Abstract
We used flow cytometry to quantify minimal residual disease (MRD) in 56 patients with acute myeloid leukemia (AML) expressing a leukemia-associated phenotype. Thirty-four patients aged 18 to 60 years were entered into the AML-10 protocol (induction, consolidation, and autologous stem-cell transplantation [ASCT]), whereas 22 patients older than 60 years received the AML-13 protocol (induction, consolidation, and consolidation II). After induction, the level of MRD that was best associated with treatment outcome was 4.5 × 10−4 residual leukemic cells. However, the outcome in patients with at least 4.5 × 10−4 cells (n = 26) was not significantly different from that in patients with fewer leukemic cells (n = 30); there were 15 (58%) relapses in the first group and 12 (40%) relapses in the second. After consolidation, the most predictive MRD cutoff value was 3.5 × 10−4cells: 22 patients had an MRD level of 3.5 × 10−4 cells or higher and 17 (77%) of these patients had relapse, compared with 5 of 29 patients (17%) with lower MRD levels (P < .001). An MRD level of 3.5 × 10−4 cells or higher after consolidation was significantly correlated with poor or intermediate-risk cytogenetic findings, a multidrug resistance 1 (MDR1) phenotype, short duration of overall survival, and short duration of relapse-free survival (P = .014, .031, .00022, and .00014, respectively). In multivariate analysis, this MRD status was significantly associated with a high frequency of relapse (P < .001) and a short duration of overall (P = .025) and relapse-free survival (P = .007). ASCT did not alter the prognostic effect of high MRD levels after consolidation: the relapse rate after transplantation was 70%. Thus, we found that an MRD level of 3.5 × 10−4 cells or higher at the end of consolidation strongly predicts relapse and is significantly associated with an MDR1 phenotype and intermediate or unfavorable cytogenetic findings.
Introduction
Complete remission (CR) rates as high as 70% to 80% have been reported in adult patients with acute myeloid leukemia (AML),1-6 but approximately 60% to 70% patients will eventually have relapse due to the persistence of residual leukemic cells surviving after chemotherapy. The persistence of residual malignant cells below the threshold of conventional morphologic findings—minimal residual disease (MRD)—may identify patients at a higher risk of relapse.
Polymerase chain reaction (PCR) and flow cytometry are the most commonly used techniques for detecting MRD in patients with AML. Reverse transcriptase–PCR studies of PML/RARα transcripts in acute promyelocytic leukemia (APL)7,8 and ofAML1/ETO transcripts in patients with AML M29have demonstrated the value of this approach for monitoring MRD. However, only a fraction of patients with AML have fusion transcripts suitable for PCR assays.10 MRD monitoring with flow cytometry relies on the idea that AML cells frequently show aberrant or leukemia-associated phenotypes resulting from asynchronous antigen expression, cross-lineage antigen expression, antigen overexpression, and aberrant light-scatter properties.10-13
In this study, we used multiparametric flow cytometry to determine the levels of MRD after induction, consolidation, and autologous stem-cell transplantation (ASCT) in AML patients in first CR after induction therapy. Our objective was to determine the effect of MRD on clinical outcome and correlate MRD with other recognized prognostic factors, such as multidrug resistance 1 (MDR1) phenotype and cytogenetic findings.14
Patients and methods
Patients and treatments
Approval for this study was obtained from the institutional review board. Informed consent was provided according to the Declaration of Helsinki. Criteria for inclusion in the MRD study were (1) diagnosis of AML other than APL (patients with APL were entered into a different protocol); (2) expression of leukemia-associated phenotype; (3) eligibility for intensive chemotherapy; (4) achievement of a morphologic CR after induction therapy. Sixty-five of 93 (70%) adult patients with de novo AML enrolled in the European Organization for Research and Treatment of Cancer/Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto (EORTC/GIMEMA) protocols AML-10 and AML-13 expressed a leukemia-associated phenotype; 56 (60%) achieved a CR and therefore had follow-up that included MRD monitoring.
The EORTC/GIMEMA AML-10 randomized trial included patients aged 18 to 60 years. The induction treatment combined cytarabine (100 mg/m2 of body-surface area given intravenously on days 1-10), etoposide (100 mg/m2 given on days 1-5), and on days 1, 3, and 5, either daunorubicin (50 mg/m2), mitoxantrone (12 mg/m2), or idarubicin (10 mg/m2), according to random assignment. As consolidation, patients received cytarabine (500 mg/m2 every 12 hours on days 1-6) and the same anthracycline given in the induction (on days 4 to 6). Patients with an HLA-compatible sibling were given allografts, whereas the others were randomly assigned to receive peripheral or bone marrow (BM) stem-cell transplantation15 (Figure 1).
Treatment design in 2 protocols for treatment of acute myeloid leukemia (AML).
The AML-10 protocol included patients aged 18 to 60 years, whereas only patients older than 60 years were included in the AML-13 protocol.
Treatment design in 2 protocols for treatment of acute myeloid leukemia (AML).
The AML-10 protocol included patients aged 18 to 60 years, whereas only patients older than 60 years were included in the AML-13 protocol.
Patients older than 60 years were entered into the EORTC/GIMEMA AML-13 randomized trial. The patients received mitoxantrone (7 mg/m2 on days 1, 3, and 5), etoposide (100 mg/m2 on days 1-3), and cytarabine (100 mg/m2on days 1-7) as induction therapy. On achievement of CR, patients were randomly assigned to receive either an intravenous or an oral consolidation program (2 cycles). Intravenous consolidation treatment consisted of idarubicin (8 mg/m2 on days 1, 3, and 5), etoposide (100 mg/m2 on days 1-3), and cytarabine (100 mg/m2 on days 1-5). Oral consolidation therapy consisted of idarubicin (20 mg/m2 on days 1, 3, and 5), etoposide (100 mg/m2 twice/day on days 1-3), and subcutaneously administered cytarabine (50 mg/m2 twice/day on days 1-5; Figure 1).
Immunophenotypic studies and detection of MRD
BM samples were collected at diagnosis, at full hematologic restoration after each treatment step (induction, consolidation I and II, and autografting) and every 3 months for the first 2 years; a total of 437 samples were evaluated. At diagnosis, studies were done on erythrocyte-lysed whole BM samples by using a broad panel of monoclonal antibodies targeting membrane antigens, as described previously.11,14,16 17 Briefly, each antibody was incubated with 1 to 2 × 106 cells in a 100-μL volume, and isotype-matched antibodies were used as negative controls. After 10 minutes of incubation, 2 mL lysing solution for fluorescence-activated cell sorting (FACS; Becton Dickinson, Mountain View, CA) was added and the sample incubated for another 10 minutes. After 2 washings in phosphate-buffered saline (PBS), cells were resuspended in 0.5 mL PBS and analyzed with a flow cytometer (Facsort; Becton Dickinson).
After the immunophenotype of the leukemic cells was established, samples from patients with leukemia-associated phenotypes were selected and reinvestigated by staining them with the (double-triple) relevant combinations of antibodies. A given combination of markers was regarded as relevant if it was expressed in more than 50% of the blasts (Table1). This step served to define a phenotypic patient profile which was in turn used to track possible residual leukemic cells during follow-up. At least 2 antibody combinations for each case were used to minimize problems caused by phenotypic switches10 that sometimes accompany relapses. CellQuest software (Becton Dickinson) was used for acquisition of flow cytometric data, with application of live gates on the forward-light–orthogonal light scatter (gate 1, blast region) and fluorescence plots (gates 2 and 3, double and triple positive events).
Leukemia-associated phenotypes and their distribution at diagnosis
| Aberrant phenotype . | Frequency (%)* . |
|---|---|
| CD34+CD56+ | 2 |
| CD34+CD14+ | 5 |
| CD34+CD11b+ | 46 |
| CD34+CD49b+ | 2 |
| CD34+CD69+ | 10 |
| CD34+Icam+ | 15 |
| C-kit+CD56+ | 6 |
| CD33+CD2+ | 6 |
| CD33+CD4+ | 15 |
| CD33+CD7+ | 51 |
| CD34+CD15+Hla-Dr+ | 11 |
| CD34+CD33+CD7+ | 47 |
| CD34+CD33+CD19+ | 5 |
| CD34+CD33+CD4+ | 8 |
| CD33−CD14+Hla-Dr+ | 17 |
| CD33−CD15+Hla-Dr+ | 19 |
| CD34+CD11b+C-kit+ | 47 |
| CD34+C-kit+CD56+ | 1 |
| Aberrant phenotype . | Frequency (%)* . |
|---|---|
| CD34+CD56+ | 2 |
| CD34+CD14+ | 5 |
| CD34+CD11b+ | 46 |
| CD34+CD49b+ | 2 |
| CD34+CD69+ | 10 |
| CD34+Icam+ | 15 |
| C-kit+CD56+ | 6 |
| CD33+CD2+ | 6 |
| CD33+CD4+ | 15 |
| CD33+CD7+ | 51 |
| CD34+CD15+Hla-Dr+ | 11 |
| CD34+CD33+CD7+ | 47 |
| CD34+CD33+CD19+ | 5 |
| CD34+CD33+CD4+ | 8 |
| CD33−CD14+Hla-Dr+ | 17 |
| CD33−CD15+Hla-Dr+ | 19 |
| CD34+CD11b+C-kit+ | 47 |
| CD34+C-kit+CD56+ | 1 |
At least 2 aberrant phenotypes were observed for each case.
Samples were then analyzed by using the Paint-a-GateProsoftware program (Becton Dickinson), as previously described.11 17-19 Briefly, this program provides multidimensional, multicolor analysis of FACS-acquired list-mode data files. It allows classification of events by painting them different colors and quantifying them as percentages of total events. This allows visualization in different plots of cell populations difficult to see in 2 dimensions. The Paint-a-Gate program is also equipped with a MouseTrax Control option that allows analysis procedures to be repeated automatically. When this option is activated, every step taken during the analysis is recorded, including the moves made with the computer mouse. Paint-a-Gate stores these sequences in a file so that the analysis can be repeated with many different data files. Thus, for each patient expressing a leukemia-associated phenotype, the MouseTrax Control option was used to set up a tailored procedure that exactly defined the phenotypic patient's profile. This procedure was memorized and run automatically to analyze BM when CR was achieved and during subsequent follow-up.
MRD studies during remission were performed on erythrocyte-lysed whole BM samples by using the same association of antibodies defining the specific patient's profile. During data acquisition, a live-gate including the lymphomonocytic and granuloblastic region and excluding debris and platelet aggregates was used, with 106 total events acquired for all samples. The acquired events were analyzed with the Paint-a-Gate program by running the MouseTrax Control option as described above. Studies of a series of normal BM samples from healthy donors created an internal standard reference for distinguishing normal from leukemic patterns.10-13
Dilution experiments were also performed to test the sensitivity of the assessment technique. Thus, leukemic blasts were mixed with normal BM samples in decreasing concentrations (10−1 to 10−6). After the instrument was cleaned, faint clusters of leukemic cells were still detected at the dilution of 10−6. However, when BM samples from patients in CR were processed, no clusters of residual leukemic cells were observed below the threshold of 10−5; therefore, we assumed that the sensitivity of the technique ranged from 10−4 to 10−5.10 11
MDR1 expression and cytogenetic studies
MDR1 expression was tested in all samples by using Moab MRK16 (Immunotech, Marseille, France), which reacts with an extracellular epitope of the human 170-kd P-glycoprotein, as described previously.20 The procedures for cytogenetic analysis were previously described in detail.21 Karyotypes were classified according to International System for Human Cytogenetic Nomenclature.22
Statistical analysis
The relation between MRD and patients' characteristics and response to treatment was assessed with use of a 2-sided χ2 or Fisher exact test (when either group included fewer than 20 cases). A P value of .05 or less was considered to represent significance. The Spearman rank correlation (r) was used to assess the relation between the level of MRD after consolidation and time to relapse. The Kaplan-Meier method23 was used to estimate overall survival (OS) and relapse-free survival (RFS). For comparisons of survival and remission duration in 2 or more groups, the log-rank test was applied. CR and relapse were defined according to standard criteria.24 OS was calculated from the date of diagnosis to the date of death or last follow-up evaluation. RFS was measured from achievement of CR until relapse. To evaluate the simultaneous effect of different variables on relapse rate and duration of OS and RFS, we performed a multivariate analysis using a stepwise regression model.
Results
Determination of MRD after induction
Clinical characteristics of the 56 patients included in the study are shown in Table 2. At the end of induction therapy, the median level of MRD was 3.5 × 10−4 residual leukemic cells (range, 0-8.1 × 10−2). The cutoff value that had the best statistical correlation with outcome was 4.5 × 10−4: 58% (15/26) of patients with 4.5 × 10−4 cells or more (the MRDInd+ group) had relapse, whereas 40% (12/30) of those with less than 4.5 × 10−4 cells (the MRDInd− group) did so (P = .097). No significant correlation was found between MRDInd+ status and the expression of MDR1 phenotype or the presence of poor- or intermediate-risk cytogenetic findings. Similarly, no significant differences in OS or RFS were observed between the MRDInd+and the MRDInd− group.
Clinical characteristics of patients according to protocol for treatment of acute myeloid leukemia (AML)
| Characteristic . | AML-10 protocol (n = 34) . | AML-13 protocol (n = 22) . | All patients (n = 56) . |
|---|---|---|---|
| Median (range) age, y | 47 (18-61) | 68 (61-78) | 54 (18-78) |
| Sex (M/F) | 17/17 | 8/14 | 25/31 |
| WBC, × 109/L | |||
| < 50 | 22 | 19 | 41 |
| 50-100 | 8 | 2 | 10 |
| > 100 | 4 | 1 | 5 |
| FAB class | |||
| M0 | 3 | 1 | 4 |
| M1 | 7 | 7 | 14 |
| M2 | 10 | 10 | 20 |
| M4 | 3 | — | 3 |
| M5 | 10 | 4 | 14 |
| M6 | 1 | — | 1 |
| Cytogenetic findings* | |||
| Favorable | 8 | 2 | 10 |
| Intermediate | 13 | 12 | 25 |
| Unfavorable | 10 | 4 | 14 |
| Induction | 34 | 22 | 56 |
| Consolidation | 33 | 18 | 51 |
| Consolidation II | — | 11 | 11 |
| ASCT | 28 | — | 28 |
| Characteristic . | AML-10 protocol (n = 34) . | AML-13 protocol (n = 22) . | All patients (n = 56) . |
|---|---|---|---|
| Median (range) age, y | 47 (18-61) | 68 (61-78) | 54 (18-78) |
| Sex (M/F) | 17/17 | 8/14 | 25/31 |
| WBC, × 109/L | |||
| < 50 | 22 | 19 | 41 |
| 50-100 | 8 | 2 | 10 |
| > 100 | 4 | 1 | 5 |
| FAB class | |||
| M0 | 3 | 1 | 4 |
| M1 | 7 | 7 | 14 |
| M2 | 10 | 10 | 20 |
| M4 | 3 | — | 3 |
| M5 | 10 | 4 | 14 |
| M6 | 1 | — | 1 |
| Cytogenetic findings* | |||
| Favorable | 8 | 2 | 10 |
| Intermediate | 13 | 12 | 25 |
| Unfavorable | 10 | 4 | 14 |
| Induction | 34 | 22 | 56 |
| Consolidation | 33 | 18 | 51 |
| Consolidation II | — | 11 | 11 |
| ASCT | 28 | — | 28 |
WBC indicates white blood cell count; FAB, French-American-British leukemia classification system; and ASCT, autologous stem-cell transplantation.
Available for 49 of 56 patients.
Determination of MRD after consolidation
Five patients with MRDInd+ status had relapse before or during consolidation and died of progressive disease; thus, 51 patients (21 MRDInd+ and 30 MRDInd−) could be evaluated for MRD at the end of consolidation. The median MRD value after consolidation was 3.1 × 10−4 residual leukemic cells (range, 0-6.8 × 10−2). A level of 3.5 × 10−4 residual malignant cells divided the 51 evaluated patients into 2 distinct groups: the MRDCons+group and the MRDCons− group, which had relapse rates of 77% (17/22) and 17% (5/29), respectively (P < .001). Of the 21 patients in the MRDInd+ group, 7 became MRDCons− and are in continuous CR. Ten (71%) of the remaining 14 patients in the MRDInd+ group who did not become MRDCons− had relapse (P = .001). Similarly, among the 30 patients in the MRDInd− group, 8 had positive findings after consolidation and 7 (87%) of them had relapse. In contrast, only 5 (23%) of the remaining 22 with MRDCons− status had relapse (P = .007).
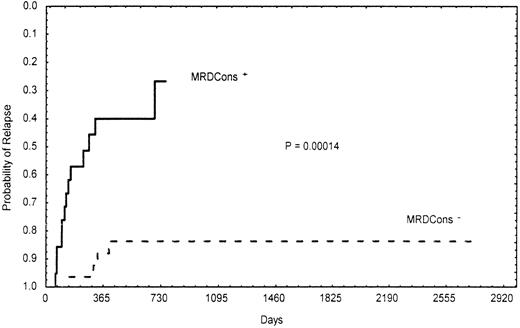
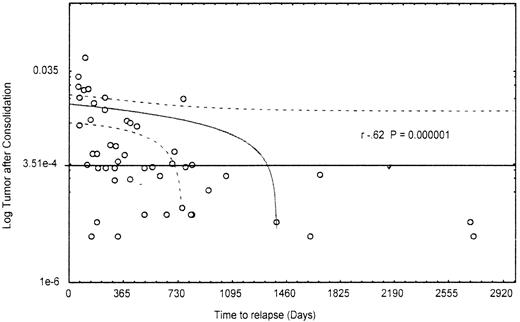
Importantly, the presence of a certain number of residual leukemic cells (≥3.5 × 10−4) after consolidation was significantly correlated with poor- and intermediate-risk cytogenetic findings and the MDR1 phenotype. In fact, 95% (19/20) of patients with MRDCons+ status had an unfavorable or intermediate karyotype, whereas 40% (10/25) of those in the MRDCons−group had favorable cytogenetic findings (P = .014). Similarly, 68% (15/22) of MRDCons+ patients expressed the MDR1 phenotype, whereas 62% (18/29) of those with MRDCons− status did not (P = .031). Also, duration of OS and RFS was significantly associated with the level of MRD at the end of consolidation. Patients in the MRDCons+group had a median OS of 10 months, a point not reached by those in the MRDCons− group (P = .00022; Figure2). Likewise, the median RFS was not reached by patients with MRDCons− status, whereas it was 7 months among those in the MRDCons+ group (P = .00014; Figure 3). The relation between MRD level after consolidation and time to relapse was also analyzed as a continuous variable by using the Spearman rank test; this approach showed an inverse correlation between the level of MRD after consolidation and time to relapse (r = −0.62;P = .000001; Figure 4).
Actuarial probability of overall survival according to MRDCons status.
Patients with MRDCons+ status had a median overall survival time of 10 months, a point not reached by those with MRDCons− status.
Actuarial probability of overall survival according to MRDCons status.
Patients with MRDCons+ status had a median overall survival time of 10 months, a point not reached by those with MRDCons− status.
Actuarial probability of relapse according to MRDCons status.
Patients with MRDCons+ status had a median relapse-free survival time of 7 months, a point not reached by those with MRDCons− status.
Actuarial probability of relapse according to MRDCons status.
Patients with MRDCons+ status had a median relapse-free survival time of 7 months, a point not reached by those with MRDCons− status.
Correlation between level of minimal residual disease after consolidation and time to relapse.
Most patients with durable CR are in the group with less than 3.5 × 10−4 residual leukemic cells at the end of consolidation.
Correlation between level of minimal residual disease after consolidation and time to relapse.
Most patients with durable CR are in the group with less than 3.5 × 10−4 residual leukemic cells at the end of consolidation.
Determination of MRD after consolidation II
Of 22 patients entered into the AML-13 protocol (Table 2), 4 with MRDInd+ status had relapse before receiving the first consolidation treatment and 7 with MRDCons+ status had relapse before receiving the second consolidation. Thus, 11 patients were given consolidation II therapy and could be evaluated for MRD. Among these 11 patients, 9 had MRDCons− status and the MRD level remained below 3.5 × 10−4 residual leukemic cells, even after consolidation II; all these patients remain in CR. The remaining 2 patients had MRDCons+ status and did not benefit from consolidation II therapy in that the level of MRD remained above the threshold of 3.5 × 10−4 residual malignant cells. One of these 2 patients had relapse after 13 months.
Determination of MRD after ASCT
Of 34 patients recruited to the AML-10 protocol (Table 2), 6 did not undergo transplantation because of patient refusal (1 patient), medical reasons (3 patients), or relapse (2 patients, 1 with MRDInd+ status before consolidation and 1 with MRDCons+ status before transplantation). The patient who chose not to undergo transplantation was in the MRDCons+group and had relapse 15 months later, whereas those excluded for medical reasons are in CR (1 has MRDCons+ status). The remaining 28 patients (10 with MRDCons+ status and 18 with MRDCons− status) underwent transplantation and subsequent MRD determinations. Among the 10 patients with MRDCons+status, 7 (70%) had relapse and, in all but 1 patient, the level of residual leukemic cells did not fall below 3.5 × 10−4, even after ASCT. Conversely, only 5 of the 18 (28%) patients with MRDCons− had relapse and, in 2 of them, disease recurrence was preceded by a gradual increase in MRD to the level of 1.4 × 10−2 leukemic cells. The difference in relapse rate between the MRDCons+ and MRDCons− groups was significant (P = .031).
Prognostic determinants
All relevant prognostic variables (age, French-American-British leukemia class, white blood cell count, MDR1 phenotype, cytogenetic findings, MRDInd+ status, and MRDCons+status) were pooled into a multivariate model to determine to what extent they independently affected outcome. MRDCons+status emerged as an independent variable that was significantly associated with a high frequency of relapse (P < .001) and a short duration of OS (P = .025) and RFS (P = .007). Cytogenetic findings were found to independently affect duration of OS and RFS (P = .036 andP = .025, respectively), whereas MDR1 phenotype was significantly associated with a high frequency of relapse (P = .03; Table 3).
Results of multivariate analysis of minimal residual disease and outcomes in AML
| . | Relapse . | Duration of overall survival . | Duration of relapse-free survival . |
|---|---|---|---|
| MRDCons+ | < .001 | .025 | .007 |
| MDR1 phenotype | .03 | NS | NS |
| Unfavorable or intermediate cytogenetic findings | NS | .036 | .025 |
| . | Relapse . | Duration of overall survival . | Duration of relapse-free survival . |
|---|---|---|---|
| MRDCons+ | < .001 | .025 | .007 |
| MDR1 phenotype | .03 | NS | NS |
| Unfavorable or intermediate cytogenetic findings | NS | .036 | .025 |
All data are P values.
MRDCons+ indicates minimal residual disease (≥ 3.5 × 10−4 residual leukemic cells) after consolidation; MDR1, multidrug resistance 1; and NS, not significant.
The results indicated that MRDCons+ status is independently associated with relapse rate, duration of overall survival, and duration of relapse-free survival.
Discussion
MRD testing in patients with AML in clinical remission is a potentially useful tool for assessing the risk of relapse and guiding therapeutic decisions. We found that the persistence of high levels of MRD (≥ 3.5 × 10−4 residual malignant cells) after consolidation, but not after induction, was associated with a significant likelihood of subsequent relapse and a short duration of OS and RFS. Thus, the magnitude of cytoreduction after consolidation appears to be critical to the clinical outcome of disease, regardless of the tumor reduction achieved with induction. The lack of a significant correlation between MRDInd+ status and the occurrence of relapse or duration of OS and RFS supports this assumption. Moreover, patients with MRDInd+ status who later had MRDCons− status had a lower probability of relapse than patients with MRDInd− status who did not enter the MRDCons− group.
Our findings appear to be at variance with those of San Miguel et al,11 who observed a correlation between level of MRD and the probability of relapse not only after intensification but also after induction. The different therapeutic regimens used in the 2 studies may explain this discrepancy. In the study of San Miguel et al, the remission-induction therapy consisted of 1 or 2 courses of an anthracycline and cytosine arabinoside (3 + 7 regimen), followed by an identical consolidation course. This was followed by 1 or 2 intensification courses consisting of intermediate- or high-dose cytosine arabinoside and either daunorubicin or idarubicin. The AML-10 and AML-13 protocols are 3-drug–based regimens in which an anthracycline is used in association with cytosine arabinoside and etoposide. In addition, in the AML-10 protocol, cytosine arabinoside was given for 10 days instead of 7 during the induction phase. Thus, in the study of San Miguel et al, a milder debulking effect achieved with a less intensive therapy may account for the higher level of MRD (5 × 10−3 leukemic cells after induction and 2 × 10−3 after intensification) at which a significant influence on disease outcome was found. This may also explain why, in their study, the number of residual leukemic cells correlated with the rate of relapse and RFS after both induction and intensification whereas, in our experience, such a correlation was found only after consolidation.
Although our patients with MRDCons+ status underwent ASCT, they had a relapse rate of 70%. These results confirm the highly predictive role of MRD status at the end of consolidation and are in keeping with those of Lahuerta et al,25 who reported a poor prognosis for patients with an MRD level of 0.8% or higher immediately before ASCT. Additional cytoreduction before ASCT or alternative strategies (use of matched, unrelated-donor transplants or full-haplotype mismatched transplants26) would be desirable in these patients. In contrast, only 28% of patients with MRDCons− status had relapse after ASCT and, in 2 of them, a pending relapse was suspected because of a gradual increase in the level of MRD during follow-up after ASCT. This observation illustrates the need for an accurate MRD determination in monitoring the quality of remission over time in patients with AML treated with ASCT. If these results are confirmed in a larger series of patients, they will indicate that an early therapeutic intervention may be required to prevent overt relapse in patients showing a trend toward an increased MRD level.
In one case in our series, the relapse was unexpected and not preceded by an increase in MRD level, nor were phenotypic switches documented at the time of recurrence. We hypothesize that the amount of MRD had remained below the threshold of the sensitivity of the assessment technique used until the morphologic relapse occurred. We believe that use of more advanced devices, such as a dual-laser–equipped cytometer, which allows 4 or more antibodies to be combined, would improve the sensitivity of the method. In 2 patients in our series, relapse occurred after peripheral ASCT and, once again, neither changes in the level of MRD nor phenotypic switches were observed before or at relapse. However, both patients had a high CD34+ cell count (≥ 10 × 106/kg) in the peripheral harvest. According to a recent report from the EORTC/GIMEMA cooperative group, mobilization of a large number of autologous stem cells in patients with AML is associated with a poor outcome.27 Therefore, “very good” mobilizers should be closely monitored for circulating residual leukemic cells. We recently started a protocol for MRD determination that is directed not only at BM but also at peripheral blood and apheresis products.
With regard to the 5 patients who are in continuous CR in spite of detectable disease after consolidation, we hypothesize that they still have AML, as confirmed by a cytometric pattern showing a uniquely identifiable cluster of leukemia cells. Thus, the limited follow-up time may explain why we have not yet observed relapse in these patients.
In conclusion, we found that detection of MRD using flow cytometry is a useful approach for predicting outcome in patients with AML treated with intensive regimens. If confirmed, our results will indicate that future clinical trials should take into account this information in order to verify the applicability of pre-emptive therapeutic intervention in patients with a high level of MRD after consolidation therapy. We also believe that MRD monitoring during follow-up after therapy is clinically useful, providing adequate surveillance in patients with residual leukemia (≥3.5 × 10−4cells) or those with a tendency toward increased MRD. On the other hand, we assume that patients with negative findings for a year after consolidation II therapy or ASCT do not need further follow-up and are likely to be cured. Moreover, the expanding knowledge about the antigenic composition of leukemic cells will make sequential follow-up suitable in most patients with AML. Finally, flow cytometric assays can efficiently complement molecular techniques in an integrated approach to determination of MRD.
Acknowledgment
The authors thank Dr Dario Campana for reviewing the manuscript and providing helpful suggestions.
Supported in part by MURST, Programmi di Ricerca di Interesse Nazionale.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Adriano Venditti, Cattedra di Ematologia, Università di Roma Tor Vergata, Divisione di Ematologia, Osp S Eugenio, P le dell'Umanesimo 10-00144, Rome, Italy; e-mail:adriano.venditti@tin.it.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal