Abstract
Human immunodeficiency virus-1 (HIV-1) infection has been shown to result in up-regulation of the urokinase-type plasminogen activator receptor (uPAR/CD87) on leukocytes in vitro and in vivo. The objective of this study was to investigate whether this up-regulation is paralleled by higher serum levels of soluble uPAR (suPAR) in patients with advanced HIV-1 disease and whether the serum level of suPAR is predictive of clinical outcome. Using an enzyme-linked immunosorbent assay, the level of suPAR was measured retrospectively in serum samples from 314 patients with HIV-1 infection. By Kaplan-Meier and Cox regression analyses, the serum suPAR levels were correlated to survival with AIDS-related death as the end point. High levels of serum suPAR (greater than median) were associated with poor overall survival, and Kaplan-Meier analysis on patients stratified by suPAR level demonstrated a continuous increase in mortality rates with higher suPAR levels. After adjustment for accepted prognostic markers—including Centers for Disease Control and Prevention–defined clinical stages, CD4 counts, viral load, β2-microglobulin, and age—the prognostic strength of suPAR remained highly significant, indicating that the serum suPAR level is a novel, strong, and independent predictor of survival in HIV-1 infection. This report is the first to demonstrate an important association between the plasminogen activator system and disease progression in HIV-1 infection.
Introduction
The urokinase-type plasminogen activator system consists of a proteinase (uPA), a receptor (uPAR), and inhibitors. The system is involved in pericellular proteolysis, cell migration, and tissue remodeling by multiple modes of action—proteolysis, signal transduction, and chemokine-like activities.1,2 Under physiological conditions, uPA and uPAR are predominantly expressed by blood cells, including neutrophils, monocytes, macrophages, and activated T-cells,3 for which they are believed to play important roles in cell activation, adhesion, migration, and extravasation.4-6
High serum levels of suPAR, the soluble form of uPAR, have recently been associated with worse overall survival rates among cancer patients,7,8 and it has been suggested that in cancer the excess of suPAR in the circulation derives from tumor cells or tumor-infiltrating macrophages,7-10 which often express high levels of uPAR.11
The fact that enhanced serum suPAR levels are indicative for up-regulated cellular uPAR levels, combined with the previously published data that HIV-1 infection leads to enhanced cell surface expression of uPAR on monocytes and T-lymphocytes in vitro and in vivo,12-14 prompted us to investigate the relation between serum suPAR levels in HIV-1–infected patients and disease progression.
Patients, materials, and methods
Patients
The Copenhagen HIV Immune Cohort (CHIC) is a seroprevalence cohort consisting of 347 HIV-1–infected patients enrolled in the Department of Infectious Diseases at Rigshospitalet, Copenhagen, Denmark, between September 1991 and October 1992, as previously described.15 The cohort was followed up from inclusion until June 1997; however, in the current study, follow-up was censored when the first patient in the cohort received a protease inhibitor (May 1, 1996). Thirty-three patients (9.5%) for whom serum samples were unavailable were excluded from this study. There were no significant differences in follow-up time, age, CD4 count, viral load, and β2-microglobulin level between patients in the current study and those excluded. At enrollment in CHIC, blood was drawn for determination of the CD4 count and other serologic parameters related to HIV-1 progression. Of the 314 serum samples available for suPAR measurement, 208 were obtained at the time of enrollment (baseline), and the remaining 106 were obtained close to the time of enrollment (median, 0.9 month before; range, 8.4 months before to 7.2 months after). All other serologic values used in this study were baseline values. Clinical staging of the patients was performed according to the 1993 Centers for Disease Control and Prevention (CDC) AIDS surveillance case definition for adolescents and adults. Of the 314 patients included in this study, 230 (73.2%) received anti-retroviral treatment with one or more nucleoside reverse transcriptase inhibitors (NRTIs) before enrollment or during follow-up. Of these patients, 140 (60.9%) received 1 NRTI, 88 (38.3%) received 2 NRTIs, and 2 (0.9%) received 3 NRTIs. No patients received 3 NRTIs simultaneously. Follow-up times were calculated from the date of enrollment to the date of death (caused by acquired immunodeficiency syndrome [AIDS)] or censored on May 1, 1996. The data were cross-checked with the Danish National Registry System, and patients who were lost during follow-up were censored as of the last day they were known to be alive.
Measurement of serum suPAR, CD4 count, viral load, and β2-microglobulin
suPAR was measured retrospectively on thawed serum samples by a sensitive enzyme-linked immunosorbent assay (ELISA) system.8,10 Samples were measured in duplicate, and interplate variation was, if necessary, corrected by the inclusion of a reference sample on all plates. With respect to the published method, the following minor modifications of the technique were used: bovine serum albumin (2% in phosphate-buffered saline) was used as a blocking reagent, and endpoint measurements were used instead of kinetic substrate development. CD4 count, viral load, and β2-microglobulin level had previously been determined in most of these patients.16 17
Statistical analysis
All statistical analyses were performed using statistical packages (SSPS version 8 and SAS version 6.12; SAS Institute, Cary, NC), and the observed differences were considered significant if P < .05. Comparisons of proportions were performed using the χ2 test. Comparison of unpaired observations for continuous variables was carried out using Kruskal-Wallis or Mann-Whitney U tests. Correlations were calculated using the Spearman rank correlation test. Kaplan-Meier curves were constructed stratifying the patients by the level of serum suPAR to generate similar numbers of patients in each stratum. Differences between Kaplan-Meier curves were analyzed by the log-rank test and by Cox regression. The ability of serum suPAR to predict mortality in the context of other known prognostic markers was formally assessed using a multivariate Cox proportional hazards model. Variables were fitted in a continuous scale using the transformations that provided the best fit as determined by the Wald χ2 value (log-scale for viral load, CD4 count and β2-microglobulin, and linear scale for suPAR and age).
Results
Levels of suPAR in serum
All 314 HIV-1–infected patients had measurable levels of serum suPAR with a median value of 3.69 ng/mL (range, 1.15-15.60 ng/mL). Division of all patients into 2 equal groups by the median suPAR level revealed several significant differences (Table1). Patients with high suPAR levels had shorter follow-up time (P < .0001) because of an increased incidence of AIDS-related death (P < .0001), lower CD4 count (P < .0001), higher viral load (P = .002), and higher β2-microglobulin level (P < .0001), and they were older (P = .007). No significant differences were observed with respect to the gender of the patients (P = .7) or the route of infection (P ≥ .2) or whether the patients received antiretroviral treatment at the time of enrollment (P = .3).
Patient characteristics
| . | All (n = 314) . | Low suPAR ≤ 3.69 ng/mL (n = 157) . | High suPAR > 3.69 ng/mL (n = 157) . | Low vs high P . |
|---|---|---|---|---|
| Clinical data | ||||
| Median follow-up time (mo) | 38 | 48 | 23 | < .00011-155 |
| (range) | (0.3-57) | (2.5-55) | (0.3-57) | |
| AIDS-caused death | 167 | 64 | 103 | < .00011-154 |
| Drug at enrollment* | 161 | 76 | 85 | .31-154 |
| Gender (M/F) | 280/34 | 141/16 | 139/18 | .71-154 |
| Median age (y) | 39 | 37 | 41 | .0071-154 |
| (range) | (16-66) | (20-60) | (16-66) | |
| Route of infection | ||||
| Homosexual contact | 241 | 123 | 118 | .71-154 |
| Heterosexual contact | 46 | 24 | 22 | .51-154 |
| Other† | 27 | 10 | 17 | .21-154 |
| Serologic variables | ||||
| Median CD4 count (cells/mm3) | 204 | 264 | 130 | |
| (range) | (0-1116) | (1-992) | (0-1116) | < .00011-155 |
| n = 3051-153 | n = 154 | n = 151 | ||
| Median viral load‡ | 5.02 | 4.78 | 5.20 | |
| (range) | (2.30-6.86) | (2.30-6.72) | (2.30-6.86) | .0021-155 |
| n = 2501-153 | n = 125 | n = 125 | ||
| Median β2-microglobulin (nmol/L) | 218 | 200 | 248 | |
| (range) | (0-1802) | (0-530) | (0-1802) | < .00011-155 |
| n = 2951-153 | n = 150 | n = 145 | ||
| Median suPAR (ng/mL) | 3.69 | 3.03 | 4.46 | |
| (range) | (1.15-15.60) | (1.15-3.69) | (3.70-15.60) | NA |
| . | All (n = 314) . | Low suPAR ≤ 3.69 ng/mL (n = 157) . | High suPAR > 3.69 ng/mL (n = 157) . | Low vs high P . |
|---|---|---|---|---|
| Clinical data | ||||
| Median follow-up time (mo) | 38 | 48 | 23 | < .00011-155 |
| (range) | (0.3-57) | (2.5-55) | (0.3-57) | |
| AIDS-caused death | 167 | 64 | 103 | < .00011-154 |
| Drug at enrollment* | 161 | 76 | 85 | .31-154 |
| Gender (M/F) | 280/34 | 141/16 | 139/18 | .71-154 |
| Median age (y) | 39 | 37 | 41 | .0071-154 |
| (range) | (16-66) | (20-60) | (16-66) | |
| Route of infection | ||||
| Homosexual contact | 241 | 123 | 118 | .71-154 |
| Heterosexual contact | 46 | 24 | 22 | .51-154 |
| Other† | 27 | 10 | 17 | .21-154 |
| Serologic variables | ||||
| Median CD4 count (cells/mm3) | 204 | 264 | 130 | |
| (range) | (0-1116) | (1-992) | (0-1116) | < .00011-155 |
| n = 3051-153 | n = 154 | n = 151 | ||
| Median viral load‡ | 5.02 | 4.78 | 5.20 | |
| (range) | (2.30-6.86) | (2.30-6.72) | (2.30-6.86) | .0021-155 |
| n = 2501-153 | n = 125 | n = 125 | ||
| Median β2-microglobulin (nmol/L) | 218 | 200 | 248 | |
| (range) | (0-1802) | (0-530) | (0-1802) | < .00011-155 |
| n = 2951-153 | n = 150 | n = 145 | ||
| Median suPAR (ng/mL) | 3.69 | 3.03 | 4.46 | |
| (range) | (1.15-15.60) | (1.15-3.69) | (3.70-15.60) | NA |
NA indicates not applicable.
Patients receiving antiretroviral drug treatment at the time of enrollment.
Six patients were infected by intravenous drug use, 7 were infected by contaminated blood transfusions, 6 were hemophilia patients, and 8 had unknown route of infection.
log10 HIV-1 RNA molecules/mL plasma.
Nine patients with missing data for CD4 count, 64 patients with missing data for viral load, and 19 patients with missing data for β2-microglobulin.
Mann-Whitney test.
χ2 test.
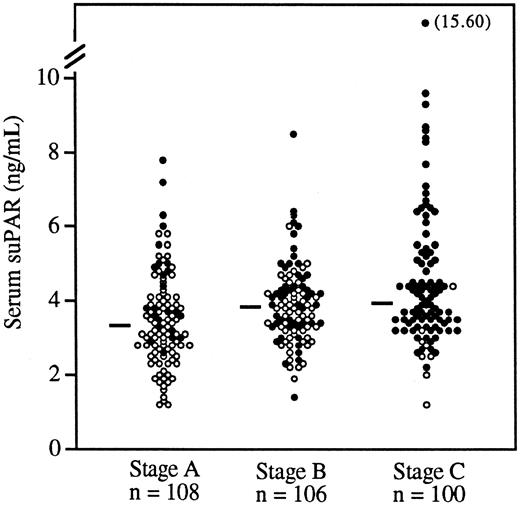
Division of the patients into 3 groups by the CDC-defined clinical stages A, B, and C (Figure 1) demonstrated that more advanced HIV-1 disease was associated with higher levels of serum suPAR (P < .0001, Kruskal-Wallis test). Within each of the clinical stages A, B, and C, the proportion of survivors was significantly (P ≤ .03, χ2test) higher among patients with suPAR levels below the median (see legend to Figure 1), indicating a possible correlation between suPAR levels and survival at different stages of HIV-1 infection.
Serum suPAR levels in patients with CDC-defined HIV-1 disease stages A, B, and C.
Each patient is indicated by a circle; ● indicates that the patient died during follow-up. Median values of suPAR within each stage are indicated by horizontal bars. The serum suPAR levels between the clinical stages were significantly different (P < .0001, Kruskal-Wallis test). Proportions of patients who survived were significantly higher in patients with low levels of suPAR (below the median in the respective group) in all 3 CDC stages (χ2test). Stage A, P = .006; stage B, P = .03; stage C, P = .03.
Serum suPAR levels in patients with CDC-defined HIV-1 disease stages A, B, and C.
Each patient is indicated by a circle; ● indicates that the patient died during follow-up. Median values of suPAR within each stage are indicated by horizontal bars. The serum suPAR levels between the clinical stages were significantly different (P < .0001, Kruskal-Wallis test). Proportions of patients who survived were significantly higher in patients with low levels of suPAR (below the median in the respective group) in all 3 CDC stages (χ2test). Stage A, P = .006; stage B, P = .03; stage C, P = .03.
Spearman rank correlation demonstrated a weak but significant negative correlation between suPAR and CD4 count (ρ = −0.33;P < .0001) and weak but significant positive correlations between suPAR and viral load (ρ = 0.28; P < 0.0001), between suPAR and β2-microglobulin (ρ = 0.45;P < .0001), and between suPAR and age (ρ = 0.18;P = .002).
Kaplan-Meier analysis
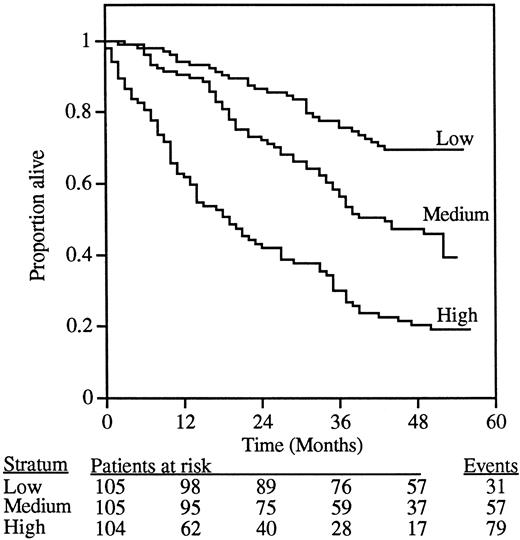
To investigate the association between suPAR levels and survival, we performed a Kaplan-Meier analysis (Figure2). In the absence of any relevant cut-off value for serum suPAR, we used the 33% and 66% percentiles of suPAR level, generating 3 groups of patients of similar size (n = 104-105 patients) representing low, medium, and high serum suPAR levels. The survival curve for patients with low serum suPAR was significantly different from that of patients with medium suPAR (P = .0008; χ2 = 11; log-rank test), which in turn was significantly different from that of patients with high suPAR (P < .0001; χ2 = 26; log-rank test).
Association between the level of suPAR in serum from 314 HIV-1–infected patients and overall survival.
Patients were divided into 3 strata based on the serum suPAR values, yielding similar numbers (n = 104-105) of patients in each stratum. The 3 curves in the figure thus represent patients with serum suPAR levels lower than 3.28 ng/mL (low), 3.28 to 4.19 ng/mL (medium), and higher than 4.19 ng/mL (high). The overall difference between the groups was highly significant (P < .0001; χ2 = 63; log-rank test). The number of patients at risk after each 12-month interval is indicated below the figure.
Association between the level of suPAR in serum from 314 HIV-1–infected patients and overall survival.
Patients were divided into 3 strata based on the serum suPAR values, yielding similar numbers (n = 104-105) of patients in each stratum. The 3 curves in the figure thus represent patients with serum suPAR levels lower than 3.28 ng/mL (low), 3.28 to 4.19 ng/mL (medium), and higher than 4.19 ng/mL (high). The overall difference between the groups was highly significant (P < .0001; χ2 = 63; log-rank test). The number of patients at risk after each 12-month interval is indicated below the figure.
Cox regression analysis
Comparison of the 3 patient groups in the Kaplan-Meier plot (Figure 2) by Cox regression analysis demonstrated a continuously increasing risk for mortality with increasing suPAR level. Compared to a low suPAR level, a medium suPAR level was associated with a hazard ratio (HR) of 2.2 (95% confidence interval [95% CI], 1.4-3.4;P < .0001) and a high suPAR level with an HR of 4.7 (95% CI, 3.1-7.2; P < .0001). Compared to a medium suPAR level, a high suPAR level was associated with an HR of 2.2 (95% CI, 1.6-3.1; P < .0001).
When suPAR was fitted as a continuous variable in a Cox model including all patients, high levels of suPAR were significantly associated with increased mortality rates (Table 2). An increase in serum suPAR of 1 ng/mL was associated with an HR of 1.6 (95% CI, 1.5-1.8; P < .0001). Other clinical and serologic variables that provided significant prognostic value in univariate Cox analysis were CD4 count, viral load, β2-microglobulin level, age, clinical stage, and drug treatment at enrollment. As expected, the well-established and clinically used prognostic marker CD4 count provided the best fit in univariate analysis, as estimated by the Wald χ2 value.
Cox regression analysis
| Variable . | Univariate . | Multivariate (n = 231†) . | ||||
|---|---|---|---|---|---|---|
| P value . | Hazard ratio (95% CI*) . | Wald χ2 . | P value . | Hazard ratio (95% CI*) . | Wald χ2 . | |
| suPAR | < .0001 | 1.6 | 107 | < .0001 | 1.5 | 33 |
| 1 ng/mL higher | (1.5-1.8) | (1.3-1.7) | ||||
| CD4 count | < .0001 | 1.6 | 200 | < .0001 | 1.3 | 15 |
| 50% lower | (1.5-1.7) | (1.1-1.5) | ||||
| Viral load | < .0001 | 3.9 | 94 | < .0001 | 2.0 | 17 |
| 10-fold higher | (3.0-5.2) | (1.4-2.7) | ||||
| β2-microglobulin | < .0001 | 3.0 | 71 | .02 | 1.3 | 5 |
| 2-fold higher | (2.3-3.8) | (1.0-1.6) | ||||
| Age | .03 | 1.2 | 5 | .06 | 1.2 | 4 |
| 10 years older | (1.0-1.4) | (1.0-1.5) | ||||
| CDC stage B vs A | < .0001 | 2.7 | 16 | .4 | 1.3 | 1 |
| (1.7-4.4) | (0.7-2.3) | |||||
| CDC stage C vs A | < .0001 | 12.3 | 113 | .005 | 2.8 | 8 |
| (7.8-20.0) | (1.4-5.7) | |||||
| Drug treatment at enrollment | < .0001 | 2.6 | 35 | .1 | 1.5 | 3 |
| (yes vs no) | (1.9-3.7) | (0.9-2.3) | ||||
| Variable . | Univariate . | Multivariate (n = 231†) . | ||||
|---|---|---|---|---|---|---|
| P value . | Hazard ratio (95% CI*) . | Wald χ2 . | P value . | Hazard ratio (95% CI*) . | Wald χ2 . | |
| suPAR | < .0001 | 1.6 | 107 | < .0001 | 1.5 | 33 |
| 1 ng/mL higher | (1.5-1.8) | (1.3-1.7) | ||||
| CD4 count | < .0001 | 1.6 | 200 | < .0001 | 1.3 | 15 |
| 50% lower | (1.5-1.7) | (1.1-1.5) | ||||
| Viral load | < .0001 | 3.9 | 94 | < .0001 | 2.0 | 17 |
| 10-fold higher | (3.0-5.2) | (1.4-2.7) | ||||
| β2-microglobulin | < .0001 | 3.0 | 71 | .02 | 1.3 | 5 |
| 2-fold higher | (2.3-3.8) | (1.0-1.6) | ||||
| Age | .03 | 1.2 | 5 | .06 | 1.2 | 4 |
| 10 years older | (1.0-1.4) | (1.0-1.5) | ||||
| CDC stage B vs A | < .0001 | 2.7 | 16 | .4 | 1.3 | 1 |
| (1.7-4.4) | (0.7-2.3) | |||||
| CDC stage C vs A | < .0001 | 12.3 | 113 | .005 | 2.8 | 8 |
| (7.8-20.0) | (1.4-5.7) | |||||
| Drug treatment at enrollment | < .0001 | 2.6 | 35 | .1 | 1.5 | 3 |
| (yes vs no) | (1.9-3.7) | (0.9-2.3) | ||||
95% CI of the hazard ratio.
Patients for whom data for one or more of the serologic variables was missing (n = 83) were excluded from the analysis (Table 1 shows details).
Patients for whom data for all the serologic variables were available (n = 231) were then fitted with suPAR in a multivariate Cox regression model (Table 2). The predictive strength of suPAR in this subset (n = 231) of patients was virtually identical to when the analysis was performed on all 314 patients (not shown). In the multivariate Cox model, suPAR, CD4 count, viral load, β2-microglobulin, and CDC stage C provided statistically significant independent prognostic information. According to the Wald χ2 value, suPAR was a stronger predictor of survival than CD4 count and viral load.
Discussion
In the current work we have provided evidence that serum suPAR is a strong independent prognostic variable in HIV-1 infection. In Kaplan-Meier survival analysis, higher serum levels of suPAR are associated with faster progression to death. After adjustment for all known relevant HIV-1 disease progression markers in multivariate Cox regression analysis, suPAR remains a highly significant independent predictor of survival. This is, to our knowledge, the first report demonstrating a connection between the urokinase plasminogen activator system and disease progression in HIV-1 infection.
One possible explanation for the prognostic value of serum suPAR in HIV-1 infection is that it derives, at least partially, from HIV-1–infected cells. Monocytes and T-lymphocytes infected with HIV-1 have been shown to express elevated levels of cell-surface uPAR,12-14 and the serum level of suPAR may correlate with the size of the reservoir of HIV-1–infected cells in the organism. This model predicts that suPAR levels will be higher in HIV-1–infected patients than in healthy controls, a possibility we have not addressed in this study. However, in accordance with this hypothesis, our data do demonstrate that the suPAR level is higher in patients with more advanced HIV-1 infection.
The striking correlation between suPAR levels and the risk of disease progression may suggest a direct relevance of uPAR in HIV-1 infection. The natural high-affinity ligand of uPAR, uPA has been shown to bind HIV-1 gp120 and to promote HIV-1 infection of macrophages in vitro.18 The interaction between uPA and gp120 involves the functionally important V3-loop of gp120 and the catalytic domain of uPA, but it leaves the ligand-binding domain of uPA free for interaction with uPAR.18 It is thus possible that uPA may form a “bridge” between gp120 on the virus and uPAR on the cell surface, which would identify uPAR as a cellular coreceptor for HIV-1. Indeed, it should be noted that the major cell types susceptible to HIV-1 infection are those in which uPAR is predominantly expressed (macrophages, activated monocytes, and T cells). Investigations addressing such an involvement now seem highly warranted.
Finally, it should be emphasized that circulating suPAR might play a direct role in the pathogenesis of HIV-1 infection. In fact, suPAR fragments have chemokine-like activities, and suPAR is capable of modulating processes such as cell adhesion, migration, and proliferation in vitro.1 2
Regardless of the biologic explanation, the serum level of suPAR remains a strong and independent prognostic predictor of disease progression in HIV-1 infection. Using a single serum suPAR measurement and arbitrary cut-off points, we were able to define patient groups associated with different hazard ratios for disease progression, suggesting that suPAR measurements may be used as a prognostic tool in HIV-1 infection.
The variables suPAR, CD4 count, and viral load were only poorly correlated mutually, and all 3 parameters provided highly significant independent prognostic information in multivariate Cox analysis. This implies that suPAR provides important additional prognostic information. In this respect it will be particularly important to determine whether suPAR is useful in determining whether and when to initiate highly active antiretroviral therapy (HAART).
There is still considerable debate, as reflected in different national guidelines, about what combination of CD4 count and viral load constitutes an indication for therapy. Virologic and immunologic arguments for early initiation of therapy must be balanced against disadvantages of treatment, such as lifelong polypharmacy, high costs, side effects, and risk for resistance development. Because the suPAR level carries strong and independent information on the progression status, it may aid in the monitoring of patients and in the important clinical decision of when to initiate HAART.
In the current study, follow-up was censored when the first patient in the cohort received a protease inhibitor, and patients therefore only received relatively light antiretroviral treatment compared to HAART. Treatment with antiretroviral drugs at the time of enrollment in CHIC was associated with poor survival in univariate Cox analysis but failed to provide significant prognostic information in multivariate Cox analysis. This most likely reflects the fact that patients who had received treatment at the time of enrollment were those with more advanced disease for whom monotherapy or dual therapy did not provide strong survival benefit. The current study is therefore likely to reflect disease development close to the natural history course of HIV-1 infection, and the possible prognostic value of suPAR in the context of HAART should be addressed in other more appropriately designed studies.
Although the current study shows a strong association between suPAR levels and disease progression, it is important to realize that the amount of data from this single study does not justify suggestions of using suPAR as a standard prognostic parameter. First, the data must be confirmed in an independent cohort. Second, because this cohort consists of patients in a European country before the HAART era, its value must be confirmed in patients with access to HAART treatment or patients in resource-poor settings, or both. In this study we have used arbitrarily chosen cut-off values and statistical methods that do not require the definition of specific cut-off values. Normal values of suPAR obtained using standardized sample techniques must be determined before routine use of suPAR can be applied.
We have recently shown that suPAR antigen can be readily quantified in urine and that the urinary suPAR level strongly correlates with the serum level.19 It is therefore possible that urine may provide an alternative to serum samples for suPAR-based HIV-1 prognosis, which would introduce an element of safety and convenience for patients and medical staff.
The introduction of HAART in the Western world has profoundly increased the life expectancy of HIV-1–infected patients. However, because of the high cost of these drugs, only a small fraction of the HIV-1–infected patients in the world have access to effective HIV-1 therapy. The measurement of suPAR is performed using a simple and inexpensive ELISA technique. After confirmation of our results in other groups of patients, the determination of suPAR in serum (or even better, urine) from HIV-1–infected patients may provide a cost-effective supplement or alternative to the currently used decision-making tools based on CD4 counts and viral load.
Acknowledgments
We thank Gunilla Høyer-Hansen (Finsen Laboratory, Rigshospitalet, Copenhagen, Denmark) for supplying antibodies. We also thank Terese Katzenstein, Jan Gerstoft, and Peter Skinhøj (Department of Infectious Diseases, Rigshospitalet) for their important contributions to the collection and analysis of patient samples.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nicolai Sidenius, Department of Molecular Pathology and Medicine, DIBIT, San Raffaele Scientific Institute, Via Olgettina 58, 20132 Milan, Italy; e-mail: nicolai.sidenius@hsr.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal