Abstract
A recent study identified a clonal expansion of CD3−CD4+cells secreting Th2-type cytokines in 4 patients with chronic hypereosinophilia. Because interferon α (IFN-α) is used in the therapy of the idiopathic hypereosinophilic syndrome, the effects of this cytokine on the survival of clonal Th2 cells isolated from the blood of 2 patients were determined. First, these cells displayed a high rate of spontaneous apoptosis on culture in cytokine-free medium and were also sensitive to Fas-mediated apoptosis induced by soluble Fas ligand. Addition of IFN-α or interleukin-2 (IL-2) to culture medium resulted in significant protection against spontaneous but not Fas-induced apoptosis. Although spontaneous apoptosis of the clonal Th2 cells was clearly associated with down-regulation of both bcl-2 and bcl-xL levels, IFN-α had no significant effect on the expression of these antiapoptotic proteins, whereas addition of IL-2 resulted in higher levels of bcl-2. On the other hand, IFN-α decreased the numbers of cells with disrupted mitochondrial transmembrane potential both during spontaneous apoptosis and after exposure to protoporphyrin IX. Thus, IFN-α might promote the survival of clonal Th2 cells, an effect that could be relevant to the therapeutic approach for patients with chronic hypereosinophilia caused by clonal expansion of Th2-type cells.
Introduction
Interferon α (IFN-α) has been used successfully to treat the idiopathic hypereosinophilic syndrome (HES).1-6 The beneficial effects of IFN-α in this disease have been attributed to the direct actions of this immunomodulatory molecule on eosinophils because it inhibits eosinophilopoiesis,7 several effector functions of eosinophils,8,9 and their recruitment into tissues.10 Moreover, it has been suggested that IFN-α might directly promote eosinophil apoptosis.11 Most clinical studies evaluating the benefits of IFN-α in the setting of the idiopathic HES have been conducted on patients presenting clinical and biologic parameters consistent with the myeloproliferative variant of this disease.1-6,12 13
We and others recently demonstrated that a subgroup of patients with idiopathic HES actually present an underlying T-lymphocyte disorder, characterized by the presence in peripheral blood of clonally expanded interleukin-5 (IL-5)–producing T cells with an aberrant surface phenotype.14-20 In our own series of 4 such patients, the clonal T cells presented a CD3−CD4+phenotype.14-17 Absent or low membrane expression of the T-cell receptor (TCR)/CD3 complex on helper T cells has been observed in several pathologic settings including (retro-)viral infections,21-23 chronic antigenic stimulation,24 and cancer.25,26 Whatever the cause of their lack of CD3/TCR expression, the pathogenic role of the clonal CD3−CD4+ cells in the induction of hypereosinophilia in our patients is highly likely. Indeed, on polyclonal activation or after contact with dendritic cells, these clonal cells secrete high levels of IL-5, together with IL-4 and IL-13, indicating their Th2 nature.14-17
We have previously demonstrated that IFN-α is a potent inhibitor of IL-5 synthesis by differentiated human Th2 clones, including cells obtained from 2 patients in our series, providing rationale for treatment of such patients with this molecule.15 However, the premalignant nature of clonal Th2 cells found in patients with chronic hypereosinophilia has been suggested by the subsequent development of T-cell lymphoma.14,17,20 We were therefore interested in investigating the influence of IFN-α on the survival of clonal Th2 cells associated with chronic hypereosinophilia. We first observed that these clonal T cells are highly prone to undergo apoptosis both spontaneously in culture with cytokine-free medium and on Fas engagement. We then determined the effects of IFN-α on spontaneous and Fas-mediated apoptosis. The observation that IFN-α protects Th2 cells against spontaneous apoptosis led us to determine the effects of this cytokine on bcl-2 and bcl-xL expression as well as on mitochondrial changes implicated in the apoptotic program, including disruption of the mitochondrial transmembrane potential (Δψm) and the production of reactive oxygen species (ROS) in those cells.27-29
Patients, materials, and methods
Patients
As previously described,15-17 patient 1 was a 20-year-old woman presenting with severe pruritus, eczema, and tenosynovitis of the right ankle. At presentation, the circulating leukocyte count was 16 900/μL, including 8923 eosinophils and 4630 lymphocytes. CD4+ T cells represented 87% of total lymphocytes and were composed of 88% CD3−CD4+cells (3545 CD3−CD4+ cells/μL) and 12% CD3+CD4+ cells. Serum IgE and IgM levels were 340 U/mL (normal, < 20) and 310 mg/dL (normal, 40-250), respectively. Treatment with IFN-α was followed by regression of both clinical manifestations and decrease of eosinophil blood count, although the clonal CD3−CD4+ population remained stable. However, recurrence of hypereosinophilia was observed less than a year after initiation of IFN-α. Subsequent evolution has been characterized by expansion of the circulating CD3−CD4+ population (reaching 4800/μL) and appearance of chromosomal abnormalities in these cells. The patient has now developed peripheral T-cell lymphoma characterized by bilateral masses in the parotid regions and a submandibular mass composed of CD3−CD4+ cells.
Patient 216 17 was a 21-year-old woman also presenting with severe pruritus and eczema, as well as cyclic angioedema. The circulating leukocyte count was 14 800/μL, including 9102 eosinophils and 3419 lymphocytes. CD4+ T cells represented 87% of total lymphocytes and were composed of 83% CD3−CD4+ cells (2469 CD3−CD4+ cells/μL) and 17% CD3+CD4+ cells. Serum IgE and IgM levels reached 15 640 U/mL and 1253 mg/dL, respectively. The clinical and biologic findings were consistent with the diagnosis of Gleich syndrome. Two-year follow-up has been characterized by good response to glucocorticoid treatment, as evidenced by normalization of eosinophil levels. Although the CD3−CD4+ cell population had decreased significantly (662/μL) over this period, tapering of steroid dosage was quickly followed by recurrence of hypereosinophilia and clinical manifestations. In both patients, bone marrow aspiration showed abundant eosinophil precursors and absence of blastic cells. They were free of treatment at the time of blood sampling for the present study.
Reagents
Human recombinant IFN-α (rIFN-α) 2b with a specific activity of 3.106 IU/mL was obtained from Schering-Plough (Innishannon, Ireland). Human recombinant IL-2 (rIL-2) with a specific activity of 3.104 IU/mL was kindly provided by K. Willard-Gallo (Université Libre de Bruxelles, Rhode Saint-Genèse, Belgium). The following monoclonal antibodies (mAb) were purchased from Becton Dickinson (Mountain View, CA): fluorescein isothiocyanate (FITC)–labeled anti-CD45RA; peridinin chlorophyll protein (PerCP)–labeled anti-CD4; phycoerythrin (PE)–labeled anti-Fas (CD95), anti-DR, and anti-CD45RO; purified anti-CD3; and anti-CD45RA. The neutralizing anti-Fas ligand (clone NOK-1) mAb was purchased from Pharmingen (San Diego, CA). The soluble human recombinant APO-1/Fas ligand (sFasL) and associated enhancer (antibody reacting with sFasL) were purchased from Alexis Corporation (Laüfelfingen, Switzerland). Mouse monoclonal anti-bcl-xL (IgG1, H-5) antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and mouse IgG1 isotype control from R & D Systems Europe (Abingdon, United Kingdom). FITC-labeled rabbit antimouse Ig used as second layers, FITC-conjugated anti-bcl-2 mAb (IgG1k, clone 124), and IgG1 control mAb for intracellular staining of bcl-2 were obtained from Dako (Glostrup, Denmark).
Isolation of CD3−CD4+ T cells from hypereosinophilic patients
Blood leukocytes were obtained from both patients by cytapheresis, after informed consent was obtained. Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation on Lymphoprep (Nycomed, Oslo, Norway). After 3 washings with Hanks balanced salt solution (HBSS) (Gibco, Life Technologies, Paisley, Scotland), cells were resuspended in cold-conservation medium composed of 50% RPMI 1640, 10% dimethylsulfoxide (DMSO) (Sigma Chemical Co, St Louis, MO), and 40% heat-inactivated fetal calf serum (FCS) (BioWhittaker, Europe, Verviers, Belgium). Cells were quickly frozen in a −80°C freezer and then stored in liquid nitrogen until use. CD3−CD4+ T cells from both patients were prepared from CD4+ T cells (see below) by selective depletion of CD3+ T cells through incubation with anti-CD3 mAb for 30 minutes at 4°C, washing, and then incubation with immunomagnetic particles coated with sheep antimouse IgG (Dynabeads, Dynal, Oslo, Norway). The resulting cell preparations contained more than 90% viable CD3−CD4+ T cells as determined by flow cytometry.
Isolation of CD3+CD4+CD45RO+ T cells from healthy volunteers
The PBMC were isolated from buffy coats of healthy individuals by density gradient centrifugation on Lymphoprep. Cells were resuspended in cold-conservation medium, quickly frozen, and then stored in liquid nitrogen until use. After 3 washings with HBSS, PBMC were resuspended in complete medium (RPMI 1640, BioWhitakker, supplemented with 10% FCS and 40 μg/mL gentamicin). CD4+lymphocytes were purified using the MACS CD4+ T cell isolation kit (Miltenyi Biotec Gmbh, Bergisch Gladbach, Germany). The CD4+ T-cell suspensions were further incubated with an anti-CD45RA mAb for 30 minutes at 4°C, washed, and incubated with immunomagnetic particles coated with sheep antimouse IgG (Dynabeads, Dynal). Coated cells were removed with a magnet, leaving purified CD3+CD4+CD45RO+ cells in suspension. The resulting cell preparations routinely contained more than 90% viable CD3+CD4+CD45RO+T cells.
Lymphocyte phenotyping
The expression of HLA-DR, CD45RO, and Fas (CD95) on CD4+ T cells was determined by flow cytometry. For that purpose, PBMC from patients and from healthy blood donors were washed in phosphate-buffered saline (PBS) and incubated for 30 minutes on ice with appropriate mAbs: FITC-, PE-, or PerCP-conjugated mAbs against CD4, CD3, CD45RO, HLA-DR, and CD95 antigens. After washing, 10 000 cells were acquired using a FACScan flow cytometer (Becton Dickinson) and analyzed with the Cellquest software (Becton Dickinson).
Cell culture and determination of apoptosis
Normal CD3+CD4+CD45RO+ and clonal CD3−CD4+ T cells were seeded at 2.105/well per 200 μL at 37°C and 5% CO2-humidified atmosphere in flat-bottomed 96-well plates (Nunc, Gibco) in complete medium in the presence or absence of IL-2 (100 U/mL) or IFN-α (104-1 IU/mL). For induction of Fas-mediated apoptosis, CD4+ T cells were incubated in the presence of sFasL at concentrations ranging from 50 to 1.5 ng/mL and associated enhancer (1 μg/mL). After 24 to 96 hours of incubation, CD4+ T cells were collected and examined for apoptosis. In most experiments, recovered viable lymphocytes were detected by flow cytometry according to their light forward and side scatter properties (FSC/SSC). Indeed, apoptotic lymphocytes were smaller in size than resting viable lymphocytes but were distinct from debris by an increase in 90° scatter. After culture, numbers of viable CD3+CD4+CD45RO+ or CD3−CD4+ T lymphocytes (FSChigh/SSClow) recovered were determined and expressed as a percentage of the original input viable cells. Based on these measurements, results were expressed as percentage of apoptotic cells. Measurement of apoptosis by the light scatter method was verified by 2 additional methods for apoptosis detection, namely annexin V binding and terminal deoxynucleotidyl transferase (TdT)–mediated deoxyuridine triphosphate (dUTP) nick end-labeling (TUNEL). FITC–annexin V binding detects phosphatidyl serine on the outer surface of cells undergoing apoptosis and was performed using a commercially available kit (Bender MedSystems, Vienna, Austria). TUNEL was performed using a kit according to the manufacturer's protocol (Boehringer Mannheim, Mannheim, Germany). We confirmed that FSClow/SSChigh cells were clearly enriched in apoptotic cells (98% annexin V positive [An+] and 97% TUNEL positive), whereas FSChigh/SSClow cells contained few apoptotic cells (9% An+ and 7% TUNEL positive). Double-labeling experiments with FITC–annexin V and propidium iodide (PI) revealed that most of the An+ cells were PI+ after 24 hours of culture (not shown).
Expression ofbcl-2 and bcl-xL
Levels of bcl-2 and bcl-xL expression were determined by flow cytometry. Intracellular staining for bcl-2 was performed as follows. Cells were washed in PBS supplemented with 10% FCS and 0.1% azide (PBS-FCS-NaN3), fixed in FACS lysing solution (Becton Dickinson) for 10 minutes at room temperature, washed again in PBS, and then incubated for 15 minutes at room temperature in permeabilizing solution (Becton Dickinson) in PBS-FCS-NaN3. Finally, cells were intracellularly stained with FITC-conjugated anti–bcl-2 mAb, FITC-isotype control mAb, nonlabeled anti–bcl-xL mAb, and isotype control for 30 minutes at room temperature. Bcl-xL–labeled cells were washed in PBS-FCS-NaN3 buffer and further incubated in presence of a FITC-labeled rabbit antimouse Ig for 30 minutes at room temperature. After washing, cells were immediately applied to a FACScan flow cytometer and analyzed with the Cellquest software (Becton Dickinson). Apoptotic cells were excluded on basis of FSC/SSC parameters. The expression of bcl-2 and bcl-xL was reported in specific molecule equivalents of soluble fluorochrome (MESF) using QuickCal beads (Flow Cytometry Standards Corp, San Juan, Puerto Rico).
Cytofluorometric analysis of Δψm and reactive oxygen species generation
To evaluate Δψm, clonal Th2 cells (106/mL) from patient 1 were incubated in complete medium with 2.5 nmol/L 3,3′-dihexyloxacarbocyanine (DiOC6; Molecular Probes, Eugene, OR) for 15 minutes at 37°C followed by analysis on a FACScan flow cytometer, according to a published protocol.30 The fluorescence of DiOC6 (green), a potential-sensitive cationic lipophilic dye that incorporates into the mitochondrial matrix, correlates with the Δψm and is oxidation independent. Therefore, DiOC6low cells correspond to cells with disrupted Δψm. Superoxide generation was also measured by flow cytometry using 0.25 μmol/L hydroethidine (HE; Molecular Probes), a dye that is oxidized into fluorescent ethidium (red) by superoxide anion.30HEhigh cells therefore correspond to cells with increased production of superoxide anions. In some experiments, the clonal Th2 cells were cultured in the presence of 30 μmol/L protoporphyrin IX (PPIX; Sigma), alone or in combination with IFN-α (104 IU/mL).
Results
Clonal CD3−CD4+ Th2 cells associated with chronic hypereosinophilia present an activated memory phenotype
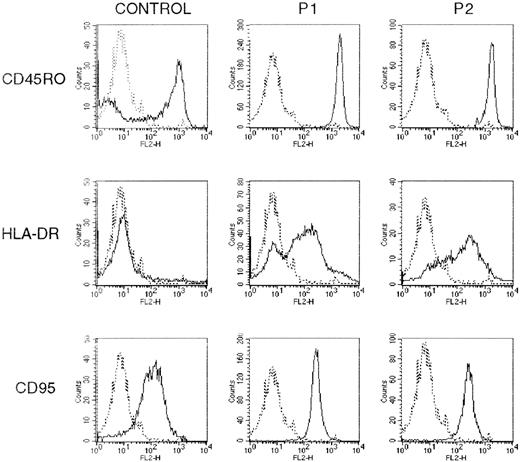
We described elsewhere the Th2-like cytokine profile of the monoclonal CD3−CD4+ T lymphocytes present in the circulation of the 2 patients with chronic hypereosinophilia.15-17 As shown in Figure1, by flow cytometry, their phenotype was further characterized by the expression of the CD45RO isoform and the activation antigen HLA-DR and by the absence of the CD7 and CD27 markers16 17 (not shown). Moreover, the clonal T cells expressed high levels of CD95 as compared with CD3+CD4+CD45RO+ T cells obtained from healthy individuals. The phenotype of the clonal Th2 cells is therefore consistent with that of activated memory T cells.
The clonal Th2 cells present an activated memory phenotype.
PBMC from 2 hypereosinophilic patients (P1 and P2) and from healthy blood donors were stained with FITC-, PE-, or PerCP-conjugated mAbs against CD4, CD3, CD45RO, HLA-DR, and CD95 antigens. Surface phenotype was determined by flow cytometry after gating on CD3+CD4+ or CD45RO+ lymphocytes from healthy subjects and clonal CD3−CD4+ Th2 cells from both patients. The figure shows histograms from 1 experiment, representative of 3. The background staining using isotype-matched control Abs is represented by the broken line.
The clonal Th2 cells present an activated memory phenotype.
PBMC from 2 hypereosinophilic patients (P1 and P2) and from healthy blood donors were stained with FITC-, PE-, or PerCP-conjugated mAbs against CD4, CD3, CD45RO, HLA-DR, and CD95 antigens. Surface phenotype was determined by flow cytometry after gating on CD3+CD4+ or CD45RO+ lymphocytes from healthy subjects and clonal CD3−CD4+ Th2 cells from both patients. The figure shows histograms from 1 experiment, representative of 3. The background staining using isotype-matched control Abs is represented by the broken line.
Interferon α protects clonal Th2 cells against spontaneous but not Fas-mediated apoptosis
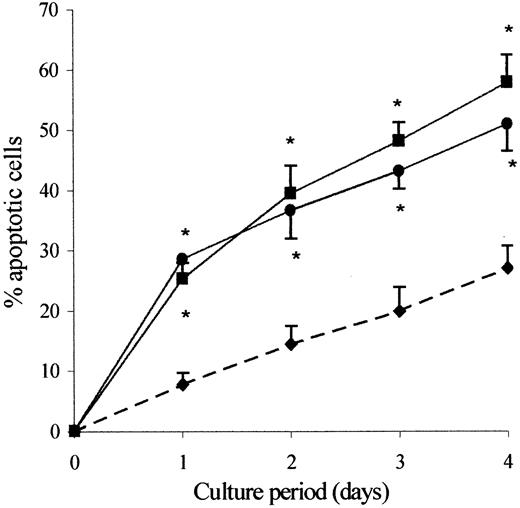
In a first series of experiments, we compared the rate of spontaneous apoptosis of clonal CD3−CD4+ cells from patients with that of CD3+CD4+CD45RO+ T cells from healthy individuals during a culture period of 1 to 4 days in the absence of any growth factor. The percentage of apoptotic cells was then quantified using flow cytometry analysis according FSC/SSC parameters, measuring DNA fragmentation using TUNEL assay and phosphatidylserine externalization using FITC–annexin V binding. The data presented in Figure 2 are a graphic representation of the flow cytometry data for FSC/SSC parameters. We found at all time points a significantly increased proportion of apoptotic cells within the clonal Th2 cells as compared with control CD3+CD4+CD45RO+ T cells. Similar results were obtained using either FITC–annexin V binding or TUNEL assay, except that in every case more apoptotic cells were detected as defined by FSC/SSC or annexin V binding than as defined by the TUNEL assay. This difference was probably due to the fact that DNA degradation is a relatively late event in apoptosis.
The clonal Th2 cells are highly prone to undergo spontaneous apoptosis.
Purified CD3+CD4+CD45RO+ cells from healthy individuals (♦) and CD3−CD4+ cells from hypereosinophilic patient 1 (●) and patient 2 (▪) were cultured in the absence of exogenous cytokine. Cells were recovered after 24, 48, 72, and 96 hours of culture. Apoptotic cells were detected by flow cytometry according to FSC/SSC parameters. Results are the means ± SEM of percent apoptotic cells of 5 experiments). *P < .005 as compared with control CD3+CD4+CD45RO+ cells from healthy individuals (ANOVA test).
The clonal Th2 cells are highly prone to undergo spontaneous apoptosis.
Purified CD3+CD4+CD45RO+ cells from healthy individuals (♦) and CD3−CD4+ cells from hypereosinophilic patient 1 (●) and patient 2 (▪) were cultured in the absence of exogenous cytokine. Cells were recovered after 24, 48, 72, and 96 hours of culture. Apoptotic cells were detected by flow cytometry according to FSC/SSC parameters. Results are the means ± SEM of percent apoptotic cells of 5 experiments). *P < .005 as compared with control CD3+CD4+CD45RO+ cells from healthy individuals (ANOVA test).
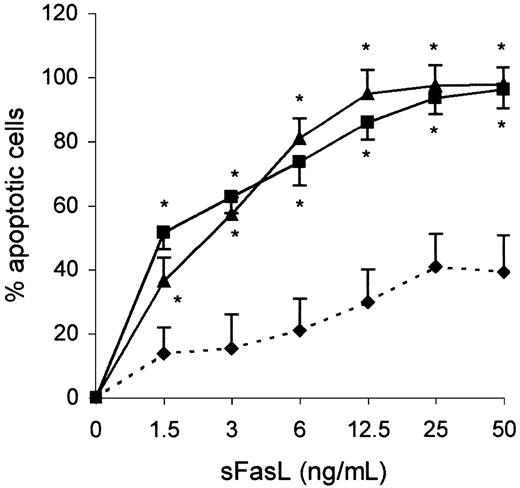
The increased expression of the CD95 antigen on the clonal Th2 cells prompted us to examine whether the propensity of these cells to undergo apoptosis could be enhanced through ligation of Fas. As shown Figure3, most of the clonal Th2 cells (> 90%) underwent apoptosis after 24 hours of incubation with 50 ng/mL sFasL, whereas less than 40% of apoptotic cells were found within control CD3+CD4+CD45RO+ T cells under the same experimental condition. The apoptosis induction by Fas engagement led us to determine whether this pathway was involved in the spontaneous apoptosis of those cells as they express mRNA encoding FasL (not shown). As shown in Table 1, addition of a blocking anti-FasL mAb did not affect the spontaneous apoptosis of the clonal Th2 cells, whereas it inhibited sFasL-induced apoptosis. These data establish that the spontaneous apoptosis of clonal Th2 cells is independent of the Fas/FasL pathway.
The clonal Th2 cells are sensitive to Fas-mediated apoptosis.
Purified CD3+CD4+CD45RO+ cells from healthy individuals (♦) and CD3−CD4+ cells from hypereosinophilic patient 1 (▴) and patient 2 (▪) were cultured in the presence of graded concentrations of sFasL. Apoptotic cells were detected by flow cytometry according to FSC/SSC parameters. Results are the means ± SEM of percent apoptotic cells of 3 experiments. *P < .05 as compared with CD3+CD4+CD45RO+ cells from healthy individuals (ANOVA test).
The clonal Th2 cells are sensitive to Fas-mediated apoptosis.
Purified CD3+CD4+CD45RO+ cells from healthy individuals (♦) and CD3−CD4+ cells from hypereosinophilic patient 1 (▴) and patient 2 (▪) were cultured in the presence of graded concentrations of sFasL. Apoptotic cells were detected by flow cytometry according to FSC/SSC parameters. Results are the means ± SEM of percent apoptotic cells of 3 experiments. *P < .05 as compared with CD3+CD4+CD45RO+ cells from healthy individuals (ANOVA test).
IFN-α and IL-2 protect the clonal Th2 cells against spontaneous but not Fas-mediated apoptosis
| Agent added . | Spontaneous . | Fas-mediated . | ||
|---|---|---|---|---|
| Patient 1 . | Patient 2 . | Patient 1 . | Patient 2 . | |
| None | 28 ± 1 | 31 ± 3 | 89 ± 4 | 96 ± 2 |
| Anti-Fas mAb | 29 ± 2 | 28 ± 1 | 30 ± 3 | 28 ± 1 |
| Control mAb | 28 ± 1 | 28 ± 1 | 90 ± 4 | 97 ± 1 |
| IL-2 (100 IU/mL) | 11 ± 0.5* | 10 ± 1* | 78 ± 7 | 94 ± 1 |
| IFN-α (104IU/mL) | 9 ± 1* | 8 ± 0.5* | 83 ± 5 | 89 ± 4 |
| Agent added . | Spontaneous . | Fas-mediated . | ||
|---|---|---|---|---|
| Patient 1 . | Patient 2 . | Patient 1 . | Patient 2 . | |
| None | 28 ± 1 | 31 ± 3 | 89 ± 4 | 96 ± 2 |
| Anti-Fas mAb | 29 ± 2 | 28 ± 1 | 30 ± 3 | 28 ± 1 |
| Control mAb | 28 ± 1 | 28 ± 1 | 90 ± 4 | 97 ± 1 |
| IL-2 (100 IU/mL) | 11 ± 0.5* | 10 ± 1* | 78 ± 7 | 94 ± 1 |
| IFN-α (104IU/mL) | 9 ± 1* | 8 ± 0.5* | 83 ± 5 | 89 ± 4 |
Purified CD3−CD4+ cells from patient 1 and patient 2 were cultured for 24 hours in absence (spontaneous) or presence (Fas-mediated) of sFasL (25 ng/mL). Anti-Fas (1 μg/mL) or control mAb (1 μg/mL), IL-2 (100 IU/mL) or IFN-α (104IU/mL) was added at the beginning of the culture. At the end of the culture, apoptotic lymphocytes were detected by flow cytometry according to their light scatter properties (FSC/SSC). Results are the mean percentages ± SD of 3 experiments.
P < .05 as compared with culture without IFN-α or IL-2 (ANOVA test).
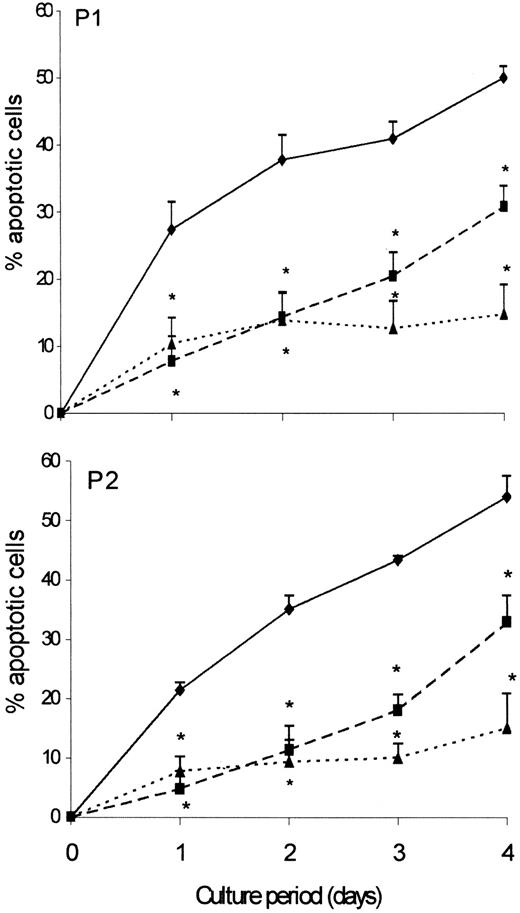
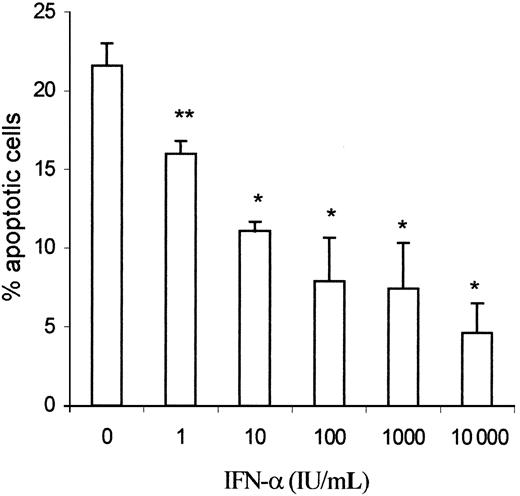
We then examined whether the clonal Th2 cells could be rescued from apoptosis by IL-2, which is known to exert antiapoptotic effects on activated T cells. As shown in Figure 4, addition of IL-2 (100 U/mL) to the culture medium rescued more than 80% of the clonal T cells from spontaneous apoptosis. Addition of rIFN-α (104 IU/mL) also exerted a clear-cut antiapoptotic action on clonal Th2 cells from both patients. As shown in Figure5, the protective effect of rIFN-α on spontaneous apoptosis was dependent on the dose, a protective effect being already observed at a concentration of 1 IU/mL. The rescuing effect of IFN-α was also confirmed using FITC–annexin V binding and TUNEL assay (not shown). Contrasting with their inhibitory effects on spontaneous apoptosis, neither rIFN-α nor IL-2 affected Fas-induced apoptosis of the clonal T cells (Table 1). In a previous study, we demonstrated that IL-2 but not IFN-α induced proliferation of the Th2 cells.16
IFN-α and IL-2 protect the clonal Th2 cells against spontaneous apoptosis.
CD3−CD4+ cells from hypereosinophilic patient 1 and patient 2 (♦) were cultured in presence of IFN-α (104 IU/mL) (▪) or IL-2 (100 IU/mL) (▴). Cells were recovered after 24, 48, 72, and 96 hours of culture. Apoptotic cells were detected by flow cytometry according to FSC/SSC parameters. Results are the means ± SEM of % apoptotic cells of 5 experiments. *P < .005 as compared with culture without IFN-α or IL-2 (ANOVA test).
IFN-α and IL-2 protect the clonal Th2 cells against spontaneous apoptosis.
CD3−CD4+ cells from hypereosinophilic patient 1 and patient 2 (♦) were cultured in presence of IFN-α (104 IU/mL) (▪) or IL-2 (100 IU/mL) (▴). Cells were recovered after 24, 48, 72, and 96 hours of culture. Apoptotic cells were detected by flow cytometry according to FSC/SSC parameters. Results are the means ± SEM of % apoptotic cells of 5 experiments. *P < .005 as compared with culture without IFN-α or IL-2 (ANOVA test).
IFN-α prevents spontaneous apoptosis of the clonal Th2 cells in a dose-dependent manner.
CD3−CD4+ cells from hypereosinophilic patient 1 were cultured in the presence of graded amounts of IFN-α (1-104 IU/mL) for 24 hours. At the end of the culture, apoptotic lymphocytes were detected by flow cytometry according to their light scatter properties (FSC/SSC). Results are the means ± SEM of 4 experiments. *P < .005, **P < .05 as compared with culture without IFN-α (ANOVA test).
IFN-α prevents spontaneous apoptosis of the clonal Th2 cells in a dose-dependent manner.
CD3−CD4+ cells from hypereosinophilic patient 1 were cultured in the presence of graded amounts of IFN-α (1-104 IU/mL) for 24 hours. At the end of the culture, apoptotic lymphocytes were detected by flow cytometry according to their light scatter properties (FSC/SSC). Results are the means ± SEM of 4 experiments. *P < .005, **P < .05 as compared with culture without IFN-α (ANOVA test).
Interferon α does not prevent bcl-2 and bcl-xLdown-regulation in clonal Th2 cells
To determine whether the susceptibility of the clonal Th2 cells to apoptosis was caused by changes in the constitutive expression of bcl-2 and bcl-xL which are known to exert antiapoptotic effects, purified clonal Th2 cells and normal CD3+CD4+CD45RO+ T cells were stained for these proteins immediately after purification and analyzed by flow cytometry. As shown in Table 2, bcl-2 expression was clearly lower among the clonal Th2 cells, whereas no significant difference was observed as far as bcl-xLexpression is concerned.
Expression of bcl-2 and bcl-xL in clonal Th2 cells
| Cells . | Agent added . | Bcl-xL . | Bcl-2 . | ||
|---|---|---|---|---|---|
| 0 . | 24 h . | 0 . | 24 h . | ||
| CD4+CD45RO+(controls) | — | 83 021 ± 6427 | 71 524 ± 4752 | 64 986 ± 5483 | 58 933 ± 4982 |
| CD3−CD4+ (patient 2) | — | 85 432 ± 5561 | — | 26 048 ± 6843* | — |
| CD3−CD4+ (patient 1) | — | 96 953 ± 11704 | 58 644 ± 10128 | 29 839 ± 5290* | 19 973 ± 2213 |
| +IFN-α (104IU/mL) | — | 62 563 ± 13723 | — | 19 911 ± 2555 | |
| +IL-2 (100 IU/mL) | — | 66 238 ± 22834 | — | 23 647 ± 3371† | |
| Cells . | Agent added . | Bcl-xL . | Bcl-2 . | ||
|---|---|---|---|---|---|
| 0 . | 24 h . | 0 . | 24 h . | ||
| CD4+CD45RO+(controls) | — | 83 021 ± 6427 | 71 524 ± 4752 | 64 986 ± 5483 | 58 933 ± 4982 |
| CD3−CD4+ (patient 2) | — | 85 432 ± 5561 | — | 26 048 ± 6843* | — |
| CD3−CD4+ (patient 1) | — | 96 953 ± 11704 | 58 644 ± 10128 | 29 839 ± 5290* | 19 973 ± 2213 |
| +IFN-α (104IU/mL) | — | 62 563 ± 13723 | — | 19 911 ± 2555 | |
| +IL-2 (100 IU/mL) | — | 66 238 ± 22834 | — | 23 647 ± 3371† | |
Intracytoplasmic bcl-xL and bcl-2 staining was performed on purified CD3+CD4+CD45RO+ cells from healthy individuals (n = 6) and CD3−CD4+ from hypereosinophilic patients (for each patient 6 determinations were performed). For patient 1, bcl-xL and bcl-2 levels were compared with those obtained after 24 hours of incubation in the absence or presence of IFN-α (104 IU/mL) or IL-2 (100 IU/mL). Results shown are the mean ± SD of bcl-xL and bcl-2 staining within live FSChigh/SSClow cells expressed in MESF.
P < .05 as compared with CD4+CD45RO+ cells from healthy individuals.
P < .05 as compared with bcl-2 expression obtained in absence of IL-2 (ANOVA test).
Interleukin-2 is known to protect activated T cells against death by increasing bcl-2 and, to a lesser extent, bcl-xLexpression. We therefore decided to compare the effects of IL-2 and IFN-α on expression levels of both bcl proteins within the clonal Th2 cells of patient 1. For this purpose, bcl-2 and bcl-xLprotein expressions were examined by flow cytometry after culture in the absence or presence of the cytokines. Apoptotic and live cells were analyzed separately on the basis of FSC/SSC parameters. As shown in Table 2, bcl-2 and bcl-xL expression by live clonal Th2 cells decreased by about 40% after 24 hours of culture in the absence of exogenous cytokines. The drop in bcl-xL protein expression was even more pronounced within apoptotic cells, resulting in a clear bimodal expression of bcl-xL by the clonal Th2 cells (not shown). On the other hand, normal CD3+CD4+CD45RO+ T cells decreased their bcl-2 and bcl-xL levels only by about 10%. Although addition of IL-2 (100 IU/mL) partially prevented the drop in bcl-2 expression in clonal Th2 cells, the antiapoptotic effect of IFN-α was not associated with any significant change in bcl-2 or bcl-xL down-regulation during the 24-hour culture period (similar data were obtained after 72 hours of culture).
Interferon α prevents Δψm dissipation in clonal Th2 cells
Loss of Δψm, generation of superoxide anions by the uncoupled respiratory chain,27-29 and phosphatidylserine exposure31 at the cell membrane are characteristic features of the apoptotic process. To get further inside the mechanism of action of IFN-α, we monitored these parameters within the clonal Th2 cells.
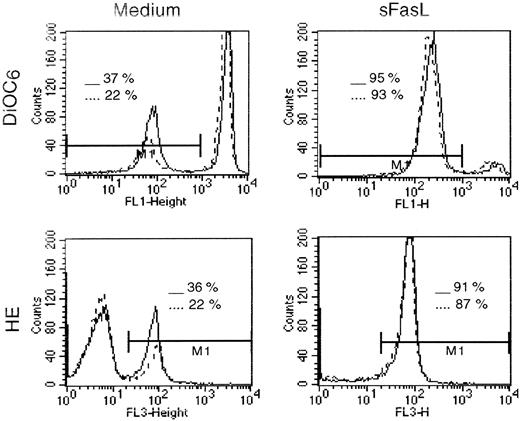
First, we observed that a fraction of Th2 cells spontaneously become DiOC6low and HEhigh after 24 hours in culture, indicating disrupted Δψm and increased superoxide production, respectively (Figure6). Double-labeling experiments (not shown) revealed that most DiOC6low cells were HEhigh, FSClow/SSChigh, and An+, confirming the association between Δψmdisruption, superoxide generation, and apoptosis. Addition of rIFN-α (104 IU/mL) to the culture medium resulted in a lower number of Th2 cells undergoing Δψm loss and associated HE+/An+ conversion. Indeed, percentages of DiOC6low/HEhigh/An+ cells after 24 hours of culture were 30.5% ± 3.3% and 16.4% ± 0.9% (mean ± SD of 5 determinations) in absence and presence of rIFN-α, respectively (P < .005 by Wilcoxon test). As shown in Figure 6, we also found that an important proportion of the clonal Th2 cells (> 90%) exhibited a drastic Δψmloss 4 hours after Fas cross-linking by sFasL (25 ng/mL). Most of these cells (> 90%) were HE+ and An+. Less than 5% of the clonal Th2 cells were rescued from Fas-mediated Δψm loss and HE+/An+ conversion by rIFN-α added at the beginning of the culture.
IFN-α inhibits generation of DiOC6low/HE+ clonal Th2 cells.
CD3−CD4+ cells from hypereosinophilic patient 1 were cultured for 24 hours in the absence or presence of sFasL (25 ng/mL). IFN-α (104 IU/mL) was added at the beginning of the culture. At the end of the culture, cells were stained for 15 minutes at 37°C with DiOC6 or HE followed by flow cytometry analysis. The histograms represent DiOC6 (upper) and HE (lower) staining of Th2 cells obtained from patient 1 after 24 hours of culture in the absence (solid lines) or presence (broken lines) of IFN-α.
IFN-α inhibits generation of DiOC6low/HE+ clonal Th2 cells.
CD3−CD4+ cells from hypereosinophilic patient 1 were cultured for 24 hours in the absence or presence of sFasL (25 ng/mL). IFN-α (104 IU/mL) was added at the beginning of the culture. At the end of the culture, cells were stained for 15 minutes at 37°C with DiOC6 or HE followed by flow cytometry analysis. The histograms represent DiOC6 (upper) and HE (lower) staining of Th2 cells obtained from patient 1 after 24 hours of culture in the absence (solid lines) or presence (broken lines) of IFN-α.
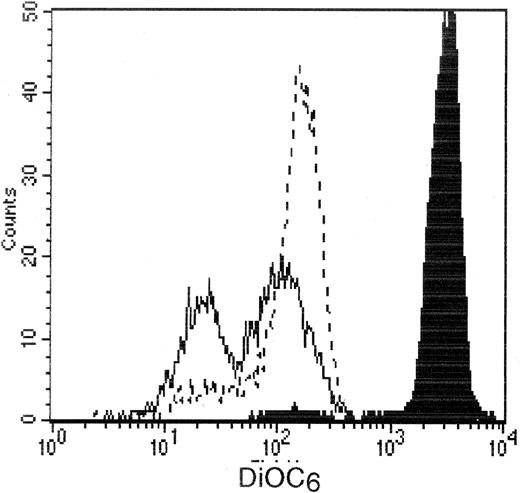
To further specify the mechanism of the antiapoptotic effect of IFN-α at the mitochondrial level, we studied the Δψmmodification induced in Th2 cells by PPIX, an agent known to trigger permeability transition (PT) pore opening after binding to benzodiazepine receptors located in the outer mitochondrial membrane.32 After incubation with this agent for 24 hours, we found that more than 60% of the clonal Th2 cells were still alive with a FSChigh/SSClow/An−phenotype. After gating on this population of viable cells, we observed 2 subpopulations of DiOC6low Th2 cells (Figure7), in agreement with a previous study on thymocytes, which demonstrated that the cell population with the lowest DiOC6 fluorescence was the one committed to apoptosis.33 As shown in Figure 7, addition of rIFN-α (104 IU/mL) to PPIX significantly decreased the numbers of cells expressing the lowest DiOC6 fluorescence. Indeed, percentages of those cells after 24 hours of culture were 40 ± 8.9% and 22.6 ± 4.8% (mean ± SEM of 8 determinations) in the absence and presence of rIFN-α, respectively (P < .01 by the Wilcoxon test). Taken together, these data indicate that IFN-α inhibits Δψm decrease induced by PT pore opening in Th2 cells.
IFN-α inhibits PPIX-induced Δψm loss in the clonal Th2 cells.
CD3−CD4+ cells from hypereosinophilic patient 1 were cultured for 24 hours in presence of PPIX (30 μmol/L). IFN-α (104 IU/mL) was added at the beginning of the culture. At the end of the culture, cells were stained for 15 minutes at 37°C with DiOC6 followed by flow cytometry analysis using gating on live cells. The histograms represent DiOC6 staining of Th2 cells obtained from patient 1 at time 0 (black histogram), after 24 hours of culture in the absence (solid lines) or presence (broken lines) of IFN-α.
IFN-α inhibits PPIX-induced Δψm loss in the clonal Th2 cells.
CD3−CD4+ cells from hypereosinophilic patient 1 were cultured for 24 hours in presence of PPIX (30 μmol/L). IFN-α (104 IU/mL) was added at the beginning of the culture. At the end of the culture, cells were stained for 15 minutes at 37°C with DiOC6 followed by flow cytometry analysis using gating on live cells. The histograms represent DiOC6 staining of Th2 cells obtained from patient 1 at time 0 (black histogram), after 24 hours of culture in the absence (solid lines) or presence (broken lines) of IFN-α.
Discussion
In this study, we first demonstrated that the clonal Th2-type cells associated with chronic hypereosinophilia displayed a high rate of spontaneous apoptosis on culture in cytokine-free medium. This increased susceptibility to apoptosis was associated with a low level of bcl-2 expression, whereas bcl-xL expression was normal as compared to control cells.
It is well known that members of the IL-2 family that share the γc as part of their receptors34 can prevent the in vitro death of activated T cells.35,36 IL-2 and IL-4 (not shown) indeed prevented spontaneous apoptosis of the clonal Th2 cells. We observed that IFN-α represents another type of survival factor for the clonal Th2 cells. Autocrine production of IL-2 did not contribute to IFN-α–induced survival of the clonal Th2 cells, because this activity was inhibited neither by anti–IL-2R-α nor anti–IL-2R-γ neutralizing antibodies (data not shown). Several studies indicate that bcl-237 and bcl-xL38 repress cell death of cytokine-deprived activated T cells.35 In this report, when clonal Th2 cells were cultured alone, bcl-2 and bcl-xLprotein expression decreased within live cells, suggesting that down-modulation of these antiapoptotic proteins could be involved in the onset of spontaneous apoptosis. Although addition of IL-2 or IFN-α resulted in an improved survival of the clonal Th2 cells, IFN-α had no significant effect on the expression of these antiapoptotic proteins, whereas addition of IL-2 resulted in higher levels of bcl-2, as expected.35 In contrast to IFN-α, IFN-γ did not influence the death rate of the clonal Th2 cells, although these cells bear receptors for IFN-γ because their expression of class I major histocompatibility complex molecules was up-regulated by IFN-γ (data not shown). Because both IFN-γ and IFN-α activate Stat1,39 whereas only IFN-α protects Th2 cells from apoptosis, it seems evident that Stat1 activation might not be sufficient to obtain the antiapoptotic effect in those cells. However, it is also possible that in the case of IFN-γ, a proapoptotic pathway is simultaneously triggered.
To get further insight into the mechanism of action of IFN-α, we evaluated the possible role of mitochondria in the spontaneous apoptosis of the clonal cells. Indeed, recent studies establish that mitochondria play a critical role in the apoptotic cascade leading to T-cell death.27-29 Early after induction of apoptosis, PT-induced pore opening results in loss of Δψm and the generation of superoxide anions by the uncoupled respiratory chain. The hierarchical relationship between these events appears complex. Along this line, Hildeman and colleagues40 established that the production of superoxide anions associated with a loss of Δψm is a decisive contributor to the in vitro death of activated T cells obtained from mice injected with superantigen. Interestingly, it was reported in another work that type I IFNs can act as a survival factor for these cells.41 Moreover, Hyde and coworkers42 demonstrated that a fibroblast-derived factor, recently recognized as IFN-β,43 enhanced survival of activated human T cells by increasing their intracellular antioxidant glutathione levels.
Others works reported that PT pore opening involved in Δψm loss would constitute a sufficient event able to trigger apoptosis in thymocytes and T cells.44 45 In the present study, the capacity of the PT pore inducer PPIX to trigger Δψm disruption as well as apoptosis in the Th2 clonal cells supported this concept. The inhibitory effect of IFN-α on Δψm changes induced by PPIX strongly suggests that IFN-α promotes the survival of the clonal cells at least in part by preventing mitochondrial changes leading to apoptosis.
Our observations are also consistent with studies demonstrating that type I IFNs display antiapoptotic activity on in vivo activated T cells obtained from human inflamed rheumatoid synovium.43,46Also, IFN-α has been reported to prevent spontaneous apoptosis of chronic lymphocytic leukemia cells47-49 and myeloma cells,50 B-cell receptor-mediated apoptosis in B cells,51 as well as Fas-mediated apoptosis of myeloma cells,52 chronic myelogenous leukemia progenitors,53 and antigen-specific human T-cell clones generated in vitro.54 The fact that IFN-α did not affect Fas-mediated apoptosis in clonal Th2 cells from our patients with hypereosinophilia indicates that the influence of IFN-α on the Fas-mediated death pathway might depend on the cell type considered.
The spontaneous apoptosis of the Th2-like cells contrasts with their persistence in vivo, in association with hypereosinophilia and hyper-IgE.14-17 Furthermore, we observed that the Th2 clonal cells showed no sign of apoptosis immediately after they were purified, suggesting that they were not yet committed to death. This might be related to their in vivo exposure to survival factors such as IL-2, IL-4, or IL-15. As shown in a previous study, IL-2 and IL-4 are produced by the clonal Th2 cells on interaction with dendritic cells.16 It is therefore possible that an autocrine loop promoting Th2-cell survival might be operative in vivo as a consequence of their contact with antigen-presenting cells. In the present study, we demonstrate that a type I IFN, IFN-α, represents another survival factor of Th2 cells that might be operative in vivo in a paracrine fashion. Along this line, recent data indicate that fibroblasts producing IFN-β keep alive activated T cells with low bcl-2 that have reverted to a quiescent memory state.55
Our findings might be clinically relevant because they suggest that the long-term effects of IFN-α therapy in HES might depend on the subtype of the syndrome considered. Initially, the rationale for use of IFN-α in the management of HES was based on its beneficial effects in patients with chronic myelogenous leukemia56 and the fact that some patients with HES presented several features suggestive of a chronic myeloproliferative disease.57,58 Although it is plausible that some patients with HES actually present a true myeloproliferative disorder, current techniques fail to provide evidence for this in most cases. On rare occasions, clonality of eosinophils has been demonstrated in patients fulfilling the diagnostic criteria of HES, leading to the more appropriate diagnosis of chronic eosinophilic leukemia.59,60 The beneficial effects observed with IFN-α in such patients61,62 may result from its suppressive action both on eosinophil progenitors7 and on differentiated eosinophils.8,9,11 The notion that clonal Th2 cells play a pathogenic role in a subgroup of patients with chronic hypereosinophilia has emerged only recently, and most studies reporting favorable effects of IFN-α in the setting of HES have been conducted on patients with no evidence of an underlying T-cell disorder.1-6,12,13,63-67 The long-term effects of IFN-α in such patients are therefore unclear. Indeed, we observed development of peripheral T-cell lymphoma in 2 of 4 patients in our series, both of whom had received IFN-α for over a year.14,17 In another study of 16 hypereosinophilic patients presenting an aberrant population of IL-5–producing cells, T-cell lymphoma was ultimately diagnosed in 3 patients.20 One of these patients had previously been treated with IFN-α, and therapeutic measures were not specified for the 2 others. In view of the antiapoptotic effects of IFN-α reported in the present paper, we suggest carefully evaluating the long-term outcome of patients receiving isolated IFN-α therapy in the context of the Th2 variant of HES.
Acknowledgments
The technical assistance of Alain Crusiaux and Martine Ducarme is gratefully acknowledged.
Supported by the Télévie program (Belgium). F.R. is a research assistant of the Fonds National de la Recherche Scientifique (Belgium).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michel Goldman, Department of Immunology, Hôpital Erasme 808, route de Lennik, B-1070 Brussels, Belgium; e-mail: mgoldman@ulb.ac.be.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal