Abstract
More than half of anaplastic large-cell lymphomas (ALCLs) have a chromosomal translocation t(2;5) that leads to the expression of a hybrid protein composed of the nucleolar phosphoprotein nucleophosmin (NPM) and the anaplastic lymphoma kinase (ALK) that exhibits an unregulated tyrosine kinase activity. We have previously identified PLC-γ as a crucial downstream signaling molecule of NPM-ALK that contributes to its mitogenic potential. Here, we show that NPM-ALK recruits the C-terminal SH2 domain of the phosphatidylinositol 3-kinase (PI 3kinase) p85 subunit. PI 3-kinase assays revealed that the kinase is activated by NPM-ALK in vivo, in turn activating PKB/Akt in NPM-ALK–expressing cells. The use of 2 specific PI 3-kinase inhibitors, wortmannin and LY294002, demonstrated the requirement of PI 3-kinase for the growth of NPM-ALK–transformed cell lines, as well as a cell line established from a patient with ALCL. Primary murine bone marrow retrovirally transduced with NPM-ALK showed a transformed phenotype that was reversible on treatment with PI 3-kinase inhibitors. Flow cytometric analysis revealed that wortmannin-treated NPM-ALK–transformed cell lines underwent apoptosis. Furthermore, apoptosis induced by overexpression of the proapoptotic molecule Bad could be partially blocked by the overexpression of NPM-ALK. Thus, NPM-ALK activates the antiapoptotic PI 3-kinase/Akt pathway, which likely contributes to the molecular pathogenesis of ALCL.
Introduction
Anaplastic large-cell lymphoma (ALCL) constitutes a subgroup of non-Hodgkin lymphoma that often express the membrane antigen CD30. Over half of these lymphomas display a typical chromosomal translocation t(2;5)(p23;q35) that fuses nucleophosmin (NPM) sequences on chromosome 5 to anaplastic lymphoma kinase (ALK) sequences on chromosome 2.1-6 NPM is an ubiquitously expressed nucleolar protein responsible for protein shuttling between the cytoplasm and the nucleus.7-10 ALK is a receptor tyrosine kinase, the expression of which is normally restricted to neural tissues.11,12 Fusion of NPM to ALK results in the dimerization and constitutive activation of the NPM-ALK oncoprotein. NPM-ALK is capable of transforming fibroblasts and inducing a lymphoma-like disease in mice.13 Thus, the NPM-ALK chimeric protein appears to be a causative agent of ALCL. Recent studies of ALCL cases revealed new variant fusions in about 20% of ALK-positive ALCL, such as TFG-ALK, TPM3-ALK, and ATIC-ALK.14-20 In all of these additional oncogenic ALK rearrangements, the truncated ALK protein with its tyrosine kinase domain is fused to a partner protein, that is capable of dimerization and subsequent activation of the ALK portion.21
It has been shown that NPM-ALK can associate with and phosphorylate the adaptor proteins SHC, Grb2, and IRS-1.21,22 However, mutational analysis of NPM-ALK that produced mutants unable to activate SHC and IRS-1 could not reveal essential biologic functions of these 2 signal transducers for the oncogenicity of NPM-ALK.21,22The biologic importance of Grb2 association with NPM-ALK has not yet been assessed. A previous study from our laboratory indicated an important role of PLC-γ for the mitogenic potential of NPM-ALK. Mutation of the PLC-γ binding site in NPM-ALK, Tyr664, resulted in an impaired transforming activity of the NPM-ALK-Y664F mutant.23 However, as implicated in this prior study, the PLC-γ activity in NPM-ALK–transformed cells does not seem to be involved in mediating antiapoptotic responses, which are likely activated separately via other signaling pathways.
Because of the sequence homology of NPM-ALK to LTK (leukocyte tyrosine kinase), it has been hypothesized that NPM-ALK, like LTK, might activate phosphatidylinositol 3-kinase (PI 3-kinase).22,24A widely distributed isoform of PI 3-kinase is a heterodimer of a 110-kd (p110) catalytic subunit and an 85-kd (p85) regulatory subunit, which is found in cellular complexes with ligand-activated growth factor receptors and nonreceptor protein tyrosine kinases (PTKs).25,26 These interactions are mediated by the Src homology 2 (SH2) domains of the p85 subunit of PI 3-kinase, which associate with specific phosphotyrosine-containing motifs present in tyrosine kinases.27-29
The association of p85 with activated tyrosine kinases is thought to provide a signal sufficient for the activation of the p110 catalytic subunit. After activation by PTK or G-protein-coupled receptors, different classes of PI 3-kinases phosphorylate the D-3 position of phosphatidylinositol (PI), phosphatidyl 4-phosphate (PI4P), and PI4,5P2.26 The 3-phosphoinositide that is generated is able to bind to Pleckstrin homology (PH) domains of signaling molecules and to activate various downstream molecules, including the serine/threonine kinase Akt/PKB.26,30-32 It has been shown that Akt is capable of phosphorylating the BCL2 family member Bad, thereby suppressing apoptosis and promoting cell survival in a variety of cellular systems.33 34
In the present work, we demonstrate a role for PI 3-kinase in the signaling pathways initiated by NPM-ALK and show its possible role in the generation of an antiapoptotic signal important for the molecular pathogenesis of ALCL.
Materials and methods
Construction of expression plasmids and site-directed mutagenesis
The NPM-ALK complementary DNA (cDNA) was cloned into pCDNA3 (Invitrogen, Groningen, The Netherlands).23 For the generation of retrovirus NPM-ALK cDNA was cloned into theHpaI site of the Mig1 vector.35 Site-directed mutagenesis was performed using the QuickChange Site-Directed Mutagenesis Kit with Pfu DNA polymerase (Stratagene, Heidelberg, Germany). All mutated cDNAs were sequenced using the ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction Kit (Perkin Elmer, Weiterstadt, Germany). The murine Bad cDNA was subcloned in pCMV-Flag-Tag (Stratagene, Heidelberg, Germany). The humanBCL2 cDNA was subcloned in pCDNA3.1 with an Xpress tag.
Antibodies
The rabbit polyclonal anti-ALK antibody (anti-ALK No. 11) has been described12 previously. The goat polyclonal anti-Akt1 and mouse monoclonal anti-Shc and anti-Cbl antibodies were from Santa Cruz Biotechnology, Santa Cruz, CA. Rabbit polyclonal anti-p85 antibody and mouse monoclonal antiphosphotyrosine 4G10 antibody were purchased from Upstate Biotechnology, Lake Placid, NY. Mouse monoclonal anti-p85 antibody was purchased from Transduction Laboratories, Los Angeles, CA. The rabbit polyclonal anti-Gab1 and anti-Gab2 and the mouse monoclonal anti-CRKL antibodies were purchased from Upstate Biotechnology. Mouse monoclonal anti-Flag M2 antibody was from Sigma, Deisenhofen, Germany, and mouse monoclonal anti-Xpress antibody was purchased from Invitrogen.
Cell culture and DNA transfection
Karpas299 is a human cell line generated from NPM-ALK–positive T-cell lymphomas of adult ALCL patients, whereas JB6, SUP-M2, and SU-DHL1 are human NPM-ALK–positive T-cell lymphoma cell lines derived from infant ALCL patients.36 Both cell lines were cultured in RPMI 1640 medium with 5% fetal calf serum (FCS) (Seromed, Berlin, Germany). The murine pro-B lymphoid cell line Ba/F3 was maintained in RPMI 1640 with 10% FCS and 1.5 ng/mL murine recombinant interleukin-3 (IL-3) (R&D Systems, DPC Bierman GmbH, Wiesbaden, Germany). DNA transfections in Ba/F3 cells were performed by electroporation using the geneZAPPER (IBI, Madison, WI) at 250 mV and 950 μF with 25 μg DNA and 5 × 106 Ba/F3 cells in cold phosphate-buffered saline (PBS). After 48 hours, the transfected cells were selected with 1 mg/mL G418 (Serva, Heidelberg, Germany) for 14 days. Cos1 cells were cultured in DMEM medium containing 10% FCS. The transient transfection of Cos1 cells was carried out with Geneporter transfection reagent, according to the manufacturer's protocol (Biozym, Oldendorf, Germany).
Glutathione-S-transferase–fusion proteins and pull-down assay
The p85 SH2 domains cloned in pGEX-2TK were a kind gift of Prof Jean Y. J. Wang, University of California, San Diego, CA. The p85 N- and C-terminal SH2 glutathione-S-transferase (GST)-fusion proteins contained aa 323-463 and aa 562-724 of mouse p85, respectively. The SH2 GST-fusion proteins were expressed in Escherichia coli HB101 and affinity purified as described37 using glutathione-Sepharose (Pharmacia Biotech, Freiburg, Germany).
For GST pull-down assays, 1 × 107 cells were solubilized in lysis buffer (10 mmol/L Tris-HCl [pH 7.4], 5 mmol/L EDTA, 130 mmol/L NaCl, 1% Triton X-100, 1 mmol/L phenylmethylsulfonyl fluoride, and 10 μg/mL each of phenantroline, aprotinin, leupeptin, and pepstatin), precleared with glutathione-beads, and incubated with 5 μg of GST-fusion proteins for 1 hour at 4°C. Protein complexes were collected on glutathione-Sepharose beads and subjected to sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Immunoprecipitation and immunoblotting
Cells were suspended in lysis buffer 10 mmol/L Tris-HCl (pH 7.5), 130 mmol/L NaCl, 5 mmol/L EDTA, 0.5% Triton X-100, 1 mg bovine serum albumin (BSA) per milliliter, 20 mmol/L sodium phosphate (pH 7.5), 10 mmol/L sodium pyrophosphate (pH 7.0), 50 mmol/L NaF, 1 mmol/L sodium orthovanadate, 1 mmol/L phenylmethylsulfonyl fluoride, 1x protease inhibitor cocktail (10 μmol/L benzamidine-HCl, 10 μg each of phenanthroline, aprotinin, leupeptin, and pepstatin per milliliter) and kept on ice for 30 minutes. Lysates were precleared by centrifugation and subjected to SDS-PAGE. Alternatively, the lysates were incubated for 2 to 12 hours with 1 to 2 μg antibody as indicated at 4°C. Immune complexes were precipitated with 30 μL protein A-Sepharose (Pharmacia Biotech, Freiburg, Germany) for 1 hour, washed 3 times with lysis buffer, and then boiled in 2% SDS sample buffer, followed by separation on SDS-polyacrylamide gels. Immunoblotting was performed with nitrocellulose membranes, and proteins were visualized by chemiluminescence, as recommended by the manufacturer (Amersham Life Science, Buckinghamshire, United Kingdom).
Phosphopeptide competition assay
The following tyrosine-phosphorylated peptides corresponding to the potential autophosphorylation sites of NPM-ALK were synthesized by BioTez GmbH, Berlin, Germany, using a SMPS 350 (Zinsser Analytik, Frankfurt, Germany): LRPQNpY17LFGCE, KADKDpY29HFKVD, AEAMNpY67EGSPI, QHLVVpY119RRKHQ, TIMTDpY152NPNYC, DYNPNpY156CFAGK, AFGEVpY191EGQVS, DIACGCQpY299LEENH, MARDIpY338RASYY, IYRASpY342YRKGG, IYRASYpY343RKG, IFSLGpY387MPYPS, LGYMPpY390PSKSN, PGPVpY418RIMTQ, IILERIEpY445CTQDP, LPIEpY461GPLVE, LWNPTpY567GSWFT, CGNVNpY644GYQQQ, NVNYGpY646QQQGL, PGAGHpY664EDTIL. p85-CSH2-GST bound on glutathione-Sepharose was first preincubated with 200 μmol/L phosphopeptide in 100 μL lysis buffer at 4°C for 1 hour, and then 1 × 106 Karpas299 cell lysate was added for an additional 1 hour. Complexes were washed 3 times, and the binding of NPM-ALK was detected by immunoblotting, as described above.
PI 3-kinase assay
PI 3-kinase activity in Ba/F3 cells was measured as described38 previously with minor modifications. Cell lysates were immunoprecipitated with antiphosphotyrosine antibody 4G10 in the lysis buffer described above for 3 hours at 4°C. Protein A-Sepharose beads were washed twice in lysis buffer, twice in buffer containing 0.2% NP-40 and 0.5 mol/L LiCl, and finally, in 0.15 mol/L NaCl and 10 mmol/L HEPES [pH 7.4]. The binding components were then eluted with 30 μL of 20 mmol/L phenyl phosphate for 30 minutes at 4°C. Kinase reaction was performed for 15 minutes at room temperature in the presence of 0.4 mg/mL phosphatidylinositol (Sigma), 40 μmol/L ATP, 30 mmol/L MgCl2, and 0.37 MBq (10 μCi) of [γ-32P] ATP. The reaction was stopped by adding 100 μL of 1 N HCl, and the lipids were first extracted with 200 μL chloroform/methanol (1:1; vol/vol), then washed once with methanol/1 N HCl (1:1; vol/vol). The lipids in organic phase were separated by thin-layer chromatrography (TLC) using silica gel 60 (Merck, Darmstadt, Germany) with chloroform/methanol/H2O/NH4OH (43:38:7:5; vol/vol) and visualized by autoradiography.
Apoptosis analysis using flow cytometry
JB6, parental Ba/F3 and NPM-ALK–transformed Ba/F3 cells were incubated with 200 nmol/L wortmannin for 3 days, with daily addition of fresh wortmannin to this concentration. The parental Ba/F3 cells were cultured with murine IL-3 as growth factor. Using a Fluorescein-FragEL DNA Fragmentation Detection Kit (Oncogene Research Products, Cambridge, MA), the exposed 3′-OH ends of DNA fragments generated in response to apoptotic signals were FITC-fluorescein–labeled by terminal deoxynucleotidyl transferase (TdT). Results were evaluated on a flow cytometer at 488 nm (Beckman Coulter, Krefeld, Germany).
Generation of retrovirus stocks
Retroviral supernatant was produced by transiently transfecting the Phoenix ecotropic producer line (Gary Nolan, Stanford, CA) with the Mig-EGFP or Mig-EGFP–NPM-ALK vectors.35 Medium was changed at 36 hours after transfection and collected after 48 hours. Titers of approximately 1 × 106helper–virus-free viral particles were obtained as measured by FACS-titering of EGFP-positive infected Rat-1 cells.
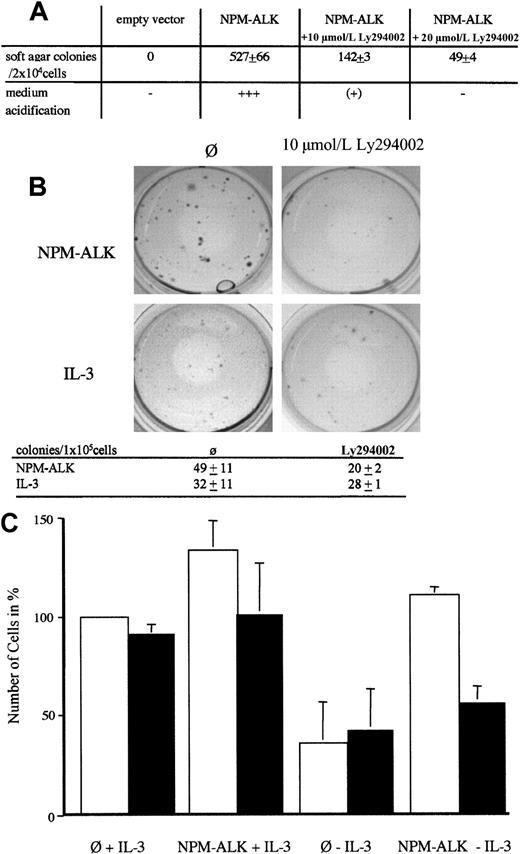
Soft agar assay
Rat-1 fibroblast cells grown in DMEM supplemented with 10% FCS, 1% glutamin, and 1% penicillin/streptomycin (Gibco, Karlsruhe, Germany) were infected in the presence of 8 μg/mL polybrene (Sigma, Deisenhofen, Germany) with viral supernatant produced as described above. Two days after infection, 2.5 × 104 cells were plated in 60-mm plates in triplicates. The soft agar medium was made of 3 layers of IMDM/agarose as described previously.23Ly294002 was added in a concentration of 10 or 20 μmol/L to the plates as indicated. Colonies were counted at 14 days after plating.
Bone marrow transformation assays
Balb/C mice were treated with 150 mg/kg 5-FU 4 days before bone marrow (BM) harvest. BM cells flushed from tibia and femur were preincubated for 1 day in BM medium (IMDM [Gibco, Berlin, Germany] supplemented with 30% FCS [Seromed], 1% BSA, 1% glutamin, 0.5% penicillin-streptomycin, 100 μmol/L 2-mercaptoethanol, and 1 μmol/L hydrocortisone) 50 ng/mL mSCF, 8 ng/mL mIL-3, and 12 ng/mL hIL-6 (all from R&D, Wiesbaden, Germany). After 1 day of preincubation, 4 × 106 BM cells were coincubated with 3 mL of viral supernatant and 1 mL of new medium, in the presence of the above mentioned growth factors and 4 μg/mL polybrene. New supernatant was added after 24 hours. Three days after infection, cells were washed in PBS. Transformation of cells in stromal-cell–dependent cultures was determined as described previously.39 Briefly, 5 × 105 infected cells were plated in BM media in 12-well plates in a volume of 2 mL with or without 100 pg/mL mIL-3. The 200 nmol/L wortmannin dissolved in DMSO or DMSO alone was added daily, as indicated and nonadherent cells were counted after 6 days.
Methylcellulose assays were performed by plating 2 × 104infected cells in 12-well plates in 1 mL methylcellulose medium (M4230, StemCell Technologies, Vancouver, British Columbia) with or without 10 pg/mL IL-3. The PI3-kinase inhibitor Ly294002 was added once as indicated at a concentration of 10 μmol/L. Colonies were counted 12 days after plating.
Results
The regulatory subunit p85 of PI 3-kinase forms a complex with NPM-ALK in vivo via the C-terminal SH2 domain of p85
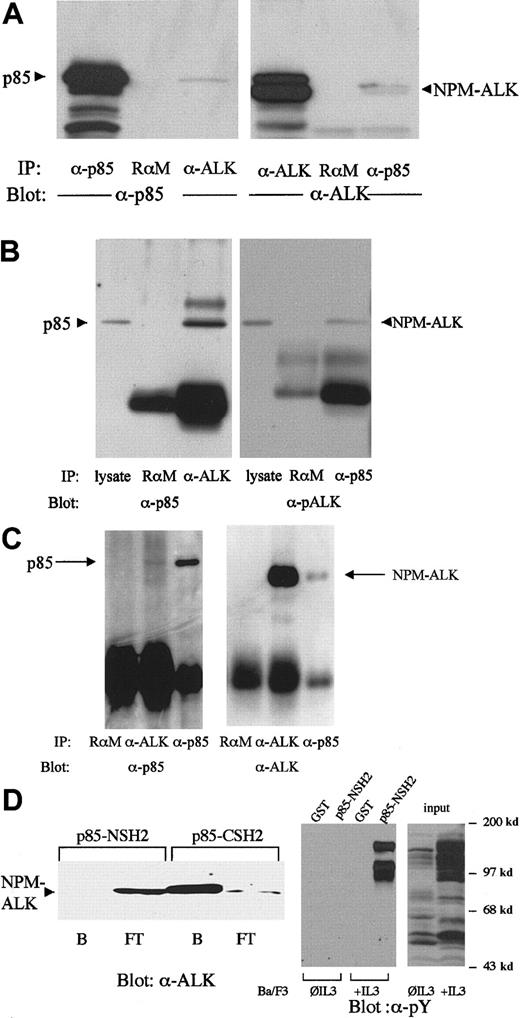
The regulatory p85 subunit of PI 3-kinase, which contains one SH3 and 2 SH2 domains, is responsible for the interaction of PI 3-kinase with activated tyrosine kinases.25 26Coimmunoprecipitation experiments in NPM-ALK–expressing Ba/F3 cells (Figure 1A) as well as in cell lines established from patients with LCAL, Karpas299 cells (Figure 1B), and SUP-M2 cells (Figure 1C) demonstrated complex formation between p85 and NPM-ALK in vivo. An anti-ALK antibody was capable to coprecipitate p85 (Figure 1A,B, left panel, third lane; Figure 1C, left panel, second lane) and an antibody against p85 could coprecipitate NPM-ALK (Figure1A,B,C, right panel, third lane) in all 3 cell lines tested. To identify the region of p85 responsible for complex formation with NPM-ALK, we carried out binding experiments with the C- and N-terminal SH2 domains of p85 in lysates of NPM-ALK–expressing Karpas299 cells. The p85-CSH2 domain, but not the p85-NSH2 domain, was shown to mediate this interaction (Figure 1D, left panel); the p85-NSH2 domain could precipitate tyrosine phosphorylated proteins (Figure 1D, right panel).
NPM-ALK and p85 form a complex in vivo via the C-terminal SH2 domain of p85.
The 2 × 107 Ba/F3 cells stably transfected with NPM-ALK (A) or 2 × 107 Karpas299 cells (B) or 2 × 107SUP-M2 cells (C) were subjected to immunoprecipitations and immunoblotting with the antibodies indicated. Association of NPM-ALK with p85 is demonstrated by coprecipitation of p85 with an NPM-ALK antibody (A and B, left panel, right lane; C, left panel, middle lane) and by coprecipitation of NPM-ALK with anti-p85 antibody (A, B, C, right panel, right lane). A rabbit antimouse antibody (RαM) served as a negative control for the immunoprecipitation. (D) Left panel: 1 × 107 Karpas299 cells were lysed, then incubated with the C- or N-terminal SH2 domain GST-fusion proteins for 1 hour at 4°C. The bound (B) and the flow-through (FT) fractions were collected with glutathione-Sepharose, subjected to 7.5% SDS-PAGE, and analyzed by anti-ALK immunoblotting. Right panel: Lysates of 1 × 107 Ba/F3 cells cultured with and without IL-3 for 2 hours as indicated were incubated with the N-terminal p85 SH2 domain or GST as control for 1 hour at 4°C and the bound fractions were collected with glutathione-Sepharose. Input and bound fractions were subjected to 7.5% SDS-PAGE, and analyzed by antiphosphotyrosine immunoblotting.
NPM-ALK and p85 form a complex in vivo via the C-terminal SH2 domain of p85.
The 2 × 107 Ba/F3 cells stably transfected with NPM-ALK (A) or 2 × 107 Karpas299 cells (B) or 2 × 107SUP-M2 cells (C) were subjected to immunoprecipitations and immunoblotting with the antibodies indicated. Association of NPM-ALK with p85 is demonstrated by coprecipitation of p85 with an NPM-ALK antibody (A and B, left panel, right lane; C, left panel, middle lane) and by coprecipitation of NPM-ALK with anti-p85 antibody (A, B, C, right panel, right lane). A rabbit antimouse antibody (RαM) served as a negative control for the immunoprecipitation. (D) Left panel: 1 × 107 Karpas299 cells were lysed, then incubated with the C- or N-terminal SH2 domain GST-fusion proteins for 1 hour at 4°C. The bound (B) and the flow-through (FT) fractions were collected with glutathione-Sepharose, subjected to 7.5% SDS-PAGE, and analyzed by anti-ALK immunoblotting. Right panel: Lysates of 1 × 107 Ba/F3 cells cultured with and without IL-3 for 2 hours as indicated were incubated with the N-terminal p85 SH2 domain or GST as control for 1 hour at 4°C and the bound fractions were collected with glutathione-Sepharose. Input and bound fractions were subjected to 7.5% SDS-PAGE, and analyzed by antiphosphotyrosine immunoblotting.
NPM-ALK activates PI 3-kinase and Akt
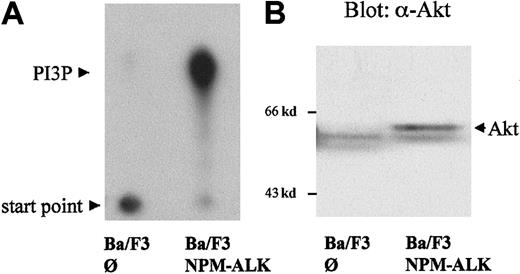
After stimulation through tyrosine kinases, PI 3-kinase generates 3-phosphoinositides (PI3P), which function as important intracellular lipid messengers.25,26 As shown in Figure2A, stable expression of NPM-ALK in Ba/F3 cells (Ba/F3 NPM-ALK), a murine pro-B-cell line, led to the generation of significant amounts of PI3P compared with parental Ba/F3 cells transfected with empty vector (Ba/F3∅) in cells cultured without IL-3. PI 3-kinase activity was measured on phosphotyrosine immunoprecipitates. Wortmannin treatment of NPM-ALK–expressing Ba/F3 cells resulted in a decrease of signal confirming that the product measured is PI3P (data not shown and Figure3D). Activation of PI 3-kinase has been shown to elicit an antiapoptotic signal through the activation of PKB/Akt.40 Therefore, we wished to determine the activation status of Akt in NPM-ALK–expressing cells. As shown in Figure 2B, a mobility shift of Akt in SDS-PAGE was demonstrable in NPM-ALK–expressing cells compared with parental cells. This mobility shift indicates phosphorylation and activation of Akt.41
Activation of PI 3-kinase and Akt by NPM-ALK.
The 5 × 106 Ba/F3 cells, stably transfected with empty vector (∅) or NPM-ALK, were starved for 4 hours in medium containing 0.5% FCS without IL-3. (A) PI 3-kinase activity was measured by incubation of antiphosphotyrosine (4G10) immunoprecipitates with phosphatidylinositol and P32-γg-ATP. P32-labeled–enzymatic products of PI 3-kinase were resolved by thin-layer chromatography (TLC) using a silica gel plate as described in “Materials and methods,” and visualized by autoradiography. (B) Cell lysates were subjected to 7.5% SDS-PAGE and immunoblotted using a goat polyclonal anti-Akt antibody. Mobility shift of Akt indicates its activation.
Activation of PI 3-kinase and Akt by NPM-ALK.
The 5 × 106 Ba/F3 cells, stably transfected with empty vector (∅) or NPM-ALK, were starved for 4 hours in medium containing 0.5% FCS without IL-3. (A) PI 3-kinase activity was measured by incubation of antiphosphotyrosine (4G10) immunoprecipitates with phosphatidylinositol and P32-γg-ATP. P32-labeled–enzymatic products of PI 3-kinase were resolved by thin-layer chromatography (TLC) using a silica gel plate as described in “Materials and methods,” and visualized by autoradiography. (B) Cell lysates were subjected to 7.5% SDS-PAGE and immunoblotted using a goat polyclonal anti-Akt antibody. Mobility shift of Akt indicates its activation.
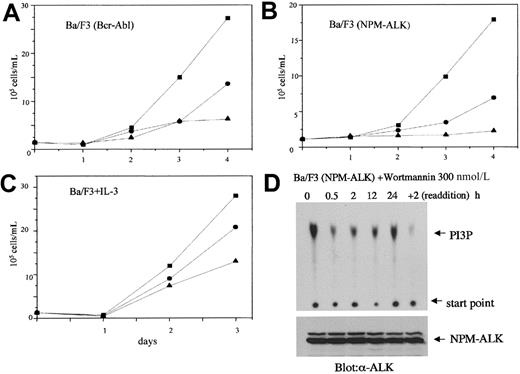
Effect of PI 3-kinase inhibitors on the proliferation of NPM-ALK–transformed cells.
Bcr-Abl–transformed Ba/F3 (A), NPM-ALK–transformed Ba/F3 (B), and parental Ba/F3 cells cultured in the presence of IL-3 (C) were incubated with wortmannin (●, 100 nmol/L; ▴, 300 nmol/L) or with DMSO as control (▪) with daily addition of wortmannin and DMSO. The Bcr-Abl– and NPM-ALK–transformed Ba/F3 cells were cultured without IL-3. The number of viable cells was determined by trypan blue staining at the days indicated. Results are representative of at least 3 independent experiments. (D) PI 3-kinase activity was measured as described in the legend to Figure 2 at the time points indicated in NPM-ALK–transformed Ba/F3 cells treated with 300 nmol/L wortmannin. After 24 hours, wortmannin was readded, and PI 3-kinase activity determined after 2 additional hours. The lower panel shows equal expression of NPM-ALK in all cell lysates tested.
Effect of PI 3-kinase inhibitors on the proliferation of NPM-ALK–transformed cells.
Bcr-Abl–transformed Ba/F3 (A), NPM-ALK–transformed Ba/F3 (B), and parental Ba/F3 cells cultured in the presence of IL-3 (C) were incubated with wortmannin (●, 100 nmol/L; ▴, 300 nmol/L) or with DMSO as control (▪) with daily addition of wortmannin and DMSO. The Bcr-Abl– and NPM-ALK–transformed Ba/F3 cells were cultured without IL-3. The number of viable cells was determined by trypan blue staining at the days indicated. Results are representative of at least 3 independent experiments. (D) PI 3-kinase activity was measured as described in the legend to Figure 2 at the time points indicated in NPM-ALK–transformed Ba/F3 cells treated with 300 nmol/L wortmannin. After 24 hours, wortmannin was readded, and PI 3-kinase activity determined after 2 additional hours. The lower panel shows equal expression of NPM-ALK in all cell lysates tested.
The growth of NPM-ALK–transformed Ba/F3 cells is suppressed by PI 3-kinase inhibitors
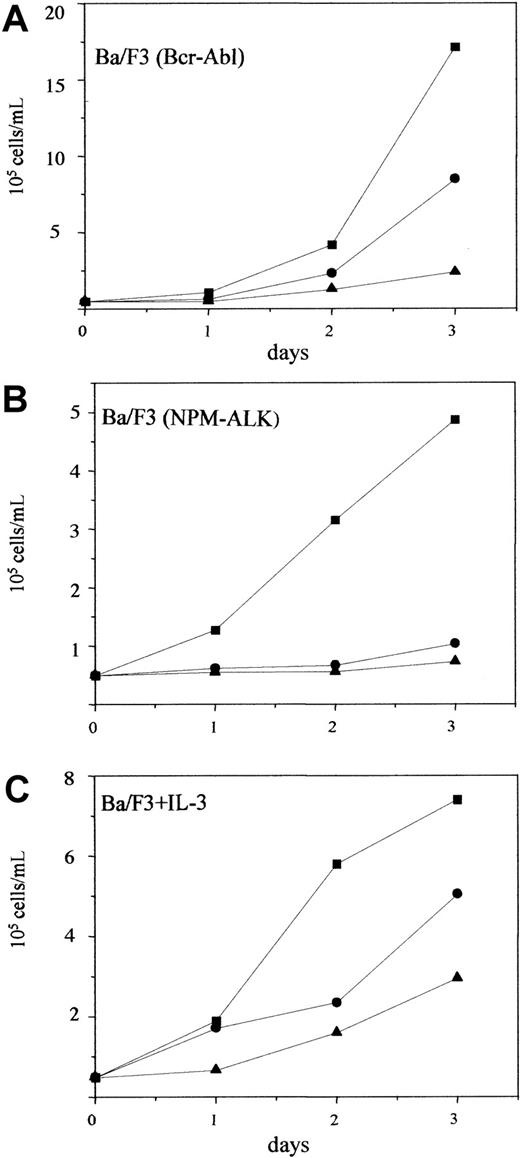
To determine the role of PI 3-kinase in NPM-ALK–initiated transformation of hematopoietic cells, we used the specific PI 3-kinase inhibitor wortmannin42 in proliferation assays with NPM-ALK–transformed Ba/F3 cells. Wortmannin has been used in a number of studies to demonstrate the importance of the PI 3-kinase/Akt pathway for the transforming ability of oncogenic tyrosine kinases. Among others, Bcr-Abl has been demonstrated to use the PI 3-kinase/Akt pathway for its transforming ability.38,43,44 As previously published, and shown in Figure 3A, wortmannin at 200 nmol/L inhibited the growth of Bcr-Abl–transformed Ba/F3 cells by 50%. Similarly, wortmannin also suppressed the growth of NPM-ALK–transformed Ba/F3 cells at comparable concentrations (Figure3B). At 300 nmol/L, wortmannin completely inhibited the growth of NPM-ALK–transformed Ba/F3 cells (Figure 3B). The PI 3-kinase/Akt/Bad pathway has been reported to function as one of the antiapoptotic signals initiated by IL-3 in Ba/F3 cells.34 Accordingly, wortmannin also inhibited the growth of parental Ba/F3 cells cultured in the presence of IL-3 (Figure 3C). However, in contrast to NPM-ALK–transformed Ba/F3 cells, the proliferation of parental Ba/F3 cells treated with 300 nmol/L wortmannin in the presence of IL-3 was reduced only by 50% (Figure 3C). Daily addition of wortmannin was sufficient to inhibit PI 3-kinase activity in NPM-ALK–expressing Ba/F3 cells (Figure 3D). These effects are likely due to the specific inhibition of the PI 3-kinase pathway because similar data were observed with another PI 3-kinase inhibitor, LY29400245(Figure 4). Thus, the strong suppression of NPM-ALK–transformed Ba/F3 cells by PI 3-kinase inhibitors emphasized the importance of the PI 3-kinase pathway in these cells.
Effect of Ly294002 on the proliferation of NPM-ALK–transformed cells.
Bcr-Abl–transformed Ba/F3 (A), NPM-ALK–transformed Ba/F3 (B), and parental Ba/F3 cells cultured in the presence of IL-3 (C) were incubated with LY294002 (●, 7 μmol/L; ▴, 10 μmol/L) or with DMSO as control (▪). The number of viable cells was determined by trypan blue staining at the days indicated. Results are representative of at least 3 independent experiments.
Effect of Ly294002 on the proliferation of NPM-ALK–transformed cells.
Bcr-Abl–transformed Ba/F3 (A), NPM-ALK–transformed Ba/F3 (B), and parental Ba/F3 cells cultured in the presence of IL-3 (C) were incubated with LY294002 (●, 7 μmol/L; ▴, 10 μmol/L) or with DMSO as control (▪). The number of viable cells was determined by trypan blue staining at the days indicated. Results are representative of at least 3 independent experiments.
NPM-ALK–mediated transformation of primary mouse bone marrow cells is suppressed by PI 3-kinase inhibitors
We have previously shown that NPM-ALK can induce soft agar growth of Rat-1 fibroblasts.23 NPM-ALK–induced soft agar growth can be significantly blocked by the single addition of LY294002 in a concentration-dependent manner (Figure5A). Primary mouse bone marrow cells (BM cells) can form colonies in methylcellulose in the presence (Figure 5B, lower left panel) but not in the absence of IL-3 (data not shown).46 Retroviral transfection of NPM-ALK into primary BM cells induces the outgrowth of colonies in the absence of IL-3 in methycellulose (Figure 5B, upper left panel). These colonies were larger than the colonies obtained with IL-3 (compare Figure 5B, upper and lower left panel). A single addition of 10 μmol/L LY294002 prevented the outgrowth of these colonies (Figure 5B, upper right panel). In contrast, addition of LY294002 decreased but did not significantly affect the growth of colonies in the presence of IL-3 (Figure 5B, compare lower right and left panel and absolute colony numbers given in the table). Thus, LY294002 suppresses the growth of NPM-ALK–transduced bone marrow in methylcellulose and this inhibitory effect is not due to unspecific toxicity.
Growth inhibition of transformed fibroblasts and primary bone marrow transduced with NPM-ALK by PI 3-kinase inhibitors.
(A) Rat-1 soft agar assay of NPM-ALK–transfected cells. Rat-1 cells were retrovirally transduced with either empty vector or NPM-ALK as described in the “Materials and methods” section. After 2 days, cells were selected for growth in soft agar medium. Colonies were scored after 12 days. Numbers of colonies represent means ± SD from triplicate experiments. (B) Primary murine bone marrow cells infected with NPM-ALK (upper 2 panels) or control-vector (lower 2 panels) were plated in methyl-cellulose without growth factors (upper 2 panels) or in the presence of 10 pg/mL IL-3 (lower 2 panels). Ten micromolars Ly290004 was added once were indicated. Representative pictures obtained after 10 days of culture from 2 independent experiments are shown. Absolute numbers of colonies are listed in the table. Data represent mean ± SD of 2 independent experiments. (C) Effect of PI3-kinase inhibitor wortmannin on growth of NPM-ALK–infected murine bone marrow (BM) cells in liquid culture. The 1 × 105 BM cells retrovirally transduced with empty vector (∅) or NPM-ALK were plated in BM-medium with or without 100 pg/mL mIL-3 as indicated. Wortmannin (200 nmol/L) was added daily to half of the wells (▪); ■, no wortmannin. The bars represent the percentage of cells present in the supernatant after 6 days of culture and the number of cells infected with control vector and supplemented with IL-3 was arbitrarily set as 100%. Data represent means ± SD of at least 2 independent experiments.
Growth inhibition of transformed fibroblasts and primary bone marrow transduced with NPM-ALK by PI 3-kinase inhibitors.
(A) Rat-1 soft agar assay of NPM-ALK–transfected cells. Rat-1 cells were retrovirally transduced with either empty vector or NPM-ALK as described in the “Materials and methods” section. After 2 days, cells were selected for growth in soft agar medium. Colonies were scored after 12 days. Numbers of colonies represent means ± SD from triplicate experiments. (B) Primary murine bone marrow cells infected with NPM-ALK (upper 2 panels) or control-vector (lower 2 panels) were plated in methyl-cellulose without growth factors (upper 2 panels) or in the presence of 10 pg/mL IL-3 (lower 2 panels). Ten micromolars Ly290004 was added once were indicated. Representative pictures obtained after 10 days of culture from 2 independent experiments are shown. Absolute numbers of colonies are listed in the table. Data represent mean ± SD of 2 independent experiments. (C) Effect of PI3-kinase inhibitor wortmannin on growth of NPM-ALK–infected murine bone marrow (BM) cells in liquid culture. The 1 × 105 BM cells retrovirally transduced with empty vector (∅) or NPM-ALK were plated in BM-medium with or without 100 pg/mL mIL-3 as indicated. Wortmannin (200 nmol/L) was added daily to half of the wells (▪); ■, no wortmannin. The bars represent the percentage of cells present in the supernatant after 6 days of culture and the number of cells infected with control vector and supplemented with IL-3 was arbitrarily set as 100%. Data represent means ± SD of at least 2 independent experiments.
We also performed cell transformation assays in stromal-cell–dependent cultures.39 In this assay, the oncogene-induced outgrowth of cells on a stromal layer in the absence of growth factors is measured. As shown in Figure 5C, wortmannin treatment does not significantly affect the outgrowth of cells in the presence of Il-3 (left 4 panels). However, in the absence of IL-3, NPM-ALK–induced outgrowth of BM cells is impaired by the treatment with wortmannin (Figure 5C, right 2 panels). Similarly, this implies that the inhibitory effect of wortmannin on NPM-ALK–expressing BM cells is specific and not due to unspecific toxicity.
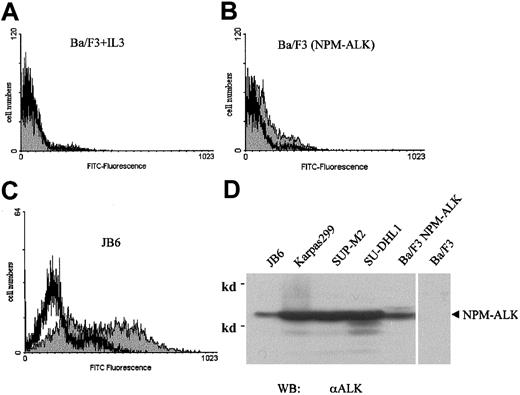
Wortmannin-induced apoptosis in NPM-ALK–transformed cells
Wortmannin has been demonstrated to block the antiapoptotic pathway related to PI 3-kinase/Akt in a variety of cells.47-49 To further investigate the growth-inhibitory effect of wortmannin on NPM-ALK–transformed cells, we performed apoptosis assays to detect fragmented DNA in wortmannin-treated cells. DNA fragments were FITC-fluorescein–labeled and quantified using a flow cytometer. Incubation of parental Ba/F3 cells grown with IL-3 in 300 nmol/L wortmannin for 3 days (the same conditions used in Figure3A-C) did not induce any detectable DNA fragmentation in this assay (Figure 6A). By contrast, the same treatment of NPM-ALK–transformed Ba/F3 cells led to a significant shift to apoptotic DNA fragmentation in these cells (compare Figure 6B and 6A). Consistent with this result, wortmannin also induced DNA fragmentation in JB6 cells, a cell line established from a patient with ALCL that has been shown to express NPM-ALK (Figure 6C). The level of NPM-ALK expression of JB-6 cells is comparable to Ba/F3 cells expressing NPM-ALK and somewhat lower than in other NPM-ALK–positive cell lines established from patients with ALCL (Figure 6D).
Wortmannin induces apoptosis in NPM-ALK–transformed cells.
Parental Ba/F3 (A), NPM-ALK–transformed Ba/F3 (B), and the ALCL cell line JB6 (C) were incubated with 300 nmol/L wortmannin (░) or DMSO as control (■) for 3 days, with daily addition of wortmannin and DMSO. The Ba/F3 cells were cultured with murine IL-3 as growth factor. The exposed 3′-OH ends of DNA fragments generated in response to apoptotic signals were FITC-fluorescein–labeled by terminal deoxynucleotidyl transferase (TdT). Results were evaluated using a flow cytometer. The expression of NPM-ALK in cells established from patients with LCAL (JB6, Karpas299, SUP-M2, SU-DHL1) and in parental Ba/F3 cells and Ba/F3 cells expressing NPM-ALK was shown by anti-ALK immunoblot (D).
Wortmannin induces apoptosis in NPM-ALK–transformed cells.
Parental Ba/F3 (A), NPM-ALK–transformed Ba/F3 (B), and the ALCL cell line JB6 (C) were incubated with 300 nmol/L wortmannin (░) or DMSO as control (■) for 3 days, with daily addition of wortmannin and DMSO. The Ba/F3 cells were cultured with murine IL-3 as growth factor. The exposed 3′-OH ends of DNA fragments generated in response to apoptotic signals were FITC-fluorescein–labeled by terminal deoxynucleotidyl transferase (TdT). Results were evaluated using a flow cytometer. The expression of NPM-ALK in cells established from patients with LCAL (JB6, Karpas299, SUP-M2, SU-DHL1) and in parental Ba/F3 cells and Ba/F3 cells expressing NPM-ALK was shown by anti-ALK immunoblot (D).
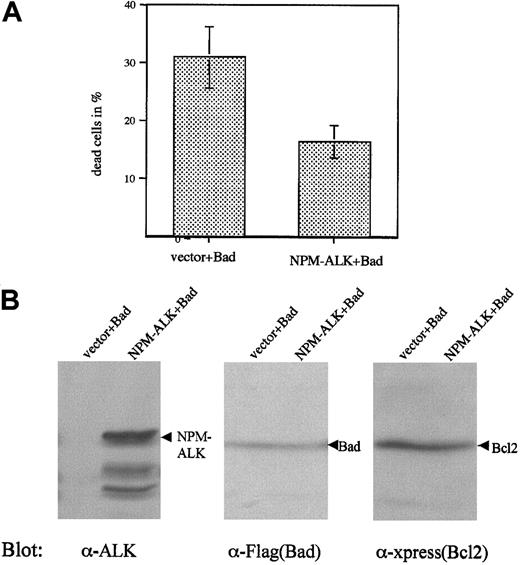
Suppression of Bad-induced apoptosis by NPM-ALK
One mechanism of apoptosis inhibition by PI 3-kinase and Akt is phosphorylation and inactivation of the proapoptotic protein Bad. Phosphorylation of Bad inhibits apoptosis by preventing the protein from binding to and inhibiting the antiapoptotic proteins Bcl2 and Bcl-xL.33,50 Thus, overexpression of Bad in cells leads to apoptosis that can be antagonized by overexpression of constitutively activated Akt or PAK (p21-activated protein kinase), both of which are able to phosphorylate Bad.51,52 Therefore, we tested the ability of NPM-ALK to block Bad-induced apoptosis. The overexpression of Bad in Cos1 cells led to almost total cell death in the early stage of the transfection (data not shown). Therefore, we cotransfected Bcl2 with Bad to obtain viable cells.34 This approach allowed us to control the expression of Bad and to rule out the possibility that differences in apoptosis might be due to inhibition of Bad expression by NPM-ALK. Even when cotransfected with Bcl2, overexpression of Bad in Cos1 cells led to death of roughly 30% of cells (Figure 7A). Coexpression of NPM-ALK could rescue part of the cells (Figure 7A). Immunoblots showed comparable expression levels of Bad and Bcl2 in these cells (Figure7B). Thus, NPM-ALK was capable of partially preventing Bad-induced apoptosis in Cos1 cells.
NPM-ALK partially rescues Cos1 cells from Bad-induced apoptosis.
Cos1 cells were transfected with Xpress-tagged Bcl2 (xp-Bcl2) and Flag-tagged Bad (Flag-Bad), plus either empty vector or NPM-ALK. Three days after the transfection, both floating and attached cells were collected and living cells determined by trypan blue staining (A). Data represent values ± SD of at least 3 independent experiments. Expression of NPM-ALK (left panel), Bad (middle panel), and cotransfected Bcl2 (right panel) was demonstrated by immunoblotting (B).
NPM-ALK partially rescues Cos1 cells from Bad-induced apoptosis.
Cos1 cells were transfected with Xpress-tagged Bcl2 (xp-Bcl2) and Flag-tagged Bad (Flag-Bad), plus either empty vector or NPM-ALK. Three days after the transfection, both floating and attached cells were collected and living cells determined by trypan blue staining (A). Data represent values ± SD of at least 3 independent experiments. Expression of NPM-ALK (left panel), Bad (middle panel), and cotransfected Bcl2 (right panel) was demonstrated by immunoblotting (B).
Phosphopeptide competition identifies NPM-ALK-Tyr418 as a potential binding site for the p85-CSH2 domain.
To further define the importance of the PI 3-kinase pathway for the transforming activity of NPM-ALK, we attempted to identify the tyrosine-containing motif of NPM-ALK responsible for recruitment and activation of PI 3-kinase. A series of 11-residue-long peptides encompassing putative tyrosine autophosphorylation sites in NPM-ALK was synthesized with Fmoc-protected phosphotyrosine, and phosphopeptide competition experiments were performed. Binding studies with the C-terminal SH2 domain of p85 and lysates of NPM-ALK–expressing Karpas299 cells were performed in the presence of 200 μmol/L competing phosphopeptides. Among 21 phosphopeptides tested, only one totally blocked the binding of the p85-CSH2 domain to NPM-ALK (data not shown). The putative NPM-ALK autophosphorylation site Y418 with the sequence PGPVpY418RIMTQ matches the high-affinity consensus-binding motif “pYXXM” for the p85-SH2 domain.53 Thus, these studies suggested that Y418 is the tyrosine residue potentially responsible for recruiting PI 3-kinase.
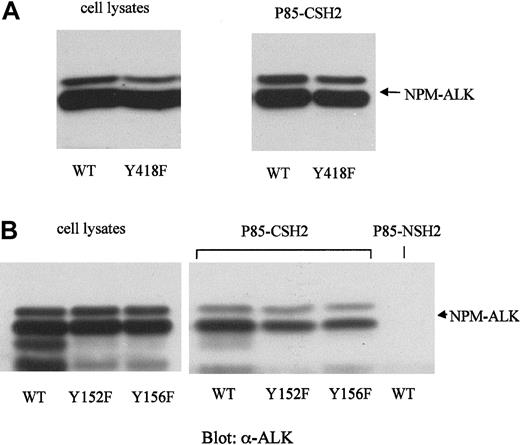
Mutation of Y418 of NPM-ALK does not prevent the binding of p85-CSH2
To test whether Y418 is in fact the binding site for the p85-CSH2 domain, we constructed a tyrosine-to-phenylalanine mutant of NPM-ALK (NPM-ALK-Y418F). WT-NPM-ALK and NPM-ALK-Y418F were stably expressed in Ba/F3 at comparable levels (Figure 8A, left panel). However, mutation of Tyr418 did not significantly affect the binding affinity of NPM-ALK to the p85-CSH2 domain (Figure 8A, right panel).
Mutations of the potential p85-binding site (Y418) and the binding site for IRS-1 (Y152 and Y156) have no effect on the binding of NPM-ALK with the p85-CSH2 domain.
Mutants of NPM-ALK were generated by site-directed mutagenesis as described in “Materials and methods.” The wild-type or mutated NPM-ALK constructs were transfected into Ba/F3 cells and stably selected by G418. The expression levels of the different NPM-ALK constructs were demonstrated by anti-ALK immunoblotting of the total cell lysates (A and B, left panels). For each binding experiment, 5 × 106 Ba/F3 cell lysates were incubated with p85-CSH2 or p85-NSH2 GST-fusion protein for 1 hour at 4°C. The bound protein complexes were collected with glutathione-beads, washed thoroughly, and resolved by 7.5% SDS-PAGE. Immunoblotting was performed with anti-ALK antibody (A and B, right panels).
Mutations of the potential p85-binding site (Y418) and the binding site for IRS-1 (Y152 and Y156) have no effect on the binding of NPM-ALK with the p85-CSH2 domain.
Mutants of NPM-ALK were generated by site-directed mutagenesis as described in “Materials and methods.” The wild-type or mutated NPM-ALK constructs were transfected into Ba/F3 cells and stably selected by G418. The expression levels of the different NPM-ALK constructs were demonstrated by anti-ALK immunoblotting of the total cell lysates (A and B, left panels). For each binding experiment, 5 × 106 Ba/F3 cell lysates were incubated with p85-CSH2 or p85-NSH2 GST-fusion protein for 1 hour at 4°C. The bound protein complexes were collected with glutathione-beads, washed thoroughly, and resolved by 7.5% SDS-PAGE. Immunoblotting was performed with anti-ALK antibody (A and B, right panels).
In addition, we constructed 2 other mutants of NPM-ALK, NPM-ALK-Y152F, and NPM-ALKY156F. Tyr156 of NPM-ALK is the published binding site for IRS-1.22,54 IRS-1 has been shown to recruit the adaptor molecules p85 and Grb2.55,56 Therefore, we speculated that NPM-ALK may activate PI 3-kinase by recruiting IRS-1. However, in GST pull-down experiments, both NPM-ALK-Y152F and -Y156F bound to the p85-CSH2 domain (Figure 8B, right panel). Furthermore, we constructed 18 additional tyrosine-to-phenylalanine mutants of NPM-ALK representing almost all potential autophosphorylation sites (data not shown).23 None of these point mutations abolished the binding of the p85 SH2 domain to NPM-ALK. Together, these results show that none of these single sites could be solely responsible for the binding of NPM-ALK to the p85-CSH2 domain.
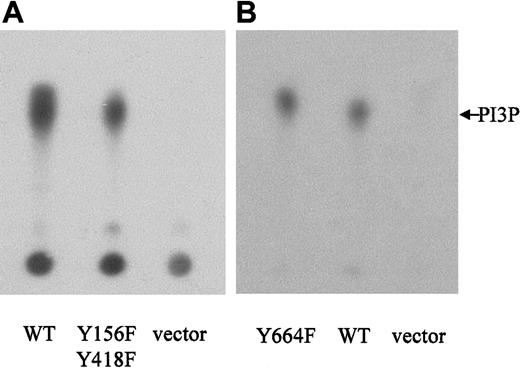
The NPM-ALK-Y156F-Y418F and -Y664F mutants activate PI 3-kinase
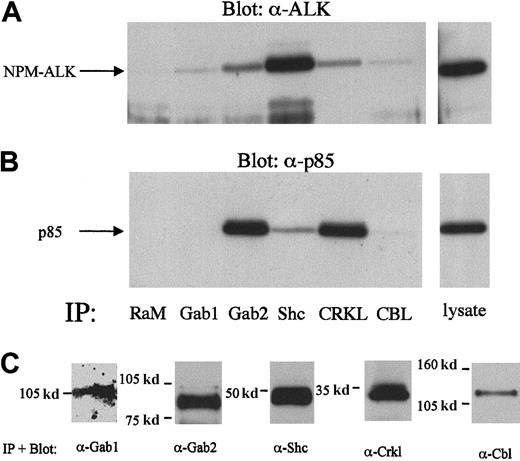
Because none of our single tyrosine-to-phenylalanine mutations was able to abolish the binding of the p85-CSH2 domain to NPM-ALK, we constructed a double mutant containing tyrosine-to-phenylalanine substitutions of both Tyr156 and Tyr418. However, PI 3-kinase activity in cells expressing the double-mutant NPM-ALK or WT-NPM-ALK was comparable (Figure 9A), indicating that these phosphorylation sites are not essential for the activation of PI 3-kinase. Therefore, it is possible that an intermediary protein is involved in the complex formation of NPM-ALK and PI 3-kinase. Indeed, among several adaptor proteins tested, Gab2, Shc, and Crkl form a complex simultanously with NPM-ALK and p85 in KARPAS299 cells (Figure10). Interestingly, Crkl and Gab2 but not Gab1 coprecipitated p85 more strongly than NPM-ALK, whereas Shc preferentially precipitated NPM-ALK. Thus, Gab2, Crkl, and Shc are candidates mediating activation of PI 3-kinase by an oncogenic tyrosine kinase.
NPM-ALK-Y156F-Y418F and NPM-ALK-Y664F retain the ability to activate PI 3-kinase.
Ba/F3 cells were stably transfected with WT-NPM-ALK, NPM-ALK tyrosine-to-phenylalanine mutants, as indicated, or pCDNA3 vector only. PI 3-kinase activity was measured by incubation of antiphosphotyrosine (4G10) immunoprecipitates with phosphatidylinositol and32P-γ-ATP. 32P-labeled–enzymatic products of PI 3-kinase were separated by TLC on a silica gel plate and visualized by autoradiography.
NPM-ALK-Y156F-Y418F and NPM-ALK-Y664F retain the ability to activate PI 3-kinase.
Ba/F3 cells were stably transfected with WT-NPM-ALK, NPM-ALK tyrosine-to-phenylalanine mutants, as indicated, or pCDNA3 vector only. PI 3-kinase activity was measured by incubation of antiphosphotyrosine (4G10) immunoprecipitates with phosphatidylinositol and32P-γ-ATP. 32P-labeled–enzymatic products of PI 3-kinase were separated by TLC on a silica gel plate and visualized by autoradiography.
Complex formation of Gab2, Shc, and Crkl with NPM-ALK and p85 in Karpas299 cell lysates.
The 2 × 107 Karpas299 cells were subjected to immunoprecipitations with the anti-Gab1,-Gab2,-Shc,-Crkl, or –Cbl antibodies and subjected to anti-NPM-ALK or anti-p85 immunoblotting as described in the “Materials and methods.” Association of NPM-ALK with Gab1, Gab2, Shc, Crkl, and Cbl are demonstrated by the anti-NPM-ALK immunoblotting (A, left panel). Anti-p85 immunoblotting showed the complex formation of Gab2, Shc, and Crkl with p85 (B, left panel). A rabbit antimouse antibody (RαM) served as a negative control for the immunoprecipitation. Immunoblotting with anti-Gab1,-Gab2,-Shc,-Crkl, or -Cbl antibodies demonstrated the immunoprecipitated proteins (C).
Complex formation of Gab2, Shc, and Crkl with NPM-ALK and p85 in Karpas299 cell lysates.
The 2 × 107 Karpas299 cells were subjected to immunoprecipitations with the anti-Gab1,-Gab2,-Shc,-Crkl, or –Cbl antibodies and subjected to anti-NPM-ALK or anti-p85 immunoblotting as described in the “Materials and methods.” Association of NPM-ALK with Gab1, Gab2, Shc, Crkl, and Cbl are demonstrated by the anti-NPM-ALK immunoblotting (A, left panel). Anti-p85 immunoblotting showed the complex formation of Gab2, Shc, and Crkl with p85 (B, left panel). A rabbit antimouse antibody (RαM) served as a negative control for the immunoprecipitation. Immunoblotting with anti-Gab1,-Gab2,-Shc,-Crkl, or -Cbl antibodies demonstrated the immunoprecipitated proteins (C).
We have previously demonstrated that Tyr664 of NPM-ALK is an in vivo autophosphorylation site that is necessary for the activation of PLC-γ. Mutation of this tyrosine residue severely impairs the mitogenicity of NPM-ALK.23 We wished to determine whether this Y664F mutant exhibited PI 3-kinase activity in Ba/F3 cells. The results illustrated in Figure 9B indicate that Tyr664, responsible for activation of PLC-γ by NPM-ALK, is not involved in the PI 3-kinase signaling pathway.
Discussion
The oncoprotein NPM-ALK has been shown to transform cells by recruiting specific signaling molecules, such as Grb2, IRS-1, Shc, and PLC-γ that are important for the regulation of growth and differentiation in hematopoietic cells.21-23,57 In a previous study, we were able to demonstrate an important role of PLC-γ for the NPM-ALK–dependent transformation of lymphoid cells.23 Experiments with NPM-ALK mutants unable to recruit and activate PLC-γ revealed that this pathway seems to be important predominantly for a mitogenic response. However, NPM-ALK expression in lymphocytes also prevented apoptosis after IL-3 withdrawal, which was not mediated by PLC-γ. Thus, an antiapoptotic pathway initiated by NPM-ALK and independent from PLC-γ is likely to exist.
PI 3-kinase and its downstream target PKB/Akt have been shown to play an important role for the antiapoptotic function of IL-3 in lymphocytes.32,58,59 Akt itself is involved in the phosphorylation of Bad, activation of NF-κB, inhibition of caspase-9, and reduction of the transcription of the Fas ligand, all of which contribute to its antiapoptotic function.60 In addition, PI 3-kinase has been demonstrated to be involved in the signaling cascade initiated by oncogenes like Bcr-Abl, leading to hematopoietic malignancies.38,40,43,44 61 To investigate a potential role for PI 3-kinase and its downstream effectors in the transforming activity of NPM-ALK, we first demonstrated the complex formation of p85 and NPM-ALK in vivo by coimmunoprecipitation. We were able to show that this complex is mediated mainly by the C-terminal SH2 domain of the regulatory subunit p85 of PI 3-kinase, whereas the N-terminal SH2 domain seems not to be important for the interaction.
PI 3-kinase assays revealed the activation of PI 3-kinase by NPM-ALK and immunoblot analysis indicated the phosphorylation/activation of Akt by NPM-ALK. The specific PI 3-kinase inhibitors wortmannin and LY294002 were able to block NPM-ALK–induced IL-3–independent proliferation of Ba/F3 lymphocytes. Furthermore, these inhibitors prevented NPM-ALK–induced IL-3–independent growth and transformation of primary mouse BM cells. In addition, a clearly recognizable population of NPM-ALK–transformed Ba/F3 cells and cells from a patient with ALCL underwent apoptosis upon wortmannin treatment. Together, these data indicate a role for PI 3-kinase in the antiapoptotic function of NPM-ALK, both in vitro and in ALCL. Interestingly, parental Ba/F3 lymphocytes and primary mouse BM cells supplemented with IL-3 were not significantly affected by wortmannin concentrations already showing an inhibitory effect on NPM-ALK–expressing cells, although the IL-3–induced antiapoptotic phosphorylation of Bad through Akt is well established.33,34 However, there are reports showing that overexpression of dominant-negative p85 or treatment with wortmannin in IL-3–dependent cells can result in inhibition of Bad phosphorylation, but fail to induce apoptosis.62 63 Thus, this pathway seems to be indispensable for NPM-ALK–induced prevention of apoptosis but not for IL-3–induced inhibition of apoptotic death.
The apoptotic cascade is triggered by the loss of integrity of the outer mitochondrial membrane accompanied by release of cytochrome c, which is able to activate protease-activating factor and subsequently the caspase cascade.64 Akt is able to prevent caspase activation by maintaining mitochondrial integrity, and presumably, at least in part, by regulation of the proapoptotic Bcl2 family member Bad.51 Akt phosphorylates Bad and thereby deactivates its proapoptotic function.33 Further, it has been shown that overexpression of Bad can lead to apoptosis in a wide variety of cell lines,51,52,65 and that this induction of apoptosis can be inhibited by overexpression of constitutively activated Akt.51 NPM-ALK counteracted the apoptotic effects of Bad and partially rescued Cos1 cells from Bad-induced death. These data clearly revealed the antiapoptotic function of NPM-ALK, which is likely initiated through the PI 3-kinase/Akt pathway.
We have also observed that Karpas299 cells are totally resistant to apoptotic signals triggered by anti-CD95 antibody although this ALCL cell line expresses CD95 after treatment with anti-CD3 antibody (R.-Y.B. and J.D., unpublished observations, 2000). This finding is consistent with previous reports showing protection of CD95-mediated apoptosis by activation of the PI 3-kinase/Akt pathway in T-leukemia cells66 and the ability of Bcr-Abl, which also activates PI 3-kinase and Akt, to block CD95-induced apoptosis.67
In our efforts to identify the binding site of the P85-CSH2 domain on NPM-ALK, we first identified NPM-ALK-Y418 as the only potential binding site in phosphopeptide competition assays. However, mutation of this site failed to abolish binding in cell lysates. A possible explanation might be that tyrosine residue 418 is not autophosphorylated or it is not accessible to the p85-SH2 domain in vivo. An indirect interaction of NPM-ALK with p85 through other adaptor molecules has to be taken into consideration. NPM-ALK-Tyr156 has been described as an IRS-1 binding site.22 IRS-1 can recruit PI 3-kinase and could thus serve as an adaptor for NPM-ALK. However, even double mutations of Tyr418F and Tyr156F did not reduce the binding affinity of NPM-ALK for p85 or the PI 3-kinase activity in NPM-ALK–expressing Ba/F3 cells. Given the numerous adaptor molecules that can function as linkers between tyrosine kinases and SH2 domains of downstream proteins, like Crk, Crkl, and Cbl,64-71 a complex involving p85, NPM-ALK, and an adaptor molecule is likely. Indeed, we can demonstrate a simultaneous complex among NPM-ALK, p85, and Gab2 or Shc or Crkl, respectively. Thus, Gab2, Crkl, and Shc are candidates linking NPM-ALK to PI 3-kinase via the regulatory subunit p85. Further studies are necessary to exactly determine the role of this adaptor proteins for NPM-ALK–mediated oncogenicity.
We have demonstrated a mechanism by which the oncoprotein NPM-ALK triggers an antiapoptotic signal via PI 3-kinase/Akt. Interestingly, the v-Akt sequence was originally isolated from murine thymic lymphomas.72 This constitutively activated v-Akt is capable of initiating thymic lymphomas after inoculation of susceptible mouse strains and revealed high oncogenicity in the T-cell line 5675.73 74 Because NPM-ALK is associated with T-cell lymphomas, the activation status of Akt in tumor material from patients with ALCL should be interesting to determine. Specific therapies designed to inhibit PI 3-kinase activity might then be of potential interest for the treatment of NPM-ALK–related ALCL.
Acknowledgments
We are grateful to K. Schwarz for her crucial help in apoptotic analysis. We thank P. Seipel for excellent technical assistance.
Supported by a grant from the Wilhelm-Sander Stiftung and Mildred-Scheel Stiftung to J.D., by SFB grant No. 456 to J.D. and C.P. and National Cancer Institute (NCI) grant CA69129 and CORE grant CA21765 to S.W.M., and the American-Lebanese Associated Charities (ALSAC), St Jude Children's Research Hospital.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Justus Duyster, Department of Internal Medicine III, Laboratory of Leukemogenesis, Technical University of Munich, Ismaningerstr 22, 81675 Munich, Germany; email:justus.duyster@lrz.tum.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal