Abstract
Although persons with hemophilia are known to be at increased risk of death, no studies have examined the source of medical care and other personal characteristics for associations with mortality. To determine death rates and to identify causes of death and predictors of mortality, we studied a cohort comprised of all hemophilic males identified by a six-state surveillance system. Data were obtained by medical record review of contacts with physicians, hemophilia treatment centers (HTCs), and other sources of care during 1993-1995 and from death certificates. Factors examined included age, race, state of residence, health insurance type, medical care source, hemophilia type/severity, presence of inhibitor, liver disease, HIV infection, and AIDS. A total of 2950 subjects were followed for an average of 2.6 years. Their median age was 22 years; 73% were white, 79% had hemophilia A, 42% had severe disease, and 67% had visited an HTC. During 7575 person years (PYs) of observation, 236 persons died—an age-adjusted mortality rate of 40.4 deaths/1000 PYs; 65% of deaths were HIV related. In addition to age, factors independently associated with increased risk of death (relative risk, P value) were the following: AIDS (33.5, <.001); HIV infection (4.7, <.001); liver disease (2.4, <.001); and Medicare/Medicaid insurance (1.4, .01). Those persons who had received care in an HTC had a significantly decreased risk of death (0.6, .002). Although HIV infection and the presence of severe liver disease remain strong predictors of mortality, survival is significantly greater among hemophilics who receive medical care in HTCs.
Hemophilia A and hemophilia B are hereditary deficiencies of factor VIII and factor IX, respectively, two glycoproteins required for normal blood clotting. Among affected persons, spontaneous bleeding or bleeding at the site of an injury is common and, if uncorrected, can lead to severe disability or death. These complications can be prevented by appropriate clinical management and treatment with preparations of factor VIII or factor IX concentrates, therapeutic clotting agents that have been available since the late 1960s.
From 1970 until the early 1980s, mortality among persons with hemophilia declined substantially.1-4 The two primary reasons usually suggested for this decline are (a) increased availability of clotting factor replacement products for treating life-threatening bleeding episodes and (b) in the United States, improved medical management provided by specialized hemophilia treatment centers (HTCs) that were established in the mid-1970s.1 5 The role of the former in reducing mortality is obvious; however, little or no data are available concerning the contribution made by HTCs toward reducing mortality among persons with hemophilia.
Because hemophilia is rare, previous studies of mortality have frequently been limited either to a relatively small number of individuals recruited from HTC populations or to analyses of national mortality data that lack individual risk factor information. No studies have directly examined the associations between mortality and factors related to medical management, such as care received in HTCs. Current economic pressures to reduce costs for medical care discourage the use of expensive, specialized care for persons with rare diseases, such as hemophilia, unless justified by demonstrated superior clinical outcomes. Thus, the extent to which HTCs contribute to the successful clinical management of individuals with hemophilia has become an important issue for health care planners.
In 1995, the Centers for Disease Control and Prevention (CDC), in collaboration with the health departments in six states, began active surveillance for all persons with hemophilia residing in those states. Data were collected on the health status and health care of persons receiving care both inside and outside of HTCs. We used data collected during the first 3 years of this surveillance project to examine the rates and causes of death among a large cohort of affected individuals. This report examines the effect of several factors, including source of care, on mortality among this population.
Materials and methods
The surveillance system, case-finding methods, and data collection procedures have been described in detail elsewhere.6 The Hemophilia Surveillance System (HSS) is a cooperative project between CDC and the health departments of Colorado, Georgia, Louisiana, Massachusetts, New York, and Oklahoma, designed to identify and collect demographic and health information on all persons with hemophilia residing in these states. The case-finding methods used, including the use of lists obtained from physicians, clinical laboratories, hospitals, HTCs, and other sources, have previously been shown to enable identification of virtually all residents with hemophilia.6
Persons were considered to have hemophilia A or B based on a physician's diagnosis. Persons with acquired inhibitors of factor VIII or IX in the absence of a genetic deficiency, persons with von Willebrand disease, and symptomatic carriers of the hemophilia gene were excluded. The level of hemophilia severity was categorized as mild, if the patient's clotting factor activity was from 6%-30%; moderate, if from 1%-5%; and severe, if <1% of normal. Clotting factor activity was documented in 98% of cases.
Data collection
From 1995 through 1997, trained data abstractors collected standardized demographic, clinical, treatment, and outcome data on persons with hemophilia from medical records obtained from HTCs, physicians' offices, laboratories, pharmacies, hospitals, emergency rooms, and outpatient clinics for the period January 1993 through December 1995. Pertinent to this analysis, detailed information was collected on (a) demographic and clinical characteristics; (b) the primary source of hemophilia care and reimbursement; (c) the results of laboratory testing; and (d) if applicable, causes of death. Information was aggregated from all settings in which patients received medical care.
Patients who were identified at any time during the 3-year interval were eligible for inclusion in the system. Information collected during the first year for which a medical record was available was used to determine the patient's date of birth, race, state of residence, and type of health insurance at baseline. Health insurance was categorized as private insurance/health maintenance organization (HMO), Medicare/Medicaid, or other/none.
HTCs provide integrated, multidisciplinary, comprehensive care to patients with hemophilia according to a set of published guidelines7 and serve as a primary source of consultative and treatment advice for enrolled patients and their physicians, even when these patients receive care in other health care settings. Although HTCs routinely schedule patient visits at least once per year, visits often occur less frequently. For this analysis, patients who had received care at an HTC any time during the study period were considered HTC users.
Persons with a peak inhibitor titer of ≥1.0 Bethesda unit during the first year of follow-up were classified as having an inhibitor; persons with liver disease-related signs and symptoms (eg, hepatomegaly, jaundice, dark urine, and clay-colored stools) were considered to have severe liver disease at baseline. HIV seropositivity was determined on the basis of a prior positive test for HIV-1 antibody. When no evidence of HIV testing could be found, patients were categorized as having an unknown HIV serostatus. Persons were classified as having AIDS if their medical records indicated conditions that met the CDC surveillance case definition for AIDS.8
Patient death data were obtained from medical records or death certificates. Deaths were categorized according to the ninth revision of the International Classification of Diseases, Injuries, and Causes of Death (ICD-9),9 using the final diagnoses by attending physicians. Up to four causes, categorized as immediate, underlying, and contributing causes, were recorded. Cause-specific analyses used the multiple-cause-of-death classification, which allows for mortality statistics based on more than one cause. A death was considered to be HIV-related if the medical record or death certificate listed at least one of the following diseases (ICD-9 codes) as the immediate or underlying cause of death: (a) HIV infection with specified conditions or other infections (042.0-044.9), (b) Pneumocystis carinii pneumonia (136.3), (c) immune deficiency (279.1, 279.3, 279.9), or (d) serologic or culture findings indicative of HIV infection (795.8). Categorization of other causes of death followed the scheme used by Chorba et al.5
Data analysis
Follow-up was from January 1 of the year of patient identification until the date of death, or until January 1 of the year during which a patient was lost to follow-up, or December 31, 1995, whichever came first.
A crude mortality rate was calculated for each demographic (age, race/ethnicity, state of residence, insurance type, and hemophilia care source) or clinical (hemophilia type and severity, inhibitor, severe liver disease, HIV serostatus, and AIDS) characteristic. This rate was expressed as the number of deaths among persons in a category of the characteristic divided by the number of years of follow-up of such persons multiplied by 1000 [deaths per 1000 person years (PYs)]. The relative risk of death between persons in different categories of the characteristic was assessed by using the ratio of these rates.10 We also calculated an age-adjusted mortality rate based on direct standardization to the U.S. male population in 1990.
Estimates of survival times and comparisons between groups of individuals were made using the survival distribution function calculated by the actuarial method.11,12 The statistical significance of differences in survival between groups was evaluated with the use of a log-rank test.13 Abridged life tables for the cohort were created with 5-year age intervals (0-4, 5-9, ..., 85+) by using the method described by Elandt-Johnson and Johnson.14 Two tables, one that included and one that excluded HIV-infected individuals, were constructed and used to estimate the life expectancy at birth and median age at death for these populations.15
Cause-specific mortality in the cohort was compared with that in the U.S. male population overall by using the ratio of the observed-to-expected mortality, or standardized mortality ratio (SMR). The number of expected deaths was calculated by multiplying the number of PYs of follow-up for each 5-year age category of the cohort by the corresponding cause-specific mortality rates in the general male population16 and summing the products over the age groups. Confidence intervals for the SMRs were calculated, based on the Poisson distribution.17
To determine characteristics independently associated with mortality, we used proportional hazards regression.18 Prior to multivariable modeling, potential predictors to be included in the model were examined for conformity with the proportionality assumption by visual examination of plots of the log of the negative log of the survival function against the log of follow-up time.13 We used a model that included all of the factors so that we could assess the simultaneous influence of all characteristics on mortality. All hypothesis testing was two-tailed, with a significance level of .05.
Results
Detailed demographic analyses of this population have been published previously.6 The characteristics and mortality rates of 2950 males with hemophilia A and B identified during the 3-year study period are shown in Table 1. Overall, 67% of identified patients received care in HTCs during the period. Of the remainder, 13% had received care primarily from private physicians or hematologists, 4% primarily from hospital- and nonhospital-based clinics, 8% received care only in hospitals or emergency rooms, and the rest received care from a variety of other sources. One half of the study population had private medical insurance or HMO coverage, whereas the remainder either were insured by Medicare/Medicaid or had no insurance. At baseline, 5% of patients had inhibitors to factor, 2% had signs or symptoms of severe liver disease, 26% were HIV infected, and 7% had AIDS (Table 1).
Characteristics and mortality rates among 2950 males with hemophilia in six US states, 1993-1995
| Characteristic . | Total* . | Deaths . | Mortality rate† . | Relative risk . | 95% CI . | ||
|---|---|---|---|---|---|---|---|
| N . | % . | N . | % . | ||||
| Age (years) | |||||||
| 0-9 | 777 | 26 | 2 | 1 | 1.0 | 1.0 | |
| 10-19 | 593 | 20 | 25 | 11 | 15.2 | 15.1 | 3.6-63.6 |
| 20-29 | 514 | 17 | 51 | 22 | 38.7 | 38.3 | 9.3-157 |
| 30-39 | 454 | 16 | 55 | 23 | 48.0 | 47.5 | 11.6-195 |
| 40-49 | 310 | 11 | 43 | 18 | 56.5 | 55.9 | 13.5-231 |
| 50-59 | 147 | 5 | 26 | 11 | 73.7 | 72.9 | 17.3-307 |
| 60-69 | 92 | 3 | 17 | 7 | 73.6 | 72.8 | 16.8-315 |
| 70+ | 63 | 2 | 17 | 7 | 118.9 | 117.6 | 27.2-509 |
| Race/Ethnicity | |||||||
| White | 2159 | 73 | 190 | 80 | 34.0 | 1.0 | |
| African-American | 403 | 14 | 30 | 13 | 28.8 | 0.8 | 0.6-1.2 |
| Hispanic | 215 | 7 | 12 | 5 | 22.6 | 0.7 | 0.4-1.2 |
| Other | 119 | 4 | 3 | 1 | 9.8 | 0.3 | 0.1-0.9 |
| Unknown | 54 | 2 | 1 | 1 | 9.0 | 0.3 | 0.04-1.9 |
| State of residence | |||||||
| New York | 1214 | 41 | 110 | 47 | 35.2 | 1.0 | |
| Georgia | 533 | 18 | 39 | 17 | 29.1 | 0.8 | 0.6-1.2 |
| Massachusetts | 423 | 14 | 30 | 12 | 29.3 | 0.8 | 0.6-1.2 |
| Louisiana | 294 | 10 | 24 | 10 | 29.6 | 0.8 | 0.5-1.3 |
| Colorado | 257 | 9 | 16 | 7 | 23.5 | 0.7 | 0.4-1.1 |
| Oklahoma | 229 | 8 | 17 | 7 | 28.4 | 0.8 | 0.5-1.3 |
| Hemophilia type | |||||||
| A | 2334 | 79 | 207 | 88 | 34.7 | 1.0 | |
| B | 616 | 21 | 29 | 12 | 18.0 | 0.5 | 0.4-0.8 |
| Disease severity | |||||||
| Mild | 902 | 31 | 39 | 17 | 17.4 | 1.0 | |
| Moderate | 717 | 24 | 41 | 17 | 21.7 | 1.2 | 0.8-1.9 |
| Severe | 1252 | 42 | 144 | 61 | 43.6 | 2.5 | 1.8-3.6 |
| Insurance type | |||||||
| Private/HMO | 1465 | 50 | 105 | 44 | 27.7 | 1.0 | |
| Medicare/Medicaid | 923 | 31 | 108 | 46 | 45.8 | 1.6 | 1.3-2.2 |
| Other/None | 146 | 5 | 5 | 2 | 14.3 | 0.5 | 0.2-1.3 |
| Hemophilia care source | |||||||
| Non-HTC | 971 | 33 | 86 | 37 | 38.3 | 1.0 | |
| HTC | 1979 | 67 | 149 | 63 | 28.1 | 0.7 | 0.6-0.9 |
| Inhibitor | |||||||
| No | 2810 | 95 | 226 | 96 | 31.4 | 1.0 | |
| Yes | 140 | 5 | 10 | 4 | 26.0 | 0.8 | 0.4-1.6 |
| Liver disease | |||||||
| No | 2897 | 98 | 215 | 91 | 28.9 | 1.0 | |
| Yes | 53 | 2 | 21 | 9 | 169.4 | 5.9 | 3.7-9.2 |
| HIV serostatus | |||||||
| Negative | 1366 | 46 | 25 | 11 | 6.8 | 1.0 | |
| Positive | 781 | 26 | 186 | 78 | 94.7 | 14.0 | 9.2-21.2 |
| Unknown‡ | 803 | 28 | 25 | 11 | 13.2 | 1.9 | 1.1-3.4 |
| AIDS | |||||||
| No | 2731 | 93 | 109 | 46 | 15.2 | 1.0 | |
| Yes | 219 | 7 | 127 | 54 | 300.9 | 19.7 | 15.3-25.5 |
| Characteristic . | Total* . | Deaths . | Mortality rate† . | Relative risk . | 95% CI . | ||
|---|---|---|---|---|---|---|---|
| N . | % . | N . | % . | ||||
| Age (years) | |||||||
| 0-9 | 777 | 26 | 2 | 1 | 1.0 | 1.0 | |
| 10-19 | 593 | 20 | 25 | 11 | 15.2 | 15.1 | 3.6-63.6 |
| 20-29 | 514 | 17 | 51 | 22 | 38.7 | 38.3 | 9.3-157 |
| 30-39 | 454 | 16 | 55 | 23 | 48.0 | 47.5 | 11.6-195 |
| 40-49 | 310 | 11 | 43 | 18 | 56.5 | 55.9 | 13.5-231 |
| 50-59 | 147 | 5 | 26 | 11 | 73.7 | 72.9 | 17.3-307 |
| 60-69 | 92 | 3 | 17 | 7 | 73.6 | 72.8 | 16.8-315 |
| 70+ | 63 | 2 | 17 | 7 | 118.9 | 117.6 | 27.2-509 |
| Race/Ethnicity | |||||||
| White | 2159 | 73 | 190 | 80 | 34.0 | 1.0 | |
| African-American | 403 | 14 | 30 | 13 | 28.8 | 0.8 | 0.6-1.2 |
| Hispanic | 215 | 7 | 12 | 5 | 22.6 | 0.7 | 0.4-1.2 |
| Other | 119 | 4 | 3 | 1 | 9.8 | 0.3 | 0.1-0.9 |
| Unknown | 54 | 2 | 1 | 1 | 9.0 | 0.3 | 0.04-1.9 |
| State of residence | |||||||
| New York | 1214 | 41 | 110 | 47 | 35.2 | 1.0 | |
| Georgia | 533 | 18 | 39 | 17 | 29.1 | 0.8 | 0.6-1.2 |
| Massachusetts | 423 | 14 | 30 | 12 | 29.3 | 0.8 | 0.6-1.2 |
| Louisiana | 294 | 10 | 24 | 10 | 29.6 | 0.8 | 0.5-1.3 |
| Colorado | 257 | 9 | 16 | 7 | 23.5 | 0.7 | 0.4-1.1 |
| Oklahoma | 229 | 8 | 17 | 7 | 28.4 | 0.8 | 0.5-1.3 |
| Hemophilia type | |||||||
| A | 2334 | 79 | 207 | 88 | 34.7 | 1.0 | |
| B | 616 | 21 | 29 | 12 | 18.0 | 0.5 | 0.4-0.8 |
| Disease severity | |||||||
| Mild | 902 | 31 | 39 | 17 | 17.4 | 1.0 | |
| Moderate | 717 | 24 | 41 | 17 | 21.7 | 1.2 | 0.8-1.9 |
| Severe | 1252 | 42 | 144 | 61 | 43.6 | 2.5 | 1.8-3.6 |
| Insurance type | |||||||
| Private/HMO | 1465 | 50 | 105 | 44 | 27.7 | 1.0 | |
| Medicare/Medicaid | 923 | 31 | 108 | 46 | 45.8 | 1.6 | 1.3-2.2 |
| Other/None | 146 | 5 | 5 | 2 | 14.3 | 0.5 | 0.2-1.3 |
| Hemophilia care source | |||||||
| Non-HTC | 971 | 33 | 86 | 37 | 38.3 | 1.0 | |
| HTC | 1979 | 67 | 149 | 63 | 28.1 | 0.7 | 0.6-0.9 |
| Inhibitor | |||||||
| No | 2810 | 95 | 226 | 96 | 31.4 | 1.0 | |
| Yes | 140 | 5 | 10 | 4 | 26.0 | 0.8 | 0.4-1.6 |
| Liver disease | |||||||
| No | 2897 | 98 | 215 | 91 | 28.9 | 1.0 | |
| Yes | 53 | 2 | 21 | 9 | 169.4 | 5.9 | 3.7-9.2 |
| HIV serostatus | |||||||
| Negative | 1366 | 46 | 25 | 11 | 6.8 | 1.0 | |
| Positive | 781 | 26 | 186 | 78 | 94.7 | 14.0 | 9.2-21.2 |
| Unknown‡ | 803 | 28 | 25 | 11 | 13.2 | 1.9 | 1.1-3.4 |
| AIDS | |||||||
| No | 2731 | 93 | 109 | 46 | 15.2 | 1.0 | |
| Yes | 219 | 7 | 127 | 54 | 300.9 | 19.7 | 15.3-25.5 |
CI indicates confidence interval; HMO, health maintenance organization; HTC, hemophilia treatment center.
Total does not always equal 100% because of missing data.
Mortality rate in deaths per 1000 person years.
Includes untested individuals.
Univariate analysis
During the study period, 236 (8%) persons with hemophilia died, corresponding to an age-adjusted mortality rate of 40.4 deaths per 1000 PYs. Mortality rates were similar among racial/ethnic groups and among residents of the six states (Table 1). Persons who received care at an HTC during follow-up were 30% less likely to die than were those who did not receive HTC care. Compared with persons with private insurance or HMO coverage, persons with Medicare or Medicaid coverage had nearly a twofold risk of death. The risk of death for persons with hemophilia B was half that for those with hemophilia A. Individuals with severe disease were more than twice as likely to die compared with those with mild disease, regardless of hemophilia type. Although no excess mortality risk was observed according to the presence of factor inhibitors, the risk of death was significantly increased for persons with severe liver disease, HIV infection, or AIDS (Table 1). The 3-year survival was 21% lower (P < .001) among HIV-infected individuals compared with noninfected persons.
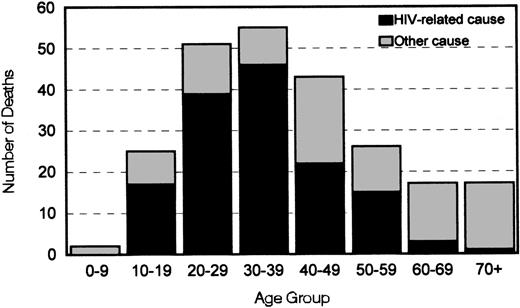
AIDS/HIV infection was the immediate cause of death for 124 (52.5%) persons (Table 2) and an underlying cause in 29 (12%) others. Among persons between the ages of 10 and 59 years who died, HIV infection was listed as an immediate or underlying cause of death for ≥50% in every 10-year age group, and it accounted for 84% of deaths among those in the 30-39 year age group (Figure1). Liver disease was responsible for an additional 8% of all deaths. According to mortality rates in US males, the number of deaths from acute myocardial infarction, cancer, liver disease, and renal disease among persons in the cohort was greater than expected (Table 2).
Causes of death and selected standardized mortality ratios (SMR)* among 2950 males with hemophilia in six US states, 1993-1995
| Cause of death . | Number (%†) of persons (N = 236) . | SMR . | 95% CI . |
|---|---|---|---|
| AIDS/Infection | 124 (52.5) | ||
| Circulatory disease | 43 (18.2) | ||
| Acute myocardial infarction | 8 (3.4) | 3.0 | 1.5-5.8 |
| Respiratory disease | 34 (14.4) | ||
| Neoplasm | 21 (8.9) | ||
| Non-HIV- or liver-related cancers | 14 (5.9) | 2.2 | 1.3-3.7 |
| Hemorrhage | 20 (8.5) | ||
| Intracerebral hemorrhage | 6 (2.5) | ||
| Liver disease | 19 (8.1) | 38 | 24.3-59.7 |
| Central nervous system disease | 17 (7.2) | ||
| Infections other than Pneumocystis or HIV | 13 (5.5) | ||
| Renal disease | 10 (4.2) | 50 | 26.8-92.8 |
| Other causes | 7 (3.0) | ||
| Trauma | 5 (2.1) | ||
| Unknown | 3 (1.3) |
| Cause of death . | Number (%†) of persons (N = 236) . | SMR . | 95% CI . |
|---|---|---|---|
| AIDS/Infection | 124 (52.5) | ||
| Circulatory disease | 43 (18.2) | ||
| Acute myocardial infarction | 8 (3.4) | 3.0 | 1.5-5.8 |
| Respiratory disease | 34 (14.4) | ||
| Neoplasm | 21 (8.9) | ||
| Non-HIV- or liver-related cancers | 14 (5.9) | 2.2 | 1.3-3.7 |
| Hemorrhage | 20 (8.5) | ||
| Intracerebral hemorrhage | 6 (2.5) | ||
| Liver disease | 19 (8.1) | 38 | 24.3-59.7 |
| Central nervous system disease | 17 (7.2) | ||
| Infections other than Pneumocystis or HIV | 13 (5.5) | ||
| Renal disease | 10 (4.2) | 50 | 26.8-92.8 |
| Other causes | 7 (3.0) | ||
| Trauma | 5 (2.1) | ||
| Unknown | 3 (1.3) |
The SMR is the ratio of the observed-to-expected number of deaths from this cause. An SMR >1.0 indicates that there were a greater number of deaths in the cohort than would be expected to occur in a cohort of similarly aged men without hemophilia. CI indicates confidence interval.
Persons may have had more than one cause of death listed.
Distribution by age of 236 deaths and relations with cause among 2950 men with hemophilia in six US states, 1993-1995.
Deaths were considered to be HIV related when according to death records the immediate, underlying, or contributing cause of death was infection with HIV.
Distribution by age of 236 deaths and relations with cause among 2950 men with hemophilia in six US states, 1993-1995.
Deaths were considered to be HIV related when according to death records the immediate, underlying, or contributing cause of death was infection with HIV.
Multivariate analysis
After adjusting for the effects of all of the studied characteristics in a multivariate analysis, medical care provided by HTCs was even more strongly associated with reduced mortality; persons who had received care in HTCs during the study period were 40% less likely to die than those who had not (Table3). In contrast, several factors were associated with increased risk of death. Mortality risk increased by 60% with each additional decade of age. Persons with severe liver disease had 2.4 times the risk of death, those persons with HIV-infection but without AIDS had nearly 5 times the risk, and persons with AIDS had 33 times the risk compared with persons without these conditions.
Associations between characteristics and death among 2950 males with hemophilia in six US states, 1993-1995
| Characteristic . | Relative risk . | 95% CI . | P . |
|---|---|---|---|
| Age (10-year increase) | 1.6 | 1.4-1.7 | <.001 |
| Race (white vs all other) | 1.1 | 0.8-1.6 | .56 |
| State of residence (vs New York) | |||
| Colorado | 0.4 | 0.2-0.7 | <.001 |
| Georgia | 0.8 | 0.6-1.2 | .33 |
| Louisiana | 1.4 | 0.9-2.3 | .13 |
| Massachusetts | 0.7 | 0.5-1.1 | .11 |
| Oklahoma | 0.8 | 0.5-1.4 | .52 |
| Hemophilia type (B vs A) | 0.8 | 0.5-1.2 | .35 |
| Disease severity (vs mild) | |||
| Moderate | 0.7 | 0.5-1.1 | .17 |
| Severe | 1.0 | 0.7-1.5 | .89 |
| Insurance type (vs private/HMO) | |||
| Medicare/Medicaid | 1.4 | 1.1-1.9 | .01 |
| Other/None | 0.9 | 0.3-2.1 | .75 |
| Inhibitor present | 1.6 | 0.8-3.0 | .18 |
| Liver disease | 2.4 | 1.5-3.9 | <.001 |
| HIV-infected without AIDS | 4.7 | 3.0-7.2 | <.001 |
| AIDS | 33.5 | 22.7-49.5 | <.001 |
| Hemophilia care source (HTC vs non-HTC) | 0.6 | 0.5-0.8 | .002 |
| Characteristic . | Relative risk . | 95% CI . | P . |
|---|---|---|---|
| Age (10-year increase) | 1.6 | 1.4-1.7 | <.001 |
| Race (white vs all other) | 1.1 | 0.8-1.6 | .56 |
| State of residence (vs New York) | |||
| Colorado | 0.4 | 0.2-0.7 | <.001 |
| Georgia | 0.8 | 0.6-1.2 | .33 |
| Louisiana | 1.4 | 0.9-2.3 | .13 |
| Massachusetts | 0.7 | 0.5-1.1 | .11 |
| Oklahoma | 0.8 | 0.5-1.4 | .52 |
| Hemophilia type (B vs A) | 0.8 | 0.5-1.2 | .35 |
| Disease severity (vs mild) | |||
| Moderate | 0.7 | 0.5-1.1 | .17 |
| Severe | 1.0 | 0.7-1.5 | .89 |
| Insurance type (vs private/HMO) | |||
| Medicare/Medicaid | 1.4 | 1.1-1.9 | .01 |
| Other/None | 0.9 | 0.3-2.1 | .75 |
| Inhibitor present | 1.6 | 0.8-3.0 | .18 |
| Liver disease | 2.4 | 1.5-3.9 | <.001 |
| HIV-infected without AIDS | 4.7 | 3.0-7.2 | <.001 |
| AIDS | 33.5 | 22.7-49.5 | <.001 |
| Hemophilia care source (HTC vs non-HTC) | 0.6 | 0.5-0.8 | .002 |
All characteristics were included in a multivariate analysis, using proportional hazards regression. Each relative risk has been adjusted for the effects of all other characteristics in the model. CI indicates confidence interval; HMO, health maintenance organization; HTC, hemophilia treatment center.
For the entire cohort, the life expectancy at birth was 38.7 years, and the median age at death was 35 years. However, when HIV-infected persons were excluded from the cohort, the life expectancy rose to 64.1 years, and the median age at death nearly doubled to 67 years.
Discussion
The finding that HTCs have a significant effect on reducing mortality in patients with hemophilia supports the effectiveness of such centers in providing specialized preventive care. The national HTC program was initiated in 1975 to provide comprehensive, specialized care to persons with bleeding disorders. Beginning in 1983, additional CDC funding was provided to improve health outcomes among this population by increasing the emphasis on risk-reduction practices. Today, approximately 130 HTCs provide a range of comprehensive services, including diagnosis, clinical management, orthopedic and dental care, along with patient education, training, and counseling, throughout the United States and its territories.
In addition to providing expertise in coagulation disorders, the HTCs also provide individual treatment plans for persons with hemophilia and place a premium on preventive medicine because the complications are extremely difficult and expensive to treat. The design of the HTC provides each patient with access to multiple medical disciplines, each of which has specific experience in hemophilia care. This integration of services maximizes both the effectiveness and the efficiency of the health care program.
The substantial socioeconomic benefits of these services for the hemophilic population, including lower cost of care, decreased health care resource utilization, and increased employment, have been documented.19 20 However, before our analyses, no outcome comparisons between patients who do and do not receive care in HTCs have been available. The 40% reduction in risk of death that we observed among persons using HTCs is even more remarkable because HTCs provide health care services to a higher proportion of severely affected patients as well as to a disproportionate share of patients with severe liver disease, HIV infection, and AIDS—the primary risk factors for mortality in this population (Table4).
Distribution of clinical characteristics by source of care among 2950 males with hemophilia in six US states, 1993-1995
| Characteristic . | HTC (%) . | Non-HTC (%) . | P . |
|---|---|---|---|
| Severity | |||
| Mild | 21.8 | 52.8 | <.001 |
| Moderate | 24.2 | 26.7 | |
| Severe | 54.0 | 20.5 | |
| Inhibitors | 6.0 | 2.3 | <.001 |
| Liver disease | 2.3 | 0.7 | .002 |
| HIV infection | 31.1 | 17.1 | <.001 |
| AIDS | 8.2 | 5.9 | .02 |
| Characteristic . | HTC (%) . | Non-HTC (%) . | P . |
|---|---|---|---|
| Severity | |||
| Mild | 21.8 | 52.8 | <.001 |
| Moderate | 24.2 | 26.7 | |
| Severe | 54.0 | 20.5 | |
| Inhibitors | 6.0 | 2.3 | <.001 |
| Liver disease | 2.3 | 0.7 | .002 |
| HIV infection | 31.1 | 17.1 | <.001 |
| AIDS | 8.2 | 5.9 | .02 |
Several possible explanations are available for lower mortality rates from HIV-related and liver diseases among persons receiving HTC care. First, clinical management assistance by infectious and hepatic disease specialists is easily accessed and integrated into the comprehensive, team approach that is a central component of the HTC standard of care. Second, because patients with hemophilia were excluded from participation in HIV treatment trials, HTCs using federal funding obtained by the National Hemophilia Foundation (NHF) were able to enroll HIV-infected hemophiliacs in special HTC-based AIDS Clinical Trials Units. Finally, in addition to the expertise provided by the HTC's infectious and liver disease specialists, a unique information center maintained by the NHF with federal support provides HTC staff with the latest treatment and prevention information by distributing medical bulletins and compendia of scientific articles and bibliographic citations related to HIV disease, hepatitis, and liver disease.
HTCs may also influence mortality through the establishment and promotion of home factor-infusion programs. Home therapy facilitates early treatment of bleeding episodes, but it requires intensive patient training and close monitoring by the health care professional. The practice was far more common among persons in our cohort who received HTC care compared with those who did not (61% versus 25%, P < .001). Other potential contributors to the effectiveness of HTCs include extensive patient and family education and expert consultation from HTC staff during periods of hemostatic stress. Further study will be required to assess the contribution of each of these factors in reducing the risk of death among persons receiving HTC care as well as to explore reasons for the geographic differences in mortality that we observed.
Compared with persons in other health insurance categories, those with Medicare/Medicaid were more likely to die; this finding did not change after adjustment for differences in the distributions of age and other risk factors. Increased mortality among individuals with Medicare/Medicaid coverage has been observed in the general US population and is attributed to selection for these programs on the basis of existing health problems or low socioeconomic status and, once enrolled, to differences in access to medical care.21 Among persons in our cohort who were ≥18 years old, 22% were either unemployed or disabled; moreover, mortality rates were 2 and 4 times higher, respectively, among individuals in these categories compared with those who were employed (results not shown). The unemployed and disabled comprised 29% of persons with Medicare/Medicaid compared with only 4% of those with private insurance or HMO coverage. Some of this observed effect of government insurance on mortality may have been mediated by poverty, but no socioeconomic data were available for study of this issue. Conversely, access to HTC care appeared not to have been a factor because the proportion of persons with and without Medicare/ Medicaid who received HTC care was similar (69% versus 63%, respectively).
Overall, the median age at death of 35 years was substantially lower than previous estimates of 49.1 and 40 years that were based on mortality rates during the 1980s.5,22 This finding suggests that the impact of AIDS on mortality among persons with hemophilia continued to increase during the first half of the 1990s. When HIV-infected individuals (those with and without AIDS) were excluded from the analysis, both life expectancy and median age at death nearly doubled. Because viral inactivation procedures have eliminated the threat of new HIV transmissions through receipt of plasma-derived factor concentrates, our estimate of 64.1 years, based on exclusion of HIV-infected individuals, more accurately approximates the current life expectancy of males presently born with hemophilia. Our figure is similar to the estimate of 64.9 years obtained by Stafford et al,23 which was based on mortality in the hemophilia population during the decade prior to the beginning of the AIDS epidemic, but it still is substantially less than the overall 72.3-year life expectancy for US males in 1994.24
The impact of HIV infection and liver disease on mortality in this cohort was not unexpected and is consistent with other published findings.22,25 26 Complications associated with HIV infection were the immediate or underlying cause of 65% of all deaths that occurred during 1993-1995. The age-adjusted mortality rate of 40.4 deaths per 1000 PYs was more than 4 times that of the US male population.
Our findings have important implications for future medical care for persons with hemophilia. On the basis of our surveillance data, one third of the hemophilia population receives no care from HTCs. The barriers to HTC utilization among persons with hemophilia have not been formally studied, but possibilities include a lack of awareness of the potential benefits of HTC care, socioeconomic factors, cultural barriers, transportation and other logistical problems, and cost concerns related to referral or reimbursement for HTC services. Formal studies of knowledge, attitudes, and beliefs among users and nonusers of HTCs, currently in the planning stages, will help to identify these barriers and may suggest strategies to increase access to this vital source of medical care.
Acknowledgments
The Hemophilia Surveillance System comprises the following persons and institutions: Centers for Disease Control and Prevention (B. Cicatello, B. Evatt, D. Jackson, J. M. Soucie); Colorado Department of Health (R. Hoffman, S. Michael, F. Nocera); Mountain States Regional Hemophilia Center (M. Manco-Johnson, R. Nuss, B. Riske, J. Stultz); Georgia Division of Public Health (N. Stroup); Emory University School of Public Health (E. Brockman, H. Hill, B. McDowell, T. Poindexter, K. Smith, S. Stein); Louisiana State Department of Health (C. Myers); Louisiana Comprehensive Hemophilia Care Center (A. Abdou, B. Bates, J. Bunting, C. Leissinger, V. Shea, K. Wulff); Massachusetts Department of Public Health (L. Livens, J. Su, D. Walker, N. Wilber); Boston Hemophilia Center (B. Ewenstein, F. Ross); New England Hemophilia Center (D. Brettler, A. Forsberg, P. Geary, D. Thibeault); New York State Department of Health (M. Arrington, J. Bartholomew, B. Connelly, B. Cushman, B. Kearney, M. Kolakoski, J. Lima, J. Linden, E. Villegas); Mt. Sinai Medical Center (E. Aulov, S. Gaynor); Oklahoma State Department of Health (S. Kinney); Oklahoma Hemophilia Treatment Center (H. Huszti, F. Kiplinger, C. Sexauer); and University of Oklahoma Health Sciences Center (N. Asal, L. Cowan, B. Erickson, L. Hudson, C. Smith-Edwards, S. Warner, M. Young).
Reprints:J. Michael Soucie, Centers for Disease Control and Prevention, 1600 Clifton Road, MS E64, Atlanta, GA 30333; e-mail:msoucie@cdc.gov.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal