Abstract
This study addresses a mechanism by which lymphocytes may promote vascular endothelial growth factor (VEGF) expression and angiogenesis in immune inflammation. Resting human umbilical endothelial cells (HUVECs) were found to express low levels of VEGF messenger RNA (mRNA) by reverse transcription polymerase chain reaction and ribonuclease protection assay with little or no change in expression following activation by cytokines, including tumor necrosis factor-α, interleukin (IL)–1, interferon γ, or IL-4. In contrast, treatment of HUVECs and monocytes with soluble CD40 ligand (sCD40L) resulted in a marked dose-dependent induction of VEGF mRNA (approximately 4-fold), which peaked between 1 and 5 hours post-stimulation. Transient transfection of HUVECs was performed with a luciferase reporter construct under the control of the human VEGF promoter. Treatment of transfected HUVECs with sCD40L was found to enhance luciferase activity (approximately 4-fold) compared with controls, similar to the relative fold induction in mRNA expression in parallel cultures. Thus, CD40-dependent VEGF expression was a result of transcriptional control mechanisms. Treatment of HUVECs with sCD40L was also found to function in vitro to promote growth and proliferation in a VEGF-dependent manner, and CD40-dependent HUVEC growth was comparable to that found following treatment with recombinant human VEGF. Furthermore, subcutaneous injection of sCD40L in severe combined immunodeficient and nude mice induced VEGF expression and marked angiogenesis in vivo. Taken together, these findings are consistent with a function for CD40L-CD40 interactions in VEGF-induced angiogenesis and define a mechanistic link between the immune response and angiogenesis.
Introduction
It is well established that leukocyte recruitment into tissues is associated with angiogenesis in a variety of inflammatory conditions, including allograft rejection.1-5 Indeed, the same factors that mediate recruitment (eg, cytokines, chemokines, and adhesion molecules) may themselves promote angiogenesis.6-8 Vascular endothelial growth factor (VEGF) is a potent angiogenesis factor and has been demonstrated to be functional in acute and chronic inflammation.2,9,10 VEGF is produced by endothelial cells, macrophages, activated T cells, and a variety of other cell types.6,11,12 It exists as 5 different isoforms composed of 206, 189, 165, 145, and 121 amino acids by alternative splicing of a single gene.12-14 VEGF binds to high-affinity tyrosine kinase receptors KDR/Flk-1 and Flt-1, resulting in endothelial proliferation in vitro and in angiogenesis and increased vascular permeability in vivo.14 In addition, VEGF binds to mononuclear cells, resulting in activation responses and chemotactic activity.15 Despite an abundance of angiogenesis factors, VEGF appears to be most critical in vivo since VEGFgene–knockout mice (including heterozygotes) fail to develop blood vessels, with resultant embryonic lethality.16 While hypoxia is the most potent stimulus for VEGF expression,17-19 other factors known to induce VEGF include some cytokines, oncogenes, prostaglandins, modulators of protein kinase C, nitric oxide, and stimulators of adenylate cyclase.6
Interactions between CD40 ligand (CD40L, also called CD154) and CD40 have been found to have pluripotent functions in inflammation, predominantly in the effector phase of the immune response.20,21 CD40, a 50 kd type I transmembrane-glycoprotein member of the tumor necrosis factor receptor (TNF-R) gene family is expressed by numerous cell types including most professional antigen-presenting cells (APCs), monocytes, and endothelial cells.21-24 CD40L, a 33-kd type II membrane-protein member of the TNF family, is predominantly expressed by activated CD4+ T cells and platelets25,26although it is also expressed on many other cell types.20Signaling via CD40 mediates immunoglobulin isotype switching in B cells; the expression of costimulatory molecules, notably B7-family molecules on APCs; the activation of monocytes; and adhesion molecule expression by endothelial cells, as well as the expression of cytokines and chemokines characteristic of the effector phase of the immune response.23,24 27
In this study, we have examined pro-inflammatory signals that mediate VEGF expression. Our data provide insight into the role of the T cell in leukocyte-induced angiogenesis and show that CD40L-CD40 interactions are potent for VEGF expression and VEGF-induced angiogenesis in vitro and in vivo.
Materials and methods
Reagents
The following monoclonal antibodies were used in these studies: anti–human CD40 (G28.5 and 220), anti–human CD40L (39-1.106) (gifts of D. Hollenbaugh, Bristol Myers Squibb, Princeton, NJ), and anti–human VEGF antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, and a gift of Genentech, South San Francisco, CA). Soluble human CD40L was used as culture supernatant (a gift of D. Hollenbaugh) or as recombinant protein (Ancell, Bayport, MN). Soluble murine CD40L, also a culture supernatant, was a gift of D. Hollenbaugh. Cytokines used in these studies were human interferon-γ (IFN-γ), human interleukin-4 (IL-4) (Genzyme, Cambridge, MA), human IL-1α (PharMingen, San Diego, CA), human IL-12 (R&D Systems, Minneapolis, MN), and human tumor necrosis factor-α (TNF-α) (a gift of Biogen, Cambridge, MA). Other reagents included Escherichia colilipopolysaccharide (LPS) (Sigma Chemical, St Louis, MO) and phytohemagglutinin (PHA) (Murex Biotech, Dartford, UK). A VEGF promoter-luciferase construct (in pGL2 basic; Promega, Madison, WI) containing the 2.6-kilobase (kb) full-length (base pairs −2361 to +298 relative to the transcription start site) VEGF promoter fragment was used in transfection assays as described.28
Cell isolation and culture
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll Hypaque gradient centrifugation from blood obtained from healthy volunteers. CD4+ T cells were purified from PBMCs by positive selection by means of CD4+ magnetic beads and Detach-a-Bead (Dynal, Lake Success, NY) according to the manufacturer's instructions. The efficiency of purification was greater than 98% as assessed by double-stain fluorescence-activated cell sorter analysis for CD3 and CD4.29 Human monocytes were purified from PBMCs by positive selection by means of anti-CD14–coated microbeads (MiniMACS separation column; Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. Monocytes were unactivated on the basis of high levels of L-selectin expression and relatively low levels of B7 expression.
Human umbilical vein endothelial cells (HUVECs) were isolated from umbilical cords as previously described30 and were cultured in M199 medium (BioWhittaker, Walkersville, MD) containing 20% fetal bovine serum (FBS) (Gibco-BRL Products, Gaithersburg, MD), endothelial cell growth supplement, 1% penicillin/streptomycin, L-glutamine, and heparin. Single-donor HUVECs were purchased from Clonetics (Walkersville, MD) and were cultured in complete endothelial medium (EGM BulletKit, Clonetics) as supplied and according to the recommended instructions. HUVECs were subcultured and used at passage 3 to 5. The human renal cell carcinoma cell line 786-O was cultured in Dulbecco modified Eagle medium containing 10% FBS, 1% penicillin/streptomycin, and L-glutamine. This cell line has been shown to express high constitutive levels of VEGF and was used in our studies as a positive control.28
RNA extraction and reverse transcription polymerase chain reaction
Transfer RNA was isolated from cultured cells by means of the Ultraspec RNA Isolation System (Biotecx Laboratories, Houston, TX) according to the manufacturer's instructions and was quantified by spectrophotometry. Complementary DNA (cDNA) was prepared by reverse transcription of RNA with the use of random hexamer primers (100 ng/μL) and Moloney murine leukemia virus reverse transcriptase (50 U/μL) (Gibco-BRL) in a 50-μL reaction. We used 10 μL cDNA for each polymerase chain reaction (PCR) amplification reaction. PCR was performed with Taq DNA polymerase with the use of the manufacturer's buffer (Boehringer Mannheim, Indianapolis, IN). Sequence-specific primers were used for amplification of the human VEGF gene (sense primer 5′-TCACCGCCTCGGCTTGTCACA-3′, antisense primer 5′-ATGAACTTTCTGCTGTCTTGG-3′).
We used β-actin (Stratagene, La Jolla, CA) as an internal control. PCR reactions were performed under the following conditions: 1 cycle at 94°C for 5 minutes followed by 40 cycles at 94°C for 1 minute, 60°C for 1 minute, and 72°C for 1 minute. The last cycle was extended to 7 minutes at 72°C. Messenger RNA (mRNA) extracted from PBMCs served as positive control. The amplified products were resolved by electrophoresis in an ethidium bromide–stained 1.5% agarose gel and were analyzed by densitometry by means of an AlphaImager 2000 system (Alpha Innotech, San Leandro, CA).
Ribonuclease protection assay
Equal amounts of mRNA were analyzed by ribonuclease protection assay (RPA) by means of the RiboQuant multiprobe template (PharMingen) according to the manufacturer's instructions. Briefly, RNA was hybridized overnight with the 32P-labeled RNA probe, which had previously been synthesized from the template set. Single-stranded RNA and free probe were digested by ribonuclease A and T1. Subsequently, protected RNA was analyzed on a 5% denaturing polyacrylamide gel. VEGF and cytokine transcripts were analyzed either by means of a PhosphorImager and ImageQuant software (Molecular Dynamics, Sunnyvale, CA) or by means of autoradiography for 4 hours to 5 days and subsequent quantification by densitometry (Alpha Innotech). For quantification, the VEGF signals for each sample of the blot were normalized by expressing the density of its signal to the sum of the corresponding signals of the housekeeping genes GAPDH and L32.
Transfection and luciferase assay
Single-donor HUVECs (Clonetics) were seeded at 2.5 × 105 cells per well in 3.0 mL EBM basal medium (Clonetics) containing 0.5% FBS, but no other supplements, in 6-well tissue culture plates for 24 hours. Transfection was then performed by lipofection by means of the GenePORTER Transfection Reagent (Gene Therapy Systems, San Diego, CA) according to the manufacturer's instructions. Briefly, 1 μg DNA and 3 μL GenePORTER were separately diluted in 500 μL EBM each and then rapidly mixed and incubated for 30 to 45 minutes at room temperature. Culture medium was aspirated, and 1 mL DNA-GenePORTER mix was carefully added to each well and incubated at 37°C. Following 3 to 4 hours, 1 mL EBM containing 1% FBS was added to each well, and the incubation was continued for 6 to 24 hours in the absence or presence of soluble CD40L (sCD40L) at 37°C in 5% CO2, with the use of duplicate wells for each condition. Subsequently, cells were lysed in buffer, and protein concentration was determined by Bradford assay.31Using equal amounts of total cellular protein, we analyzed luciferase activity at room temperature by means of a MicroLumat LB96P luminometer (EG & G Berthold, Bad Wildbad, Germany) for 10 seconds according to the manufacturer's instructions (Promega).
In vivo models of VEGF expression and angiogenesis
We evaluated the effect of human sCD40L in vivo in human skin that was transplanted onto CB.17 severe combined immune deficient (SCID) mice (obtained from Massachusetts General Hospital, Boston, MA) as described.2 32 Briefly, the mice were anesthetized by means of ketamine and xylazine, according to the guidelines of the Animal Care and Use Committee, Children's Hospital, Boston, MA, and received approximately 1 cm2 full-thickness human neonatal foreskin grafts. After 4 to 6 weeks, when the human skin had engrafted, a total volume of 60 μL, containing 40 μL Matrigel (Becton Dickinson, Bedford, MA), together with 5 μg human sCD40L (Ancell) was injected into the human skin. After 7 days, the skin was harvested, snap-frozen in isopentane/liquid nitrogen, and analyzed by immunohistochemistry for the expression of VEGF. An adjacent portion of the human skin was fixed in formalin, embedded in paraffin, and processed for von Willebrand factor (vWF) staining.
We also used a well-established in vivo model of angiogenesis in athymic NCr nude mice as described33 34 to determine the effect of CD40L-CD40 interaction on angiogenesis. Briefly, mice received subcutaneous injection of 35 μL Matrigel together with 35 μL murine sCD40L in one flank, and 35 μL Matrigel together with 35 μL heat-inactivated murine sCD40L in the other flank as control. The site of each injection was marked with indelible ink. After 7 days, the undersurface of the skin was exposed and photographed for evaluation of angiogenesis. In addition, all skin samples were formalin-fixed, paraffin-embedded, and processed for routine histology.
Immunohistochemistry
Immunohistochemistry was performed on 4-μm cryostat sections or on 10-μm–thick paraffin-embedded sections as described.2,32 35 Briefly, specimens were fixed in acetone (frozen) or were deparaffined, and primary antibody diluted in phosphate-buffered saline (PBS) was applied to the specimens for 1 hour at room temperature or overnight at 4°C in a humidified container. Optimal concentration of the antibody was determined by simultaneous staining of positive control tissues (human tonsil or inflamed skin). Subsequently, specimens were incubated with a species-specific peroxidase-conjugated secondary antibody (Jackson Immunoresearch, Westgrove, PA) and were developed with 0.25 mg/mL 3-amino-ethylcarbazole in 2% N, N-dimethylformamide and 0.1 mol/L sodium acetate buffer (pH 5.2) with 0.03% hydrogen peroxide to produce a rose-colored reaction product. Negative controls were skin sections incubated with an isotype-matched primary antibody or in PBS alone. Finally, specimens were counterstained in hematoxylin and were mounted in glycerol gelatin.
Results
Effects of inflammatory cytokines on VEGF expression
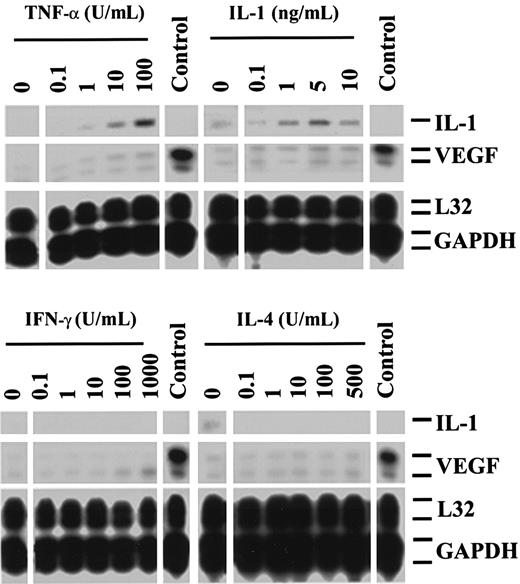
To investigate mechanisms by which CD4+ T cells may stimulate VEGF-induced angiogenesis in immune reactions, we initially assessed the expression of VEGF mRNA in resting HUVECs, or HUVECs cultured with optimal concentrations of the cytokines IL-1α (0.1 to 10 ng/mL), TNF-α (0.1 to 100 U/mL), IL-4 (0.1 to 500 U/mL), or IFN-γ (0.1 to 1000 U/mL). By reverse transcription PCR (RT-PCR) and RPA, we found that resting HUVECs express low and variable levels of VEGF mRNA and that activation of HUVECs with cytokines induced, at best, a weak enhancement in VEGF expression, even in concentrations that markedly induced the expression of E-selectin, intracellular adhesion molecule 1, and vascular cell adhesion molecule 1 in control cultures (Figure 1).
Expression of VEGF by human endothelial cells stimulated with cytokines.
RPAs were performed on RNA samples harvested from resting HUVECs or HUVECs stimulated for 5 hours with increasing concentrations of TNF-α, IL-1α, IFN-γ, or IL-4. Illustrated is the variable low-level expression of VEGF in resting HUVECs with minimal change in expression following cytokine-dependent activation. The expression of VEGF in a renal carcinoma cell line is illustrated as a positive control (Control), and the expression of L32 and GAPDH serves as internal housekeeping gene controls. The expression of IL-1 is also illustrated. All cytokines were assessed for bioactivity in separate studies (not shown). The autoradiographs are representative of 3 experiments with similar results.
Expression of VEGF by human endothelial cells stimulated with cytokines.
RPAs were performed on RNA samples harvested from resting HUVECs or HUVECs stimulated for 5 hours with increasing concentrations of TNF-α, IL-1α, IFN-γ, or IL-4. Illustrated is the variable low-level expression of VEGF in resting HUVECs with minimal change in expression following cytokine-dependent activation. The expression of VEGF in a renal carcinoma cell line is illustrated as a positive control (Control), and the expression of L32 and GAPDH serves as internal housekeeping gene controls. The expression of IL-1 is also illustrated. All cytokines were assessed for bioactivity in separate studies (not shown). The autoradiographs are representative of 3 experiments with similar results.
Ligation of CD40 induces VEGF expression in endothelial cells and monocytes
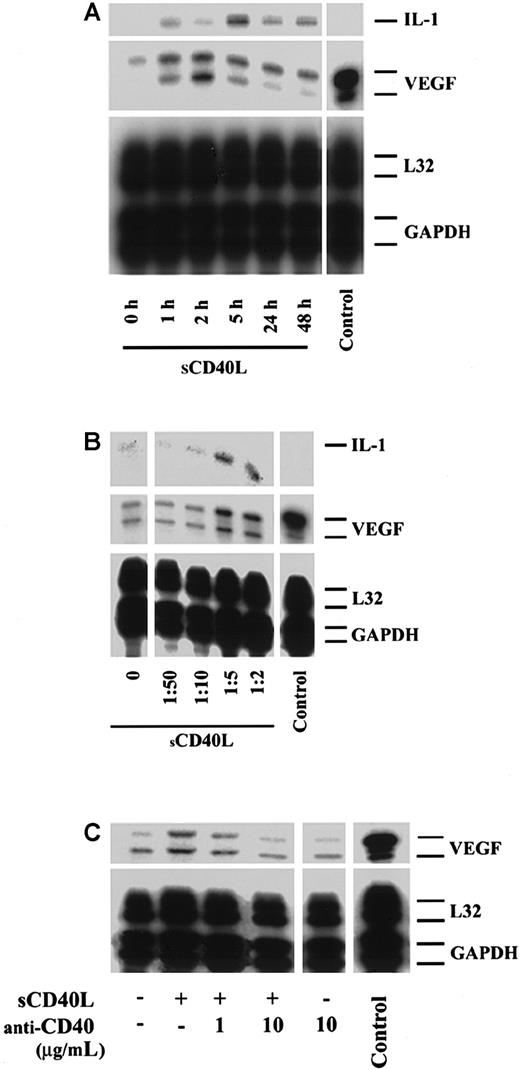
We next wished to define whether a cell-surface molecule on activated CD4+ T cells mediates VEGF expression. In initial studies, using semiquantitative RT-PCR, we found that treatment of HUVECs with cell membranes derived from 6-hour PHA-activated CD4+ T cells resulted in a 2- to 2.5-fold induction in VEGF mRNA expression (not shown). We next assessed the temporal expression of VEGF following stimulation of HUVECs with sCD40L. Our HUVECs,35 as well as others,36 37 express CD40 constitutively as well as following stimulation in a homogenous distribution. HUVECs were treated with increasing concentrations of sCD40L, and VEGF mRNA was examined by RPA. As illustrated in Figure2A, we found a marked increase in VEGF expression as early as 1 hour following activation with sCD40L, with peak expression occurring between 2 and 5 hours and expression remaining elevated for 48 hours. Furthermore, CD40-dependent VEGF expression was dose-dependent (Figure 2B) and was inhibited by coculture in the presence of neutralizing monoclonal antibodies to CD40 (Figure 2C) or CD40L (not shown). Parallel experiments, using purified recombinant human sCD40L (Ancell), instead of culture supernatant (Bristol Myers Squibb), yielded equivalent results.
Effect of sCD40L on the expression of VEGF by human endothelial cells.
HUVECs were cultured until confluence and were treated with sCD40L (Bristol Myers Squibb) as detailed below. RNA was harvested from HUVECs, and the expression of VEGF was analyzed by RPA. Unless indicated, sCD40L was used as a culture supernatant in all assays at a concentration found to have optimal bioactivity (equal to a dilution of 1:5) in positive controls. (A) Temporal expression of VEGF in resting HUVECs or HUVECs stimulated with sCD40L. The expression of VEGF is markedly induced 1 hour following treatment with sCD40L and peaks in expression between 2 and 5 hours following activation. (B) Effect of increasing concentrations of sCD40L (represented by dilutions of culture supernatant) on VEGF expression after 5 hours. (C) HUVECs cultured in the absence or presence of sCD40L and a blocking anti-CD40 antibody to determine the specificity of sCD40L for CD40-dependent VEGF expression. In these studies, identical effects of sCD40L on the induction of VEGF were obtained when purified recombinant sCD40L (Ancell) was used at a concentration of 1 to 10 μg/mL. The expression of VEGF in a renal carcinoma cell line served as a positive control (Control), and the expression of L32 and GAPDH served as internal housekeeping gene controls. For panels A and B, the expression of IL-1 is also illustrated. All autoradiographs are representative of 3 experiments with similar results.
Effect of sCD40L on the expression of VEGF by human endothelial cells.
HUVECs were cultured until confluence and were treated with sCD40L (Bristol Myers Squibb) as detailed below. RNA was harvested from HUVECs, and the expression of VEGF was analyzed by RPA. Unless indicated, sCD40L was used as a culture supernatant in all assays at a concentration found to have optimal bioactivity (equal to a dilution of 1:5) in positive controls. (A) Temporal expression of VEGF in resting HUVECs or HUVECs stimulated with sCD40L. The expression of VEGF is markedly induced 1 hour following treatment with sCD40L and peaks in expression between 2 and 5 hours following activation. (B) Effect of increasing concentrations of sCD40L (represented by dilutions of culture supernatant) on VEGF expression after 5 hours. (C) HUVECs cultured in the absence or presence of sCD40L and a blocking anti-CD40 antibody to determine the specificity of sCD40L for CD40-dependent VEGF expression. In these studies, identical effects of sCD40L on the induction of VEGF were obtained when purified recombinant sCD40L (Ancell) was used at a concentration of 1 to 10 μg/mL. The expression of VEGF in a renal carcinoma cell line served as a positive control (Control), and the expression of L32 and GAPDH served as internal housekeeping gene controls. For panels A and B, the expression of IL-1 is also illustrated. All autoradiographs are representative of 3 experiments with similar results.
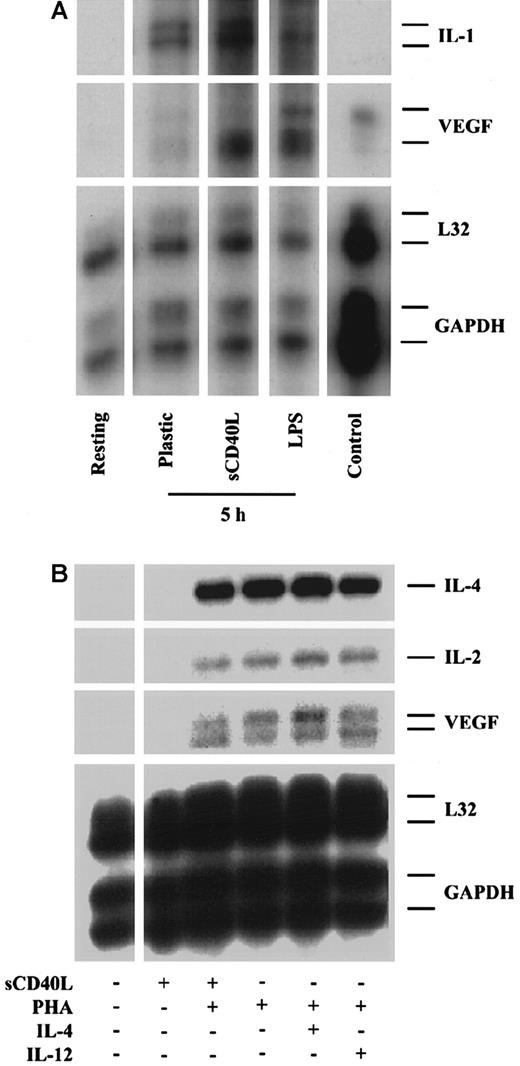
Since leukocyte subsets, especially monocytes and CD4+ T cells, are known to be a potent source of VEGF in vitro and in vivo,11,38,39 we also wished to assess the effect of CD40 ligation on VEGF expression by these cells. After purification, monocytes were cultured in the absence or presence of sCD40L, and mRNA expression was again assessed by RPA. Our findings were that resting monocytes (immediate harvest) express low but variable levels of VEGF that was induced moderately by plastic adherence and in a more pronounced way by treatment with LPS (1 μg/mL) (Figure3A). Treatment with sCD40L resulted in a marked induction of VEGF mRNA expression as described above for endothelial cells. In contrast, while activation of CD4+ T cells with PHA induced VEGF mRNA expression, treatment with sCD40L alone or in combination with PHA had no effect on CD4+T-cell VEGF expression (Figure 3B). This is consistent with the low level of expression of CD40 on CD4+ T cells and the lack of dependence of CD4+ T cells on CD40 signaling for activation response.21
Expression of VEGF by human monocytes and CD4+ T cells.
CD14+ monocytes and CD4+ T cells were purified from human PBMCs. (A) VEGF expression in resting monocytes and in monocytes following activation by plastic adherence or treatment with sCD40L (Ancell, 10 μg/mL) or LPS (1 μg/mL) for 5 hours illustrate a marked induction in VEGF expression following stimulation with sCD40L. (B) VEGF expression in resting CD4+ T cells, and CD4+ T cells stimulated for 5 hours with sCD40L (Bristol Myers Squibb), PHA (1 μg/mL), or PHA in combination with either sCD40L, IL-4 (500 U/mL), or IL-12 (10 ng/mL). As illustrated, there is a low-level expression of VEGF in resting CD4+ T cells, and expression is markedly induced in PHA-activated CD4+ T cells. Treatment with sCD40L, IL-4, or IL-12 does not further enhance this effect. Treatment with IL-4 or IL-12 alone failed to induce any VEGF expression above baseline (not shown). The expression of VEGF in a renal carcinoma cell line is illustrated as a positive control (Control), and the expression of L32 and GAPDH serves as internal housekeeping gene controls. The expression of IL-2 and IL-4 is also illustrated. For both monocytes and CD4+ T cells, similar results were obtained with both sources of sCD40L. Representative autoradiographs of 3 experiments.
Expression of VEGF by human monocytes and CD4+ T cells.
CD14+ monocytes and CD4+ T cells were purified from human PBMCs. (A) VEGF expression in resting monocytes and in monocytes following activation by plastic adherence or treatment with sCD40L (Ancell, 10 μg/mL) or LPS (1 μg/mL) for 5 hours illustrate a marked induction in VEGF expression following stimulation with sCD40L. (B) VEGF expression in resting CD4+ T cells, and CD4+ T cells stimulated for 5 hours with sCD40L (Bristol Myers Squibb), PHA (1 μg/mL), or PHA in combination with either sCD40L, IL-4 (500 U/mL), or IL-12 (10 ng/mL). As illustrated, there is a low-level expression of VEGF in resting CD4+ T cells, and expression is markedly induced in PHA-activated CD4+ T cells. Treatment with sCD40L, IL-4, or IL-12 does not further enhance this effect. Treatment with IL-4 or IL-12 alone failed to induce any VEGF expression above baseline (not shown). The expression of VEGF in a renal carcinoma cell line is illustrated as a positive control (Control), and the expression of L32 and GAPDH serves as internal housekeeping gene controls. The expression of IL-2 and IL-4 is also illustrated. For both monocytes and CD4+ T cells, similar results were obtained with both sources of sCD40L. Representative autoradiographs of 3 experiments.
CD40-signals induce VEGF promoter activity
Having established that ligation of CD40 induces VEGF mRNA expression in endothelial cells and monocytes, we next wished to assess whether CD40 signals regulate transcriptional activity of VEGF. For these experiments, HUVECs were transiently transfected with a full-length (2.6 kb) luciferase reporter construct under the control of the human VEGF promoter by means of a standard lipofection procedure as described.40 41 Luciferase activity was assessed in transfected cell lysates following a 6- to 24-hour incubation in the presence or absence of human sCD40L. Consistently, we found enhanced luciferase activity following treatment with sCD40L (Figure4A). Furthermore, the relative fold induction in luciferase activity was similar to the relative fold induction in mRNA expression (Figure 4B), suggesting a predominant transcriptional control mechanism for CD40-dependent regulation of VEGF.
Ligation of CD40 results in enhanced VEGF promoter activity.
(A) HUVECs were first incubated for 24 hours in 0.5% FBS containing EBS. Subsequently, a 2.6-kb full-length VEGF promoter-luciferase construct (1 μg DNA per milliliter) was introduced into HUVECs by lipofection as detailed in “Materials and methods.” HUVECs were then cultured for 24 hours in the absence or presence of sCD40L (Bristol Myers Squibb) in duplicate wells for each condition. Luciferase activity was analyzed with the use of equal amounts of total cellular protein for each sample. Illustrated is the luciferase activity in lysates in transfected but unstimulated HUVECs and in HUVECs following stimulation with sCD40L. Mock-transfected HUVECs served as negative control. Luciferase activity was consistently induced (approximately 4-fold) in sCD40L-treated cells compared with untreated cells. Representative of 3 independent experiments performed in duplicate cultures. (B) The expression of VEGF mRNA in the same HUVECs used in panel A, either untreated or following treatment with sCD40L for 5 and 24 hours. The induced expression of VEGF mRNA was similar (approximately 4-fold) to the induced luciferase activity illustrated in panel A.
Ligation of CD40 results in enhanced VEGF promoter activity.
(A) HUVECs were first incubated for 24 hours in 0.5% FBS containing EBS. Subsequently, a 2.6-kb full-length VEGF promoter-luciferase construct (1 μg DNA per milliliter) was introduced into HUVECs by lipofection as detailed in “Materials and methods.” HUVECs were then cultured for 24 hours in the absence or presence of sCD40L (Bristol Myers Squibb) in duplicate wells for each condition. Luciferase activity was analyzed with the use of equal amounts of total cellular protein for each sample. Illustrated is the luciferase activity in lysates in transfected but unstimulated HUVECs and in HUVECs following stimulation with sCD40L. Mock-transfected HUVECs served as negative control. Luciferase activity was consistently induced (approximately 4-fold) in sCD40L-treated cells compared with untreated cells. Representative of 3 independent experiments performed in duplicate cultures. (B) The expression of VEGF mRNA in the same HUVECs used in panel A, either untreated or following treatment with sCD40L for 5 and 24 hours. The induced expression of VEGF mRNA was similar (approximately 4-fold) to the induced luciferase activity illustrated in panel A.
Functional effect of CD40 ligation for VEGF-induced angiogenesis
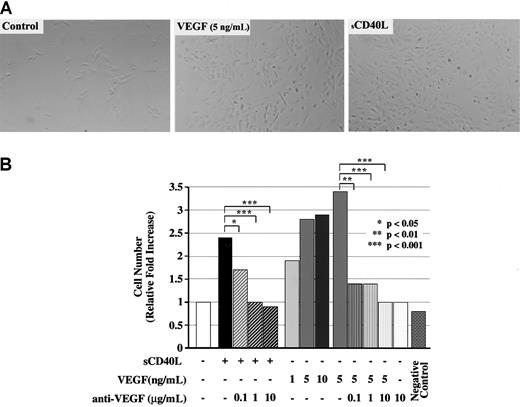
To assess the functionality of CD40-dependent VEGF expression in vitro, we developed a modified endothelial cell growth assay. HUVECs were seeded in gelatin-coated tissue culture plates in medium containing 5% FBS but no endothelial cell growth supplement overnight. Subsequently, HUVECs were cultured with sCD40L or recombinant human VEGF (1 to 10 ng/mL) in the absence or presence of a neutralizing anti–human VEGF antibody (0.1 to 10 μg/mL). As illustrated in Figure5, we found marked growth of HUVECs and increased HUVEC density following treatment with sCD40L for 48 to 72 hours. Furthermore, we found that neutralizing anti-VEGF antibody completely inhibited the effect of sCD40L on HUVEC growth. Positive control cultures treated with VEGF resulted in a similar increase in cell density, and the effect of optimal concentrations of sCD40L was similar to that found for VEGF at a concentration of 5 ng/mL (Figure5A-B). Negative control cultures treated with heat-inactivated sCD40L or murine sCD40L (generated identically as human sCD40L) were similar to untreated controls. Thus, CD40-dependent VEGF expression is of functional consequence for HUVEC proliferation in vitro.
Function of CD40 ligation for VEGF-induced angiogenesis.
HUVECs were cultured in 24-well tissue culture plates in 5% FBS for 48 hours in the absence or presence of sCD40L or VEGF. HUVECs were seeded at 5 × 104 cells per well in 0.75 mL medium, and cell numbers were counted daily by means of a calibrated grid (100 × magnification). Treatment of HUVECs with VEGF resulted in enhanced survival of HUVECs and promoted growth (as described13) and served as positive control. Treatment of HUVECs with heat-inactivated sCD40L supernatant served as negative control. (A) Representative photomicrographs of HUVEC cultures after 48 hours in medium alone, VEGF (5 ng/mL), and sCD40L (Bristol Myers Squibb). (B) Relative increase in number of HUVECs compared with control (medium alone) after 48 hours of culture in the presence of sCD40L or VEGF, in the absence or presence of a neutralizing anti-VEGF antibody. The median values of 4 independent experiments performed in duplicate cultures are illustrated. There is enhanced growth of HUVECs following treatment with sCD40L, and this effect is abrogated by addition of a neutralizing anti-VEGF antibody into cell cultures. Controls for anti-VEGF were cultures of VEGF in the absence or presence of antibody. Additional negative controls (not shown) were cultures in the presence of murine sCD40L (Bristol Myers Squibb) generated in the same manner as the human sCD40L used in these experiments.
Function of CD40 ligation for VEGF-induced angiogenesis.
HUVECs were cultured in 24-well tissue culture plates in 5% FBS for 48 hours in the absence or presence of sCD40L or VEGF. HUVECs were seeded at 5 × 104 cells per well in 0.75 mL medium, and cell numbers were counted daily by means of a calibrated grid (100 × magnification). Treatment of HUVECs with VEGF resulted in enhanced survival of HUVECs and promoted growth (as described13) and served as positive control. Treatment of HUVECs with heat-inactivated sCD40L supernatant served as negative control. (A) Representative photomicrographs of HUVEC cultures after 48 hours in medium alone, VEGF (5 ng/mL), and sCD40L (Bristol Myers Squibb). (B) Relative increase in number of HUVECs compared with control (medium alone) after 48 hours of culture in the presence of sCD40L or VEGF, in the absence or presence of a neutralizing anti-VEGF antibody. The median values of 4 independent experiments performed in duplicate cultures are illustrated. There is enhanced growth of HUVECs following treatment with sCD40L, and this effect is abrogated by addition of a neutralizing anti-VEGF antibody into cell cultures. Controls for anti-VEGF were cultures of VEGF in the absence or presence of antibody. Additional negative controls (not shown) were cultures in the presence of murine sCD40L (Bristol Myers Squibb) generated in the same manner as the human sCD40L used in these experiments.
In vivo induction of VEGF expression and angiogenesis by sCD40L
We next wished to assess the effect of CD40L-CD40 interactions on VEGF expression and angiogenesis in vivo. First, we evaluated the effect of human sCD40L (used in the in vitro studies described above) for VEGF expression in vivo. For these studies, we used a model in SCID mice bearing human skin transplants as described.2 32 We injected human sCD40L into the human skin grafts (n = 4) and evaluated the expression of VEGF after 7 days. As illustrated in Figure6, we found a marked induction in VEGF expression in all sCD40L-treated skins compared with controls (nontransplanted skin, and transplanted but untreated skin) (n = 5). In addition, the number of vWF-expressing vessels appeared to be more numerous (2- to 3-fold by semiquantitative grid counting) in the sCD40L-treated skin specimens compared with untreated controls (not shown).
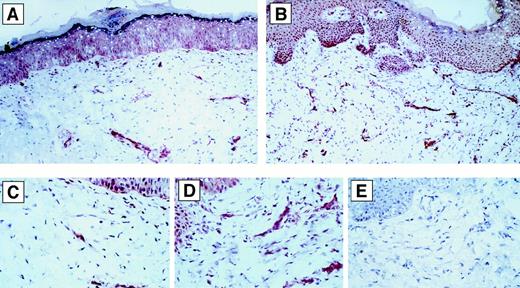
Effect of human sCD40L on VEGF-expression in vivo.
Immunohistochemical staining of VEGF in vivo in representative human skin transplants on SCID mice following injection of Matrigel without (panels A and C) or with (panels B and D) human sCD40L. Representative areas of panels A and B (200 × magnification) are viewed at high-power magnification (800 ×) in panels C and D, respectively. Consistently, there was enhanced expression of VEGF in sCD40L-treated human skins in multiple cells. There was no staining of the specimens with medium alone or isotype control antibody. Panel E represents the negative control staining of the same sample as in Panel D (800 ×).
Effect of human sCD40L on VEGF-expression in vivo.
Immunohistochemical staining of VEGF in vivo in representative human skin transplants on SCID mice following injection of Matrigel without (panels A and C) or with (panels B and D) human sCD40L. Representative areas of panels A and B (200 × magnification) are viewed at high-power magnification (800 ×) in panels C and D, respectively. Consistently, there was enhanced expression of VEGF in sCD40L-treated human skins in multiple cells. There was no staining of the specimens with medium alone or isotype control antibody. Panel E represents the negative control staining of the same sample as in Panel D (800 ×).
We next used an established model in the athymic nude mouse33 34 to determine the effect of sCD40L on angiogenesis. Mice (n = 7) received subcutaneous injections of Matrigel containing murine sCD40L on one flank and Matrigel containing heat-unactivated murine sCD40L on the contralateral flank. Additional control animals received Matrigel implants with saline only instead of sCD40L. On day 7, animals were anesthetized, and the undersurface of the injection site was exposed (Figure7). Consistently, there was more bleeding around the incision site of the sCD40L-treated skin (Figure 7B) compared with control skin (Figure 7A). As illustrated, in the sCD40L-treated skins, there was an increased number of grossly visible small tortuous blood vessels with multiple bifurcations and trifurcations as compared with negative control implants with heat-inactivated murine sCD40L or saline (not shown). Findings were highly consistent within each of the groups. These observations confirm that ligation of CD40 in vivo results in VEGF expression and angiogenesis.
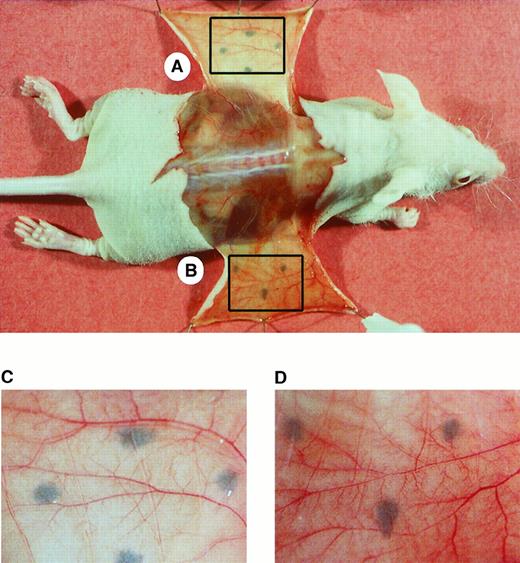
Effect of sCD40L in a murine model of angiogenesis.
Murine sCD40L in Matrigel was injected subcutaneously into athymic nude mice. The site of injection was marked with indelible ink. Illustrated is a representative photograph of the exposed undersurface of the injection site after 7 days. (A) Site injected with heat-inactivated sCD40L. (B) Site injected with active sCD40L. The lower panels A and B represent the boxed areas of the skin viewed at higher magnification. Following injection of sCD40L, multiple tortuous vessels were found with bifurcations and trifurcations that are typical findings of active angiogenesis. Representative of 1 of 7 animals with similar results.
Effect of sCD40L in a murine model of angiogenesis.
Murine sCD40L in Matrigel was injected subcutaneously into athymic nude mice. The site of injection was marked with indelible ink. Illustrated is a representative photograph of the exposed undersurface of the injection site after 7 days. (A) Site injected with heat-inactivated sCD40L. (B) Site injected with active sCD40L. The lower panels A and B represent the boxed areas of the skin viewed at higher magnification. Following injection of sCD40L, multiple tortuous vessels were found with bifurcations and trifurcations that are typical findings of active angiogenesis. Representative of 1 of 7 animals with similar results.
Discussion
In this study, we define the ability of CD40L-CD40 interactions for the expression of VEGF and angiogenesis in vitro and in vivo. Our findings establish a mechanistic association among the inflammatory response and VEGF-induced angiogenesis and are consistent with the ability of early activated T cells and monocytes to initiate angiogenesis.2,5,8,11,12,38,39 42
There are several pathophysiologic implications of our findings for immune inflammation, including allograft rejection. One possibility is that CD40L expressed by early activated T cells (and perhaps by activated platelets) stimulates local endothelial cell and tissue monocyte/macrophage production of VEGF in the course of recruitment into local sites of inflammation. This possibility is consistent with our recent observations that angiogenesis occurs very early in the course of inflammation and is temporally and spatially associated with T-cell infiltrates.2 Furthermore, our data are consistent with the original studies by Auerbach,3-5 in which it was concluded that leukocyte-induced angiogenesis is mediated by CD4+ T cells, or products of CD4+ T cells that have biologic effects on local cells in the course of acute inflammation. The expression of CD40 and CD40L has been previously reported to be prominent in pathologic processes known to be associated with high levels of VEGF, such as tumors, at sites of chronic inflammation and in allografts undergoing rejection.10,12,20,21,35,38,43 Indeed, the prominent expression of CD40 at sites of inflammation is consistent with a potent function in the effector angiogenesis response,35 44 and we have also found a spatial correlation between the expression of CD40 and the expression of VEGF in skin allografts undergoing rejection (M.M. and D.M.B., unpublished observations, December 1999 to March 2000).
Activated leukocytes that are recruited into inflammatory sites may already produce VEGF as well as other angiogenesis factors as a function of their state of activation.6,11,38 It is well established that monocytes known to be critical mediators of leukocyte-induced angiogenesis do so via the induced expression of many angiogenesis factors, and monocyte activation may occur in the absence of CD40 ligation.1,8,39 Thus, the effect of CD40 signals on angiogenesis in vivo observed in this study may be redundant. However, a large body of literature has concluded that CD40 signals are most potent for monocyte activation responses and that CD40 signals may promote the expression of additional angiogenesis factors.21,45 Furthermore, blockade of CD40L-CD40 interactions has been found to inhibit acute inflammation, chronic inflammation, and chronic diseases such as atherosclerosis and rheumatoid arthritis known to be dependent on monocyte activation responses and characterized by profound angiogenesis.21 46 Thus, it is likely that the biological effects of CD40L-CD40 interactions for the angiogenesis response in chronic inflammation is of great pathologic significance.
It is possible that the effects of sCD40L for angiogenesis in vivo observed in our studies may be in part a result of non-VEGF mechanisms and positive-feedback loops. Ligation of CD40 results in the expression of multiple cytokines, chemokines, and adhesion molecules that themselves may have effects on VEGF expression and the angiogenesis reaction.21,45 For instance, the production of TNF-α and transforming growth factor–β has been reported to augment VEGF expression in monocytes,38 and TNF-α has been reported to up-regulate VEGF receptor expression by other cells, including endothelial cells.47 Thus, while CD40 signals may directly activate VEGF expression, additional CD40-regulated genes may also promote angiogenesis.
In our studies, the overall increase in VEGF mRNA expression (approximately 4-fold induction) was similar to that previously reported for hypoxic-induced transcriptional regulation of VEGF.19 In addition, the relative fold induction of VEGF mRNA expression in HUVECs and in monocytes was similar to that found following CD40-dependent activation of our VEGF promoter construct, suggesting a predominant transcriptional control mechanism. To date, hypoxia is the best-characterized stimulus for VEGF expression and for the VEGF-induced angiogenesis response in vivo.17-19 Hypoxia stimulates VEGF expression by both transcriptional and post-transcriptional mechanisms.19 Transcriptional regulation is mediated by well-described hypoxia-inducible factor-1 (HIF-1) and Sp-1 binding elements. In addition, several transactivating factor intermediaries including mutant src and ras oncogenes have been described to regulate VEGF expression.17,48 Ligation of CD40 is known to regulate multiple genes via transcriptional mechanisms.21,49 CD40 signals result in the activation of the TNF-receptor–associated factor (TRAF) family molecules, the nuclear factor (NF)-κB and c-Jun amino-terminal kinase/activator protein (AP)–1 transactivating factor(s) and resultant signaling. But the VEGF promoter does not contain putative NF-κB binding sites. Thus, it is likely that CD40-dependent regulation of VEGF transcription involves additional elements, such as an AP-1–dependent mechanism.19 Alternatively, it is possible that NF-κB may regulate or associate with another transcriptional regulatory element that is functional for VEGF expression.
In summary, these studies define that CD40L-CD40 interactions promote VEGF expression and angiogenesis in vitro and in vivo. Our findings have important implications for the understanding of acute and chronic inflammation, including atherosclerosis and allograft rejection, in which CD40L-CD40 interactions are thought to play a pathophysiologic role.
Acknowledgments
Dr Harald F. Dvorak, Dr Donald R. Senger, and Dr Karen Moulton for helpful discussions. M.M. received the TEAM Management Young Investigator Award of the American Society of Transplantation at the 18th Annual Scientific Meeting on May 16, 1999, for this work.
Supported by National Institute of Health grants DK53606 and AI46756 and the National Kidney Foundation (Massachusetts affiliate).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
David M. Briscoe, Division of Nephrology, Children's Hospital, 300 Longwood Ave, Boston, MA 02115; e-mail:briscoe@a1.tch.harvard.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal