Abstract
During acute inflammatory processes, β2 and β1 integrins sequentially mediate leukocyte recruitment into extravascular tissues. We studied the influence of VLA-4 (very late antigen-4) (4β1) engagement on β2 integrin activation-dependent cell-to-cell adhesion. Ligation of VLA-4 by the soluble chimera fusion product vascular cell adhesion molecule-1 (VCAM-1)–Fc or by 2 anti-CD29 (β1 chain) monoclonal antibodies (mAb) rapidly induced adhesion of myelomonocytic cells (HL60, U937) to human umbilical vein endothelial cells (HUVECs). Cell adhesion was mediated via β2 integrin (LFA-1 and Mac-1) activation: induced adhesion to HUVECs was inhibited by blocking mAbs anti-CD18 (70%-90%), anti-CD11a (50%-60%), or anti-CD11b (60%-70%). Adhesion to immobilized ligands of β2 integrins (intercellular adhesion molecule-1 [ICAM-1], fibrinogen, keyhole limpet hemocyanin) as well as to ICAM-1–transfected Chinese hamster ovary cells, but not to ligands of β1 integrins (VCAM-1, fibronectin, laminin, and collagen), was augmented. VCAM-1–Fc binding provoked the expression of the activation-dependent epitope CBRM1/5 of Mac-1 on leukocytes. Clustering of VLA-4 through dimeric VCAM-1–Fc was required for β2 integrin activation and induction of cell adhesion, whereas monovalent VCAM-1 or Fab fragments of anti-β1 integrin mAb were ineffective. Activation of β2 integrins by 4β1 integrin ligation (VCAM-1–Fc or anti-β1 mAb) required the presence of urokinase receptor (uPAR) on leukocytic cells, because the removal of uPAR from the cell surface by phosphatidylinositol-specific phospholipase C reduced cell adhesion to less than 40%. Adhesion was reconstituted when soluble recombinant uPAR was allowed to reassociate with the cells. Finally, VLA-4 engagement by VCAM-1–Fc or anti-β1 integrin mAb induced uPAR-dependent adhesion to immobilized vitronectin as well. These results elucidate a novel activation pathway of β2 integrin–dependent cell-to-cell adhesion that requires 4β1 integrin ligation for initiation and uPAR as activation transducer.
Leukocyte recruitment is a key step in the pathogenesis of inflammatory diseases such as atherosclerosis.1,2 It is a highly coordinated multistep process that requires activation of different adhesion receptors in a cascade-like fashion.3 The families of selectins and integrins subsequently become activated to allow adhesion of leukocytes to endothelium followed by transmigration into the vessel wall. The integrin family of adhesion receptors is critically involved in this process by mediating cell-to-cell and cell-to-extracellular matrix contacts of various strength and duration.4 Integrins are heterodimeric transmembranous receptors that do not only function as adhesive proteins but also can transduce cellular signals in a bidirectional manner4-6: Membrane-bound nonintegrin components such as integrin-associated protein (IAP/CD47) or the glycolipid-anchored molecules CD14 as well as urokinase receptor (CD87) appear to be required for inside-out and outside-in signaling.7-9 In particular, the integrin VLA-4 (very late antigen-4) (α4β1) has previously been shown to transduce several intracellular activation signals via tyrosine phosphorylation of intracellular kinases resulting in nuclear translocation of nuclear factor κB as well as tissue factor translocation to the cell surface.10 11
VLA-4 engagement occurs at several stages of cell-to-cell contacts between various cell types: apart from the selectin family of adhesion receptors, VLA-4 is engaged in tethering of monocytes, lymphocytes and, potentially, neutrophils to the vessel wall by binding vascular cell adhesion molecule-1 (VCAM-1) on vascular endothelium.12-15 In addition, VLA-4–VCAM-1 contacts may occur when leukocytes migrate along or through smooth muscle cell layers, which highly express VCAM-1 when activated during atherogenesis.16-18 Within the blood stream, soluble VCAM-1, which is elevated under inflammatory conditions such as atherosclerosis,19 myocardial infarction,20 or stroke,21 may bind to elevated VLA-422 on leukocytes. Yet, consequences of VLA-4 engagement for adhesive events have not been reported.
In this study, we sought to investigate the hypothesis that engagement of VLA-4 on monocytic cells by its natural ligand VCAM-1 or by anti-β1 integrin monoclonal antibody (mAb) can trigger enhanced adhesiveness via β2 integrins. We provide evidence that the 2 major β2 integrins LFA-1 (leukocyte function–associated-1) and Mac-1 become activated in response to VLA-4 engagement and contribute equally to the induced adhesiveness of leukocytic cells to the endothelium. In addition, we demonstrate that the urokinase receptor directly becomes involved upon VLA-4 engagement and controls the transdominant activation between VLA-4 and β2 integrins.
Materials and methods
Reagents
Phorbol 12-myristate 13-acetate (PMA) was from Gibco (Paisley, Scotland, UK). Fibrinogen, fibronectin, laminin, collagen, and keyhole limpet hemocyanin (KLH) were from Sigma (Munich, Germany). Vitronectin was purified from human plasma as described.23 Soluble human recombinant intercellular adhesion molecule-1 (ICAM-1) was kindly provided by Dr Carl Figdor (Nijmegen, The Netherlands); soluble human recombinant VCAM-1 was from R & D Systems (Wiesbaden, Germany). Human recombinant VCAM-1–Fc was kindly provided by Dr Dietmar Seiffge (Hoechst, Frankfurt, Germany). Recombinant phosphatidylinositol-specific phospholipase C was from Oxford Glyco-Systems (Abingdon, UK). Recombinant soluble urokinase receptor (uPAR) was produced as described24,25and was generously provided by Dr Niels Behrendt (Finsen Laboratory, Copenhagen, Denmark). Recombinant soluble tissue factor pathway inhibitor was from American Diagnostica (Pfungstadt, Germany). Mouse antihuman CD29 (β1 integrin chain) mAbs K20 and LIA1/2 as well as mAb antihuman CD49d (α4 chain, HP2.1) were from Immunotech (Hamburg, Germany); HP2.1 was described to block cell-to-cell adhesion via VLA-4–VCAM-1 interactions. F(ab) fragments of mAb K20 were generated by digestion with immobilized papain followed by protein A–Sepharose affinity chromatography (Pierce, Rockford, IL), and the purity was confirmed by polyacrylamide gel electrophoretic analysis. Cross-linking of F(ab) fragments of mAb K20 was achieved using affinipure F(ab)2 fragment goat antimouse immunoglobulin G (IgG; Dianova, Hamburg, Germany). KIM185 is a mAb that binds and directly activates the common β2 integrin chain CD1826 and was kindly provided by Dr Marc Robinson (Celltech Ltd, Slough, England). Mouse antihuman β2-chain (CD18) mAb 60.3 (IgG2a-type) blocks β2 integrin–mediated leukocyte adhesion to endothelium27 and was generously provided by Dr John Harlan (Seattle, WA). Murine antihuman LFA-1 (anti-CD11a) L15 was kindly provided by Dr Carl Figdor. Monoclonal antibody CBRM1/5, which recognizes only the activated epitope of CD11b and blocks Mac-1–dependent adhesion,28 was generously provided by Dr Timothy Springer (Boston, MA). Blocking mAb anti-αvβ3 (LM609) was from Chemicon (Temecola, CA). Murine antihuman IgG2a (Sigma) was used as isotype-matched control antibody.
Cells
Human myeloid HL60 and U937 cell lines (German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany) were cultured in RPMI-1640 supplemented with 10% (vol/vol) fetal calf serum, 1% sodium pyruvate, 1-mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (all from Gibco). Twenty-four hours prior to the experiments, monocytic differentiation was induced by addition of 50-ng/mL 1α,25-dihydroxyvitamin D3 and 1 ng/mL transforming growth factor-β1 (Biomol, Hamburg, Germany). Peripheral blood polymorphonuclear neutrophils were isolated by discontinuous density gradient centrifugation using Histopaque-1119 and -1077 (Sigma) as described by the manufacturer. An enrichment of at least 95% neutrophils was obtained as controlled by flow cytometry using forward and side scatter analysis and staining for CD15. Human umbilical vein endothelial cells (HUVECs) were isolated as described29 and cultured (for 2 to 4 passages) in low serum endothelial cell growth medium (PromoCell, Heidelberg, Germany) on gelatin-coated tissue culture plastic. Chinese hamster ovary (CHO) cells transfected with ICAM-1 were purchased from American Type Culture Collection (Rockville, MD) and cultured in RPMI-1640 with 10% fetal calf serum.
Adhesion assays
Cell-to-cell adhesion.
HUVECs or ICAM-1–transfected CHO (CHO–ICAM-1) cells were seeded onto gelatin-coated 48-well plates (Costar, Badhoevedorp, The Netherlands) 48 hours prior to the experiment. Confluency was confirmed by microscopic inspection before each experiment. HL60 or U937 cells were differentiated to monocytic cells (see “Cells”) for 24 hours. These monocytic cells or freshly isolated neutrophils were washed twice in adhesion medium (serum-free RPMI-1640/HEPES 25 mM), followed by various pretreatments (described in figure legends) and mixed with the chromophore BCECF-am (Molecular Probes, Eugene, OR). After washing, they were added (7 × 105/mL adhesion medium) to the prewashed HUVECs or CHO–ICAM-1 monolayers in the absence or presence of blocking mAb. After 30 minutes of coincubation (37°C, 5% CO2, 90% humidity), the plates were gently washed twice with adhesion buffer to remove nonadherent cells. Remaining adherent cells were lysed with 1-mol/L NaOH and quantified in a fluorescence plate reader (TEKAN, Crailsheim, Germany). At least triplicate wells were run per test substance, and results are expressed as mean values ± SEM; experiments were repeated at least twice.
Cell adhesion to immobilized integrin ligands.
Ninety-six–well plates were coated with human fibrinogen, fibronectin, laminin, type I/III collagen, vitronectin (each 20 μg/mL), KLH (100 μg/mL), soluble recombinant ICAM-1 or VCAM-1 (20 μg/mL), respectively, for 2 hours at 37°C and blocked with 1% (wt/vol) bovine serum albumin for 30 minutes at 25°C. Pretreated leukocytes were seeded at 70 000 cells/well in the absence or presence of blocking mAb for 30 minutes. After removal of nonadherent cells by 2 washing steps, adhesion was quantified by peroxidase reaction using p-nitrophenol as a substrate in an enzyme-linked immunosorbent assay reader (Bio-Rad, Munich, Germany).
Flow cytometry
Cells (2.5 × 105) were washed twice with HEPES-buffered saline and incubated with fluorescein-conjugated mAb (as specified in “Results”) at a dilution of 1:50. Mean fluorescence of 5000 cells was measured in a flow cytometer (Becton Dickinson, Heidelberg, Germany). Nonspecific fluorescence was determined using an isotype-matched mouse IgG.
Statistical analysis
Comparisons between group means were performed using ANOVA. Data represent mean ± SEM. A value of P < .05 was regarded as significant.
Results
VLA-4 engagement by VCAM-1–Fc or anti-β1 integrin mAb induces leukocyte adhesion to human endothelial cells
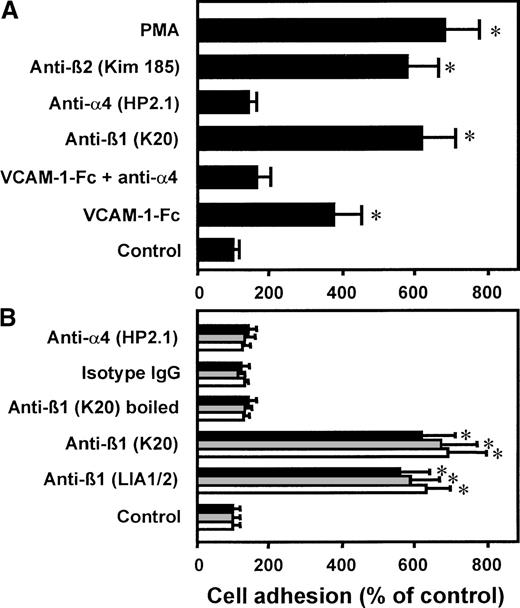
Preincubation of the myelomonocytic cell line HL60 with an antibody (mAb K20) directed against the β1 integrin chain of VLA-4 or with human soluble recombinant VCAM-1–Fc, respectively, resulted in strong cell adhesion to HUVECs (Figure 1A). A similar maximal level of adhesion was reached with PMA or with the known β2 integrin–activating mAb KIM185. Control isotype-matched mAb IgG2a as well as an antibody directed against the α chain of VLA-4 did not induce leukocyte adherence. The specificity of VCAM-1–Fc binding to VLA-4 was confirmed by competition with mAb anti–VLA-4 (clone HP2.1), which abrogated cell adhesion induced by VCAM-1–Fc. When β1 integrins were ligated by the same procedure on the myelomonocytic cell line U937, HL60, or isolated neutrophils, comparable cell-to-cell adhesion was achieved (Figure 1B). Preincubation with mAb anti-β1 LIA1/2 had a similar proadhesive effect as observed with mAb K20. Boiling of mAb K20 prior to incubation with the cells totally abrogated its stimulatory capacity. Because all reagents were found to be endotoxin-free, this excludes the possibility that bacterial contamination may be responsible for cellular activation. Isolated neutrophils, which do not express VLA-4, did not respond to VCAM-1–Fc incubation (not shown).
Effect of VCAM-1–Fc and anti-β1 integrin mAbs on leukocyte adhesion to endothelium.
(A) Myelomonocytic HL60 cells were preincubated with medium alone (control), VCAM-1–Fc (40 μg/mL) with or without mAb anti–VLA-4 (α4 chain, HP2.1, 20 μg/mL), mAbs anti-β1integrin (K20), anti-α4 integrin (HP2.1), or the β2 integrin–activating mAb KIM185 (20 μg/mL each) or PMA (10 ng/mL) for 30 minutes at 37°C. After washing, adhesion to endothelial cell monolayers was performed. (B) HL60 cells (filled bars), U937 cells (gray bars), or isolated neutrophils (open bars) were preincubated with medium alone, VCAM-1–Fc (40 μg/mL), mAbs anti-β1 integrin LIA1/2 or K20, boiled mAb K20, isotype-matched irrelevant IgG2a, or anti-α4 integrin mAb HP2.1 for 30 minutes at 37°C. After washing, adhesion to endothelial cell monolayers was performed. Values are displayed as percentage of control and represent the mean ± SEM of at least 3 independent experiments. *Indicates P < .01 compared with control.
Effect of VCAM-1–Fc and anti-β1 integrin mAbs on leukocyte adhesion to endothelium.
(A) Myelomonocytic HL60 cells were preincubated with medium alone (control), VCAM-1–Fc (40 μg/mL) with or without mAb anti–VLA-4 (α4 chain, HP2.1, 20 μg/mL), mAbs anti-β1integrin (K20), anti-α4 integrin (HP2.1), or the β2 integrin–activating mAb KIM185 (20 μg/mL each) or PMA (10 ng/mL) for 30 minutes at 37°C. After washing, adhesion to endothelial cell monolayers was performed. (B) HL60 cells (filled bars), U937 cells (gray bars), or isolated neutrophils (open bars) were preincubated with medium alone, VCAM-1–Fc (40 μg/mL), mAbs anti-β1 integrin LIA1/2 or K20, boiled mAb K20, isotype-matched irrelevant IgG2a, or anti-α4 integrin mAb HP2.1 for 30 minutes at 37°C. After washing, adhesion to endothelial cell monolayers was performed. Values are displayed as percentage of control and represent the mean ± SEM of at least 3 independent experiments. *Indicates P < .01 compared with control.
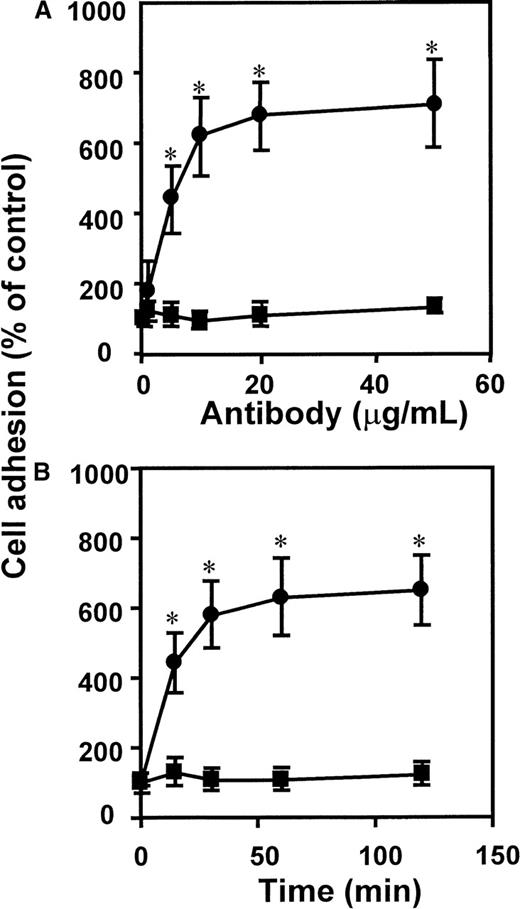
The dose- and time-dependent response to antibody ligation demonstrated that adhesion was rapidly induced by lowconcentrations of mAb K20, reaching a maximum after 30 to 60 minutes (Figure2). Thus, engagement of VLA-4 seems to serve as a fast and effective stimulus of leukocyte adhesion.
Kinetics of mAb anti-CD29–induced leukocyte adhesion to endothelium.
The mAb K20 (circles) or isotype control mAb IgG2a (squares) were added to myelomonocytic HL60 cells (A) at different concentrations as indicated for 30 minutes at 37°C, or (B) for different time intervals as indicated at a concentration of 10 μg/mL. Following washing, adhesion to endothelial cell monolayers was performed as described. Values (mean ± SEM) are displayed as percentage of control (no antibody added). One representative experiment (of 3) is shown. *Indicates P < .01 compared with control (no mAb added).
Kinetics of mAb anti-CD29–induced leukocyte adhesion to endothelium.
The mAb K20 (circles) or isotype control mAb IgG2a (squares) were added to myelomonocytic HL60 cells (A) at different concentrations as indicated for 30 minutes at 37°C, or (B) for different time intervals as indicated at a concentration of 10 μg/mL. Following washing, adhesion to endothelial cell monolayers was performed as described. Values (mean ± SEM) are displayed as percentage of control (no antibody added). One representative experiment (of 3) is shown. *Indicates P < .01 compared with control (no mAb added).
VLA-4–induced adhesion requires integrin clustering
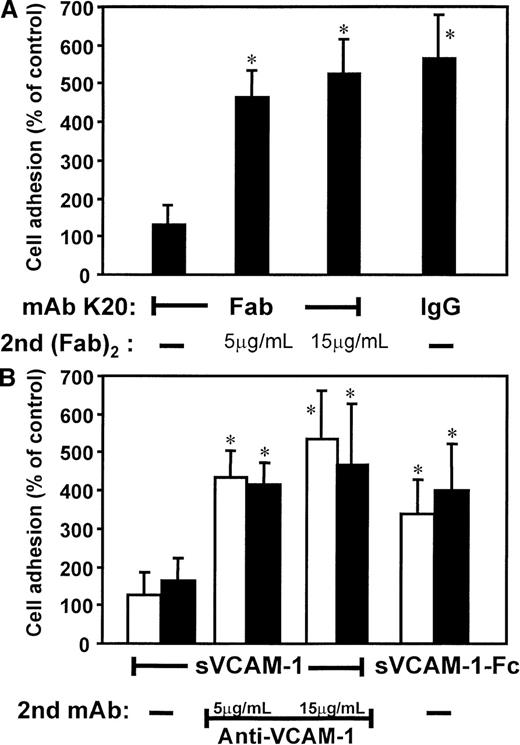
Based on these findings, we studied the proadhesive effects of ligation versus clustering of VLA-4 (Figure3). Isolated, monovalent Fab fragments of mAb K20 alone (in contrast to the intact mAb) did not induce adhesion. However, when these monovalent fragments were cross-linked by goat (Fab)2 antimouse Fab, strong adhesion was induced (Figure3A). Secondary (Fab)2 antimouse Fab fragments alone (without the primary mAb K20) did not affect cell adhesion (not shown). Likewise, preincubation with soluble recombinant monomeric VCAM-1 did not affect adhesion of otherwise untreated cells, whereas secondary clustering by mAb anti–VCAM-1 resulted in a 5-fold increase of cell-to-cell adhesion (Figure 3B). Consistently, the bivalent human soluble recombinant VCAM-1–Fc induced a 4-fold increase of monocytic cell adhesion to HUVECs. Thus, cross-linking of VLA-4 accounted for enhanced cell adhesion.
Effects of clustering versus ligation of VLA-4 on HL60 cell adhesion to HUVECs.
(A) Myelomonocytic HL60 cells were preincubated with medium alone (control, not shown), isolated Fab fragments of mAb K20 (5 μg/mL), or mAb K20 (20 μg/mL) for 30 minutes at 37°C. Cells were washed, and cells preincubated with K20 Fab were further incubated with increasing concentrations of a secondary goat (Fab)2 antimouse Fab for 30 minutes at 37°C. After washing, adhesion to endothelial cell monolayers was performed. (B) HL60 cells were preincubated (20 minutes, 37°C) with medium (open bars) or human IgG (20 μg/mL, filled bars) to saturate Fc receptors. Medium alone (control, not shown), soluble VCAM-1 (sVCAM-1; 40 μg/mL), or soluble VCAM-1–Fc (sVCAM-1–Fc; 40 μg/mL) was added for 30 minutes at 37°C. Cells were washed, and cells preincubated with soluble VCAM-1 were further incubated with increasing concentrations of mAb anti–VCAM-1 for 30 minutes at 37°C. Following washing, adhesion to endothelial cell monolayers was performed. Values are displayed as percentage of control and represent the mean ± SEM of at least 3 independent experiments. *Indicates P < .01 compared with the respective control.
Effects of clustering versus ligation of VLA-4 on HL60 cell adhesion to HUVECs.
(A) Myelomonocytic HL60 cells were preincubated with medium alone (control, not shown), isolated Fab fragments of mAb K20 (5 μg/mL), or mAb K20 (20 μg/mL) for 30 minutes at 37°C. Cells were washed, and cells preincubated with K20 Fab were further incubated with increasing concentrations of a secondary goat (Fab)2 antimouse Fab for 30 minutes at 37°C. After washing, adhesion to endothelial cell monolayers was performed. (B) HL60 cells were preincubated (20 minutes, 37°C) with medium (open bars) or human IgG (20 μg/mL, filled bars) to saturate Fc receptors. Medium alone (control, not shown), soluble VCAM-1 (sVCAM-1; 40 μg/mL), or soluble VCAM-1–Fc (sVCAM-1–Fc; 40 μg/mL) was added for 30 minutes at 37°C. Cells were washed, and cells preincubated with soluble VCAM-1 were further incubated with increasing concentrations of mAb anti–VCAM-1 for 30 minutes at 37°C. Following washing, adhesion to endothelial cell monolayers was performed. Values are displayed as percentage of control and represent the mean ± SEM of at least 3 independent experiments. *Indicates P < .01 compared with the respective control.
Possible involvement of Fc receptors in these activation pathways was excluded: (a) cross-linking of Fab fragments of mAb K20 was performed by antimouse (Fab)2 in the absence of any Fc fragments; (b) saturation of Fc receptors by human IgG (Figure 3B) or mAb anti-CD16 and anti-CD32 (not shown) prior to the experiment did not affect the results.
VLA-4 clustering induces adhesion via activated β2integrins LFA-1 and Mac-1
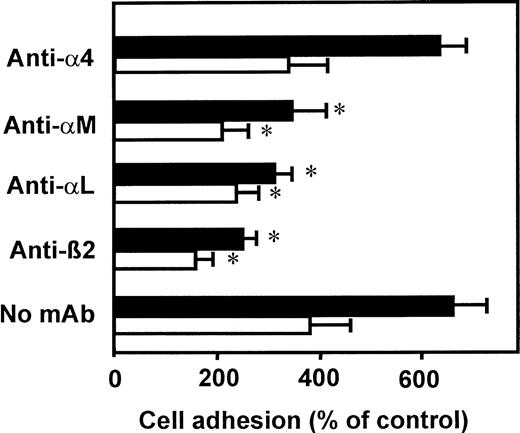
To identify the adhesion receptors that become activated upon VLA-4 clustering, we investigated cell adhesion to HUVECs in the presence of specific blocking mAbs (Figure 4). Leukocyte adhesion induced by both VCAM-1–Fc and by mAb K20 was abrogated in the presence of the blocking mAb 60.3, directed against the common β2 integrin chain. In addition, mAbs directed against the respective α chain of LFA-1 (αLβ2) or Mac-1 (αMβ2) significantly inhibited adhesion to a similar extent. Thus, both LFA-1 and Mac-1 seem to become activated upon engagement of VLA-4 and appear to contribute equally to enhanced leukocyte adhesion to endothelial cells. An antibody directed against the α4 chain of VLA-4, known to effectively block VLA-4 binding to VCAM-1, did not affect cell adhesion in response to mAb K20 or VCAM-1–Fc. Similar results were obtained when myelomonocyte cell adhesion to ICAM-1–transfected CHO cells was studied after VLA-4 engagement (5-fold and 3.5-fold increase in response to adhesion of mAb K20 and VCAM-1–Fc, respectively).
VLA-4–induced adhesion is mediated by β2integrins LFA-1 and Mac-1.
Myelomonocytic HL60 cells were pretreated with medium (control, not shown), soluble VCAM-1–Fc (40 μg/mL, open bars), or mAb K20 (20 μg/mL, filled bars) for 60 minutes at 37°C. After washing, adhesion to endothelial cell monolayers was performed in the presence or absence of blocking mAbs anti-α4 (mAb HP2.1, 50 μg/mL), anti-αM (mAb CBRM1/5, 50 μg/mL), anti-αL (mAb L15, 20 μg/mL), or anti-β2 integrin (mAb 60.3, 20 μg/mL). Values (mean ± SEM) are displayed as percentage of control adhesion and represent the mean of at least 3 independent experiments. *Indicates P < .01 compared with the respective control (no mAb).
VLA-4–induced adhesion is mediated by β2integrins LFA-1 and Mac-1.
Myelomonocytic HL60 cells were pretreated with medium (control, not shown), soluble VCAM-1–Fc (40 μg/mL, open bars), or mAb K20 (20 μg/mL, filled bars) for 60 minutes at 37°C. After washing, adhesion to endothelial cell monolayers was performed in the presence or absence of blocking mAbs anti-α4 (mAb HP2.1, 50 μg/mL), anti-αM (mAb CBRM1/5, 50 μg/mL), anti-αL (mAb L15, 20 μg/mL), or anti-β2 integrin (mAb 60.3, 20 μg/mL). Values (mean ± SEM) are displayed as percentage of control adhesion and represent the mean of at least 3 independent experiments. *Indicates P < .01 compared with the respective control (no mAb).
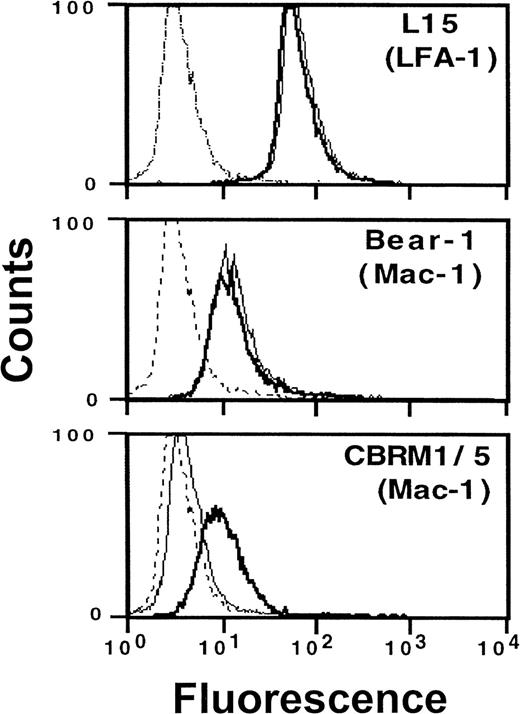
Consistent with these functional data, flow cytometry measurements directly revealed Mac-1 activation following VLA-4 engagement: preincubation with VCAM-1–Fc resulted in the expression of a cellular Mac-1 epitope, which was expressed only on activated leukocytes and was detected by mAb CBRM1/5 (Figure 5). No quantitative changes in expression of β2integrin were found when mAbs were used that detect LFA-1 (L15) or Mac-1 (Bear-1) irrespective of their activation state. Thus, conformational rather than quantitative changes of the β2integrins LFA-1 and Mac-1 appear to account for enhanced cell adhesivity in response to VLA-4 occupancy. No changes of integrin expression were found after incubation of cells with soluble monomeric VCAM-1 or human IgG, which was used to exclude the involvement of Fc receptors. In addition, saturation of Fc receptors by human IgG prior to the experiment did not influence the activating effect of VCAM-1–Fc on the expression of epitope CBRM1/5.
Induction of an active β2 integrin conformation by VCAM-1–Fc binding to VLA-4.
Myelomonocytic HL60 cells were pretreated with medium (thin line) or soluble VCAM-1–Fc (solid line) (40 μg/mL) for 30 minutes at 37°C. After washing, fluorescence-activated cell sorter analysis was performed as described in “Materials and methods” using mAbs anti-αL (L15) and anti-αM (Bear-1), which both detect the respective integrin independent of its activation state, as well as mAb CBRM1/5 (anti-αM), which only binds to the activated αM chain of Mac-1.28 The broken line represents the nonspecific control mAb.
Induction of an active β2 integrin conformation by VCAM-1–Fc binding to VLA-4.
Myelomonocytic HL60 cells were pretreated with medium (thin line) or soluble VCAM-1–Fc (solid line) (40 μg/mL) for 30 minutes at 37°C. After washing, fluorescence-activated cell sorter analysis was performed as described in “Materials and methods” using mAbs anti-αL (L15) and anti-αM (Bear-1), which both detect the respective integrin independent of its activation state, as well as mAb CBRM1/5 (anti-αM), which only binds to the activated αM chain of Mac-1.28 The broken line represents the nonspecific control mAb.
The specificity of VLA-4–promoted leukocyte adhesion was further defined by cell adhesion to immobilized components of the extracellular matrix as well as to the immobilized recombinant human integrin counter-receptors ICAM-1 and VCAM-1 (Table1). After preincubation with mAb K20, cell adhesion to the immobilized ligands of β1 integrins, collagen and laminin, as well as to the specific ligands of VLA-4, fibronectin and VCAM-1, was unaffected. In contrast, adhesion to ICAM-1, the common ligand of the β2 integrins LFA-1 and Mac-1, as well as to fibrinogen and KLH, which are specific ligands for Mac-1, was significantly induced. The specificity of these interactions for β2 integrin–dependent cell adhesion was corroborated by the findings that mAb K20-induced adhesion to the latter β2 integrin ligands was totally abolished by the blocking anti-β2 integrin mAb (60.3) but not by anti–VLA-4 (HP2.1).
Induction of β2-integrin mediated cell adhesion by β1-integrin engagement
| Preincubation . | Blocking mAb . | Adhesion to ligands . | ||||||
|---|---|---|---|---|---|---|---|---|
| β2-integrins . | β1-integrins . | |||||||
| KLH . | Fibrinogen . | ICAM-1 . | Fibronectin . | Laminin . | Collagen . | VCAM-1 . | ||
| Medium | No mAb | 0.14 ± 0.01 | 0.13 ± 0.01 | 0.22 ± 0.05 | 0.31 ± 0.10 | 0.29 ± 0.03 | 0.24 ± 0.02 | 0.22 ± 0.05 |
| Anti-β2 | 0.09 ± 0.02 | 0.12 ± 0.02 | 0.17 ± 0.01 | 0.37 ± 0.12 | 0.31 ± 0.05 | 0.24 ± 0.03 | 0.25 ± 0.04 | |
| Anti-α4 | 0.14 ± 0.01 | 0.14 ± 0.01 | 0.23 ± 0.04 | 0.27 ± 0.12 | 0.30 ± 0.04 | 0.25 ± 0.03 | 0.20 ± 0.04 | |
| mAb K20 | No mAb | 0.29 ± 0.05 | 0.27 ± 0.03 | 0.39 ± 0.05 | 0.37 ± 0.04 | 0.26 ± 0.04 | 0.25 ± 0.04 | 0.24 ± 0.05 |
| Anti-β2 | 0.07 ± 0.02 | 0.08 ± 0.01 | 0.17 ± 0.07 | 0.43 ± 0.05 | 0.27 ± 0.06 | 0.24 ± 0.05 | 0.25 ± 0.05 | |
| Anti-α4 | 0.27 ± 0.04 | 0.26 ± 0.04 | 0.41 ± 0.06 | 0.34 ± 0.06 | 0.23 ± 0.03 | 0.23 ± 0.04 | 0.21 ± 0.04 | |
| Preincubation . | Blocking mAb . | Adhesion to ligands . | ||||||
|---|---|---|---|---|---|---|---|---|
| β2-integrins . | β1-integrins . | |||||||
| KLH . | Fibrinogen . | ICAM-1 . | Fibronectin . | Laminin . | Collagen . | VCAM-1 . | ||
| Medium | No mAb | 0.14 ± 0.01 | 0.13 ± 0.01 | 0.22 ± 0.05 | 0.31 ± 0.10 | 0.29 ± 0.03 | 0.24 ± 0.02 | 0.22 ± 0.05 |
| Anti-β2 | 0.09 ± 0.02 | 0.12 ± 0.02 | 0.17 ± 0.01 | 0.37 ± 0.12 | 0.31 ± 0.05 | 0.24 ± 0.03 | 0.25 ± 0.04 | |
| Anti-α4 | 0.14 ± 0.01 | 0.14 ± 0.01 | 0.23 ± 0.04 | 0.27 ± 0.12 | 0.30 ± 0.04 | 0.25 ± 0.03 | 0.20 ± 0.04 | |
| mAb K20 | No mAb | 0.29 ± 0.05 | 0.27 ± 0.03 | 0.39 ± 0.05 | 0.37 ± 0.04 | 0.26 ± 0.04 | 0.25 ± 0.04 | 0.24 ± 0.05 |
| Anti-β2 | 0.07 ± 0.02 | 0.08 ± 0.01 | 0.17 ± 0.07 | 0.43 ± 0.05 | 0.27 ± 0.06 | 0.24 ± 0.05 | 0.25 ± 0.05 | |
| Anti-α4 | 0.27 ± 0.04 | 0.26 ± 0.04 | 0.41 ± 0.06 | 0.34 ± 0.06 | 0.23 ± 0.03 | 0.23 ± 0.04 | 0.21 ± 0.04 | |
Myelomonocytic HL60 cells were pretreated with mAb anti-β1 (K20) or control IgG (10 μg/mL each) for 30 minutes at 37°C. Cells were washed and added to the wells precoated with the specific β2-integrin ligands keyhole limpet hemocyanin (KLH), fibrinogen, or human recombinant ICAM-1, or with β1-integrin ligands fibronectin, laminin, collagen, or human recombinant VCAM-1. Adhesion was performed in the presence or absence of blocking mAbs anti-β2 (60.3), or anti-α4 (HP2.1) (20 μg/mL each). The assay was performed as described in “Materials and methods.” Values are displayed as mean absorbance ± SEM of at least 3 independent experiments. Brackets indicate significant differences (P < .01).
VLA-4 engagement induces uPAR-mediated adhesion
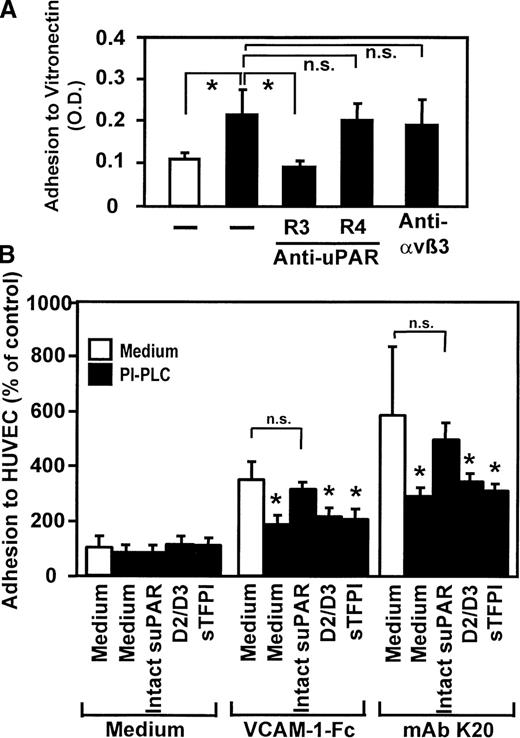
The uPAR forms functional complexes with integrins on the cell surface and has been shown to control integrin activities.30-32 Thus, the involvement of uPAR in VLA-4–induced adhesion was studied: preincubation with mAb K20 (Figure6A) or soluble VCAM-1–Fc (but not unclustered soluble VCAM-1, not shown) directly resulted in significant monocytic cell adhesion to immobilized vitronectin, an extracellular matrix protein that specifically binds to uPAR. Induced cell adhesion was inhibited in the presence of a blocking anti-uPAR mAb (R3), whereas the nonblocking anti-uPAR mAb R4 or the integrin-blocking mAb anti-αvβ3 (LM609) had no effect. Flow cytometric analysis showed no quantitative changes of uPAR surface expression (not shown), indicating that enhanced adhesion was due to conformational activation of uPAR rather than quantitative up-regulation.
Requirement of uPAR for leukocyte adhesion in response to VLA-4 ligation.
(A) Myelomonocytic HL60 cells were treated with medium alone (open bar) or with mAb K20 (20 μg/mL, filled bars) for 30 minutes at 37°C. Following washing, adhesion to immobilized vitronectin was performed in the presence or absence of mAbs anti-uPAR R3 (blocking), R4 (nonblocking), or anti-αvβ3 LM609 (20 μg/mL) as described. Values represent the mean ± SEM of 3 independent experiments. (B) Myelomonocytic HL60 cells were treated with medium alone (open bars) or pretreated with phosphatidylinositol-specific phospholipase C (0.5 U/mL) for 90 minutes at 37°C (filled bars), washed, and incubated for 10 minutes in the absence or presence of soluble intact uPAR (D1-D3, 16 nmol/L), the truncated form of uPAR (D2/D3, 20 nmol/L) lacking domain 1, or with soluble tissue factor pathway inhibitor (sTFPI; 16 nmol/L), followed by incubation for 20 minutes with medium, soluble VCAM-1–Fc (40 μg/mL), or mAb K20 (15 μg/mL) as indicated. Following washing, adhesion to endothelial cell monolayers was performed as described. Values are displayed as percentage of control (no pretreatment, 100%) and represent the mean ± SEM of 3 independent experiments. *Indicates P < .01 compared with the respective medium control; n.s., not significant (P > .05).
Requirement of uPAR for leukocyte adhesion in response to VLA-4 ligation.
(A) Myelomonocytic HL60 cells were treated with medium alone (open bar) or with mAb K20 (20 μg/mL, filled bars) for 30 minutes at 37°C. Following washing, adhesion to immobilized vitronectin was performed in the presence or absence of mAbs anti-uPAR R3 (blocking), R4 (nonblocking), or anti-αvβ3 LM609 (20 μg/mL) as described. Values represent the mean ± SEM of 3 independent experiments. (B) Myelomonocytic HL60 cells were treated with medium alone (open bars) or pretreated with phosphatidylinositol-specific phospholipase C (0.5 U/mL) for 90 minutes at 37°C (filled bars), washed, and incubated for 10 minutes in the absence or presence of soluble intact uPAR (D1-D3, 16 nmol/L), the truncated form of uPAR (D2/D3, 20 nmol/L) lacking domain 1, or with soluble tissue factor pathway inhibitor (sTFPI; 16 nmol/L), followed by incubation for 20 minutes with medium, soluble VCAM-1–Fc (40 μg/mL), or mAb K20 (15 μg/mL) as indicated. Following washing, adhesion to endothelial cell monolayers was performed as described. Values are displayed as percentage of control (no pretreatment, 100%) and represent the mean ± SEM of 3 independent experiments. *Indicates P < .01 compared with the respective medium control; n.s., not significant (P > .05).
We further tested the relevance of the uPAR on integrin-mediated myelomonocyte adhesion in response to VLA-4 engagement by both mAb K20 or VCAM-1–Fc, respectively (Figure 6B). Removal of uPAR from the leukocyte surface by preincubation with phosphatidylinositol-specific phospholipase C resulted in a significant inhibition of cell adhesion stimulated by VCAM-1–Fc (64% inhibition) or mAb K20 (62% inhibition). Adhesion was almost completely restored when soluble recombinant uPAR was added prior to the cell adhesion assay. Control proteins such as the truncated form of uPAR, which lacks domain 1, or recombinant tissue factor pathway inhibitor, which represents another glycolipid–linked protein on monocytes, did not restore adhesion (Figure 6B). These findings demonstrate that, upon VLA-4 engagement, uPAR is activated and can either directly bind to vitronectin or serve a superior regulatory function particularly in the inductionof β2integrin–dependent cellular adhesive interactions provoked by VLA-4 occupancy.
Discussion
The established chain reaction of adhesive events necessary for leukocyte adhesion and transmigration includes directed activation between selectins, β2 integrins, and β1/α4 integrin. The present study demonstrates that clustering of VLA-4 on the surface of monocytic cells by VCAM-1 enhances cell adhesion to human vascular endothelium via β2 integrins. VLA-4–mediated cell adhesion was a result of activation of the 2 major β2 integrins, LFA-1 and Mac-1. The activated urokinase receptor (uPAR) is substantially involved in this activation pathway. Thus, bidirectional integrin transactivation including the action of uPAR appears to be plausible for VLA-4–VCAM-1 interactions, particularly during the fast process of firm leukocyte adhesion and migration, and may stabilize and promote leukocyte infiltration via rapid activation of β2integrins.
The specificity of cell-to-cell adhesion was confirmed by a variety of approaches, including specific extracellular matrix ligands, ICAM-1–transfected CHO cells, and blocking antibodies. In particular, cellular interactions induced by VLA-4 occupancy were blocked by mAb against the common β2 integrin chain CD18 as well as by mAbs against the respective α chain of LFA-1 (CD11a) or Mac-1 (CD11b). Thus, LFA-1 and Mac-1 appear to contribute equally to the adhesive strength of leukocytes, comparably to physiologic situations.33 Three additional findings suggest that VLA-4 engagement may activate β2 integrins via conformational changes rather than quantitative up-regulation: (1) flow cytometry did not show any changes in quantitative surface expression of LFA-1 or Mac-1 in response to VLA-4 engagement; (2) direct Mac-1 activation was confirmed by flow cytometry using mAb CBRM1/5 that only detects Mac-1 in its activated, binding state28; (3) functional relevance of this activation-dependent epitope on Mac-1 and of an activated LFA-1 was proven in adhesion assays, because mAb CBRM1/5 or mAb L15 effectively blocked cell adhesion in response to VLA-4 engagement.
The role of VLA-4 was elucidated by using specific ligands such as soluble VCAM-1 as well as a function blocking mAb anti–VLA-4. When mAb anti–VLA-4 was allowed to compete with soluble VCAM-1–binding to the cells, binding of VCAM-1 and subsequent cell adhesion were blocked. In contrast, mAb anti–VLA-4 did not affect induced adhesion when added following VLA-4 clustering by VCAM-1. Therefore, in the described activation pathway, adhesion to HUVECs is inducedvia VCAM-1–VLA-4 interaction and mediated via β2integrin (LFA-1 and Mac-1)–ICAM-1 interaction.
The specificity of this transdominant activation of integrins was further confirmed by the finding that cell adhesion to the immobilized β2 integrin ligand ICAM-1, but not to the VLA-4-ligand VCAM-1, was stimulated by antibody ligation of VLA-4. Consistently, adhesion to immobilized fibrinogen, an adhesive extracellular matrix protein that selectively binds Mac-1, was induced, whereas adhesion to specific β1-integrin ligands such as fibronectin, laminin, or collagen remained unaffected. The fact that freshly isolated neutrophils as well as myelomonocytic cells (U937, HL60) reacted with 2 different ICAM-1–expressing cell types (HUVEC and CHO–ICAM-1) in a similar manner after β1 integrin engagement suggests that the functional interaction of β1and β2 integrins is not restricted to a particular cell type. Consistently, activation of LFA-1 via VLA-4–VCAM-1 interactions has recently been described on T cells.34 Circulating neutrophils strongly express β1 integrins α5β1 (VLA-5) and α6β1 (VLA-6) but no VLA-4 (α4β1). Consistent with published results, fluorescence-activated cell sorter analysis showed strong expression of the β1 integrin chain as well as α5 and α6 (not shown) but no surface expression of α4 (mAb HP2.1). As expected, VCAM-1–Fc did not induce neutrophil adhesion (not shown), whereas mAb anti-β1(K20) effectively induced neutrophil adhesion to HUVECs (Figure 1B). We were able to identify the natural receptor-ligand pair (VLA-4–VCAM-1) that activates β2-integrin function on monocytic cells. Which natural ligands of β1 integrins are able to activate neutrophil β2 integrins remains to be clarified. Because VLA-4 is also expressed on neutrophils under certain circumstances (eg, after transmigration)35 the described activation pathway may be operative on these cells in vivo.
Relative surface expression as well as the activation status of the involved integrins may influence the respective adhesive response of different leukocyte subtypes. For example, LFA-1 activation may dominate on T cells,36 whereas Mac-1 activation may be of importance on monocytes. In addition, chemokines may selectively activate VLA-4 on a specific cell type, such as eosinophils,37 thereby inducing an activation cascade that results in β2 integrin–mediated adhesion.
Engagement of VLA-4 was achieved by soluble forms of VCAM-1 as well as mAb directed against VLA-4. To distinguish between effects of binding only versus integrin cross-linking, bivalent VCAM-1–Fc as well as Fab fragments of mAb K20 were used. In addition, VLA-4–bound VCAM-1 was secondarily clustered by mAb anti–VCAM-1. Evidence is provided that intracellular signals following VLA-4 cross-linking may account for β2 integrin activation and cell-to-cell adhesion: clustering of VLA-4 was required for the observed cell-to-cell adhesion, because Fab fragments of mAb K20 induced adhesion only if cross-linked by secondary mAb. Similarly, soluble monovalent VCAM-1 on its own had no effect, whereas cross-linking by secondary mAb anti–VCAM-1 strongly enhanced adhesion. Consistently, soluble bivalent VCAM-1–Fc effectively induced the expression of an activation-inducible epitope on Mac-1 and enhanced cell adhesion via β2 integrin activation. Intracellular signal transduction in response to VLA-4 cross-linking has been previously described.10 11 Further studies need to elucidate the role of intracellular and extracellular molecular crosstalks that are required for β1 integrin–dependent transdominant activation of β2 integrins. At present, the putative signaling mechanism leading to β2 integrin activation in response to VLA-4 engagement seems to differ from previously published pathways because preliminary experiments revealed that tyrosine phosphorylation was not involved (unpublished observations). We could rule out a potential involvement of Fc receptors in this activation pathway, because β1 integrin clustering was achieved in the absence of Fc fragments (Figure 3A) and saturation of Fc receptors prior to clustering of VLA-4 by VCAM-1–Fc or VCAM-1/anti–VCAM-1 did not affect adhesion (Figure 3B).
The glycolipid-anchored cell surface glycoprotein uPAR plays a central role for both proteolytic and adhesive cellular functions.7,32,38 By forming a functional unit in a yet poorly understood manner, uPAR modulates β2-integrin function.31,32,39,40 Specific binding of soluble uPAR to a variety of human hematopoietic cells (including HL60 cells, monocytes, and neutrophils) has been previously demonstrated.41Functional relevance of soluble uPAR for cell activation has been shown by others30,42 and our group.32,40 In the present study, a central role for uPAR in VLA-4–induced cell adhesivity is emphasized, because uPAR was required for β2 integrin–mediated leukocyte adhesion to endothelium following VLA-4 engagement: while the removal of uPAR hardly allowed adhesion, full cell adhesivity was regained by addition of soluble uPAR. In addition, apart from β2 integrin activation, direct activation of uPAR was noted after VLA-4 clustering, as measured by enhanced specific monocytic cell adhesion to immobilized vitronectin, an extracellular matrix protein that specifically binds to uPAR. Thus, clustering of VLA-4 by VCAM-1 activates leukocyte adhesion to extracellular matrix or vitronectin via activated uPAR as well as to endothelial cells via activated β2 integrins. Apart from uPAR activation and direct binding to vitronectin, uPAR appears to control the described integrin crosstalk. These findings confirm the concept that uPAR plays a key role for integrin regulation and is essential for an adequate function of both LFA-1 and Mac-1.32
The relevance of monocyte rolling for lesion development in early atherogenesis has recently been reviewed.43 VLA-4 interaction with VCAM-1 was shown to stabilize rolling and prolonged transit time of monocytes in early atherosclerotic lesions.44 Our study demonstrates that this stabilization may not only be due to VLA-4–VCAM-1 binding, but also through the exchange of subsequent activation signals leading to engagement of β2 integrins LFA-1 and Mac-1. Subsequent β2integrin–dependent firm cell adhesion and transmigration could thus be regulated by anti–β1-integrin strategies. Interruption of signals that result in “downstream” cell activation and enhanced cell adhesivity via β2 integrins may, therefore, additionally account for protective effects of direct VLA-4–VCAM-1 blockade. Apart from atherogenesis, leukocyte recruitment is fundamental for the development of restenosis following percutaneous coronary interventions45-47 as well as for myocardial damage after ischemia and reperfusion.48 These entities are characterized by enhanced surface expression of adhesion molecules on monocytes and neutrophils as well as on vascular endothelium. Our findings can assist in the design of new therapeutic strategies for prevention of unwanted leukocytic cell recruitment during inflammatory cardiovascular processes by employing specific antagonists against the VLA-4–VCAM-1 and the urokinase receptor systems.
Acknowledgments
We gratefully acknowledge the excellent technical assistance of Monika Hölderle and Thomas Schmidt. We thank Drs Marc Robinson, Carl Figdor, John Harlan, and Timothy Springer for the generous supply of antibodies. We are grateful to Dr Dietmar Seiffge for kindly providing VCAM-1–Fc and Dr Niels Behrendt for soluble recombinant urokinase receptor. We also thank Dr Triantefyllos Chavakis (Giessen, Germany) for critically reading the manuscript.
Supported in part by grants from Novartis-Foundation (Nürnberg, Germany), the Deutsche Forschungsgemeinschaft (Ne 540/1-2, Bonn, Germany), and the GTH (Gesellschaft für Thrombose und Hämostaseforschung).
Reprints:Andreas E. May, Deutsches Herzzentrum, Technische Universität München, Lazarettstr. 36, D-80636 München, Germany; e-mail: may@dhm.mhn.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal