Abstract
Infection with hepatitis C virus (HCV) or human immunodeficiency virus (HIV) or both is common in hemophiliac patients due to putative transmission through clotting factor concentrates. Recently, highly active antiretroviral therapy (HAART) has been found to markedly improve viremia and immunologic parameters in patients infected with HIV. This report considers interactions between these viral infections, the immune system, and antiretroviral therapy. A total of 130 male hemophiliac patients were grouped according to type of viremia (HCV, HIV, both, or neither). Along with 30 healthy men age-matched to viremic patients, these groups were compared with respect to viral load and immunologic parameters. Thirty-five patients treated as above for HIV were serially followed up. HCV infection was associated with reduced peripheral B-cell and CD4+-cell counts and with increased serum IgG and IgM levels, whereas HIV infection was associated with reduced peripheral CD4+-cell counts and increased serum IgG and IgA levels. In patients with both viruses, HCV and HIV RNA load correlated inversely with peripheral B-cell and CD4+-cell counts, respectively. HAART reduced levels of both viruses in the blood. Of the 25 patients with both viruses, HAART eliminated HCV in 2. In conclusion, immunologic dynamics differed between hemophiliac patients infected with HCV, HIV, or both. The relative dynamics of HCV viral load, peripheral B-cell count, and serum IgM level were similar to those of HIV viral load, CD4+-cell count, and serum IgA.
Introduction
Clotting factor concentrates such as factor VIII and factor IX have been used as replacement therapy in hemophiliac patients to control hemorrhagic episodes. Because of viral contamination of clotting factor concentrates with such viruses as hepatitis C virus (HCV) and human immunodeficiency virus (HIV), many hemophiliac patients were infected with the viruses in Japan before blood product screening tests for both viruses were implemented in 1986 for HIV and in 1989 for HCV. As a result, infection with HCV, HIV, or both is common among hemophiliac patients.
Previous studies1 have shown that in patients coinfected with HCV and HIV, HIV infection accelerates the clinical and histologic course of chronic hepatitis C. Recently, combination antiretroviral therapy including a protease inhibitor, termed highly active antiretroviral therapy (HAART), has been found to markedly improve prognosis for patients with HIV infection.2,3 The consequent dramatic improvement in immune status may result in significant survival benefit. As a result, the relative importance of HCV-related liver disease as a cause of morbidity and mortality in individuals coinfected with HCV and HIV is increasing. Unfortunately, accumulated data indicate that antiretroviral therapy for HIV infection cannot alter replication of HCV in patients with both viruses even when immunologic improvement is achieved.4-7
Certain kinds of viruses can achieve persistent infection in a host by mechanisms that permit them to evade the host immune system. For example, HIV directly infects CD4+ cells, which are central to host immune defenses, hepatitis B virus (HBV)–related chronic infection is established in infants whose immune system is immature, and human herpes simplex virus becomes latent within nerve cells, so that immune cells cannot detect it. However, the mechanism permitting persistent infection with HCV is incompletely understood. This report describes 3 informative sets of observations in hemophiliac patients: differing immunologic dynamics between patients infected with HCV, HIV, or both; beneficial effects of HAART on HCV in coinfected patients, which contrasts with previous reports; and an account of 2 patients whose HCV viremia was resolved with HAART. In general, the host immune system interferes with viral infection of the target organ in a complex manner, obscuring the basic interaction between the virus and its cellular target. Immune impairment, as in HIV infection, can partly unmask this interaction. From this viewpoint, the present observations may shed light on the mechanism of HCV persistence.
Patients, materials, and methods
Patients
Male hemophiliac patients treated at our hospital were screened for serum antibody to HIV, and presence of HIV was confirmed in antibody-positive patients by the detection of HIV RNA in serum, as described below. All patients with anti-HIV antibody also had detectable HIV RNA with quantitative assay. Given the possibility that HIV infection might alter the anti-HCV antibody response,8both anti-HCV antibody and serum HCV RNA were measured in all patients. Although no anti-HCV–negative patients were positive for HCV RNA, some anti-HCV–positive patients were negative for HCV RNA. Because all of the latter patients repeatedly showed normal serum transaminase levels and remained negative for HCV RNA for at least 3 years, we concluded that they had been infected transiently in the past and that the viremia had resolved. We considered them to be free from HCV infection (ie, HCV negative). A total of 130 hemophiliac patients were divided into 4 groups according to their HCV and HIV viremia status (Table1, groups A1, B, C, and D). Because HCV- and HIV-negative patients (group A1) were significantly younger than patients in the other groups, we also recruited 30 age-matched healthy men who were undergoing health screening at our hospital (group A2). Twenty-five of 29 HCV and HIV coinfected hemophiliac patients and all 10 HCV-negative, HIV-positive patients were treated with HAART over 12 months, and these patients were recruited for serial follow-up study. Treatment was performed according to a protocol modified from an international panel recommended by Carpenter and colleagues.9 Informed consent was obtained from all individuals.
Profiles of hemophiliac patients and healthy individuals studied
| Group (HCV/HIV RNA) . | No. . | Age (y)* . | Hemophilia A/B . | Anti-HCV (%) . |
|---|---|---|---|---|
| Hemophiliac patients | ||||
| A1 (−/−) | 31 | 17.3 ± 2.4† | 27/4 | 11 (35.5) |
| B (+/−) | 60 | 32.9 ± 1.4 | 50/10 | 60 (100.0) |
| C (−/+) | 10 | 29.2 ± 2.4 | 7/3 | 10 (100.0) |
| D (+/+) | 29 | 29.6 ± 1.8 | 19/10 | 29 (100.0) |
| Total | 130 | 28.2 ± 1.1 | 103/27 | 110 (84.6) |
| Healthy individuals | ||||
| A2 (−/−) | 30 | 29.3 ± 0.8 | 0/0 | 0 (0.0) |
| Group (HCV/HIV RNA) . | No. . | Age (y)* . | Hemophilia A/B . | Anti-HCV (%) . |
|---|---|---|---|---|
| Hemophiliac patients | ||||
| A1 (−/−) | 31 | 17.3 ± 2.4† | 27/4 | 11 (35.5) |
| B (+/−) | 60 | 32.9 ± 1.4 | 50/10 | 60 (100.0) |
| C (−/+) | 10 | 29.2 ± 2.4 | 7/3 | 10 (100.0) |
| D (+/+) | 29 | 29.6 ± 1.8 | 19/10 | 29 (100.0) |
| Total | 130 | 28.2 ± 1.1 | 103/27 | 110 (84.6) |
| Healthy individuals | ||||
| A2 (−/−) | 30 | 29.3 ± 0.8 | 0/0 | 0 (0.0) |
HCV indicates hepatitis C virus; HIV, human immunodeficiency virus.
Represented by mean ± SE.
P < .05 versus groups B, C, and D.
Methods
Serum antibodies to HCV and HIV were detected with a second-generation HCV enzyme-linked immunosorbent assay (ELISA, Chiron, Emeryville, CA) and with an HIV chemiluminescent enzyme immunoassay (Lumipulse Ortho HIV-1/2, Ortho-Clinical Diagnostics, Tokyo, Japan), respectively. HCV and HIV viremia were quantified using the second version of a commercially available branched DNA probe (bDNA) assay (Quantiplex HCV RNA, Chiron) and the Amplicore HIV monitor test (Roche Molecular Systems, Somerville, NJ), which have detection limits of 0.5 × 106 genome equivalents per milliliter (Eq/mL) and 400 RNA copies per milliliter (copies/mL), respectively. When the values obtained by bDNA assays were less than the detection limit, a qualitative nested reverse transcription-polymerase chain reaction (RT-PCR) for HCV was performed according to Okamoto and associates.10 Genotype of HCV was also determined by a RT-PCR method according to Okamoto and coworkers.11 Serum concentrations of alanine aminotransferase (ALT) and immunoglobulins (IgG, IgA, IgM) as well as peripheral lymphocyte counts were measured by routine clinical laboratory methods. The distributions of B-cell and CD4+-cell markers among lymphocytes were determined with indirect immunofluorescence followed by flow cytometric analysis, and the corresponding cell counts were calculated based on total lymphocyte counts. Whole blood was sampled serially from the patients treated with HAART before and after this therapy was started.
Data are expressed as the mean ± SE. The Student ttest was used to compare the means of normally distributed values, and the Wilcoxon 2-sample test was used to analyze variables that were not normally distributed such as changes in viral RNA levels. In the comparisons of time-dependent values after initiation of HAART, a paired-sample t test was used. Correlations between values were tested with the Spearman rank correlation coefficient.P values less than .05 were considered statistically significant. A statistical software package, Statview 5.0 (SAS Institute, Cary, NC), was used.
Results
Clinical characteristics of patients infected with hemophilia
Patients in the HCV- and HIV-negative group (A1) were significantly younger (P < .05) than those in the other 3 groups (groups B, C, and D) whose ages were similar. Healthy men who were age-matched to these 3 groups contributed an additional virus-free control group (group A2), which was used to confirm baseline values in this study. Among the 130 patients, 110 (84.6%) and 39 (30.0%) were positive for anti-HCV and anti-HIV antibody, respectively. Of note, all the anti-HIV–positive patients were positive for anti-HCV antibody. Among anti-HCV–positive patients, 89 (80.9%) were positive for HCV RNA; the remaining 21 (19.1%) were repeatedly negative.
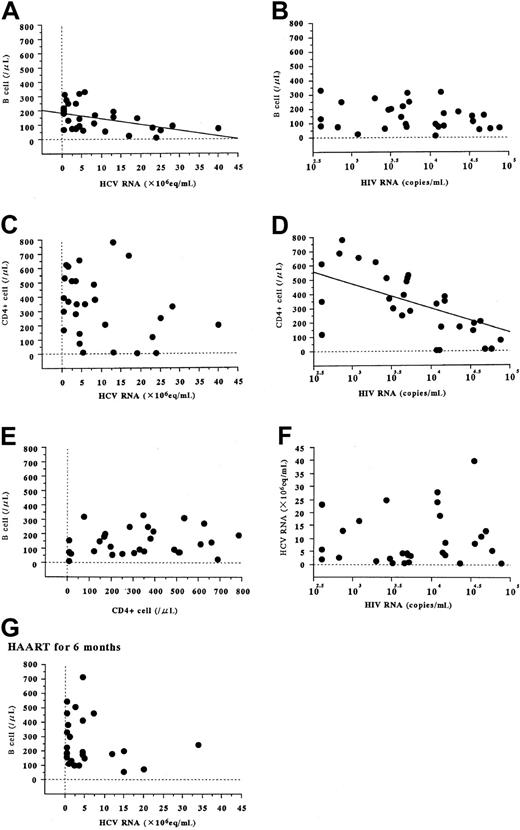
Comparison among groups B, C, and D revealed that HCV coinfection did not result in significant changes in the HIV load (Figure1B), whereas HIV coinfection correlated with increased serum HCV load (Figure 1A, P = .02) and ALT concentration (Figure 1C, P = .005). Comparisons among groups A2, B, and D revealed that HCV infection was associated with a reduction in numbers of peripheral B cells (Figure 1G,P = .0008) and CD4+ cells (Figure 1H,P = .01), and an increase in serum IgG level (Figure 1D,P < .0001). These alterations were intensified further by coinfection with HIV (P = .002 for B cells,P < .0001 for CD4+ cells,P = .01 for IgG level). HCV infection was also associated with increased serum IgM level (Figure 1F, P = .001), which was not significantly increased in association with HIV coinfection. Comparisons among groups A2, C, and D showed that HIV infection was related to a reduction in peripheral CD4+-cell count (Figure 1H, P < .0001), and an increase of serum IgG (Figure 1D, P < .0001) and IgA levels (Figure 1E, P < .0001), whereas coinfection with HCV was not related to a significant accentuation of these changes.
Serum viral RNA levels and immunologic parameters of patients infected with HCV or HIV or both.
Four groups of 130 hemophiliac patients, defined according to HCV and HIV viremia, as well as a group of 30 healthy individuals age-matched to patients with viremia, were studied. The letter designations of the groups represented by the columns are defined in the left column of Table 1. In panels A and B, HCV and HIV viremia was quantified using a bDNA probe assay and the Amplicore HIV monitor test. When the HCV RNA level measured by bDNA assay was below the detection limit (0.5 × 106 Eq/mL), the value was taken as equal to it. Serum levels of ALT (C), immunoglobulin (D, IgG; E, IgA; F, IgM), and peripheral lymphocyte counts were measured by routine ordinary methods. Percentages of B cells and CD4+ cells were determined by indirect immunofluorescence followed by flow cytometric analysis, and respective cell counts (G,H) were calculated from total lymphocyte counts. The columns and vertical bars indicate mean values and SEs, respectively. No significant variations between group A1 and A2 were noted for any immunologic parameter.
Serum viral RNA levels and immunologic parameters of patients infected with HCV or HIV or both.
Four groups of 130 hemophiliac patients, defined according to HCV and HIV viremia, as well as a group of 30 healthy individuals age-matched to patients with viremia, were studied. The letter designations of the groups represented by the columns are defined in the left column of Table 1. In panels A and B, HCV and HIV viremia was quantified using a bDNA probe assay and the Amplicore HIV monitor test. When the HCV RNA level measured by bDNA assay was below the detection limit (0.5 × 106 Eq/mL), the value was taken as equal to it. Serum levels of ALT (C), immunoglobulin (D, IgG; E, IgA; F, IgM), and peripheral lymphocyte counts were measured by routine ordinary methods. Percentages of B cells and CD4+ cells were determined by indirect immunofluorescence followed by flow cytometric analysis, and respective cell counts (G,H) were calculated from total lymphocyte counts. The columns and vertical bars indicate mean values and SEs, respectively. No significant variations between group A1 and A2 were noted for any immunologic parameter.
In patients infected with HCV and HIV (group D), HCV RNA levels correlated inversely with B-cell counts (Figure2A, r = −0.445, P = .02) but not with CD4+-cell counts (Figure 2C, r = −0.340,P = .07). In contrast, HIV RNA levels correlated inversely with CD4+-cell counts (Figure 2D, r = −0.536,P = .005) but not with B-cell counts (Figure 2B, r = −0.220, P = .24). No significant correlation was evident between peripheral B-cell and CD4+-cell counts (Figure 2E, r = 0.204, P = .28) or between serum HCV and HIV RNA levels (Figure 2F, r = 0.108, P = .57).
Correlations between serum viral RNA levels and peripheral B-cell and CD4+-cell counts in patients with both HCV and HIV infections.
These patients compose group D in Table 1. Viral levels and cell numbers were measured as described in Figure 1. When the HCV RNA level measured by bDNA assay was below the detection limit (0.5 × 106 Eq/mL), the value was taken as equal to it. Correlations of B-cell counts with HCV RNA (A, r = −0.445,P = .02) and HIV RNA (B, r = −0.220,P = .24), and correlations of CD4+-cell counts with HCV RNA (C, r = −0.340, P = .07) and HIV RNA (D, r = −0.535, P = .005) in HCV- and HIV-positive patients (group D in Table 1) were analyzed before HAART was started. We further investigated correlations between B-cell and CD4+-cell counts (E, r = 0.204, P = .28), and between HCV and HIV RNA load (F, r = 0.108, P = .57). Correlation between B-cell counts and HCV RNA at 6 months after initiation of HAART (G, r = −0.335, P = .10) is also indicated.
Correlations between serum viral RNA levels and peripheral B-cell and CD4+-cell counts in patients with both HCV and HIV infections.
These patients compose group D in Table 1. Viral levels and cell numbers were measured as described in Figure 1. When the HCV RNA level measured by bDNA assay was below the detection limit (0.5 × 106 Eq/mL), the value was taken as equal to it. Correlations of B-cell counts with HCV RNA (A, r = −0.445,P = .02) and HIV RNA (B, r = −0.220,P = .24), and correlations of CD4+-cell counts with HCV RNA (C, r = −0.340, P = .07) and HIV RNA (D, r = −0.535, P = .005) in HCV- and HIV-positive patients (group D in Table 1) were analyzed before HAART was started. We further investigated correlations between B-cell and CD4+-cell counts (E, r = 0.204, P = .28), and between HCV and HIV RNA load (F, r = 0.108, P = .57). Correlation between B-cell counts and HCV RNA at 6 months after initiation of HAART (G, r = −0.335, P = .10) is also indicated.
In HCV-positive, HIV-negative patients (group B), no significant correlation between B-cell count and HCV RNA load was noted (data not shown).
HAART for HIV infection alters replication of HCV
Irrespective of HCV infection, the peripheral CD4+-cell count was increased after HAART (Figure3D), and this increase was accompanied by a marked decline in HIV RNA load (Figure 3B). In HCV coinfected patients but not in others, HAART was associated with an increase in peripheral B-cell counts (Figure 3C) accompanied by a decrease in the HCV RNA load (Figure 3A); in HCV-negative patients, HAART did not result in variation in B-cell counts (Figure 3C). HAART was associated with a decrease in serum IgM level in HCV-positive patients but not in HCV-negative patients (Figure 3G). In patients coinfected with HCV and HIV, HAART appeared to alter HCV replication, peripheral B-cell counts, and serum IgM levels, but not serum ALT (Figure 3H). At 6 months after initiation of HAART, HCV RNA levels no longer correlated inversely with B-cell counts (Figure 2G).
Mean serum viral RNA levels and immunologic parameters in serial determinations in 35 HIV-infected patients treated with HAART.
Twenty-five of 29 hemophiliac patients infected with both HCV and HIV (●) and all 10 HCV-negative, HIV-positive patients (○) were treated with HAART, and underwent follow-up examinations before and at 6 and 12 months after initiation of HAART. Serum viral RNA levels and various immunologic parameters were measured as described in Figure 1. When the viral RNA level was below the detection limit of the quantitative assays (0.5 × 106 Eq/mL for HCV; 400 copies/mL for HIV), the value was taken to be equal to the limit. Serial changes of serum HCV and HIV RNA levels (A,B), peripheral B-cell and CD4+-cell counts (C,D), serum immunoglobulin levels (E, IgG; F, IgA; G, IgM), and serum ALT levels (H) are indicated as means and SE.
Mean serum viral RNA levels and immunologic parameters in serial determinations in 35 HIV-infected patients treated with HAART.
Twenty-five of 29 hemophiliac patients infected with both HCV and HIV (●) and all 10 HCV-negative, HIV-positive patients (○) were treated with HAART, and underwent follow-up examinations before and at 6 and 12 months after initiation of HAART. Serum viral RNA levels and various immunologic parameters were measured as described in Figure 1. When the viral RNA level was below the detection limit of the quantitative assays (0.5 × 106 Eq/mL for HCV; 400 copies/mL for HIV), the value was taken to be equal to the limit. Serial changes of serum HCV and HIV RNA levels (A,B), peripheral B-cell and CD4+-cell counts (C,D), serum immunoglobulin levels (E, IgG; F, IgA; G, IgM), and serum ALT levels (H) are indicated as means and SE.
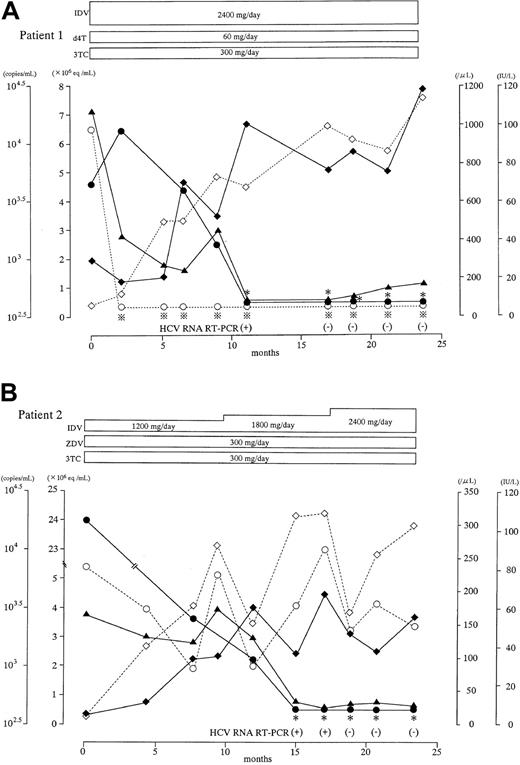
HAART for HIV infection achieved HCV clearance in 2 cases
Patient 1 (Figure 4A) was a 16-year-old boy with hemophilia B who was coinfected with HCV genotype 2a and HIV. Two months after HAART initiation, the serum HIV RNA had decreased to quantitatively undetectable levels. Accompanied by an increase in peripheral B-cell and CD4+-cell counts, serum HCV RNA level had decreased from 4.6 × 106 Eq/mL to below the sensitivity threshold of the bDNA assay at 11 months. No serum HCV RNA could be detected by nested RT-PCR at 17 months.
Laboratory course of 2 patients coinfected with HCV and HIV whose HCV viremia was resolved after immune improvement with HAART.
Serum viral RNA levels and various immunologic values were measured as described in Figure 1. ∗ and indicate that the viral RNA level was below the respective detection limit (0.5 × 106 Eq/mL for HCV; 400 copies/mL for HIV). When the HCV RNA level was below the limit, nested RT-PCR was performed. Serial changes in serum HCV and HIV RNA levels (● and ○, respectively), peripheral B-cell and CD4+-cell counts (▪ and ■, respectively), and serum ALT concentrations (▴) are indicated. Antiretroviral drugs are abbreviated as follows: IDV, indinavir; d4T, stavudine; 3TC, lamivudine; ZDV, zidovudine.
indicate that the viral RNA level was below the respective detection limit (0.5 × 106 Eq/mL for HCV; 400 copies/mL for HIV). When the HCV RNA level was below the limit, nested RT-PCR was performed. Serial changes in serum HCV and HIV RNA levels (● and ○, respectively), peripheral B-cell and CD4+-cell counts (▪ and ■, respectively), and serum ALT concentrations (▴) are indicated. Antiretroviral drugs are abbreviated as follows: IDV, indinavir; d4T, stavudine; 3TC, lamivudine; ZDV, zidovudine.
Laboratory course of 2 patients coinfected with HCV and HIV whose HCV viremia was resolved after immune improvement with HAART.
Serum viral RNA levels and various immunologic values were measured as described in Figure 1. ∗ and indicate that the viral RNA level was below the respective detection limit (0.5 × 106 Eq/mL for HCV; 400 copies/mL for HIV). When the HCV RNA level was below the limit, nested RT-PCR was performed. Serial changes in serum HCV and HIV RNA levels (● and ○, respectively), peripheral B-cell and CD4+-cell counts (▪ and ■, respectively), and serum ALT concentrations (▴) are indicated. Antiretroviral drugs are abbreviated as follows: IDV, indinavir; d4T, stavudine; 3TC, lamivudine; ZDV, zidovudine.
indicate that the viral RNA level was below the respective detection limit (0.5 × 106 Eq/mL for HCV; 400 copies/mL for HIV). When the HCV RNA level was below the limit, nested RT-PCR was performed. Serial changes in serum HCV and HIV RNA levels (● and ○, respectively), peripheral B-cell and CD4+-cell counts (▪ and ■, respectively), and serum ALT concentrations (▴) are indicated. Antiretroviral drugs are abbreviated as follows: IDV, indinavir; d4T, stavudine; 3TC, lamivudine; ZDV, zidovudine.
Patient 2 (Figure 4B) was a 14-year-old boy with hemophilia B who was coinfected with HCV genotype 1a and HIV. The effect of HAART was not as dramatic in this patient as it was in patient 1; serum HIV RNA level decreased less markedly and recovery of B-cell and CD4+-cell counts was slow and limited. However, the serum HCV RNA level had decreased from 2.4 × 107 Eq/mL to below the sensitivity threshold of the bDNA assay at 15 months, and a nested RT-PCR was negative at 18 months.
Discussion
The finding that the younger hemophiliac patients whom we studied were negative for both HCV and HIV (data not shown) reflects the rarity of viral infection after initiation of viral screening of clotting factor concentrates. In contrast, almost all patients born before availability of such testing were positive for antibody to at least one of these viruses, indicating that viral transmission clearly occurred via clotting factor concentrates. Accordingly, when we grouped patients according to viremia, the mean age differed significantly between the infection-free group A1 and the virally infected groups B to D (Table1). Because the age difference could affect baseline values of immunologic parameters such as B-cell and CD4+-cell counts, and immunoglobulin levels, we recruited a healthy control group age-matched to virally infected groups. However, we did not detect an immunologic difference between the younger hemophiliac patients (group A1) and the healthy subjects who were age-matched with infected patients (group A2). For confirmation, we used both groups A1 and A2 as control groups in each comparison study with groups B to D, and we obtained very similar results (data for group A1 not shown).
The HCV RNA load was higher in HIV-coinfected patients than in HIV-negative patients (Figure 1A), as reported previously.9,12,13 This finding is consistent with a report of Eyster and coworkers14 indicating that HCV RNA levels increased 8 times faster in HIV-positive patients than in HIV-negative patients, which suggests a correlation between HCV replication and HIV infection. However, no correlation was seen between serum HCV and HIV load (Figure 2F), which suggests that HCV replication is independent of the level of HIV viremia. Recently developed HAART regimens can improve T-cell immune responses of HIV-infected individuals to a clinically relevant degree by improving CD4+ and CD8+ cell counts and functioning.15 This immunologic improvement has been reported to result in a reduction or resolution of coinfections with HBV16 and hepatitis G virus,17 as well as human herpes virus 8,18,19 which has been implicated in the pathogenesis of Kaposi sarcoma associated with acquired immunodeficiency syndrome.20 Despite these observations, much clinical evidence concerning the HCV status of patients coinfected with HCV and HIV unexpectedly has indicated that antiretroviral therapy does not decrease HCV viremia even after immunologic improvement.4-7 Recently, Fialaie and coworkers21 described 2 exceptional HCV-HIV–infected hemophiliac patients whose HCV viremia resolved during HAART. Even in that report, other HCV-HIV–infected patients showed no significant reduction of HCV viremia. Although HAART has been considered by many to be ineffective against HCV viremia, our observations strongly contradict this opinion. In our study, a marked reduction of HCV viremia was associated with increased CD4+-cell counts, as one might expect from the correlation between HCV replication and host immune condition. Although the disparity between previous findings4-7 and ours is as yet unexplained, 2 possible explanations can be suggested. With the advent of blood product screening, hemophiliac patients are no longer exposed to reinfection with the viruses, unlike many parenteral drug users and homosexual men. Additionally, racial differences may be important. Reddy and colleagues22 reported marked racial variation in virologic response to interferon (IFN) in chronic hepatitis C. Long-term sustained responses to IFN are much more common among Asian patients than among patients of African or Caucasian descent, which suggests that viral elimination might occur more readily in Asians such as our study subjects.
Infection with HCV was associated with reduced peripheral B-cell counts, and HIV coinfection resulted in further B-cell reduction (Figure 1G). Moreover, in coinfected patients, serum HCV RNA load correlated inversely with peripheral B-cell counts (Figure 2A). When we further investigated the possibility of this correlation in HCV-positive, HIV-negative hemophiliac patients (group B), and in outpatients with common forms of chronic hepatitis who did not have hemophilia (data not shown), such a correlation could not be detected in patients without HIV infection. These relations may occur only with immunosuppressive status. Even in HCV and HIV coinfected patients, this correlation was obscured after initiation of HAART (Figure 2G). After HAART was started in coinfected patients, low B-cell counts increased to nearly normal (Figure 3C), accompanied by a reduction of HCV viremia (Figure 3A). This change was particularly dramatic in a patient whose HCV viremia was resolved with HAART (Figure 4A). HIV infection was associated with B-cell counts much less than HCV infection was (Figure1G), and neither HIV RNA load nor CD4+-cell counts correlated with B-cell counts (Figure 2B,E). HCV, then, greatly affects peripheral B-cell counts, whereas HIV does not.
Hepatitis C virus RNA can be detected in peripheral blood mononuclear cells (PBMCs) of patients with HCV infection.23-25 An investigation involving the use of an in situ RT-PCR technique found that at most 8.1% of PBMCs become infected with HCV.25According to other previous reports,26,27 negative-strand (intermediate replicative) forms of HCV RNA can be detected in B cells but not in CD4+ or CD8+ cells. In addition, Nakajima and colleagues28 have established a long-term HCV replication system that has functioned for more than a year with the use of cultured Daudi cells, which are B cells. Recently, one of the receptors that bind HCV has been identified as CD81, a protein with 4 membrane-spanning domains that is expressed by various cells including hepatocytes and B cells.29 These findings indicate that the B cell is one of the target cells to which HCV binds, then infects, and replicates, just as the CD4+ cell is a target for HIV. In the serum of coinfected patients, HCV and HIV RNA levels correlated inversely with B-cell and CD4+-cell counts, respectively (Figure 2A,D). Considering that these cells are the target cells for the respective viruses, infection or replication by these viruses would progressively reduce target cell numbers with increasing viral load. One possible explanation of this reduction is that infected cells may undergo apoptotic cell death as a result of cytopathic effects of the virus.30 Both persistent viruses considered here have target cells participating in host immune function. These sequences and relations suggest 2 hypotheses. First, HCV infection alters host B-cell function through direct infection of these cells. Second, the abnormal B-cell function interferes with host immune status, resulting in HCV persistence. This latter scenario is analogous to that with HIV and CD4+ cells. If these hypotheses are correct, HCV could be considered a kind of immunodeficiency virus. As we have described in a previous report,31 patients with chronic hepatitis C with cirrhosis who are in a terminal stage of persistent HCV infection have impaired immune function.
Serum gamma globulin elevation is a well-known phenomenon in many cases of chronic HCV infection, and this elevation is greatest in the cirrhotic stage. HCV infection may be associated with a benign lymphoproliferative disorder, mixed cryoglobulinemia.32,33Based on findings from bone marrow examinations, some researchers consider this disorder to be a variant of low-grade B-cell non-Hodgkin lymphoma.34,35 Persistence of HCV may chronically stimulate B cells to produce excessive amounts of immunoglobulin. This may cause clonal expansion of these immunoglobulin-secreting cells and may eventually result in malignant B-cell lymphoproliferative disorders. Of note, B-cell expansion associated with HCV infection has been reported to frequently involve IgG and IgM secretion. In our present study, chronic infection with HCV or HIV-related chronic infection was associated with increased serum IgG levels (Figure 1D), which would be expected because viral antigen can continuously stimulate IgG production. Even with ordinary viral infections, IgG against viral antigen is produced for a long time afterward. Additionally, chronic HCV or HIV infection is likely to result in specific persistent excesses of IgM or IgA, respectively (Figure 1F or E). After ordinary viral infections, these immunoglobulins show elevation only for a restricted period. Serum IgM levels were increased after HAART in HCV-positive patients but not in HCV-negative patients (Figure 3G); this finding supports a specific correlation between HCV infection and IgM elevation. Overall, chronic HCV infection appears to result in not only B-cell reduction in the peripheral blood but also expansion of IgG or IgM-secreting B-cell populations in the bone marrow. Thus, HCV infection affects B-cell dynamics in a host and may alter the characteristics of functioning B cells. The correlation between HIV infection and IgA has been described elsewhere.36
Persistence of HCV has been explained in terms of a delayed antibody response against the N-terminal portion of the HCV envelope 2 protein (hypervariable region-1; HVR-1),37 the main target of neutralizing antibodies in patient sera.38 Sequences of HVR-1 present at early time points during infection rapidly undergo mutation during chronic infection, which is followed by new specific anti–HVR-1 antibody responses.37 HCV persistence could reflect failure of the neutralizing antibody response to keep pace with such viral mutations, possibly as a result of B-cell impairment caused by HCV infection. We were surprised to discover that HCV infection was associated with a reduction in CD4+ cells. This reduction was certainly less than that noted in HIV infection (Figure 1H). According to previous reports,26,27 no intermediate replicative form of HCV is detected in CD4+ cells, which suggests that this CD4+-cell reduction may have a cause other than direct viral cytopathic effect. Immunologic factors are likely to be involved in this interesting phenomenon. Recently proposed mechanisms for HCV persistence involve impaired function of dendritic cells,39 which are a type of antigen-presenting cell (APC), and T helper 2 (Th2) dominance in the host Th1/Th2 balance.40 These mechanisms could favor viral evasion of specific cytotoxic T lymphocytes (CTL) that play a crucial role in the clearance of viral infection. APCs are critical in induction of appropriate CTL. Th2 dominance implies diminished Th1 function, namely, reduced cell-mediated immune function including CTL activity. Thus, both factors could lead to viral persistence through CTL impairment. In addition to secretion, B cells can themselves act as APCs as well as influencing host Th1/Th2 balance by secreting interleukin-12, which acts to favor Th1 dominance. B-cell impairment may be an important factor in the interference with appropriate CTL induction in HCV-infected patients.
When HBV infection of a healthy adult induces hepatitis, clearance of the virus follows in almost all cases. In contrast, HCV infection in a healthy adult leads to chronic infection with continuous viremia in approximately 85% of cases. Among our 110 anti-HCV–positive hemophiliac patients, 89 (80.9%) were positive for HCV RNA (Table 1), and the rest (19.1%) were repeatedly negative for HCV RNA and had normal transaminase concentrations. Even though hemophiliac patients typically had repeated exposures to the virus until blood products were screened, viremia was considered resolved in 19.1%. These features of infection suggest that HCV elimination is difficult even in patients with normal immune function. However, they also suggest that HCV can be eliminated under restricted conditions that probably depend on individual factors, as in our 2 patients whose HCV viremia was eliminated during HAART (Figure 4). The immune improvement associated with HAART apparently can help to reduce HCV viremia in HCV and HIV-coinfected patients, sometimes eliminating the virus. Interestingly, HCV viremia was eliminated in our patient 2 even though the peripheral CD4+-cell count did not greatly improve; so, HCV elimination is possible, even with suboptimal improvement in immune function. Both patients whose HCV was resolved were considerably younger than the others; thus, age-related factors may be important although we did not detect differences in immune function between our 2 control groups (A1 and A2) that differed in age.
Highly active antiretroviral therapy has dramatically improved the life expectancy of HIV-infected patients, resulting in a proportional increase in HCV-related liver disease as an important cause of morbidity and mortality in HIV-infected individuals. IFN therapy remains the main treatment to control HCV viremia; however, responsiveness to IFN is reportedly less in HIV-positive than in HIV-negative patients.41 Our observation that HAART induces a decline in HCV viremia is an encouraging finding for coinfected patients because responsiveness to IFN therapy is believed to correlate inversely with serum HCV RNA levels.42,43Moreover, a combination of IFN and the oral antiviral agent ribavirin recently has been shown to substantially increase the response rate.44 45 Considering these points, coinfected patients should be treated with HAART to improve host immune status, followed by treatment with IFN and ribavirin. Such combined therapy may improve outcomes in these hemophiliac patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Junki Takamatsu, Department of Transfusion Medicine, Nagoya University, School of Medicine, 65 Tsuruma-cho, Showa-ku, Nagoya, 466-8550, Japan.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal