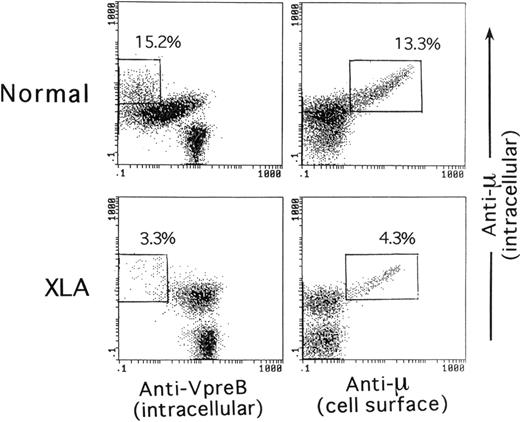
As illustrated in Figure 1A, a combination of CD19 and VpreB expression ensured that the analysis was capable of covering the vast majority of B-lineage cells in BM cells. Figure 1B shows the relationship of VpreB to the μH chain and TdT expression by the B-lineage cells gated on the basis of CD19 and VpreB expression. Smaller populations of μ−SLhigh cells, seemingly coexpressing TdT, were identified in normal BM, whereas a large proportion of B-lineage cells in XLA BM and the vast majority of the cells in SCID BM were identified as μ−VpreB+ and TdT+VpreB+. The μ−SLhigh cells in BM lymphoid cells appeared to represent the pro–B-cell compartment. A question was raised on whether discrimination between pre-B cells and B cells in permeabilized BM samples was possible. We then performed a comparative analysis between intracellular expression of the μH chain and intracellular VpreB or surface μH chain expression in B-lineage cells in BM of controls and XLA patients (Figure2). The figure indicates that mature B cells expressed the μH chain at higher levels than the presumed pre–B-cell population without surface μH chain expression.
Comparative expression analysis of the intracellular μH chain, intracellular VpreB, or cell surface μH chain by BM B-lineage cells.
The analysis compared a 5-year-old healthy child and an XLA patient (P3). The intracellular staining of BM cells for VpreB and the μH chain in combination with CD19 was performed as described in the legend of Figure 1. For the intracellular versus cell surface expression of the μH chain by BM B-lineage cells, viable BM cells were first treated with a PE-labeled antihuman μH chain Ab, fixed, permeabilized, and subsequently stained with a PC5-labeled anti-CD19 mAb and FITC-antihuman μH chain Ab. The open square denotes mature B cells that showed more intense expression of the μH chain than the presumed pre–B-cell population.
Comparative expression analysis of the intracellular μH chain, intracellular VpreB, or cell surface μH chain by BM B-lineage cells.
The analysis compared a 5-year-old healthy child and an XLA patient (P3). The intracellular staining of BM cells for VpreB and the μH chain in combination with CD19 was performed as described in the legend of Figure 1. For the intracellular versus cell surface expression of the μH chain by BM B-lineage cells, viable BM cells were first treated with a PE-labeled antihuman μH chain Ab, fixed, permeabilized, and subsequently stained with a PC5-labeled anti-CD19 mAb and FITC-antihuman μH chain Ab. The open square denotes mature B cells that showed more intense expression of the μH chain than the presumed pre–B-cell population.
Analysis of various stages of B-lineage cells in BM
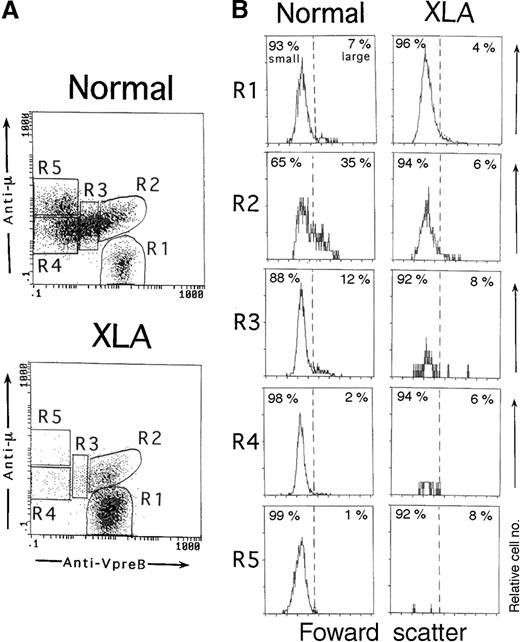
As described above, a cellular gating based on both CD19 and VpreB expression in the permeabilized cell samples helped identify the whole population of B-lineage cells in BM. Using this system, we next examined whether various stages of B-lineage cells in BM could be discriminated by the degree of VpreB and μH chain expression. Figure3A shows typical profiles of VpreB and μH chain expression by BM B-lineage cells in a control and an XLA patient. The μ− cells expressing VpreB intensely (μ−SL++) were considered to be pro-B cells (R1). The VpreB− cells expressing higher levels of the μH chain (μhighSL−) were assessed to contain mature B cells (R5), although some mature B cells overlapped with a small fraction of pre-B cells. The pre–B-cell population with the lower μH chain expression was distributed from the VpreB+ (R2) to VpreB− (R4) populations. We designated the VpreB+ pre–B-cell population (μlowSL+∼++) as pre-B1 cells (R2 and R3), and the VpreB− pre–B-cell population (μlowSL−) was designated as pre-B2 cells (R4). The VpreB+ pre-B cells were further divided into the 2 populations, namely, the cells with intense VpreB expression (μlowSL++, R2) and the ones with decreasing VpreB expression (μlowSL+, R3). We termed the former pre-B1a cells and the latter pre-B1b cells. This nomenclature was justified by the cell size analysis of each population in normal BM (Figure 3B). In normal BM, large cells were more enriched in pre-B1a cells as compared with pre-B1b cells (n = 7; 29.2% ± 5.3% versus 12.9% ± 4.0% [P < .001]). The other B-lineage cells were largely composed of small-sized cells. In XLA BM, the pre-B1a cells were identifiable, whereas the pre-B1b cells was markedly decreased (Figure 3A). It should be stressed that in contrast to normal BM, the pre-B1a cells in XLA BM were largely made up of small cells (n = 5; large cells, 7.0% ± 3.6%) (Figure 3B).
Three-color immunofluorescence analysis of different stages of B-lineage cells in BM.
BM cells from an 11-month-old healthy infant and an XLA patient (P6) were fixed, permeabilized, and then stained with PE-labeled anti-VpreB and PC5-labeled anti-CD19 mAbs in combination with an FITC-labeled antihuman μH chain Ab. B-lineage cells in BM lymphoid cells were gated by a combination of VpreB and CD19 expression, as described in Figure 1. (A) According to the degree of VpreB and μH chain expression, BM B-lineage cells were arbitrarily divided into 5 fractions: μ−SL++ (R1), μlowSL++ (R2), μlowSL+ (R3), μlowSL− (R4), and μhighSL− (R5) cells. R1, R2, R3, R4, and R5 fractions comprise 7.6%, 9.9%, 19.0%, 38.8%, and 24.6%, respectively, of the normal BM or 76.8%, 14.8%, 3.9%, 3.1%, and 1.0% of the XLA BM. (B) The cell size of each fraction of BM-lineage cells was assessed by forward scatter. In normal BM, large cells were more enriched in the R2 fraction. By contrast, all fractions were mainly composed of small cells in XLA BM.
Three-color immunofluorescence analysis of different stages of B-lineage cells in BM.
BM cells from an 11-month-old healthy infant and an XLA patient (P6) were fixed, permeabilized, and then stained with PE-labeled anti-VpreB and PC5-labeled anti-CD19 mAbs in combination with an FITC-labeled antihuman μH chain Ab. B-lineage cells in BM lymphoid cells were gated by a combination of VpreB and CD19 expression, as described in Figure 1. (A) According to the degree of VpreB and μH chain expression, BM B-lineage cells were arbitrarily divided into 5 fractions: μ−SL++ (R1), μlowSL++ (R2), μlowSL+ (R3), μlowSL− (R4), and μhighSL− (R5) cells. R1, R2, R3, R4, and R5 fractions comprise 7.6%, 9.9%, 19.0%, 38.8%, and 24.6%, respectively, of the normal BM or 76.8%, 14.8%, 3.9%, 3.1%, and 1.0% of the XLA BM. (B) The cell size of each fraction of BM-lineage cells was assessed by forward scatter. In normal BM, large cells were more enriched in the R2 fraction. By contrast, all fractions were mainly composed of small cells in XLA BM.
Analysis of pro-B, pre-B, and B cells in BM of XLA patients and controls
These observations indicated that there was maturation block in the B-cell differentiation in XLA patients. We accumulated data on BM samples, including B-lineage cells, from XLA patients and controls (Tables 2 and 3). The analysis of normal BM indicated that the relative frequencies of total B-lineage cells in BM lymphoid cells were higher in all ages of children and declined with advancing age. In XLA patients, the frequencies of total B-lineage cells in BM lymphoid cells were significantly lower compared with the frequencies in children. Each B-lineage cell in BM was similarly identified in infants, children, and adults, reflecting the function of BM as the provider of B cells after birth. It was noted that the percentages of pro-B cells within B-lineage cells in XLA BM were significantly higher than those in controls of all ages. Variable proportions of pre-B1a cells were identified in XLA BM, but their percentages were not so increased. The frequencies of pre-B1b, pre-B2, and B cells were extremely reduced in XLA BM.
B-lineage cells in BM samples of XLA patients
| Patient no. . | B-lineage cells in BM lymphoid cells, total % . | Percentage of cell types within B-lineage cells . | ||||||
|---|---|---|---|---|---|---|---|---|
| μ−SL++ (pro-B cells), % . | μlowSL++ (pre-B1a cells), % . | μlowSL+ (pre-B1b cells), % . | μlowSL− (pre-B2 cells), % . | μhighSL− (B cells), % . | ||||
| P1 | 26.2 | 88.7 | 7.2 | 3.0 | 0.2 | 0.8 | ||
| P2 | 21.0 | 62.3 | 21.2 | 7.2 | 6.7 | 2.3 | ||
| P3 | 27.8 | 72.8 | 20.5 | 2.8 | 1.1 | 2.7 | ||
| P4 | 41.5 | 77.8 | 15.4 | 4.1 | 1.8 | 0.1 | ||
| P5 | 9.8 | 56.2 | 20.0 | 9.0 | 7.7 | 4.9 | ||
| P6 | 18.5 | 76.8 | 14.8 | 3.9 | 3.1 | 1.0 | ||
| P7 | 29.1 | 66.2 | 17.5 | 8.2 | 5.7 | 1.8 | ||
| P8 | 19.3 | 71.2 | 15.0 | 5.0 | 6.6 | 1.1 | ||
| P9 | 18.9 | 67.3 | 20.2 | 6.7 | 2.7 | 1.7 | ||
| P10 | 14.3 | 73.2 | 10.0 | 5.2 | 5.1 | 6.4 | ||
| P11 | 11.0 | 82.6 | 11.3 | 3.6 | 1.7 | 0.2 | ||
| P12 | 10.4 | 91.6 | 5.0 | 2.0 | 0.1 | 0.7 | ||
| P13 | 8.9 | 80.0 | 10.6 | 2.5 | 0.9 | 0.7 | ||
| Patient no. . | B-lineage cells in BM lymphoid cells, total % . | Percentage of cell types within B-lineage cells . | ||||||
|---|---|---|---|---|---|---|---|---|
| μ−SL++ (pro-B cells), % . | μlowSL++ (pre-B1a cells), % . | μlowSL+ (pre-B1b cells), % . | μlowSL− (pre-B2 cells), % . | μhighSL− (B cells), % . | ||||
| P1 | 26.2 | 88.7 | 7.2 | 3.0 | 0.2 | 0.8 | ||
| P2 | 21.0 | 62.3 | 21.2 | 7.2 | 6.7 | 2.3 | ||
| P3 | 27.8 | 72.8 | 20.5 | 2.8 | 1.1 | 2.7 | ||
| P4 | 41.5 | 77.8 | 15.4 | 4.1 | 1.8 | 0.1 | ||
| P5 | 9.8 | 56.2 | 20.0 | 9.0 | 7.7 | 4.9 | ||
| P6 | 18.5 | 76.8 | 14.8 | 3.9 | 3.1 | 1.0 | ||
| P7 | 29.1 | 66.2 | 17.5 | 8.2 | 5.7 | 1.8 | ||
| P8 | 19.3 | 71.2 | 15.0 | 5.0 | 6.6 | 1.1 | ||
| P9 | 18.9 | 67.3 | 20.2 | 6.7 | 2.7 | 1.7 | ||
| P10 | 14.3 | 73.2 | 10.0 | 5.2 | 5.1 | 6.4 | ||
| P11 | 11.0 | 82.6 | 11.3 | 3.6 | 1.7 | 0.2 | ||
| P12 | 10.4 | 91.6 | 5.0 | 2.0 | 0.1 | 0.7 | ||
| P13 | 8.9 | 80.0 | 10.6 | 2.5 | 0.9 | 0.7 | ||
Different cell types within the B-lineage cells in BM samples were analyzed by 3-color fluorescence in flow cytometry, as shown in the legend of Figure 3. B-lineage cells were discriminated from other lymphoid cells by a combined assessment of CD19 and VpreB expression.
Percentage of B-lineage cells in BM samples of XLA patients and healthy controls of various ages
| Subjects . | B-lineage cells in BM lymphoid cells, total % . | Percentage of cell types within B-lineage cells . | ||||||
|---|---|---|---|---|---|---|---|---|
| μ−SL++ (pro-B cells), % . | μlowSL++ (pre-B1a cells), % . | μlowSL+ (pre-B1b cells), % . | μlowSL− (pre-B2 cells), % . | μhighSL− (B cells), % . | ||||
| XLA patients, n = 13 | 19.7 ± 9.54-150 | 74.4 ± 10.14-151 | 14.5 ± 5.3‡ | 4.9 ± 2.74-153 | 3.3 ± 2.74-155 | 1.9 ± 1.84-155 | ||
| Infants, n = 10 | 70.8 ± 12.6 | 8.7 ± 2.3 | 11.9 ± 3.0 | 19.7 ± 5.9 | 34.5 ± 4.7 | 24.0 ± 7.6 | ||
| Children, n = 10 | 50.7 ± 11.8 | 10.8 ± 6.7 | 10.9 ± 3.0 | 17.6 ± 4.6 | 29.5 ± 8.1 | 30.6 ± 15.2 | ||
| Adults, n = 5 | 20.3 ± 3.3 | 7.9 ± 3.1 | 4.4 ± 1.4 | 9.3 ± 4.8 | 28.1 ± 5.5 | 49.6 ± 9.4 | ||
| Subjects . | B-lineage cells in BM lymphoid cells, total % . | Percentage of cell types within B-lineage cells . | ||||||
|---|---|---|---|---|---|---|---|---|
| μ−SL++ (pro-B cells), % . | μlowSL++ (pre-B1a cells), % . | μlowSL+ (pre-B1b cells), % . | μlowSL− (pre-B2 cells), % . | μhighSL− (B cells), % . | ||||
| XLA patients, n = 13 | 19.7 ± 9.54-150 | 74.4 ± 10.14-151 | 14.5 ± 5.3‡ | 4.9 ± 2.74-153 | 3.3 ± 2.74-155 | 1.9 ± 1.84-155 | ||
| Infants, n = 10 | 70.8 ± 12.6 | 8.7 ± 2.3 | 11.9 ± 3.0 | 19.7 ± 5.9 | 34.5 ± 4.7 | 24.0 ± 7.6 | ||
| Children, n = 10 | 50.7 ± 11.8 | 10.8 ± 6.7 | 10.9 ± 3.0 | 17.6 ± 4.6 | 29.5 ± 8.1 | 30.6 ± 15.2 | ||
| Adults, n = 5 | 20.3 ± 3.3 | 7.9 ± 3.1 | 4.4 ± 1.4 | 9.3 ± 4.8 | 28.1 ± 5.5 | 49.6 ± 9.4 | ||
The percentages are given as the mean plus or minus SD. The B-lineage cells were assessed as described in the footnotes of Table 2.
Significantly reduced when compared with infants (P < .0001) and children (P < .0001), but not with adults.
Markedly increased when compared with infants (P < .0001), children (P < .0001), and adults (P < .0001).
Not significantly different from infants and children.
Significantly reduced when compared with infants (P < .0001), children (P < .0001), and adults (P < .03).
Extremely lower when compared with infants (P < .0001), children (P < .0001), and adults (P < .0001).
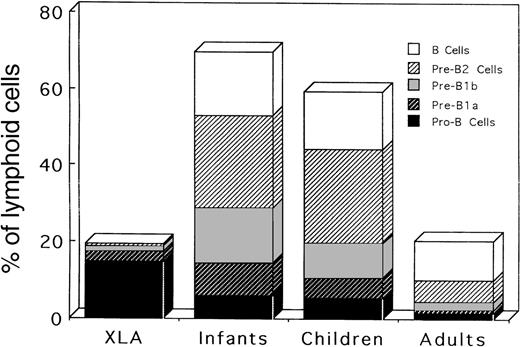
The relative frequencies of B-lineage cells among BM lymphoid cells must be more informative in elucidating the nature of B-cell defects in XLA patients, and the frequencies were further calculated from the results of Table 3 (Table4). Interestingly, age-dependent changes in the distribution of respective B-lineage cells in BM were seen in the controls. All stages of B-lineage cells, from pro-B cells to B cells, were present in abundant numbers in the BM of children of all ages, indicating the active B lymphopoiesis during these periods in human life. On the other hand, adult BM exhibited the predominance of B cells and pre-B2 cells, which is concomitant with a decrease in other earlier B-lineage cells. Characteristically, pro-B cells in XLA BM were markedly expanded compared with the controls of any ages, and the number of pre-B1a cells was reduced in XLA patients compared with the number in younger children. Importantly, this expansion of pro-B cells was also found in XLA patients in the adult period (P11-P13). However, adult controls exhibited lower frequencies of all BM lineage cells than children controls. Later stages of B-lineage cells (pre-B1b, pre-B2, and B cells) were negligible, although identifiable, in XLA patients. Figure4 depicts the mean percentages of respective B-lineage cells among BM lymphoid cells in XLA patients and controls. This figure clearly demonstrates an expansion of pro-B cells in XLA BM as well as age-dependent changes in BM B-lineage cells. The results suggest that the transition of pro-B cells beyond the earlier pre–B-cell stage to the later stage of pre-B cells might be severely impaired in XLA patients.
Frequencies of B-lineage cells among BM lymphoid cells in XLA patients and healthy controls of various ages
| Subjects . | μ−SL++ (pro-B cells), % . | Percentage of each B-lineage cell among BM lymphoid cells . | |||||
|---|---|---|---|---|---|---|---|
| μlowSL++ (pre-B1a cells), % . | μlowSL+ (pre-B1b cells), % . | μlowSL− (pre-B2 cells), % . | μhighSL− (B cells), % . | ||||
| XLA patients, n = 13 | 14.7 ± 7.43-150 | 3.0 ± 1.93-151 | 1.0 ± 0.63-152 | 0.6 ± 0.53-153 | 0.3 ± 0.33-153 | ||
| Infants, n = 10 | 6.1 ± 1.0 | 8.4 ± 2.5 | 14.3 ± 6.4 | 24.3 ± 4.7 | 16.7 ± 5.4 | ||
| Children, n = 10 | 5.2 ± 2.5 | 5.6 ± 1.8 | 9.1 ± 3.5 | 15.6 ± 7.1 | 15.1 ± 5.9 | ||
| Adults, n = 5 | 1.7 ± 0.6 | 0.9 ± 0.3 | 1.8 ± 1.1 | 5.6 ± 1.2 | 10.3 ± 3.6 | ||
| Subjects . | μ−SL++ (pro-B cells), % . | Percentage of each B-lineage cell among BM lymphoid cells . | |||||
|---|---|---|---|---|---|---|---|
| μlowSL++ (pre-B1a cells), % . | μlowSL+ (pre-B1b cells), % . | μlowSL− (pre-B2 cells), % . | μhighSL− (B cells), % . | ||||
| XLA patients, n = 13 | 14.7 ± 7.43-150 | 3.0 ± 1.93-151 | 1.0 ± 0.63-152 | 0.6 ± 0.53-153 | 0.3 ± 0.33-153 | ||
| Infants, n = 10 | 6.1 ± 1.0 | 8.4 ± 2.5 | 14.3 ± 6.4 | 24.3 ± 4.7 | 16.7 ± 5.4 | ||
| Children, n = 10 | 5.2 ± 2.5 | 5.6 ± 1.8 | 9.1 ± 3.5 | 15.6 ± 7.1 | 15.1 ± 5.9 | ||
| Adults, n = 5 | 1.7 ± 0.6 | 0.9 ± 0.3 | 1.8 ± 1.1 | 5.6 ± 1.2 | 10.3 ± 3.6 | ||
The frequencies are given as the mean plus or minus SD. Based on the data in Table 3, the relative frequencies of individual B-lineage cells among BM lymphoid cells were calculated as follows: their percentages within the total B-lineage cells multiplied by the percentages of total B-lineage cells in BM lymphoid cells divided by 100.
Significantly increased when compared with infants (P < .01), children (P < .01), and adults (P < .002).
Significantly reduced when compared with infants (P < .0001) and children (P < .01).
Significantly reduced when compared with infants (P < .0001), children (P < .0001), and adults (P < .05).
Significantly reduced when compared with infants (P < .0001), children (P < .0001), and adults (P < .0001).
Relative frequencies of different stages of B-lineage cells among BM lymphoid cells in XLA patients and in infant, children, and adult controls.
The mean values shown in Table 4 are figured.
Relative frequencies of different stages of B-lineage cells among BM lymphoid cells in XLA patients and in infant, children, and adult controls.
The mean values shown in Table 4 are figured.
Discussion
Many studies in mice and humans have examined how B cells are committed from hematopoietic stem cells and generated through discrete developmental stages that are defined by sequential Ig gene rearrangements and intracellular or cell surface expression of various B-cell–related molecules. However, the phenotypic criteria and nomenclatures for distinguishing the different stages of B-cell development, especially in humans, remain controversial.42Besides B cell lines, B-cell neoplasmas, and normal BM, the genetic deficit of B-cell development would provide some complementary information in the understanding of human B-cell development.
In this study, we attempted to subcharacterize the B-cell lineage cells in human BM using VpreB, a component of the SL chains, which seemed a suitable marker to specifically delineate early B-cell affiliation. The cell surface expression of the SL chains is usually expressed at very low levels and seen, even if detectable, only in a small proportion of BM B-lineage cells. The chains are intracellularly synthesized in plentiful amounts in early B-lineage cells before the cell surface expression of the μH chain appears.8,9,21,22 Thus, we investigated the distribution of different stages of BM B-lineage cells defined by VpreB expression in the permeabilized BM cell samples. We disclosed that a combination of VpreB and CD19 expression could identify the presumably whole population of B-lineage cells in BM. Consistent with previous observations,43 mature B cells were found to express the μH chain at higher levels than the pre–B-cell population did. We also observed that these presumed mature B cells with enhanced levels of μH chain, but not other BM B-lineage cells, expressed L chains (data not shown). Although a small fraction of mature B cells was, to some extent, overlapped with pre-B cells, the density of the μH chain expression appeared to discriminate between both populations, even in permeabilized BM cells. The flow cytometric analysis of BM samples from controls of different ages and from XLA patients revealed that the B-lineage cells in BM were divided into several populations at discrete stages. The earliest B-lineage cells, termed pro-B cells, which were seemingly TdT+, expressed relatively high levels of VpreB and lacked the μH chain expression.
Some other cell surface antigens, such as CD34 and CD10, have been used to identify the pro-B cells in BM.7,19,35-39 Our preliminary observations indicated that the putative pro-B cells in XLA BM appeared to express CD34 as well CD10 relatively intensely, whereas normal BM pro-B cells showed variable expression of both antigens. The combined use of intracellular VpreB with the cytoplasmic μH chain might be useful in identifying the pro–B-cell population in BM. According to the classical criteria,2 the pre-B cells were defined here as the cells containing the cytoplasmic μH chain. The flow cytometric analysis showed that the expression of VpreB in the pre–B-cell population tended to decline with maturation. We demonstrated that pre-B cells without VpreB production were present in BM. These pre-B cells lacking VpreB expression have been identified in murine BM.8 We distinguished the pre–B-cell population with VpreB expression, named pre-B1 cells, from the pre-B2 cells without VpreB expression. It seemed that pre-B1 cells could further be divided into 2 subpopulations, termed pre-B1a or pre-B1b cells with VpreB expression comparable to or lower than that seen in pro-B cells. The cell size analysis showed that pre-B1a cells were more enriched with large cells as compared to pre-B1b cells, indicating the active cycling state in the former cells.
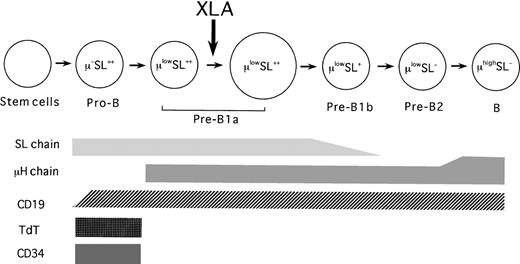
Although the synthesis of the L chains associated with L chain gene rearrangement is generally believed to begin beyond the stage of pre-B cells,42 Guelpa-Fonlupt et al10 have proposed that the L chains can be coexpressed on a minor population of μ+VpreB+ B-lineage cells in BM, possibly at the later stage of pre-B cells. However, our flow cytometric evaluation revealed that the expression of the L chains and VpreB seemed to occur independently in BM B-lineage cells (data not shown). This discrepancy may be partially due to a difference of antibodies used. A tentative model of the human B-cell differentiation pathway is depicted in Figure5.
Tentative model of the B-cell differentiation pathway in healthy human BM and the B-cell defect in XLA BM.
The arrow indicates the major blockage in XLA patients.
Tentative model of the B-cell differentiation pathway in healthy human BM and the B-cell defect in XLA BM.
The arrow indicates the major blockage in XLA patients.
Despite recent availability of early B-cell specific markers, the ontogenic features of B lymphopoiesis in human BM remains to be precisely evaluated. Nuñez et al43 have used CD19 as the pan–B-cell marker, in addition to TdT and the μH chain, to examine the frequencies of pro-B, pre-B, and B cells in fetal tissues and BM samples from controls. They have elegantly demonstrated that BM in humans functions as the major site of B-cell generation after birth.43 They have also described that the relative frequencies of the B-lineage cells in BM decline with advancing age; mature B cells predominate in adult BM, resulting from an age-dependent reduction in the ratio of B precursors to mature B cells.43 Such an age-dependent decrease in total BM B-lineage cells, with the increasing frequencies of mature B cells, has also been observed by other investigators.20 Similarly, we demonstrated that B lymphopoiesis was more active in young children than in adults. We found that B precursors (or pro-B cells) identified as μ−SL++ cells were obviously reduced in frequency in adult BM. Such information about the age-related changes in the compartment of BM B-lineage cells would be very helpful in evaluating the specific block of B-cell differentiation in primary B-cell defect conditions.
There is now increasing evidence that Btk participates in signal transduction after B-cell receptor-mediated activation of mature B cells.44 But the issue of how the genetic defect in XLA affects early B-cell development is somewhat confusing. Earlier studies have used fluorescence microscopy to examine BM pre-B cells containing the cytoplasmic μH chain in a gammaglobulinemic males with presumed XLA. Pearl et al33described XLA patients as having a normal frequency of pre-B cells in BM and speculated that the pre–B-cell to B-cell transition might be impaired in XLA. In contrast, later studies demonstrated that there is more heterogeneity in the number of pre-B cells in XLA BM than earlier recognized.34,35 While pre-B cells are more or less detectable in XLA BM, it has been shown that the majority of XLA patients have a substantial number of pro-B cells in BM, resulting in an increased ratio of pro-B to pre-B cells.19,35-39 Conley et al45 have proposed that the defect in XLA might not be limited to the pre–B-cell to B-cell transition, and instead, the defect might interfere with multiple stages of B-cell differentiation. Mutations in genes other than Btk result in a clinical phenotype resembling XLA.18,19,37-39,46 Although approximately 90% of males with presumed XLA have mutations in theBtk gene,47 it is likely that a heterogeneity in XLA BM might be partly due to the inclusion of some non-XLA cases in previous studies.
In this study, we enrolled the XLA patients, all of whom had identified Btk mutations, to analyze the B-lineage cells in XLA BM. The flow cytometric analysis disclosed that while the frequencies of total B-lineage cells were appreciably decreased, pro-B cells were markedly expanded in XLA BM, regardless of the present age. We detected variable numbers of pre-B cells in XLA BM. It should be noted that the pre-B cells seen in XLA BM were largely composed of the earliest pre-B population, namely, pre-B1a cells. In contrast to the controls, pre-B1a cells in XLA BM were constituted with small cells. Similarly, previous studies have observed that pre-B cells identified in XLA patients were mainly nonproliferating small cells.35 The important point was that the relative frequencies of pre-B1a cells in XLA BM were significantly lower than those seen in normal BM. The later stages of B-lineage cells, including pre-B1b as well as pre-B2 cells, were markedly reduced in XLA BM. As depicted in Figure 5, these results suggest that the genetic defect in XLA might interrupt the proliferation and survival of pre-B1a cells, resulting in an expansion of pro-B cells with a few pre-B1a cells in BM.
A leaky generation of mature B cells is often seen in most XLA patients. Supporting this fact, the Epstein-Barr virus (EBV) is capable of rescuing these leaked B cells.48-51 While most of the EBV-transformable B cells from XLA patients produce IgM, Anker et al50 revealed that an EBV infection could establish B cell lines expressing all Ig isotypes in XLA. Nonoyama et al52demonstrated that leaky B cells in XLA have the capability to undergo the final differentiation into the cells producing all Ig subclasses in vivo as well as in vitro. In addition, some XLA cases have been shown to exhibit a mild phenotype, which is manifested with delayed disease recognition, nonlethal bacterial infection, and higher than expected concentrations of serum Ig.32,53,54 In contrast to typical XLA, the murine Btk mutations, as seen in Btk-null as well as Xid mice, caused the milder phenotype, which exhibited a generation of near normal numbers of B cells with substantial Ig levels in the serum.55-57 Although unable to make antibodies to T-independent antigens, mice with Btk mutations respond to the specified T-dependent antigens.67–69 Kerner et al57 demonstrated the existence of an impaired expansion of pro-B cells in Btk-deficient mice. They proposed that the role of Btk in early B-cell development is the same in humans and mice, and the consequences of Btk deficiency differ only qualitatively between both groups. It is tempting to suppose that B-cell differentiation may occur in Btk-dependent andBtk-independent manners, and the largest proportion of B-cell generation in humans, unlike mice, may depend on the molecular function of Btk.
In conclusion, the results presented in this study have delineated both the nature of the B-cell defect caused by a Btk mutation as well as the human B-cell differentiation pathway. It appears thatBtk mutations in XLA impede the maturational evolution of pro-B cells beyond the earlier pre–B-cell stage to the later stage of pre-B cells, resulting in a marked reduction in mature B cells. The major B-cell defect in XLA may exit at the transition of pro-B cells to earlier pre-B cells. This is verified by observations that there is a random inactivation of the X chromosome among pro-B cells in female XLA carriers, whereas the carriers usually exhibit skewing of X inactivation in peripheral B cells.58 Given that the pre–B-cell receptor is essentially involved in proliferation and survival of early B precursors, there is a possibility that Btk may contribute to intracellular signaling downstream of the pre–B-cell receptor. This intriguing point will be clarified by further studies using pro–B cell lines derived from XLA BM.
Acknowledgments
We thank all the patients and families for their generous cooperation in this study, Drs Yuichi Adachi and Shoichi Koizumi for helpful discussion, and Chikako Sakai and Hitoshi Moriuchi for technical assistance.
Supported by Grant-in-Aid for Scientific Research 10470176 from the Ministry of Education, Science and Culture of Japan, Japan; a grant from the Ministry of Health and Welfare of Japan, Japan; and a grant from the Uehara Foundation, Tokyo, Japan.
Reprints:Toshio Miyawaki, Department of Pediatrics, Faculty of Medicine, Toyama Medical and Pharmaceutical University, 2630 Sugitani, Toyama 930-0194, Japan; e-mail:toshio65@ms.toyama-mpu.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal