Abstract
Polymorphonuclear leukocytes (PMNLs) are thought to be terminally differentiated, short-lived, and unable to actively synthesize new proteins or to interact with T cells. In the current study, it was found that PMNLs incubated with supernatants of phytohemagglutinin (PHA)-stimulated peripheral blood mononuclear cells (PHA-sup) expressed high levels of CCR6 mRNA. Neutralization with IgG against several cytokines revealed that tumor necrosis factor (TNF)-α was largely responsible for the PHA-sup–induced CCR6 mRNA expression. Among recombinant cytokines, TNF-α induced high levels of CCR6 mRNA expression, whereas interferon (IFN)-γ induced low levels. The 2 cytokines together exhibited a considerable synergy. Cytokine-activated PMNLs expressed functional CCR6, as detected by the binding of sodium iodide I 125–labeled liver and activation-regulated chemokine (LARC) and dose-dependent migration toward LARC. The induction of CCR6 suggested that these cytokine-activated PMNLs have more similarities with dendritic cells (DCs) that express CCR6 in an immature stage. In fact, the activation of PMNLs with TNF-α and IFN-γ induced the expression of CD83, a dominant cell-surface marker of DCs. When PMNLs were activated with granulocyte macrophage–colony-stimulating factor, TNF-α, and IFN-γ, these cells expressed CD40 and HLA-DR in addition to CD83. Taken together, PMNLs, under appropriate conditions, can undergo a differentiation process characterized by the acquisition of new phenotypes and functions, and such differentiated PMNLs may play more active roles in the adaptive immune response.
Introduction
Polymorphonuclear leukocytes (PMNLs) are the most abundant white blood cells and comprise approximately two thirds of circulating leukocytes in humans. In response to inflammatory stimuli, PMNLs immediately migrate to inflamed tissues and contribute to the clearance of pathogens by phagocytosis and by releasing cytotoxic compounds. Circulating PMNLs are thought to be terminally differentiated, short-lived, unable to actively synthesize new proteins, and unable to interact with T cells. However, it is now known that PMNL survival can be greatly extended in the tissues after exposure to microenvironmental signals involved in infection and immunity.1-3 Furthermore, PMNLs are capable of synthesizing and releasing immunoregulatory cytokines after activation with inflammatory cytokines.3 Low-level expression of major histocompatibility complex class II molecules was also observed in PMNLs after in vitro activation with granulocyte macrophage–colony-stimulating factor (GM-CSF), interferon (IFN)-γ, or IL-3, and in PMNLs from patients after GM-CSF or IFN-γ administration.4-6 PMNLs stimulated with GM-CSF and IFN-γ have the capacity to present Ag to naive T cells.7Those PMNLs with antigen-presenting capacity are thought to be a relatively mature population.5
We recently reported that PMNL expressed and produced the CC-chemokine monocyte chemoattractant protein-1 (MCP-1) after in vitro culture with crude culture supernatants of phytohemagglutinin (PHA)-stimulated peripheral blood mononuclear cells (PBMCs) (designated as PHA-sup).8 Unlike other chemokines, such as IL-8 and MIP-1s, whose mRNA could be induced in PMNLs within 1 hour of stimulation, the expression of MCP-1 was always delayed, and at least 9 hours of stimulation were necessary to detect significant increases in MCP-1 mRNA. Early protein synthesis and tyrosine phosphorylation were involved in the expression of MCP-1. Expression of MCP-1 in PMNL appears to be regulated by a novel mechanism that consists of 2 steps: priming with an unidentified factor that alters the responsiveness of PMNLs to TNF-α and subsequent activation of the cells with TNF-α.9 Thus, our studies of MCP-1 production suggested that PMNLs could differentiate and acquire new functions to promote a chronic inflammatory response after priming with selected cytokines.
To better understand the molecular events occurring in cytokine-stimulated PMNLs and to identify the functions PMNLs may acquire, we recently screened approximately 7000 human genes with a cDNA microarray (unpublished) and found that the CC-chemokine receptor CCR6, which is exclusively expressed in immature dendritic cells (DCs), T cells, and B cells, was one of the highly up-regulated genes in PHA-sup–stimulated PMNLs. This finding prompted us to further investigate whether PMNLs could undergo phenotypic and functional changes similar to those observed during the maturation of DCs. In the current study, we demonstrate that human PMNLs activated with PHA-sup, or cytokines such as TNF-α and IFN-γ, express functional CCR6 on the cell surface. A dominant DC marker, CD83, was also induced in PMNLs activated with TNF-α and IFN-γ. Furthermore, PMNLs activated with TNF-α, IFN-γ, and GM-CSF expressed CD40 and HLA-DR, in addition to CD83. Taken together, our study demonstrates that selected cytokines can induce PMNLs to further differentiate and to acquire new phenotypes and new functions. Our study also suggests that in addition to mediating acute innate host defense, PMNLs may play an active role in promoting the conversion of acute inflammation to chronic inflammation and adaptive immunity.
Materials and methods
Reagents
Human recombinant TNF-α (2.5 × 107 U/mg), IFN-γ (1 × 107 U/mg), GM-CSF (2 × 107U/mg), IL-4 (1 × 107 U/mg), IL-1β (1 × 107 U/mg), and neutralizing antibodies against human TNF-α, IFN-γ, or GM-CSF were purchased from R&D Systems (Minneapolis, MN). Human recombinant LARC, MCP-1, and IL-8 were from Peprotech (Rocky Hill, NJ). Sodium iodide I 125–labeled LARC/MIP-3α (specific activity, 2000 Ci/mmol) and Dextran T500 were from Amersham Pharmacia Biotech (Piscataway, NJ); α-[32P]dCTP was from ICN (Costa Mesa, CA); [3H]-TdR was from NEN (Boston, MA); human CCR6 cDNA was from Genome System (St Louis, MO); human β-actin cDNA was from Clontech (Palo Alto, CA); TRIzolreagent was from Life Technologies (Gaithersburg, MD); fetal calf serum (FCS) was from HyClone (Logan, UT); PHA, paraformaldehyde, formamide, and triethanolamine were from Sigma (St Louis, MO); lipopolysaccharide (LPS) was from Difco (Detroit, MI). Accu-Prep was from Accurate Chemical & Scientific (Westbury, NY). Monoclonal antibodies against human CD40, CD83, CD86, and HLA-DR were from Immunotech (Marseille, France). Fluorescein isothiocyanate (FITC)–conjugated rabbit F(ab′)2 antimouse IgG was from DAKO (Carpinteria, CA). Proteinase K, RNase A, and anti-DIG fluorescein Fab fragments were from Boehringer Mannheim (Indianapolis, IN). In Situ Hyb Buffer was from Ambion (Austin, TX).
Preparation of PMNLs
Polymorphonuclear leukocytes were obtained from the heparinized blood of healthy volunteers (10 U heparin/mL blood) by mixing 1 vol 5% Dextran T500 in phosphate-buffered saline (PBS) with 3 vol blood in 50-mL tubes for sedimentation of red blood cells. After 30 minutes at room temperature, the leukocyte-rich plasma was overlaid onto Accu-prep and centrifuged at 800g for 20 minutes at room temperature. The cell pellets were treated with 0.2% NaCl, washed twice with RPMI 1640 with 10% fetal bovine serum, and resuspended in the same media at a density of 1 × 107 cells/mL. PMNLs were also prepared from granulocytapheresis collections supplied by the Department of Transfusion Medicine (Clinical Center, National Institutes of Health, Bethesda, MD). Contamination of mononuclear leukocytes in PMNL preparations obtained by this method was less than 0.1% by morphologic examination after staining with Diff-Quick. Cell viability was higher than 99% by trypan blue staining.
Activation of PMNLs and Northern blot analysis
Fifteen million PMNLs were cultured in 3 mL RPMI 1640 supplemented with 10% FCS in the presence or absence of appropriate stimulants in 6-well plates (Costar, Cambridge, MA). Total RNA was extracted from each cultured PMNL using TRIzol Reagent. Northern blot analysis was performed as previously described.10 Blots were hybridized with human CCR6 or β-actin cDNA probe labeled with [α-32P]dCTP. The intensity of mRNA expression was quantified by densitometry.
Chemotaxis assay
Chemotaxis assay was performed by using a 48-well microchemotaxis chamber (Neuroprobe, Cabin John, MD). After incubation under appropriate conditions, PMNLs were rinsed 3 times and added to the upper wells of the chambers that were separated from the lower wells containing chemoattractants by a polycarbonate membrane with 5-μm diameter pores. The number of PMNLs migrating through the pores during a 60-minute incubation was counted. Results were presented as chemotactic index denoting the fold increase of cell migration in response to stimulants over control.
Receptor-ligand binding assay
Ten million PMNLs were incubated with 125I-labeled LARC with increasing amounts of unlabeled LARC for 1 hour at room temperature. Cells were then centrifuged through 0.8 mL of 10% sucrose cushion in microcentrifuge tubes. The tips of the tubes containing cell pellets were incised, and cell-associated radioactivities were counted by a gamma counter. Binding data were analyzed by the LIGAND program.11
FACS analysis
PMNLs were first washed 3 times with PBS supplemented with 1% FCS and 0.02% NaN3, and then they were incubated with each primary antibody or control IgG at room temperature for 1 hour. Cells were washed 3 times and incubated with FITC-conjugated rabbit antimouse IgG for 30 minutes and fixed with 1% paraformaldehyde in PBS, and the expression of antigen was analyzed by a FACScan flow cytometer (Becton Dickinson, San Jose, CA).
In situ hybridization
Cytospin preparations of PMNLs were subjected to nonradioactive in situ hybridization described previously with some modifications.12 Briefly, digoxigenin-labeled antisense and sense cRNA probes for human CCR6 were synthesized using the DIG RNA labeling kit (Boehringer Mannheim). Cells fixed in 4% paraformaldehyde were treated with proteinase K, denatured with 0.2 N HCl, acetylated with 0.1 mol/L triethanolamine and 0.25% acetic anhydride, and hybridized for 16 hours at 50°C with In Situ Hyb Buffer containing cRNA probes (20 ng/30 μL). After hybridization, the cells were washed with 2 × SSC and 50% formamide and treated with RNase A for digestion of unhybridized probes. Hybridized probe was detected immunologically using anti-digoxigenin–fluorescein Fab fragments under a fluorescein microscope.
Reverse transcription–polymerase chain reaction
Reverse transcription–polymerase chain reaction (RT-PCR) was performed using Superscript II 1 Step RT-PCR System (Life Technologies) with total RNA extracted from fresh or stimulated PMNLs. Primers used were 5′-CTGTGGACAAAGCCAACTTG-3′ and 5′-ACGTTCTCTGTAGTCTCTGG-3′ for HLA-DR α-chain; 5′-CTCCGAAGATGTGGACTTGC-3′ and 5′-ATGCCAGCTTTAGAAAAATC-3′ for CD83; and 5′-GCAGGACCAGGAAAACTTGG-3′ and 5′-AGAAAGGTGAAGATAAAAGC -3′ for CD86.
Results
TNF-α and IFN-γ synergistically induce expression of CCR6 mRNA in PMNLs
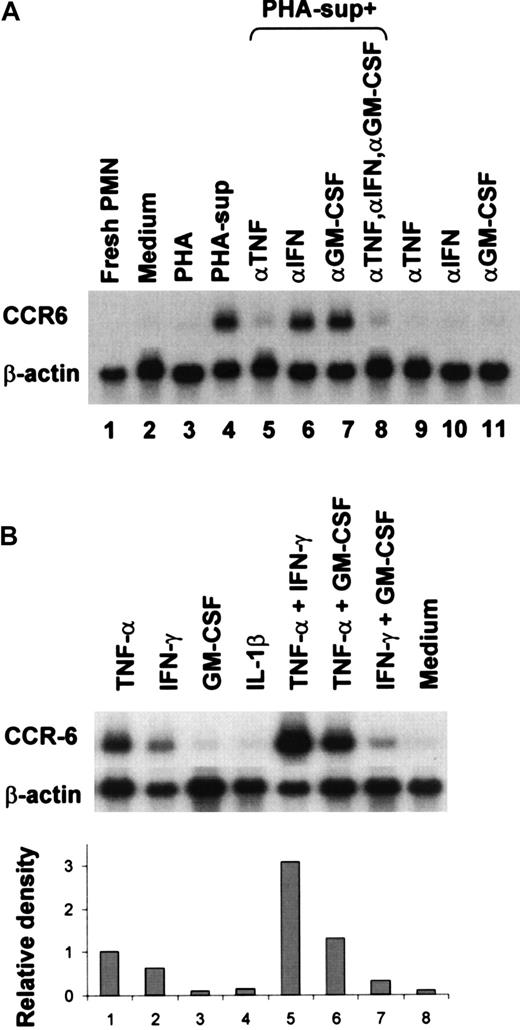
We first clarified that PHA-sup could indeed induce CCR6 mRNA expression in PMNLs by Northern blotting. As shown in Figure1, panel A, freshly isolated unstimulated PMNL did not express CCR6 mRNA (lane 1). A low level of CCR6 mRNA expression was detected in PMNL after incubation for 6 hours in RPMI 1640 containing 10% FCS (lane 2), and the addition of PHA did not affect the level of CCR6 mRNA expression (lane 3). However, a high level of CCR6 mRNA expression was detected in PMNLs incubated with PHA-sup (lane 4). To identify the factors responsible for CCR6 induction, we incubated PMNLs with the PHA-sup for 6 hours with or without neutralizing antibodies against various cytokines. The expression of CCR6 mRNA was almost completely inhibited by the addition of a neutralizing IgG against TNF-α (lane 5), but not IFN-γ (lane 6) or GM-CSF (lane 7). The addition of all 3 IgGs did not further increase the inhibitory effect obtained by anti–TNF-α IgG (lane 8). The addition of each IgG did not induce CCR6 mRNA expression (lanes 9-11). These results indicated that TNF-α contained in the PHA-sup played a major role in the induction of CCR6 mRNA expression.
Up-regulation of CCR6 mRNA expression in PHA-sup–stimulated or cytokine-stimulated PMNLs.
(A) PMNLs were cultured with medium alone, PHA (2.5 μg/mL), or PHA-sup in the presence or absence of neutralizing IgG (5 μg/well) against TNF-α, IFN-γ, or GM-CSF, for 6 hours. (B) PMNLs were cultured with LPS (1 ng/mL), TNF-α (1 ng/mL), IFN-γ (2.5 U/mL), GM-CSF (0.05 ng/mL), or IL-1β (0.05 ng/mL), rTNF-α (1 ng/mL), and IFN-γ (2.5 U/mL), TNF-α (1 ng/mL), and GM-CSF (0.05 ng/mL), or IFN-γ (2.5 U/mL) and GM-CSF (0.05 ng/mL) for 6 hours. Total RNA was extracted and subjected to Northern blot analysis. The blots were hybridized with 32P-labeled human CCR6 or β-actin cDNA probe and exposed to x-ray film. Data were also analyzed by densitometry. Representative of several experiments with similar results.
Up-regulation of CCR6 mRNA expression in PHA-sup–stimulated or cytokine-stimulated PMNLs.
(A) PMNLs were cultured with medium alone, PHA (2.5 μg/mL), or PHA-sup in the presence or absence of neutralizing IgG (5 μg/well) against TNF-α, IFN-γ, or GM-CSF, for 6 hours. (B) PMNLs were cultured with LPS (1 ng/mL), TNF-α (1 ng/mL), IFN-γ (2.5 U/mL), GM-CSF (0.05 ng/mL), or IL-1β (0.05 ng/mL), rTNF-α (1 ng/mL), and IFN-γ (2.5 U/mL), TNF-α (1 ng/mL), and GM-CSF (0.05 ng/mL), or IFN-γ (2.5 U/mL) and GM-CSF (0.05 ng/mL) for 6 hours. Total RNA was extracted and subjected to Northern blot analysis. The blots were hybridized with 32P-labeled human CCR6 or β-actin cDNA probe and exposed to x-ray film. Data were also analyzed by densitometry. Representative of several experiments with similar results.
The effect of several recombinant cytokines on the CCR6 mRNA expression was next investigated. As shown in Figure 1, panel B, a high level of CCR6 mRNA was detected in PMNLs stimulated with TNF-α for 6 hours (lane 1), whereas only a low level of CCR6 mRNA was expressed after incubation in the medium alone (lane 8). IFN-γ also induced CCR6 mRNA expression, but the level of CCR6 mRNA expression induced by IFN-γ was approximately 60% of that induced by TNF-α (lane 2). In contrast, neither GM-CSF nor IL-1β induced CCR6 mRNA expression at a concentration range up to 100 U/mL (lanes 3, 4). There was a synergistic effect between TNF-α and IFN-γ (lane 5). GM-CSF did not increase CCR6 mRNA expression induced with either TNF-α or IFN-γ (lanes 6, 7).
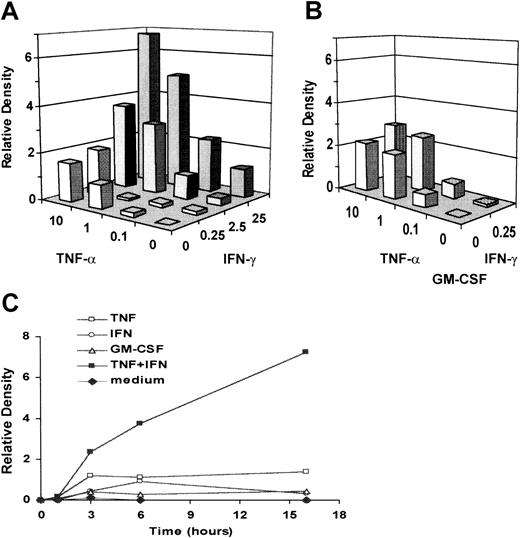
The effects of TNF-α and IFN-γ on PMNL CCR6 mRNA expression were dose-dependent (Figure 2A). When PMNLs were cultured with both TNF-α and IFN-γ, CCR6 mRNA expression was 3- to 5-fold higher than that induced with TNF-α alone. Although GM-CSF did not induce CCR6 mRNA expression by itself or increase CCR6 mRNA expression induced with either TNF-α or IFN-γ, the addition of GM-CSF augmented the synergistic effect at lower concentrations of TNF-α and IFN-γ (Figure 2B).
Dose-dependent induction and kinetics of CCR6 mRNA expression in cytokine-activated PMNLs.
(A) PMNLs were cultured with increasing concentrations of TNF-α (0-10 ng/mL), IFN-γ (0-25 U/mL), or both for 6 hours. (B) PMNLs were cultured with increasing concentrations of TNF-α (0-10 ng/mL), IFN-γ (0-0.25 U/mL), or both in the presence of GM-CSF (0.05 ng/mL). (C) PMNLs were cultured in the presence or absence of TNF-α (1 ng/mL), IFN-γ (2.5 U/mL), or both for 1, 3, 6, and 16 hours. Total RNA was extracted and subjected to Northern blot analysis. The blots were hybridized with 32P-labeled human CCR6 or β-actin cDNA probe. Autoradiographic signals were quantified, standardized against the levels of β-actin, and presented as relative density. The level of expression induced by 1 ng/mL TNF-α equals 1 in the chart. Representative of several experiments with similar results.
Dose-dependent induction and kinetics of CCR6 mRNA expression in cytokine-activated PMNLs.
(A) PMNLs were cultured with increasing concentrations of TNF-α (0-10 ng/mL), IFN-γ (0-25 U/mL), or both for 6 hours. (B) PMNLs were cultured with increasing concentrations of TNF-α (0-10 ng/mL), IFN-γ (0-0.25 U/mL), or both in the presence of GM-CSF (0.05 ng/mL). (C) PMNLs were cultured in the presence or absence of TNF-α (1 ng/mL), IFN-γ (2.5 U/mL), or both for 1, 3, 6, and 16 hours. Total RNA was extracted and subjected to Northern blot analysis. The blots were hybridized with 32P-labeled human CCR6 or β-actin cDNA probe. Autoradiographic signals were quantified, standardized against the levels of β-actin, and presented as relative density. The level of expression induced by 1 ng/mL TNF-α equals 1 in the chart. Representative of several experiments with similar results.
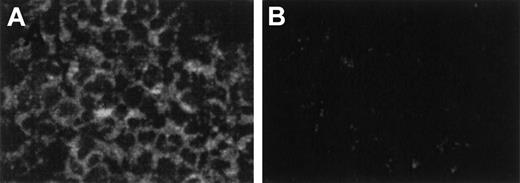
A study of the kinetics of the response showed that the peak CCR6 mRNA expression was detected at 3 hours and was sustained up to 16 hours after activation with TNF-α alone (Figure 2C). The maximal effect of IFN-γ was also detected at 6 hours. When PMNLs were cultured with both TNF-α and IFN-γ, CCR6 mRNA expression was markedly enhanced at 3 hours, in comparison with that with TNF-α or IFN-γ alone. The expression level reached the highest level at 16 hours and was 7.2-fold higher than the peak level induced by TNF-α alone. By in situ hybridization, CCR6 mRNA was detected in most PMNLs with the DIG-labeled antisense probe (Figure 3A) but not with the DIG-labeled sense probe (Figure 3B), indicating that CCR6 mRNA expression was induced in most PMNLs.
Detection of CCR6 mRNA in cytokine-activated PMNLs by in situ hybridization.
PMNLs were incubated with TNF-α (1 ng/mL) and IFN-γ (25 U/mL) for 6 hours. (A) Hybridized with FITC-labeled antisense cRNA probe. Cytoplasm of PMNLs was stained positive for CCR6 mRNA. (B) Hybridized with FITC-labeled sense probe used as negative control. Magnification, 400×.
Detection of CCR6 mRNA in cytokine-activated PMNLs by in situ hybridization.
PMNLs were incubated with TNF-α (1 ng/mL) and IFN-γ (25 U/mL) for 6 hours. (A) Hybridized with FITC-labeled antisense cRNA probe. Cytoplasm of PMNLs was stained positive for CCR6 mRNA. (B) Hybridized with FITC-labeled sense probe used as negative control. Magnification, 400×.
125I-labeled LARC specifically binds to PMNLs activated with TNF-α and IFN-γ
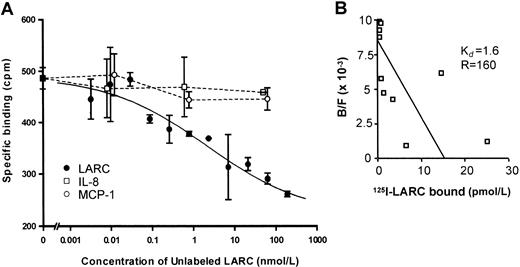
To confirm the expression of CCR6 on the cell surface of PMNLs, a receptor-ligand binding assay was performed. Neither freshly isolated PMNLs nor PMNLs cultured in the absence of cytokines exhibited specific binding of 125I-LARC, the ligand for CCR6 (data not shown). However, after activation of PMNLs with both TNF-α and IFN-γ, there was a significant binding of 125I-LARC that was competitively inhibited by the addition of increasing amounts of unlabeled LARC, but not by IL-8 or MCP-1 (Figure4A). The estimated equilibrium dissociation constant (Kd) of LARC-PMNL binding and the number of binding sites were 1.6 nmol/L and 160 sites per cell, respectively (Figure 4B). There was no specific binding of125I-LARC after overnight incubation, suggesting that the cell-surface expression of CCR6 was transient (data not shown).
Binding of 125I-labeled LARC to the cell surfaces of PMNLs.
(A) PMNLs were incubated with both TNF-α (1 ng/mL) and IFN-γ (25 U/mL) for 6 hours. Activated PMNLs were incubated with 0.05 nmol/L of125I-labeled LARC in the presence of increasing amounts of unlabeled LARC (0.003-562.5 nmol/L), IL-8 (0.01-63 nmol/L), or MCP-1 (0.01-63 nmol/L). (B) Scattered plot analysis.Kd, equilibrium dissociation constant (nmol/L); R, LARC-binding sites per cell. Representative of 3 different experiments with almost identical results is shown.
Binding of 125I-labeled LARC to the cell surfaces of PMNLs.
(A) PMNLs were incubated with both TNF-α (1 ng/mL) and IFN-γ (25 U/mL) for 6 hours. Activated PMNLs were incubated with 0.05 nmol/L of125I-labeled LARC in the presence of increasing amounts of unlabeled LARC (0.003-562.5 nmol/L), IL-8 (0.01-63 nmol/L), or MCP-1 (0.01-63 nmol/L). (B) Scattered plot analysis.Kd, equilibrium dissociation constant (nmol/L); R, LARC-binding sites per cell. Representative of 3 different experiments with almost identical results is shown.
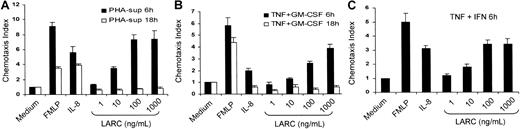
Cytokine-activated PMNLs dose-dependently migrate to LARC
To examine whether CCR6 on cytokine-activated PMNLs was functional, migration of PMNLs to LARC was evaluated by an in vitro chemotaxis assay. Freshly isolated PMNLs responded to IL-8 or fMLP but did not respond to any concentrations of LARC (data not shown). However, PMNLs activated with the PHA-sup (Figure5A), TNF-α and GM-CSF (Figure 5B), or TNF-α and IFN-γ (Figure 5C) for 6 hours exhibited a potent migration toward LARC in a dose-dependent manner. There was no significant decrease in the number of migrating cells at 10 μg/mL LARC (data not shown). In contrast, PMNLs cultured overnight in the presence of either PHA-sup or a combination of TNF-α and GM-CSF did not migrate toward LARC, suggesting that the expression of CCR6 was transient (Figure 5A,B). LARC did not induce calcium flux in PMNLs at a concentration range up to 10 μg/mL after 6 hours of incubation with the PHA-sup (data not shown).
Migration of cytokine-activated PMNLs toward LARC.
PMNLs were incubated with PHA-sup (A), TNF-α (1 ng/mL), and GM-CSF (0.05 ng/mL) (B), or TNF-α (1 ng/mL) and IFN-γ (25 U/mL) (C) for 6 hours. Activated PMNLs were tested for their ability to respond to various concentrations of LARC (1-1000 ng/mL). fMLP (10−8mol/L) and IL-8 (100 ng/mL) were used as positive controls.
Migration of cytokine-activated PMNLs toward LARC.
PMNLs were incubated with PHA-sup (A), TNF-α (1 ng/mL), and GM-CSF (0.05 ng/mL) (B), or TNF-α (1 ng/mL) and IFN-γ (25 U/mL) (C) for 6 hours. Activated PMNLs were tested for their ability to respond to various concentrations of LARC (1-1000 ng/mL). fMLP (10−8mol/L) and IL-8 (100 ng/mL) were used as positive controls.
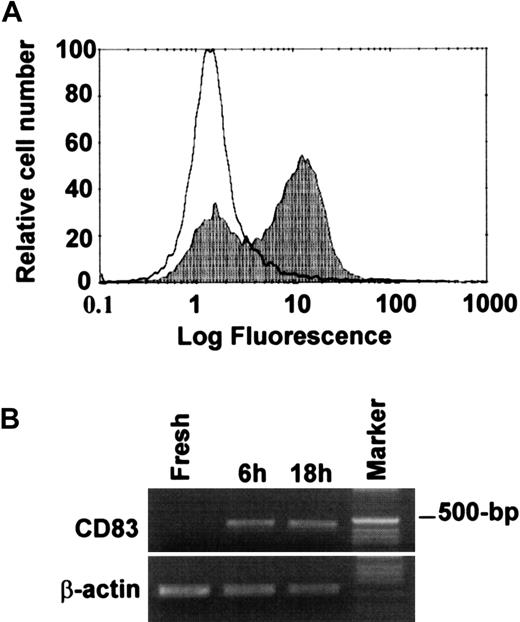
TNF-α and IFN-γ activation induce expression of CD83
Detection of functional CCR6 expression on PMNLs led us to hypothesize that these cells might be in the process of acquiring DC-like phenotypes. We examined the expression of several cell-surface molecules, including CD40, CD83, CD86, and HLA-DR, by FACS. As shown in Figure 6, panel A, we detected immunoreactive CD83 on cytokine-activated PMNL after 1-day culture. Approximately two thirds of activated PMNLs were CD83-positive. RT-PCR also revealed the expression of CD83 mRNA in cytokine-activated, but not in freshly isolated, PMNLs (Figure 6B). Almost all PMNLs became CD83-positive after 2-day culture (data not shown). There was no significant expression of CD40, CD86, or HLA-DR after activation of the cells up to 2 days. A combination of GM-CSF, TNF-α, and IFN-γ induced higher levels of CD40 and CD83 and a high level of HLA-DR, but no significant CD86 expression (data not shown).
Expression of CD83 in PMNLs activated with TNF-α and IFN-γ.
PMNLs were incubated with TNF-α (1 ng/mL) and IFN-γ (25 U/mL) for 18 hours. (A) Expression of CD83 on resting or activated PMNLs was analyzed by flow cytometry. White area represents PMNLs kept at 4°C for 18 hours; shaded area represents PMNLs activated with both TNF-α and IFN-γ for 18 hours. (B) Detection of CD83 mRNA by RT-PCR. Representative of 3 experiments with similar results.
Expression of CD83 in PMNLs activated with TNF-α and IFN-γ.
PMNLs were incubated with TNF-α (1 ng/mL) and IFN-γ (25 U/mL) for 18 hours. (A) Expression of CD83 on resting or activated PMNLs was analyzed by flow cytometry. White area represents PMNLs kept at 4°C for 18 hours; shaded area represents PMNLs activated with both TNF-α and IFN-γ for 18 hours. (B) Detection of CD83 mRNA by RT-PCR. Representative of 3 experiments with similar results.
Expression of CCR7 mRNA is not induced in PMNLs
We additionally examined whether cytokine-activated PMNLs express the CC-chemokine receptor CCR7 that has been reported to be expressed on mature DCs. There was no detectable CCR7 mRNA expression in activated PMNLs by RT-PCR up to 4 days (data not shown), supporting that the differentiated PMNL are distinct from “mature” DCs.
Discussion
The current study has, for the first time, clearly shown that PMNLs could be induced to express functional CCR6 after activation with selected cytokines. TNF-α or IFN-γ were inducers of CCR6 mRNA expression in PMNLs, and there was a synergistic effect between the 2 cytokines. In contrast, other PMNL-activating cytokines, including IL-1β and GM-CSF, did not induce CCR6 expression. Radiolabeled LARC, a ligand for CCR6, specifically bound to the activated PMNLs, as detected by a ligand-receptor binding assay. TheKd of LARC-PMNL binding of approximately 1.6 nmol/L was equivalent to the previously reportedKd of LARC-CCR6 binding.13 14 The expression level of CCR6 mRNA was high, as determined by Northern blot analysis, but the number of the receptors expressed on the surfaces of PMNLs was approximately 160 sites per PMNL. This might explain why we did not detect significant CCR6 expression by FACS analysis (data not shown). Nevertheless, activated PMNLs migrated toward LARC in a dose-dependent manner, indicating that even the low level expression of CCR6 on the cell surfaces was sufficient for chemotactic function but probably not for detectable calcium flux.
There is considerable evidence that the expression of CCR6 on DCs occurs during an earlier stage of maturation and accounts for the trafficking of immature DCs to inflammatory sites where LARC is produced.15 CD34+ progenitor cell-derived DCs expressed CCR6 during in vitro culture by day 6, but the expression of CCR6 was undetectable at day 14.16 Immature DCs generated from monocytes in the presence of GM-CSF, IL-4, and TGF-β1 also expressed CCR6 and responded to LARC. However, both CCR6 expression and responsiveness to LARC were lost when the cells completely matured to DCs in the presence of TNF-α and instead began to express CCR7.14 Thus, CCR6 is expressed on only immature DCs. In the current study, PMNLs activated with either PHA-sup, a combination of TNF-α and GM-CSF, or a combination of TNF-α and GM-CSF for 6 hours responded well to LARC, whereas the cells activated with the same stimuli overnight did not respond. In agreement with the chemotaxis result, specific binding of 125I-LARC was not detected after overnight activation of the cells, suggesting that PMNL expression of CCR6 was also transient. However, we did not detect CCR7 mRNA in cytokine-activated PMNLs by RT-PCR, even in the presence of GM-CSF, up to 4 days, which differentiates activated PMNLs from mature DCs.
It was previously reported that IFN-γ was a potent inducer of CCR1 and CCR3 expression in PMNLs but not of CCR2, CCR4, and CCR5.17 Up-regulation of CCR1 and CCR3 expression appeared to be due to the prolongation of mRNA stability after IFN-γ treatment, suggesting that the augmentation of CCR6 mRNA expression by IFN-γ might be due to its ability to increase the stability of CCR6 mRNA. Thus, although TNF-α and IFN-γ are each capable of regulating the induction of chemokine receptor expression in PMNLs, they appear to have a different role in the induction of selective CC chemokine receptors in PMNLs, which may in part account for their synergistic interactions.
The capacity of certain chemokines to recruit and activate certain leukocyte populations can be altered by up- or down-regulating the expression of chemokine receptors due to proinflammatory cytokines produced during inflammation. For example, PMNLs from rats with chronic inflammatory vasculitis expressing CCR1 and CCR2 migrated toward MCP-1.18 CCR1 and CCR3 expressed on IFN-γ–stimulated PMNLs were also functional.17 In contrast to these CC-chemokine receptors, the expression of CXCR1 and CXCR2 were down-regulated after TNF-α activation of PMNLs.19Despite the apparent induction of functional CCR6 after cytokine activation, the biologic significance of CCR6 expression in PMNLs remains unknown. Because TNF-α is secreted from various cells in an early phase of the inflammatory response, infiltrating PMNLs are likely to express CCR6 at sites of inflammation. For CCR6 expressed on PMNLs to be able to play a role, the ligand also has to be present locally. Induction of LARC expression was previously detected in LPS-stimulated PBMCs and TNF-α–stimulated immortalized human umbilical cord vein endothelial cells.20 LARC mRNA was significantly elevated in the rat spinal cord after contusion injury where PMNLs were infiltrating,21 suggesting a possible interaction between CCR6-expressed PMNLs and LARC at sites of inflammation. Our future study will be directed to identify the biologic significance of CCR6 expressed by activated PMNLs.
Oehler et al22 previously reported that lactoferrin-positive immediate precursors of end-stage PMNLs could be driven to acquire characteristics of DCs, including HLA-DR, CD40, CD80, and CD86, after activation with GM-CSF, TNF-α, and IL-4. Additional activation with CD40 ligand induced the expression of CD83 and up-regulated CD80, CD86, and HLA-DR. Because the cells used in their study were collected from patients with chronic myeloid leukemia and patients with leukocytosis and left-shifted differential blood counts due to bacterial infection or GM-CSF treatment, it was still unclear whether similar differentiation could be induced in normal PMNLs. In the current study, we demonstrated that normal human PMNLs stimulated with both TNF-α and IFN-γ expressed high levels of CD83 by FACS analysis. Up-regulation of CD83 mRNA expression was also detected by RT-PCR. These results suggest that PMNLs expressing CCR6 may be in the process of acquiring certain phenotypes of DCs and that CCR6 could be one of the cell-surface markers expressed during the process.
Another important feature of mature DCs is to present antigens to T cells. PMNLs activated with either PHA-sup or a combination of TNF-α and IFN-γ for up to 2 days expressed no significant HLA-DR, though we detected the up-regulation of HLA-DR α-chain mRNA expression by RT-PCR (data not shown). In contrast, when PMNLs were incubated in the presence of GM-CSF, TNF-α, and IFN-γ for 2 days, the cells expressed CD40 and HLA-DR in addition to CD83. It has been reported that PMNLs stimulated with GM-CSF and IFN-γ not only express HLA-DR but also are capable of presenting antigen to T cells.7 We investigated whether cytokine-activated PMNLs could stimulate allogenic MLR. PMNLs activated with TNF-α and IFN-γ never stimulated allogenic MLR. However, in a few experiments, PMNLs activated for 2 days with GM-CSF, TNF-α, and IFN-γ stimulated a modest allogenic MLR (data not shown). Because the contamination of PBMCs, mostly small lymphocytes, in our PMNL preparations was less than 0.1%, it is highly unlikely that stimulation of allogenic MLR was due to the contaminated DCs or to DC progenitor cells. The inconsistent results we obtained may be owing to the variation that appears to exist among donors as previously described.7 Regardless, we have not been able to conclude whether activated PMNLs are capable of considerably stimulating an allogenic MLR, and it appears that the capacity of activated PMNLs to present antigens is limited.
The importance of PMNLs in the development of delayed-type hypersensitivity (DTH) was previously demonstrated in vivo. Depletion of PMNLs inhibited the infiltration of monocytes and lymphocytes in a murine model of DTH.23,24 Injection of anti–IL-8 antibody inhibited the development of DTH in a rabbit DTH model.25In a rat DTH model, MCP-1, an important chemokine regulating the infiltration of monocytes in DTH, was detected by immunohistochemistry in early infiltrating PMNLs, and neutralization of MCP-1 activity with anti–MCP-1 antibody inhibited the development of DTH.26In support of these observations, we previously reported that human PMNLs could be induced to express and produce MCP-1 in vitro.8 We recently suggested that the priming of PMNLs with a product(s) of PHA-stimulated PBMCs could functionally change the ability of PMNLs to express MCP-1.9 In the current study, we have shown further evidence indicating the capacity of PMNLs to acquire phenotypic and functional changes after activation with selected cytokines, including TNF-α, IFN-γ, and GM-CSF. Taken together, the function of inflammatory PMNLs may differ depending on the availability of selected cytokines. Under appropriate conditions, newly emigrated PMNLs can acquire new phenotypes along with new functions and can play a key role in mobilizing or initiating adaptive immunity by producing important proinflammatory cytokines and chemokines such as MCP-1, and perhaps by presenting antigens to T cells.
Acknowledgments
We thank Dr Joost J. Oppenheim for his encouragement and invaluable comments throughout this study. We also thank Ms Nancy Dunlop for her technical assistance.
Supported by the Intramural Research Support Program, SAIC (Frederick, MD) (W.-H.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Teizo Yoshimura, Laboratory of Molecular Immunoregulation, National Cancer Institute-Frederick Cancer Research and Development Center, Bldg 559, Rm 1, Frederick, MD 21702; e-mail:yoshimur@mail.ncifcrf.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal