Abstract
Over time, the epidemiologic and demographic characteristics of AIDS have changed in the United States, while the use of highly active antiretroviral therapy has changed the natural history of the disease. The goal of the study was to ascertain any changes in the epidemiologic, immunologic, pathologic, or clinical characteristics of AIDS-related lymphoma (ARL) over the course of the AIDS epidemic. Records of 369 patients with ARL diagnosed or treated at a single institution from 1982 through 1998 were reviewed. Single institutional data were compared to population-based data from the County of Los Angeles. Significant changes in the demographic profile of patients with newly diagnosed ARL have occurred, with the later time intervals associated with a higher prevalence in women (P = .25), in Latino/Hispanic individuals (P < .0001), and in those who acquired human immunodeficiency virus (HIV) heterosexually (P = .01). These changes were similar in both countywide, population-based analyses and in those from the single institution. The median CD4+ lymphocyte count at lymphoma diagnosis has decreased significantly over the years, from 177/dL in the earliest time period (1982-1986), to 53/dL in the last time period from 1995 to 1998 (P = .0006). The pathologic spectrum of disease has also changed, with a decrease in the prevalence of small noncleaved lymphoma (P = .0005) and an increase in diffuse large cell lymphoma (P < .0001). Despite changes in the use of antiretroviral or chemotherapy regimens, the median survival has not significantly changed.
Introduction
Lymphoma primary to the central nervous system (CNS) was considered a manifestation of AIDS from the onset of the AIDS epidemic,1 and systemic high- or intermediate-grade lymphomas became AIDS defining in 1985.2 AIDS-related lymphoma (ARL) is usually a late manifestation of infection by the human immunodeficiency virus (HIV), with a predilection for widespread, extra nodal disease, involvement of the CNS, and poor prognosis.3-8
Over time, the epidemiologic and demographic characteristics of AIDS have changed in the United States. The prevalence of AIDS has increased among minority populations, in women, and in those who acquired HIV by heterosexual means.9-11 The widespread use of highly active antiretroviral therapy (HAART) has also changed the natural history of the disease,12-14 with a 73% decrease in AIDS-defining opportunistic infections and a 49% decrease in mortality between 1994 and 1996.15 Although HAART has been associated with a dramatic decrease in the incidence of opportunistic infections15,16 and Kaposi sarcoma,16-18 the effect of HAART on ARL is still unclear, although early data suggest no significant decline.16,19 20 Because of the changing characteristics of AIDS in general, we wished to ascertain if there were any changes in the epidemiologic, immunologic, pathologic, or clinical aspects of ARLs over the course of the AIDS epidemic, from 1982 to 1998.
Patients, materials, and methods
This is a retrospective study of 369 patients diagnosed and/or treated at Los Angeles County–University of Southern California (LAC-USC) Medical Center and the USC/Norris Cancer Hospital and Research Institute between 1982 and 1998. All patients were HIV seropositive by enzyme-linked immunosorbent assay with confirmation by Western blot. All patients had biopsy or cytologically proven non-Hodgkin lymphoma (NHL) and were staged prior to treatment, using the Ann Arbor staging system.21
A complete history, physical examination, and routine blood work, as well as T-cell subtypes in the peripheral blood were performed at the time of diagnosis. Routine staging was performed in all patients when possible, which included chest radiographs; computed axial tomography (CAT) scans of the chest, abdomen, and pelvis; lumbar puncture with cerebrospinal fluid (CSF) analysis; and bone marrow biopsies and aspirates. A total of 358 (97%) of 369 patients underwent bone marrow aspirate and biopsy, which was performed from bilateral sites. Lumbar puncture with analysis of CSF was performed in 353 (95.6%) patients. CAT scans were performed in all but 7 (98%) patients. Hospital records, bone marrow pathology reports, laboratory data, treatment regimens, and response to therapy were reviewed. All cases underwent uniform pathologic review by one of us (B.N.).
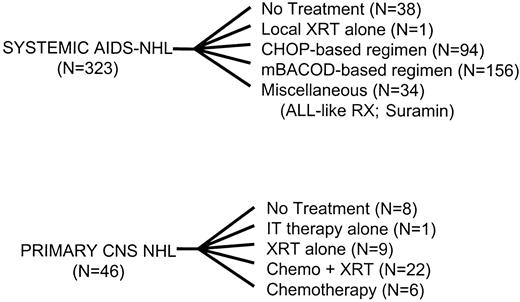
The specific regimens used to treat the 323 patients with systemic lymphoma and the 46 patients with primary CNS lymphoma are provided in Figure 1.22-30 In total, 215 patients (67% of treated patients) were enrolled in a prospective therapeutic protocol.
Patients were treated with a variety of regimens.
XRT indicates radiation therapy; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; mBACOD, methotrexate, bleomycin, doxorubicin, cyclophosphamide, vincristine, dexamethasone; IT, intrathecal; ALL, adult lymphoblastic leukemia; RX, regimen; chemo, chemotherapy.
Patients were treated with a variety of regimens.
XRT indicates radiation therapy; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; mBACOD, methotrexate, bleomycin, doxorubicin, cyclophosphamide, vincristine, dexamethasone; IT, intrathecal; ALL, adult lymphoblastic leukemia; RX, regimen; chemo, chemotherapy.
Demographic data from patients managed at our institution were compared with the population-based data in the AIDS case registry maintained by the HIV Epidemiology Program in the Department of Health Services for the entire County of Los Angeles31 to evaluate the possibility of referral bias. The Los Angeles County data include all ARL cases diagnosed from 1982 through 1998 in adults (n = 754). ARL cases with a missing date of diagnosis or those occurring in children were excluded.
Statistical analysis
Patients were categorized into 4 time periods, depending on the year of ARL diagnosis. The 4 periods were selected, based on the general concepts of therapy for HIV disease during these periods. Zidovudine was licensed for use as the first antiretroviral agent in 1987, and didanosine (ddI) was licensed in 1991. Combination antiretroviral therapy became widely used in 1994-1995,13,14 and protease inhibitors became widely available in 1995-1996.12 32 Time periods thus included 1982 through 1986, 1987 through 1990, 1991 through 1994, and 1995 through 1998.
Results
Patient characteristics
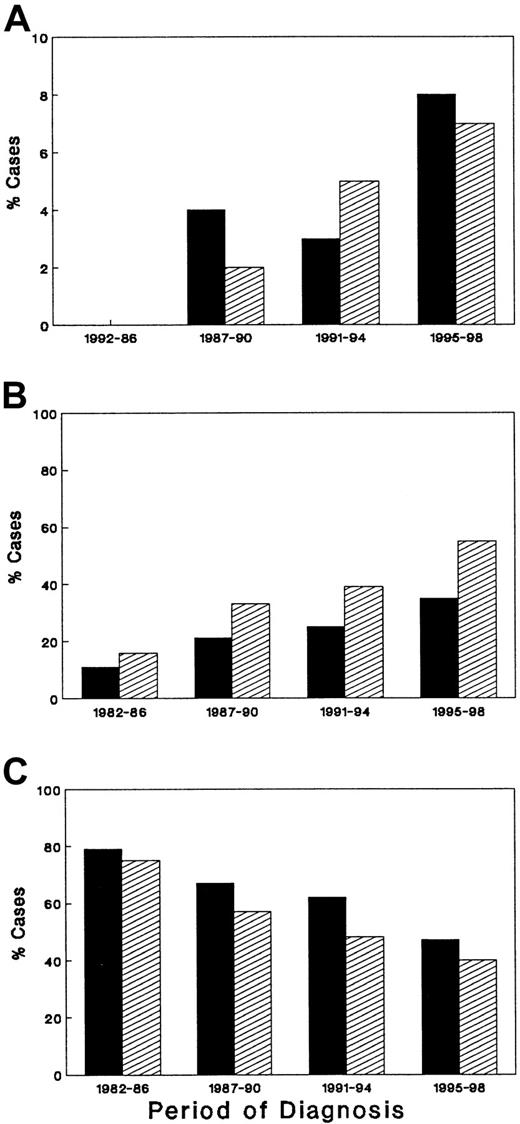
A total of 369 HIV-seropositive patients were diagnosed or referred with a diagnosis of NHL between 1982 and December 1998 (Table1). The group consisted of 354 males and 15 females. As shown, the median age at the time of ARL diagnosis has remained relatively constant over the 4 time periods, ranging from 36 to 40 years. Although the number of females with ARL is small, resulting in unstable estimates, the number of females with ARL has increased over time, ranging in the institutional cases from 0% in the earliest time period to 7% in the latest interval (P = .25), and from 0% to 8% of the cases in the County of Los Angeles (P = .075). Overall, Caucasians composed 51% of the entire group, whereas 39% were Hispanic. Over time, the prevalence of ARL has decreased in Caucasians (P = .0007) and increased in Hispanics (P < .0001). As shown in Figure 2, the demographic changes seen in ARL patients at LAC-USC Medical Center mirrored those reported for the County of Los Angeles.
Demographic profile of 369 patients with AIDS-related lymphoma over different time intervals
| . | 1982-1986 (%) . | 1987-1990 (%) . | 1991-1994 (%) . | 1995-1998 (%) . | Total (%) . | P value . |
|---|---|---|---|---|---|---|
| No. patients | 44 | 88 | 132 | 105 | 369 | |
| Median age (y) | 40 | 36 | 38 | 39 | 38 | .18 |
| Sex | .25 | |||||
| Female | 0 (0) | 2 (2) | 6 (5) | 7 (7) | 15 (4) | |
| Male | 44 (100) | 86 (98) | 126 (95) | 98 (93) | 354 (96) | |
| Race | .001 | |||||
| Caucasian | 33 (75) | 50 (57) | 64 (48) | 42 (40) | 189 (51) | * |
| Hispanic | 7 (16) | 29 (33) | 51 (39) | 58 (55) | 145 (39) | † |
| Black | 4 (9) | 4 (5) | 17 (13) | 5 (5) | 30 (8) | |
| Asian | 0 (0) | 5 (6) | 0 (0) | 0 (0) | 5 (1) | |
| Risk | .039 | |||||
| MSM | 37 (84) | 67 (76) | 105 (80) | 69 (66) | 278 (75) | ‡ |
| IDU ± MSM | 3 (7) | 7 (8) | 4 (3) | 3 (3) | 17 (5) | |
| Hetero | 2 (5) | 4 (5) | 13 (10) | 19 (18) | 38 (10) | 1-153 |
| Transfusion | 0 | 3 (3) | 1 (0.5) | 4 (4) | 8 (2) | |
| Unknown | 2 (5) | 7 (8) | 9 (7) | 10 (10) | 28 (8) | |
| KPS | .0008 | |||||
| > 80% | 14 (32) | 28 (32) | 75 (57) | 45 (43) | 162 (44) | |
| < 80% | 30 (68) | 60 (68) | 57 (43) | 60 (57) | 207 (56) | |
| Prior OI1-154 | 14 (32) | 40 (45) | 58 (44) | 53 (50) | 165 (45) | .22 |
| Prior KS1-154 | 2 (5) | 13 (15) | 11 (8) | 14 (13) | 40 (11) | .20 |
| Median CD41-155 | 177 | 113 | 54 | 53 | 66 | .0006 |
| Range | 0-1703 | 2-1927 | 0-710 | 0-700 | 0-1927 |
| . | 1982-1986 (%) . | 1987-1990 (%) . | 1991-1994 (%) . | 1995-1998 (%) . | Total (%) . | P value . |
|---|---|---|---|---|---|---|
| No. patients | 44 | 88 | 132 | 105 | 369 | |
| Median age (y) | 40 | 36 | 38 | 39 | 38 | .18 |
| Sex | .25 | |||||
| Female | 0 (0) | 2 (2) | 6 (5) | 7 (7) | 15 (4) | |
| Male | 44 (100) | 86 (98) | 126 (95) | 98 (93) | 354 (96) | |
| Race | .001 | |||||
| Caucasian | 33 (75) | 50 (57) | 64 (48) | 42 (40) | 189 (51) | * |
| Hispanic | 7 (16) | 29 (33) | 51 (39) | 58 (55) | 145 (39) | † |
| Black | 4 (9) | 4 (5) | 17 (13) | 5 (5) | 30 (8) | |
| Asian | 0 (0) | 5 (6) | 0 (0) | 0 (0) | 5 (1) | |
| Risk | .039 | |||||
| MSM | 37 (84) | 67 (76) | 105 (80) | 69 (66) | 278 (75) | ‡ |
| IDU ± MSM | 3 (7) | 7 (8) | 4 (3) | 3 (3) | 17 (5) | |
| Hetero | 2 (5) | 4 (5) | 13 (10) | 19 (18) | 38 (10) | 1-153 |
| Transfusion | 0 | 3 (3) | 1 (0.5) | 4 (4) | 8 (2) | |
| Unknown | 2 (5) | 7 (8) | 9 (7) | 10 (10) | 28 (8) | |
| KPS | .0008 | |||||
| > 80% | 14 (32) | 28 (32) | 75 (57) | 45 (43) | 162 (44) | |
| < 80% | 30 (68) | 60 (68) | 57 (43) | 60 (57) | 207 (56) | |
| Prior OI1-154 | 14 (32) | 40 (45) | 58 (44) | 53 (50) | 165 (45) | .22 |
| Prior KS1-154 | 2 (5) | 13 (15) | 11 (8) | 14 (13) | 40 (11) | .20 |
| Median CD41-155 | 177 | 113 | 54 | 53 | 66 | .0006 |
| Range | 0-1703 | 2-1927 | 0-710 | 0-700 | 0-1927 |
MSM indicates men who have sex with men; IDU, injection drug use; Hetero, heterosexual risk factor for HIV; KPS, Karnofsky performance status; OI, opportunistic infection; KS, Kaposi sarcoma.
P value = .0007, comparing Caucasians versus all other races.
P value < .0001, comparing Hispanics versus all other races.
P value = .045, comparing MSM with all other HIV risk groups.
P value = .011, comparing heterosexual transmission with all other HIV risk groups.
Patients without a diagnosis of OI or KS prior to development of lymphoma presented with lymphoma as the first AIDS-defining condition.
CD4 count at time of diagnosis of AIDS-related lymphoma.
There has been a change in the demographic profile of patients with AIDS-related lymphoma in the United States.
Percentage of female (A), Hispanic (B), and Caucasian (C) patients are shown for Los Angeles County (▪) and the University of Southern California Medical Center (▨).
There has been a change in the demographic profile of patients with AIDS-related lymphoma in the United States.
Percentage of female (A), Hispanic (B), and Caucasian (C) patients are shown for Los Angeles County (▪) and the University of Southern California Medical Center (▨).
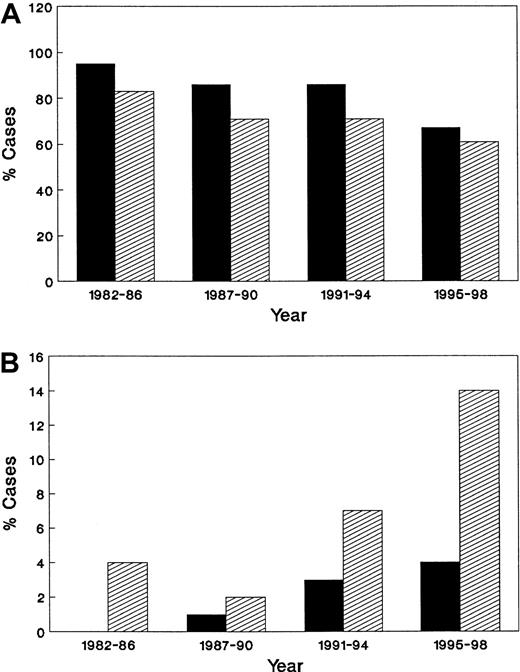
Risk factors for HIV infection included male-to-male sexual transmission in 278 (75%) patients, and heterosexual transmission in 38 (10%). Relatively few patients reported a history of injection drug use as the only risk factor for HIV. Over time, greater numbers of patients with ARL reported heterosexual contact as their route of initial HIV transmission (P = .011), a trend confirmed in the population-based data from Los Angeles County (Figure 3).
There has been a change in HIV transmission risk group among patients with AIDS-related lymphoma.
Percentage of men who have sex with men (A) and heterosexuals (B) among patients in Los Angeles County (▪) and the University of Southern California Medical Center (▨).
There has been a change in HIV transmission risk group among patients with AIDS-related lymphoma.
Percentage of men who have sex with men (A) and heterosexuals (B) among patients in Los Angeles County (▪) and the University of Southern California Medical Center (▨).
HIV-related characteristics
In the group as a whole, 165 (45%) patients had a history of opportunistic infection(s) prior to the diagnosis of ARL, and 40 (11%) had a history of Kaposi sarcoma. No significant change was observed over the various time periods in terms of prior AIDS diagnoses. Of interest, however, the median CD4 cell count at the time of diagnosis of lymphoma has declined steadily over the years, from a median level of 177 dL during 1982 through 1986, to 53 dL in the latest time period (P = .0006).
Characteristics of lymphoma at diagnosis
Specific characteristics of the lymphomas are provided in Table2. Systemic lymphoma was diagnosed in 323 (88%) patients, and 46 (12%) had primary CNS lymphoma. No change in the prevalence of primary CNS lymphoma was apparent over time.
Comparison of pathologic and clinical findings on 369 patients with AIDS-related lymphoma over different time intervals
| . | 1982-1986 (%) . | 1987-1990 (%) . | 1991-1994 (%) . | 1995-1998 (%) . | Total (%) . | P value . |
|---|---|---|---|---|---|---|
| No. of patients | 44 | 88 | 132 | 105 | 369 | |
| Lymphoma type | .28 | |||||
| Systemic | 38 (86) | 76 (86) | 121 (92) | 88 (84) | 323 (88) | .0001 |
| SNC | 21 (55) | 35 (46) | 49 (40) | 18 (20) | 123 | |
| IBL | 12 (32) | 17 (22) | 36 (30) | 29 (33) | 94 | |
| HGNOS | 1 (3) | 11 (14) | 9 (7) | 7 (8) | 28 | |
| DLC | 0 (0) | 7 (9) | 26 (21) | 30 (34) | 63 | |
| Other | 4 (11) | 6 (8) | 1 (< 1) | 4 (5) | 15 | |
| Primary CNS | 6 (14) | 12 (14) | 11 (8) | 17 (16) | 46 (12) | .05 |
| SNC | 3 (50) | 2 (17) | 1 (9) | 5 (29) | 11 | |
| IBL | 3 (50) | 2 (17) | 1 (9) | 5 (29) | 11 | |
| HGNOS | 0 | 5 (42) | 4 (36) | 0 | 9 | |
| DLC | 0 | 1 (8) | 1 (9) | 4 (24) | 6 | |
| Other | 0 | 2 (17) | 4 (36) | 3 (18) | 9 | |
| Cell type | .08 | |||||
| T cell* | 0 (0) | 3 (3) | 1 (1) | 6 (6) | 10 (3) | |
| B cell | 44 (100) | 85 (97) | 131 (99) | 99 (94) | 359 (97) | |
| Stage† | .23 | |||||
| I | 9 (20) | 22 (25) | 38 (29) | 33 (33) | 102 (28) | |
| II | 0 | 6 (7) | 6 (5) | 1 (1) | 13 (4) | |
| III | 4 (9) | 10 (11) | 11 (8) | 5 (5) | 30 (8) | |
| IV | 30 (70) | 49 (56) | 76 (58) | 62 (61) | 217 (60) | |
| Sites of stage IV | ||||||
| Bone marrow | 10 (23) | 16 (17) | 12 (9) | 21 (20) | 59 (16) | .20 |
| CSF | 5 (11) | 9 (11) | 9 (8) | 10 (10) | 33 (9) | .39 |
| Liver | 7 (16) | 19 (22) | 3 (18) | 18 (18) | 67 (19) | .84 |
| Stomach‡ | 2 (5) | 9 (10) | 21 (16) | 6 (6) | 38 (11) | .05 |
| Pleura | 1 (2) | 7 (8) | 10 (8) | 9 (9) | 27 (8) | .56 |
| Rectum | 6 (14) | 6 (7) | 14 (11) | 1 (1) | 27 (8) | .014 |
| Oral cavity | 2 (5) | 6 (7) | 11 (8) | 7 (7) | 26 (7) | .83 |
| Colon | 9 (21) | 4 (5) | 7 (5) | 6 (6) | 26 (7) | .35 |
| Lung | 3 (7) | 5 (6) | 4 (3) | 7 (7) | 19 (5) | .54 |
| Kidney | 1 (2) | 4 (5) | 5 (4) | 5 (5) | 15 (4) | .89 |
| Skin | 1 (2) | 2 (2) | 3 (2) | 5 (5) | 11 (3) | .41 |
| B symptoms | 37 (84) | 50 (60) | 77 (63) | 63 (65) | 227 (65) | .008 |
| Median survival (mo) | 6.9 | 5.4 | 6.9 | 6.3 | 6.3 | .41 |
| Range | 1.0-134+ | 0.2-142+ | 0.32-79+ | 0.2-47+ | 0.2-142+ |
| . | 1982-1986 (%) . | 1987-1990 (%) . | 1991-1994 (%) . | 1995-1998 (%) . | Total (%) . | P value . |
|---|---|---|---|---|---|---|
| No. of patients | 44 | 88 | 132 | 105 | 369 | |
| Lymphoma type | .28 | |||||
| Systemic | 38 (86) | 76 (86) | 121 (92) | 88 (84) | 323 (88) | .0001 |
| SNC | 21 (55) | 35 (46) | 49 (40) | 18 (20) | 123 | |
| IBL | 12 (32) | 17 (22) | 36 (30) | 29 (33) | 94 | |
| HGNOS | 1 (3) | 11 (14) | 9 (7) | 7 (8) | 28 | |
| DLC | 0 (0) | 7 (9) | 26 (21) | 30 (34) | 63 | |
| Other | 4 (11) | 6 (8) | 1 (< 1) | 4 (5) | 15 | |
| Primary CNS | 6 (14) | 12 (14) | 11 (8) | 17 (16) | 46 (12) | .05 |
| SNC | 3 (50) | 2 (17) | 1 (9) | 5 (29) | 11 | |
| IBL | 3 (50) | 2 (17) | 1 (9) | 5 (29) | 11 | |
| HGNOS | 0 | 5 (42) | 4 (36) | 0 | 9 | |
| DLC | 0 | 1 (8) | 1 (9) | 4 (24) | 6 | |
| Other | 0 | 2 (17) | 4 (36) | 3 (18) | 9 | |
| Cell type | .08 | |||||
| T cell* | 0 (0) | 3 (3) | 1 (1) | 6 (6) | 10 (3) | |
| B cell | 44 (100) | 85 (97) | 131 (99) | 99 (94) | 359 (97) | |
| Stage† | .23 | |||||
| I | 9 (20) | 22 (25) | 38 (29) | 33 (33) | 102 (28) | |
| II | 0 | 6 (7) | 6 (5) | 1 (1) | 13 (4) | |
| III | 4 (9) | 10 (11) | 11 (8) | 5 (5) | 30 (8) | |
| IV | 30 (70) | 49 (56) | 76 (58) | 62 (61) | 217 (60) | |
| Sites of stage IV | ||||||
| Bone marrow | 10 (23) | 16 (17) | 12 (9) | 21 (20) | 59 (16) | .20 |
| CSF | 5 (11) | 9 (11) | 9 (8) | 10 (10) | 33 (9) | .39 |
| Liver | 7 (16) | 19 (22) | 3 (18) | 18 (18) | 67 (19) | .84 |
| Stomach‡ | 2 (5) | 9 (10) | 21 (16) | 6 (6) | 38 (11) | .05 |
| Pleura | 1 (2) | 7 (8) | 10 (8) | 9 (9) | 27 (8) | .56 |
| Rectum | 6 (14) | 6 (7) | 14 (11) | 1 (1) | 27 (8) | .014 |
| Oral cavity | 2 (5) | 6 (7) | 11 (8) | 7 (7) | 26 (7) | .83 |
| Colon | 9 (21) | 4 (5) | 7 (5) | 6 (6) | 26 (7) | .35 |
| Lung | 3 (7) | 5 (6) | 4 (3) | 7 (7) | 19 (5) | .54 |
| Kidney | 1 (2) | 4 (5) | 5 (4) | 5 (5) | 15 (4) | .89 |
| Skin | 1 (2) | 2 (2) | 3 (2) | 5 (5) | 11 (3) | .41 |
| B symptoms | 37 (84) | 50 (60) | 77 (63) | 63 (65) | 227 (65) | .008 |
| Median survival (mo) | 6.9 | 5.4 | 6.9 | 6.3 | 6.3 | .41 |
| Range | 1.0-134+ | 0.2-142+ | 0.32-79+ | 0.2-47+ | 0.2-142+ |
AIDS indicates acquired immune deficiency syndrome; SNC, small noncleaved lymphoma (Burkitt or Burkitt-like); IBL, immunoblastic lymphoma; HGNOS, high-grade lymphoma, not otherwise specified; DLC, diffuse large cell lymphoma; CSF, cerebrospinal fluid; B symptoms, fever, night sweats, and/or weight loss in excess of 10% normal body weight.
T-cell lymphomas did not include any case of cutaneous T-cell lymphoma.
Excludes 7 patients who did not undergo full staging.
Stomach involvement was ascertained by endoscopy and biopsy.
The vast majority (97%) of patients were diagnosed with B-cell lymphomas, although minor increases in T-cell lymphomas have recently occurred. Although the most common pathologic subtype of disease (ie, high-grade, small noncleaved lymphoma, diagnosed in 36% overall) has decreased over time from 55% in 1982 through 1986 to 22% of all cases in 1995 through 1998 (P = .0005), the pathologic subtype of diffuse large cell lymphoma has increased significantly over time from 0% in the earlier period to 32% of all cases in the latter time period (P < .0001). As shown in Table 2, although the pathologic spectrum of systemic ARL has changed over time with a significant decline in small noncleaved lymphoma (P = .03) and a significant increase in diffuse large cell lymphoma (P < .0001), the same is not true for patients with primary CNS lymphoma, in which the pathologic diagnoses have not varied significantly over the years of study.
In patients with systemic ARL, presence of extranodal lymphomatous disease was commonly found in all time intervals, documented in 56% to 70% of newly diagnosed patients. Further, the specific sites of extranodal disease have not changed appreciably over time. Lymphoma in the bone marrow was seen in 59 (16%) of the whole group, whereas involvement of the CSF occurred in 33 (9%). Liver involvement was found in 67 (19%), and involvement of the rectum occurred in 27 (8%) patients overall. Other sites of extranodal lymphomatous disease are provided (Table 2).
Survival
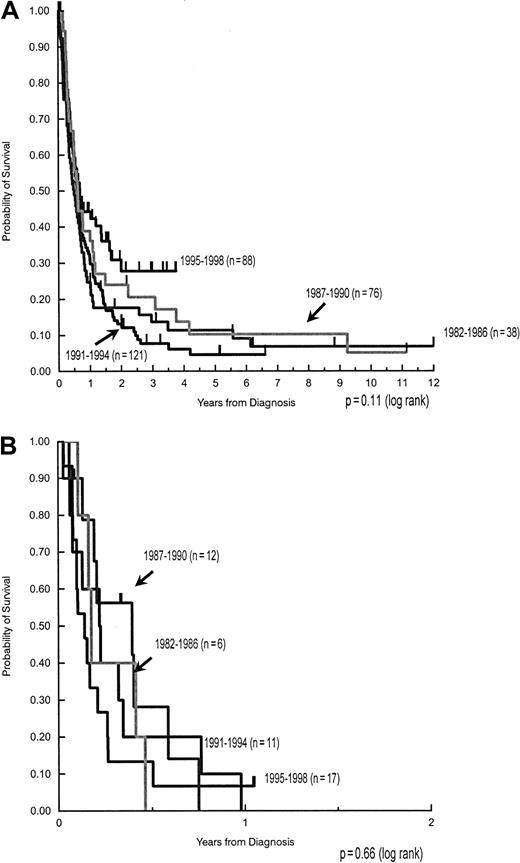
Despite changes in median CD4 cell count at ARL diagnosis, in patterns of antiretroviral use, and in various chemotherapeutic regimens, the median survival has remained stable, at approximately 6 months for patients with systemic lymphoma (Figure4A) and 2 months for those with primary CNS lymphoma (Figure 4B). Nonetheless, patients with systemic lymphoma treated in the latter time interval (1995-1998) may be doing somewhat better, with a median survival of 8.2 months versus 7.4 months in 1982 through 1986, 5.7 months in those treated from 1987 through 1990, and 7.2 months for those treated between 1991 and 1994 (P = .11). No such trend is apparent in patients with primary CNS lymphoma, whose median survival was 2.0 months in the earliest time period and 1.7 months in the latest (P = .66).
Despite changes in CD4 cell count and antiretroviral therapy at time of AIDS-lymphoma diagnosis, no change in median survival has occurred between 1982 and 1998.
(A) Survival of patients with systemic AIDS-NHL. (B) Survival of patients with primary CNS NHL.
Despite changes in CD4 cell count and antiretroviral therapy at time of AIDS-lymphoma diagnosis, no change in median survival has occurred between 1982 and 1998.
(A) Survival of patients with systemic AIDS-NHL. (B) Survival of patients with primary CNS NHL.
In patients with systemic lymphoma, there was no statistical difference in median survival based on stage of disease. Thus, the median survival for stage I patients was 7.8 months versus 13.8 months for those with stage II, 7.7 months for stage III, and 6.5 months for stage IV (P = .23). Further, there were no differences in overall survival during the various time periods when stratifying by stage. Last, there was no difference in the stage of lymphomatous disease at any particular time period.
Population-based demographic data of ARL reported in the County of Los Angeles
Comparison of demographic data from ARL patients diagnosed and treated at USC and its hospitals and for the County of Los Angeles as a whole is presented in Figures 2 and 3. As shown, trends noted at USC were similar to those in the population-based AIDS registry. When comparing cases reported in 1982 through 1986 to those in the latter time period (1995-1998), a strong trend favoring an increase of ARL in women was seen in the county as a whole (P = .075), with significant increases in Hispanics (P = .007) and a significant decrease in Caucasians (P = .002). There was no significant change in ARL cases reported in African Americans, either in the county of Los Angeles (P = .68) or at the USC Hospitals.
Discussion
The widespread use of HAART has been associated with a marked reduction in the development of opportunistic infections and a remarkable improvement in overall survival among patients with AIDS.15,16 Similarly, the incidence of Kaposi sarcoma has fallen dramatically in the United States.16-20 The effect of HAART on the incidence of ARL is less clear. Although the majority of studies have failed to demonstrate a major decline in the incidence of systemic ARL,16,17,19,20 other studies18,36have shown a decrease in the incidence of lymphoma primary to the CNS. Nonetheless, in contrast to Kaposi sarcoma,16 a major decline in the incidence of ARL has not been observed.
Although the incidence of ARL has not changed substantially, the current study indicates that the demographic characteristics of disease have evolved toward a greater prevalence of the disease among women and minority populations and among those who acquired HIV by heterosexual contact. The current study represents data from a single institution and would, therefore, potentially be biased by the specific referral patterns in this center. Nonetheless, the LAC-USC Medical Center is a government-operated facility, is the largest hospital in the United States, and serves a largely indigent patient population, who are not specifically referred to our center. The inclusion of patients from the USC/Norris Cancer Hospital (n = 61), a private facility with a large referral base, serves to broaden the scope of patients comprising this report. Furthermore, population-based data from the County of Los Angeles indicate that the changing demographic profile of ARL in the county is quite similar to what we have reported in our own institution. Of interest, the changing demographic profile of patients with AIDS in the United States is also consistent with what we have reported in patients with ARL.11 Thus, the proportion of women among all AIDS cases in the United States has increased from 7% in 1982 to 14% in 1992 and to 23% in 1998. The proportion of Hispanics with AIDS has increased from 13% of all AIDS cases in 1982 to 20% of all AIDS cases in the United States in 1998. Further, the proportion of AIDS among those who acquired HIV by heterosexual means has increased in males from 3.5% in 1992 to 7% in 1997 and in females from 41.3% to 52.6% over the same time interval.1,2 9-11These data, taken together, would suggest that the demographic changes occurring in patients with ARL are not distinct to the lymphoma, but rather represent part of the larger changes occurring in the AIDS epidemic itself.
With the use of the large database from our center, we have also seen a change in the immunologic profile of patients presenting with ARL. In the early years of the epidemic, the median CD4 cell count of our patients at the time of ARL diagnosis was significantly higher than that seen in the more recent time intervals, falling from 177/dL to 53/dL. Lymphoma has been considered a late manifestation of HIV disease,37-40 the incidence of which did not increase substantially until approximately 4 years after the first cases of AIDS had been described.1,2,41 The significant fall in median CD4 cell count in patients with newly diagnosed ARL is consistent with the profound degree of immune suppression that is expected in this disease.4-8,42 Of interest, a fall in median CD4 cell count over recent years may also be seen in reports of several large, prospective national trials conducted through the AIDS Clinical Trials Group (ACTG).26,27 Thus, patients with newly diagnosed AIDS lymphoma accrued to a Phase II study of low-dose methotrexate alternating with standard agents (m-BACOD) between June 1987 and November 1988 had a median CD4 cell count of 150 dL (range 16-1079).26 A subsequent ACTG study, which compared low-dose with standard-dose m-BACOD, accrued patients between February 1991 and October 1994 and had similar eligibility requirements but reported a median study entry CD4 cell count of 100 dL for the low-dose arm and 107 dL for the full-dose arm.27 A substantial increase in CD4 cell count is expected with effective HAART therapy.12 32 The lower CD4 cell count in these recent patients with newly diagnosed ARL may indicate that HAART was ineffective, that HAART was not employed, or that HAART therapy has simply delayed the onset of opportunistic infections or other illnesses, allowing these patients to live long enough to actually develop ARL.
The current study has also demonstrated a change in the pathologic spectrum of lymphomatous disease over the years. Although lymphoma pathology may be complex, with substantial interobserver discrepancy,43 all cases in this study have been reviewed and diagnosed by a single, expert hematopathologist (B.N.N.). It is thus unlikely that changes in pathologic diagnosis over time could have occurred because of differing opinions or criteria for diagnosis of these disorders. In patients with systemic ARL, the prevalence of small noncleaved lymphoma has decreased significantly over the time periods, whereas the prevalence of diffuse large cell lymphoma has increased. Similar trends were observed in patients with primary CNS disease. Of interest, small noncleaved NHL has been associated with higher CD4 cell count and earlier onset of lymphoma,40 whereas diffuse large cell or immunoblastic lymphomas have been associated with more profound immune deficiency and lower CD4 cell count.40,44 The current data are consistent with these concepts and support the hypothesis that different pathogenetic mechanisms may be involved in the various pathologic subtypes of ARL.45
In contrast to expectations in HIV-negative intermediate and high-grade lymphoma,46 increasing stage of disease was not associated with poorer prognosis in our patients with ARL. Furthermore, despite changes in specific chemotherapeutic regimens employed over the years, we found no statistically significant difference in median survival times, either for patients with systemic or primary CNS disease. Although the current study does not represent a prospective therapeutic trial, approximately 67% of all treated patients in this series were enrolled in such trials. Of importance, these prospective therapeutic studies have shown no major differences in complete remission rates or overall survival in patients treated with low-dose or standard-dose chemotherapy.26,27,47 Further, although no prospective study has yet compared the use of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) with methotrexate, bleomycin, doxorubicin, cyclophosphamide, vincristine, and dexamethasone (m-BACOD) (the 2 regimens used most commonly in our series), outcome data in patients with systemic ARL appear similar with the 2 regimens.27,47 48 The statistically similar median survival times of our patients over the years would be consistent with the concept that no major differences in outcome are expected in patients treated with low-dose or standard-dose therapy, or with m-BACOD versus CHOP chemotherapy in these patients.
Despite the use of more effective antiretroviral therapies over time, the median CD4 cell count of our patients has decreased, indicating that these patients may simply have lived long enough to eventually develop lymphoma as a long-term complication of HIV infection. Although median survival is not statistically different when one compares earlier to later time intervals, recent trends would suggest a possible improvement in survival of those with systemic ARL treated in the latter time interval (1995-1998), despite the fact that these patients had significantly lower CD4 cell counts at the time that lymphoma was diagnosed. Because there are no prospective therapeutic data to suggest that the chemotherapeutic regimens were associated with differences in outcome,26,27,47,48 these data would imply that use of HAART may have a substantial beneficial effect on the outcome of patients receiving chemotherapy for ARL, as has been suggested in other studies.49 In this regard, preliminary data would suggest that HAART may be used safely in conjunction with combination chemotherapy for ARL.48 Further study of HAART used together with combination chemotherapy will clearly be of importance.
Supported in part by grants NOA-A1-62540, R01-CA50850, RO1-CA-55510, and UO1 CA 70072, National Institute of Allergy and Infectious Diseases and National Cancer Institute, National Institutes of Health, Public Health Service, Department of Health and Human Services.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alexandra M. Levine, USC/Norris Cancer Hospital, 1441 Eastlake Ave, Rm 3468, NOR 3431, Los Angeles, CA 90033; e-mail: hornor@hsc.usc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal