Abstract
Donor T cells after stem cell transplantation reconstitute by 2 different pathways: by expansion from grafted, mature T cells and by intrathymic maturation from progenitor cells. This study characterized thymic-dependent reconstitution of CD4+ T cells following different transplant modalities in patients with severe combined immunodeficiency (SCID). Three groups of patients were studied: one group after transplantation from human leukocyte antigen (HLA)–identical siblings with unmanipulated grafts without conditioning, a second group after transplantation from HLA-nonidentical parents with T-cell–depleted grafts without preconditioning, and a third group with prior conditioning. Reconstitution of the T-cell compartment was monitored by determining the expression of CD45 isoforms by developing CD4+ cells in the peripheral blood and in discriminating expanded (CD45RO+) and newly generated (CD45RA+) T cells. Concomitantly, changes in the size of the thymus were evaluated sequentially by ultrasonography. Reconstitution of CD4+CD45RA+ cells was delayed in all patients for several months, including patients after HLA-identical transplantation, and was always paralleled by normalization of the size of the thymus. No engraftment of donor progenitor cells was observed, as studied in one patient transplanted without conditioning. CD4+CD45RO+ cells were detected early after transplantation only in patients given unmanipulated grafts. The study showed that thymic-dependent T-cell maturation in these patients with SCID runs an autonomous course, independent of graft manipulation, of major HLA disparities, and of whether conditioning is used or not. In addition, thymic maturation may not require engraftment of donor-derived CD34+ cells in the marrow.
Introduction
T cells after stem cell transplantation may develop from two independent sources.1,2 Mature donor T cells contained in the graft can undergo substantial and rapid antigen-triggered expansion. These memory cells are responsible for the potential induction of graft-versus-host disease (GvHD) and also may mediate protective immunity early after transplantation. Alternatively, T cells generate from engrafted stem cells preferentially within the host thymic microenvironment, recapitulating normal T-cell ontogenesis and giving rise to naive T cells. Expression of the different CD45 isoforms allows one to distinguish naive T cells and antigen-triggered, memory T cells. The high molecular weight isoform, CD45RA, is predominantly expressed by naive T cells, concomitantly with other markers like CD62L, whereas the low molecular weight isoform, CD45R0, is a characteristic marker of previously activated or of memory T cells.1,2,3 Although cells may revert to a CD45RA phenotype, determination of CD45 isoforms has proven a useful tool to follow reconstitution of CD4+ cells in the context of stem cell transplantation.1 2 Here, the appearance of CD4+CD45RA+ cells indicates newly generated T cells of thymic origin.
T-cell reconstitution in patients with severe combined immunodeficiency (SCID) after stem cell transplantation offers the opportunity to address questions related to mechanisms of thymic T-cell development. SCID constitutes a group of disorders with profound impairment of lymphocyte differentiation, characterized by a small thymus, which is embryonal in architecture and lymphocyte depleted.4 Reconstitution of the T-cell system in these patients is achieved by stem cell transplantation.5,6Three different transplant modalities are used: First, if the donor is human leukocyte antigen (HLA)–matched, transplantation with an unmanipulated stem cell graft is performed, which contains mature donor T cells and usually results in complete functional reconstitution of the T- and B-cell compartment without prior conditioning. Second, transplantation of stem cells from an HLA-disparate donor is used, which requires vigorous T-cell depletion prior to grafting to prevent GvHD. This modality frequently results in incomplete immune reconstitution characterized by normal function of the T-cell system, whereas B-cell deficiency may persist.5,6 In these cases engraftment of donor cells is restricted to the T-cell compartment.7 The use of cytoreductive conditioning prior to transplantation can overcome these limitations, representing a third treatment approach.5
In this study, we characterized thymic-dependent reconstitution of CD4+ T cells in patients with SCID, following these different transplant modalities. We show that newly generated T cells develop with a similar delay, independent of the respective transplant modality. Furthermore, we demonstrate that this maturation can occur in the absence of demonstrable donor CD34+ cells in the marrow, raising the intriguing question of the level of engraftment of T-cell precursors.
Patients, materials, and methods
Patients
Ten patients with SCID were selected for this study with a B+ SCID variant, characterized by the absence of T cells but the presence of circulating, nonfunctioning B cells. In addition, patients fulfilled the following criteria: absence or extremely low numbers (< 50 μL) of maternal T cells secondary to maternal-fetal transfusion, no severe infections after transplantation, and lack of complications from GvHD. Approval for this study was obtained by the institutional review board, and informed consent according to the Declaration of Helsinki was provided to patients. Characteristics of patients, details of transplantation, and outcome of immunologic reconstitution are shown in Table 1. Three patients were transplanted with unmanipulated bone marrow from HLA-identical siblings, the other 7 patients received T-cell–depleted grafts from HLA-nonidentical parents. In 4 of these patients, transplantation was performed without preconditioning regimen and in 3 patients following cytoreductive treatment. Grafts in 2 of these 7 cases were bone marrow cells, depleted of T cells by soybean agglutination and E-rosetting as previously described.8 In the other 5 patients, CD34+ peripheral blood progenitor cells were used. Donors in these patients were treated for 5 days with recombinant human granulocyte colony-stimulating factor (rHuG-CSF) (Lenogastrim, Rhône-Poulenc-Rorer, Cologne, Germany) to mobilize CD34+ cells. Blood leukocytes were collected by apheresis, and CD34+ cells were purified by positive selection and subsequent T-cell depletion as previously described.9
Characteristics of patients, details of transplantation, and outcome of immunologic reconstitution
| Patient characteristics . | Details of transplantation . | Stem cell graft . | T-cell reconstitution . | Chimerism . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UPN/sex . | SCID variant/ Gen-Def . | Age (mo) . | Stem cell donor . | HLA-MM . | CR . | Source . | T-cell depletion . | Nucleated cells 106/kg . | CD34+ cells 106/kg . | CD3+ cells 106/kg . | CD3 > 500/μL . | PHA > 100 SI . | MLR > 10 SI . | T cells . | B cells . | Monocytes . |
| day after transplantation . | ||||||||||||||||
| 314/M | B+/nd | 12 | Sister | 0 | No | BM | No | 2000 | nd | nd | 17 | 53 | 53 | D | nd | nd |
| 304/F | B+/nd | 6 | Sister | 0 | No | BM | No | 900 | nd | nd | 10 | 18 | 18 | D | nd | nd |
| 58/M | B+/nd | 39 | Brother | 0 | No | BM | No | 240 | nd | nd | 57 | 32 | 57 | D | nd | nd |
| 281/F | B+/nd | 2 | Mother | 3 | No | PBPC | CD34+CD2/3− | 27 | 27 | < 0.01 | 105 | 92 | 120 | D | R | R |
| 273/M | B+/yc | 8 | Mother | 1 | No | PBPC | CD34–CD2/3- | 24 | 24 | < 0.01 | 133 | 79 | 120 | D | R | R |
| 88/M | B+/yc | 1 | Mother | 2 | No | BM | SBA-ER− | 90 | nd | < 0.09 | 133 | 109 | 281 | D | R | R |
| 84/M | B+/yc | 1 | Father | 3 | No | BM | SBA-ER− | 60 | nd | < 0.06 | 124 | 124 | 168 | D | R | R |
| 346/M | B+/yc | 3 | Father | 3 | Bu | PBPC | CD34+CD2/3− | 13 | 12.6 | 0.02 | 155 | 155 | 155 | D | D/R | D/R |
| 328/M | B+/yc | 2 | Mother | 3 | Bu, Cy | PBPC | CD34+CD2/3− | 5 | 5 | < 0.01 | 166 | 78 | 112 | D | D | D |
| 271/M | B+/nd | 14 | Father | 3 | Bu, Cy | PBPC | CD34+CD2/3− | 33 | 33 | < 0.01 | 228 | 193 | 228 | D | D/R | D/R |
| Patient characteristics . | Details of transplantation . | Stem cell graft . | T-cell reconstitution . | Chimerism . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UPN/sex . | SCID variant/ Gen-Def . | Age (mo) . | Stem cell donor . | HLA-MM . | CR . | Source . | T-cell depletion . | Nucleated cells 106/kg . | CD34+ cells 106/kg . | CD3+ cells 106/kg . | CD3 > 500/μL . | PHA > 100 SI . | MLR > 10 SI . | T cells . | B cells . | Monocytes . |
| day after transplantation . | ||||||||||||||||
| 314/M | B+/nd | 12 | Sister | 0 | No | BM | No | 2000 | nd | nd | 17 | 53 | 53 | D | nd | nd |
| 304/F | B+/nd | 6 | Sister | 0 | No | BM | No | 900 | nd | nd | 10 | 18 | 18 | D | nd | nd |
| 58/M | B+/nd | 39 | Brother | 0 | No | BM | No | 240 | nd | nd | 57 | 32 | 57 | D | nd | nd |
| 281/F | B+/nd | 2 | Mother | 3 | No | PBPC | CD34+CD2/3− | 27 | 27 | < 0.01 | 105 | 92 | 120 | D | R | R |
| 273/M | B+/yc | 8 | Mother | 1 | No | PBPC | CD34–CD2/3- | 24 | 24 | < 0.01 | 133 | 79 | 120 | D | R | R |
| 88/M | B+/yc | 1 | Mother | 2 | No | BM | SBA-ER− | 90 | nd | < 0.09 | 133 | 109 | 281 | D | R | R |
| 84/M | B+/yc | 1 | Father | 3 | No | BM | SBA-ER− | 60 | nd | < 0.06 | 124 | 124 | 168 | D | R | R |
| 346/M | B+/yc | 3 | Father | 3 | Bu | PBPC | CD34+CD2/3− | 13 | 12.6 | 0.02 | 155 | 155 | 155 | D | D/R | D/R |
| 328/M | B+/yc | 2 | Mother | 3 | Bu, Cy | PBPC | CD34+CD2/3− | 5 | 5 | < 0.01 | 166 | 78 | 112 | D | D | D |
| 271/M | B+/nd | 14 | Father | 3 | Bu, Cy | PBPC | CD34+CD2/3− | 33 | 33 | < 0.01 | 228 | 193 | 228 | D | D/R | D/R |
UPN indicates unique patient number; SCID, severe combined immunodeficiency disease; Gen-Def, underlying genetic defect; HLA-MM, number of human leukocyte antigen mismatches; CR, conditioning regimen; PHA, phytohemagglutinin stimulation; SI, stimulatory index; MLR, mixed lymphocyte reaction; nd, not determined; yc, mutation of common-y-chain; Bu, busulfan 8 mg/kg; Cy, cyclophosphamide 200 mg/kg; BM, bone marrow; PBPC, peripheral blood progenitor cells; CD34+CD2/3−, PBPC after positive selection for CD34+ cells followed by E-rosetting as described in reference 9; SBA-ER−, T-cell-depleted bone marrow by lectin agglutination and E-rosetting, as described in Schreiner et al8; ne, not evaluated; D, donor; R, recipient.
Immunologic studies
Phenotyping of circulating peripheral blood mononuclear cells (PBMCs) was performed by 3-color immunofluorescence (IF) with flow cytometry (Epics XL, Coulter, Krefeld, Germany), using monoclonal antibodies specific for CD3, CD4, CD8, CD45RA, CD45R0 (DAKO, Hamburg, Germany), and CD62L (Pharmingen, Hamburg, Germany). T-cell function was determined in vitro by proliferative responsiveness to phytohemagglutinin stimulation and to allogeneic cells as described.10
Chimerism analysis
Chimerism for T cells (CD3+), B cells (CD19+), and monocytes (CD14+) in patients after HLA-nonidentical transplantation was determined by indirect IF, using major histocompatibility complex (MHC) class I-specific mouse monoclonal antibodies (One Lambda, Krefeld, Germany) that allowed us to discriminate between patient and donor cells. For chimerism of CD34+ cells, 2 to 3 mL of bone marrow was aspirated, and CD34+ cells were isolated by positive selection with the use of magnetic separation (Miltenyi, Bergisch-Gladbach, Germany), yielding CD34+ cells with more than 90% purity and absolute numbers of 1 to 2 × 106 cells. Cells were stained with anti-CD34 (HPCA2, Becton Dickinson, Heidelberg, Germany) and with recipient-specific, MHC-class-restricted monoclonal antibodies, including appropriate control antibodies.
Evaluation of thymic size by ultrasound
The size of the thymus was determined by mediastinal ultrasound.11 The apical part and the left retrosternal lobe of the thymus were evaluated. Detection of an initially completely absent retrosternal lobe with a diameter of more than 3 cm was considered as normalization of thymic size. These studies were performed at different time points after transplantation.
Results
Reconstitution of CD4+ cells after HLA-identical transplantation
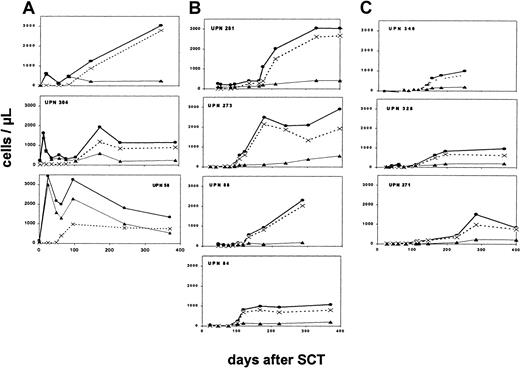
Reconstitution of CD4+ cells after HLA-identical transplantation (Figure 1A) was characterized by an early appearance of CD45R0+ cells during the second week, concomitantly with development of positive T-cell functions. In contrast, naive CD4+ cells remained absent for at least 2 months, and these cells, expressing CD45RA as well as in a majority CD62L, developed during the third and the fourth month after transplantation, respectively. This delayed appearance of thymus-derived naive T cells gave rise to a secondary marked increase in the total number of CD4+ cells.
Reconstitution of CD4+ subpopulations after stem cell transplantation.
Development of absolute numbers of CD4+ cells ●, CD4+CD45RA+ cells ×, and CD4+CD45R0+ cells ▴ up is shown to 400 days after transplantation. Findings in patients after HLA-identical transplantation (A), after HLA-nonidentical transplantation without conditioning (B), and after HLA-nonidentical transplantation with conditioning regimen (C).
Reconstitution of CD4+ subpopulations after stem cell transplantation.
Development of absolute numbers of CD4+ cells ●, CD4+CD45RA+ cells ×, and CD4+CD45R0+ cells ▴ up is shown to 400 days after transplantation. Findings in patients after HLA-identical transplantation (A), after HLA-nonidentical transplantation without conditioning (B), and after HLA-nonidentical transplantation with conditioning regimen (C).
Reconstitution of CD4+ cells after HLA-nonidentical transplantation without conditioning
In patients receiving T-cell–depleted stem cell grafts without conditioning (Figure 1B), circulating T cells remained completely absent for several months after transplantation. CD4+ cells appeared during the fourth to fifth month, coinciding with the development of positive T-cell functions. These CD4+ cells were predominantly naive cells, expressing CD45RA as well as CD62L. CD4+CD45RO+ cells reconstituted the T-cell compartment with comparable kinetics, but absolute numbers remained significantly lower as compared to CD4+CD45RA+cells.
Reconstitution of CD4+ cells after HLA-nonidentical transplantation with conditioning
The kinetics of T-cell reconstitution was similar in preconditioned patients as in SCID patients after HLA-nonidentical transplantation without prior conditioning (Figure 1C). T-cell reconstitution did not develop until the fourth month after transplantation, and reconstituting CD4+ cells expressed predominantly CD45RA. The subsequent recovery of CD4+ cells during the following months appeared slower in these conditioned patients as compared to patients in the other 2 groups.
Evaluation of thymic size
To detect an increase in thymic size and to correlate this change with peripheral reconstitution of the T-cell compartment, sequential studies of the thymus by ultrasound were performed in 9 of the 10 patients in whom T-cell reconstitution was studied. An example of the visualization of the thymus by ultrasound before and after transplantation is shown in Figure 2. A normally sized thymus with a retrosternal lobe more than 3 cm in diameter was observed during the third to fifth month in the patients, coinciding with increasing numbers of CD4+CD45RA− cells in the blood (Figure3). In 5 patients, the time point of ultrasound studies allowed us to detect an early increase of the thymic size, as defined by a visible but still abnormally small retrosternal lobe (< 3 cm). This coincided in 3 of the 5 patients with the initial appearance of very low numbers of CD45RA+ cells, whereas in 2 patients these cells appeared only shortly thereafter. These findings indicate that the increase of the thymic size preceded the appearance of CD4+ cells.
Evaluation of thymic size by ultrasound.
The size of the left retrosternal thymic lobe as visualized by ultrasound is circumscripted: before transplantation with a diameter of 1 cm (A) and after transplantation with a diameter of more than 4 cm (B). Note the enhanced density before transplantation with normalization after transplantation.
Evaluation of thymic size by ultrasound.
The size of the left retrosternal thymic lobe as visualized by ultrasound is circumscripted: before transplantation with a diameter of 1 cm (A) and after transplantation with a diameter of more than 4 cm (B). Note the enhanced density before transplantation with normalization after transplantation.
Development of the retrosternal thymic lobe and absolute numbers of CD4+CD45RA+ cells/μL after transplantation.
Patients are indicated according to the transplant procedure used, receiving T-cell–containing grafts (▴) as well as T-cell–depleted grafts without conditioning (■) and with preconditioning (●).
Development of the retrosternal thymic lobe and absolute numbers of CD4+CD45RA+ cells/μL after transplantation.
Patients are indicated according to the transplant procedure used, receiving T-cell–containing grafts (▴) as well as T-cell–depleted grafts without conditioning (■) and with preconditioning (●).
Chimerism analysis
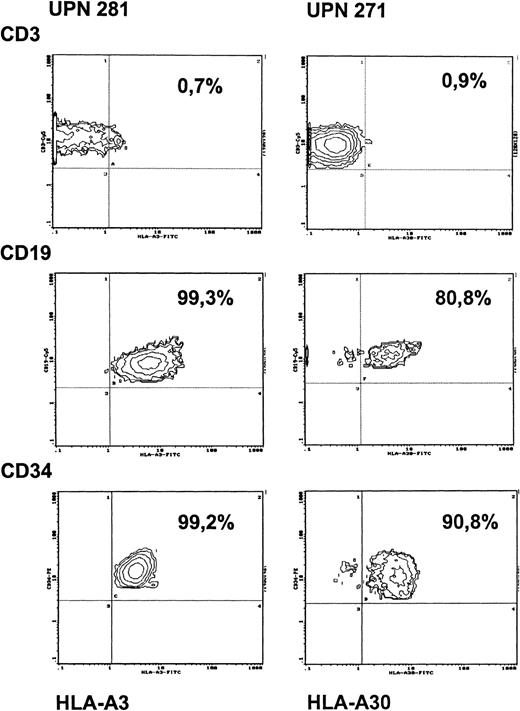
Development of a thymus coinciding with the appearance of naive CD4+ cells in all patients implicated engraftment of donor-derived precursor cells. To address the question of the level of engraftment, bone marrow aspirates were obtained from 2 patients transplanted with HLA-nonidentical stem cells either with or without cytoreductive treatment. The proportion of donor- and recipient-derived progenitor cells in the marrow was evaluated by analyzing CD34+ cells for the respective HLA-class I expression. As shown in Figure 4, in the patient without preconditioning (unique patient number [UPN] 281), who was studied at 6 months after transplantation, more than 99% of the CD34+cells were of recipient origin. Simultaneous analysis of PBMCs in this patient showed that donor engraftment, as in other nonconditioned patients, was restricted exclusively to the T-cell lineage, whereas B cells and monocytes remained of recipient origin (Figure 4, Table 1). Identical findings were obtained when this patient was restudied 2 years after transplantation. Analysis of the origin of CD34+ bone marrow cells in a SCID patient transplanted with prior conditioning (UPN 271) led to different results. In this patient, who was studied 2 years after transplantation, 91% of the CD34+ cells were of recipient origin and 9% of donor origin (Figure 4). In this patient, PBMCs were found to be exclusively of donor origin initially after transplantation, whereas subsequent studies revealed mixed chimerism for B cells and monocytes, indicating recovery of autologous hemopoietic cell lineages in this patient.
Chimerism of PBMCs and of bone marrow CD34+cells after HLA-nonidentical transplantation in a patient without conditioning (UPN 281) and in a patient with preconditioning (UPN 271).
Antibodies against HLA-A3 (UPN 281) and against HLA-A30 (UPN 271) were used as recipient-specific markers. PBMCs from respective donors were used as negative controls (staining < 1% with the recipient-specific HLA-antibodies) and from opposite parents as positive controls (staining > 99% with recipient-specific HLA antibodies).
Chimerism of PBMCs and of bone marrow CD34+cells after HLA-nonidentical transplantation in a patient without conditioning (UPN 281) and in a patient with preconditioning (UPN 271).
Antibodies against HLA-A3 (UPN 281) and against HLA-A30 (UPN 271) were used as recipient-specific markers. PBMCs from respective donors were used as negative controls (staining < 1% with the recipient-specific HLA-antibodies) and from opposite parents as positive controls (staining > 99% with recipient-specific HLA antibodies).
Discussion
T-cell reconstitution after stem cell transplantation may generate via 2 major pathways: peripheral expansion of mature T cells contained in the graft give rise to CD45RO+ cells and the intrathymic maturation of precursor cells results in development of naive, CD45RA+ T cells.1,2 Several studies12-15 have previously shown a contribution of both pathways for T-cell reconstitution. In addition, a marked heterogeneity in the development of CD45RA+ cells was observed, presumably related to differences of age, intensity of conditioning, and immunosuppression for prophylaxis and treatment of GvHD. Indeed, in animal models these factors have been demonstrated to influence intrathymic maturation.16-21
In the present study, we addressed a number of specific questions, selecting a homogeneous population of SCID patients. Besides evaluating the respective roles of thymic-dependent and thymic-independent T-cell reconstitution in a well-defined patient population, our study offered the opportunity to investigate the relevance of HLA disparity, conditioning, and the presence of mature donor T cells in the graft for intrathymic T-cell maturation. The unique situation in patients with SCID, in which T-cell reconstitution develops without previous conditioning, allowed us in addition to ask if permanent marrow engraftment of donor-derived CD34+ cells is necessary for development of thymic function.
In patients who received T-cell–depleted grafts, donor CD4+ cells developed with a delay of 3 to 4 months. These newly appearing CD4+ cells predominantly expressed a naive phenotype, indicating their thymic origin. Strikingly, a similarly delayed appearance of naive CD4+ cells was observed in patients after transplantation of unmodified, T-cell–containing marrow grafts. The suggestion that CD4+CD45RA+ cells were thymic derived was strongly supported by our observation that normalization of the thymic size paralleled the appearance of these cells. CD4+CD45R0+ cells only contributed to an early and initial CD4+ cell reconstitution in patients transplanted with unmanipulated marrow grafts. Our results also confirm that the thymus in SCID patients can reconstitute as previously demonstrated by histomorphological analysis and by demonstrating normalization of secretory function.22 23
The interval from transplantation to appearance of naive CD4+ cells likely reflects the time required for intrathymic maturation of lymphoid precursor cells. This highly complex process requires continued interaction of developing thymocytes with thymic microenvironment.24,25 In SCID patients transplanted without conditioning, the thymic microenvironment probably remains of recipient origin. This results in an unusual chimeric situation after HLA-nonidentical transplantation, raising the question to what extent HLA disparities affect intrathymic maturation. Our observation that the kinetics of intrathymic T-cell maturation was comparable in all groups of patients would indicate that HLA disparities, including full haplotype mismatches, did not adversely affect intrathymic maturation in these patients. This would strengthen recent data showing that CD4+CD45RA+ cell reconstitution after transplantation from siblings versus unrelated donors is comparable in children.26 The delayed onset of thymic-dependent T-cell reconstitution that we observed in our study could also be due to the immature morphology of the thymus in patients with SCID. This particular situation might require complete rebuilding of the thymic architecture before the thymus acquires full function. However, we observed a similar time frame of thymic-dependent T-cell reconstitution in infants treated successfully by T-cell–depleted HLA-nonidentical stem cell transplantation for other, nonimmunologic disorders, characterized by normal thymus function.27Furthermore, our data suggest that the use of conditioning regimen does not affect the kinetics of intrathymic maturation, as reflected by normalization of thymic size and appearance of circulating CD4+CD45RA+ cells. However, we selected a patient population with uncomplicated transplant courses, who also received a comparatively mild conditioning regimen. Furthermore, we observed a slower long-term recovery of normal numbers of circulating CD4+CD45RA+ cells in patients with conditioning as compared to nonconditioned patients (data not shown), and this finding may reflect some impairment of the thymus induced by the conditioning.
Several animal models have demonstrated that intrathymic homeostasis may be influenced by mature T cells. In settings not at risk for GvHD, maturation of thymocytes was found to be enhanced.28 Our study allowed us to address the role of mature T cells on intrathymic maturation in the human system. The similar pattern of thymic function following transplantation of unmanipulated and T-cell–depleted grafts, which we observed, would argue against a positive influence of mature T cells on intrathymic maturation. However, one patient developed low numbers of CD4+CD45RA+ cells already at day +60 following an unmanipulated transplant. Further studies in more patients are required to answer this question.
Surprisingly, the kinetics of thymic-dependent T-cell development was similar, regardless whether marrow engraftment of donor CD34+ cells was observed or not, as based on our findings in 2 patients transplanted without and with conditioning. Our failure to observe engraftment in the former patient could indicate that this is not a prerequisite for intrathymic maturation. However, we can not rule out transitory engraftment or an extremely low number of donor CD34+ cells in the marrow, as indeed previously reported in a similar nonconditioned patient with B− SCID in which donor-derived CD34+ marrow cells were detected at a level of 2%.29 If our result is confirmed in a larger patient group, this finding would suggest that T-lineage-committed precursor cells may seed the thymus directly. The possible consequence of this lack of marrow engraftment of CD34+ donor cells for long-term thymic function in transplanted SCID patients remains at present unknown. Marrow lymphoid precursors would not continuously be available for immigration into the thymus, raising the concern that these patients could be at risk for late complications due to T-cell exhaustion. Data from animal models have indeed shown that thymic function was lost in the absence of a continuous supply of progenitor cells from the marrow.30
The strikingly uniform time frame for development of effective thymic-dependent T-cell reconstitution was unexpected. The regulation of this process is poorly understood. Development of strategies to achieve effective and more rapid immunologic reconstitution will be of significant importance to improve the outcome of stem cell transplantation.
Acknowledgments
We would like to thank G. Holländer and K. Schwarz for helpful reading and discussion of the manuscript and A. Hänsler and K. Freudenreich for excellent technical assistance.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Susanna M. Müller, Department of Pediatrics, University Hospital of Ulm, Prittwitzstr. 43, 89075 Ulm, Germany.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal